Abstract
In May of 2013, porcine epidemic diarrhea virus (PEDV) was detected in swine for the first time in North America. It spread rapidly, in part due to contaminated livestock trailers. The objective of this study was to test the efficacy of an accelerated hydrogen peroxide disinfectant for inactivating PEDV in the presence of feces on metal surfaces, such as those found in livestock trailers. Three-week-old barrows were inoculated intragastrically with 5 mL of PEDV-negative feces for the negative control, 5 mL of untreated PEDV-positive feces for the positive control, and 5 mL or 10 mL of PEDV-positive feces that was subjected to treatment with a 1:16 or 1:32 concentrations of accelerated hydrogen peroxide disinfectant for a contact time of 30 min at 20°C. These pigs served as a bioassay to determine the infectivity of virus following treatment. Rectal swabs collected from the inoculated pigs on days 3 and 7 post-inoculation were tested by using PEDV-specific real-time reverse transcription polymerase chain reaction and the proportion of pigs in each group that became infected with PEDV was assessed. None of the pigs used for the bioassay in the 4 treatment groups and the negative control group became infected with PEDV, which was significantly different from the positive control group (P < 0.05) in which all pigs were infected. The results suggest that the application of the accelerated hydrogen peroxide under these conditions was sufficient to inactivate the virus in feces found on metal surfaces.
Résumé
En mai 2013, pour la première fois en Amérique du Nord, le virus de la diarrhée épidémique porcine (VDEP) fut détecté chez le porc. Il se répandit rapidement, en partie à cause des remorques pour animaux contaminées. L’objectif de la présente étude était de tester l’efficacité d’un désinfectant à base de peroxyde d’hydrogène accéléré pour inactiver le VDEP en présence de fèces sur des surfaces métalliques, telles que celles retrouvées dans les remorques pour animaux. Des mâles castrés âgés de 3 semaines ont été inoculés par voie intra-gastrique avec 5 mL de fèces VDEP-négatives pour les témoins négatifs, 5 mL de fèces VDEP-positives non traitées pour les témoins positifs, et 5 mL ou 10 mL de fèces VDEP-positives soumises à un traitement au désinfectant à base de peroxyde d’hydrogène accéléré à une concentration de 1:16 ou 1:32 avec un temps de contact de 30 min à 20 °C. Les porcs ont servi de bioessai afin de déterminer l’infectivité du virus suite au traitement. Des écouvillons rectaux prélevés des porcs inoculés aux jours 3 et 7 post-inoculation ont été testés par réaction d’amplification en chaine en temps réel utilisant la transcriptase réverse et spécifique au VDEP, et la proportion de porcs devenus infectés par le VDEP dans chaque groupe fut déterminée. Aucun des porcs utilisés pour le bioessai dans les quatre groupes de traitement ainsi que dans le groupe témoin négatif ne devint infecté par le VDEP ce qui était significativement différent des animaux du groupe témoin positif (P < 0,05) qui devinrent tous positifs. Ces résultats suggèrent que l’application du peroxyde d’hydrogène accéléré dans les conditions testées était suffisante pour inactiver le virus présent dans les fèces sur les surfaces métalliques.
(Traduit par Docteur Serge Messier)
Introduction
Porcine epidemic diarrhea (PED) was first described in England in 1971 in growing pigs (1), and the causative agent, porcine epidemic diarrhea virus (PEDV), was identified in 1978 (2,3). In May of 2013, PEDV was identified in swine for the first time in the United States and North America. The virus caused severe diarrhea in sows and piglets, with high mortality in neonatal piglets across a wide geographical area of the United States (4). Although the original route of entry remains unknown, contaminated livestock trailers, especially those that haul pigs to harvest facilities, represent a significant risk for movement of the virus among herds (5).
Historically, this risk has been mitigated with sanitation and decontamination procedures that include trailer washing, disinfection, and natural drying or thermo-assisted drying and decontamination (TADD) systems. Disinfection, natural drying, or TADD systems are more effective when applied to a clean trailer with little or no remaining organic matter. Much of the research on procedures to sanitize livestock trailers has been done for porcine reproductive and respiratory syndrome virus (PRRSV), which is a single-strand enveloped RNA virus like PEDV. All of the studies evaluating sanitation practices to reduce the risk of contaminated livestock trailers for PRRSV have included washing as the first step. However, none of the studies demonstrated that washing alone was sufficient (6–10). Overnight drying and several disinfectants have been shown to effectively inactivate PRRSV after washing. Disinfectants reported to reduce the risk of PRRSV transmission associated with contaminated trailers at temperatures above 0°C include a phenolic compound (Tek-Trol; Biotek Industries, Atlanta, Georgia, USA) in combination with drying (6), 2 quaternary ammonium and glutaraldehyde combinations [Synergize; Preserve International, Atlanta, Georgia, USA (7,10) and Aseptol 2000; SEC Repro, Quebec (9)], and an accelerated hydrogen peroxide (AHP) disinfectant (Accel; Virox Technologies, Oakville, Ontario) (10). Consequently, the industry standard trailer sanitation and decontamination today consists of washing, disinfection, and drying either naturally or with a TADD system.
As the industry standard, a complete wash, disinfect, and dry is always the preferred option. The industry standard, however, takes time and requires specialized facilities. Unfortunately, there are not enough of these facilities in North America to serve the large volume of livestock trailers transporting pigs across the country every day. As an alternative to doing nothing, sanitation and decontamination procedures that involve scraping to remove as much organic material as possible followed by disinfection, heating, drying, or some combination of these without a thorough washing, if demonstrated to effectively inactivate PEDV, could be used when a complete wash, dry, and disinfection is not possible. In a previous study using PEDV-positive feces contaminated aluminum coupons as a model of full-sized livestock trailers, heating to 71°C for 10 min or allowing them to sit for 7 d at 20°C was sufficient to prevent transmission of PEDV present in feces as determined by a bioassay (11).
Accel is an AHP disinfectant registered as a disinfectant cleaner that is virucidal at dilution rates of 1:16 to 1:64, in the presence of 200 ppm hard water, 5% serum load, and a 5 min contact time. The active ingredient is hydrogen peroxide, which is an oxidizing agent. It produces a long-lasting foam when delivered through a foaming tip and contains food-grade anionic and non-ionic surfactants, which act with hydrogen peroxide to increase microbiocidal activity and may make it a candidate to work in the presence of some organic matter. The aim of this study was to test the efficacy of an AHP disinfectant for inactivating PEDV in the presence of feces on metal surfaces such as those found in livestock trailers.
Materials and methods
For this study, 15.24 cm × 15.24 cm aluminum trays with 2.54 cm high sides and a material thickness of 0.32 cm were used as “coupons” to represent the surface of livestock trailers used to haul pigs. The coupons were used in a previous study to evaluate the efficacy of different combinations of time and temperature to inactivate PEDV (11). To simulate the runoff that occurs in full-sized livestock trailers as the AHP disinfectant is transformed from foam to a liquid, the coupons were modified for this study by drilling 4 holes in each coupon that were 8 mm in diameter at the junction of the bottom of the coupon and 1 side. The experimental unit was a single contaminated coupon that was matched to a single 3-week-old pig intragastrically inoculated with the contents of the coupon, as a bioassay to determine if the treatment applied to the contaminated coupon effectively inactivated PEDV.
The null hypothesis for the study was that there was no difference between the positive control group and the treatment groups in the proportion of pigs infected by the inoculum collected from the coupons as measured by the proportion of pigs that were positive for PEDV. The PEDV status of the pigs was assessed by testing rectal swabs collected at 3 and 7 d post-inoculation for PEDV by a nucleocapsid (N) gene-based quantitative real-time reverse transcription polymerase chain reaction (real-time RT-PCR) at the Iowa State University Veterinary Diagnostic Laboratory (ISU VDL). The primers and probe of the PEDV real-time RT-PCR have been described previously (5,12,13). Each PCR was set up in a 25 μL total reaction using an RT-PCR kit (Path-ID Multiplex One-Step RT-PCR Kit; Thermo Fisher Scientific, Waltham, Massachusetts, USA): 12.5 μL of 2 × multiplex RT-PCR buffer, 2.5 μL of 20 × multiplex enzyme, 0.5 μL of forward primer at 20 μM, 0.5 μL of reverse primer at 20 μM, 0.12 μL of probe at 25 μM, 3.88 μL of nuclease-free water, and 5 μL of nucleic acid extract. Amplification reactions were done using a real-time thermal cycler (ABI 7500 Fast instrument; Thermo Fisher Scientific) with the following conditions: 1 cycle of 48°C for 10 min, 1 cycle of 95°C for 10 min, and 45 cycles of 95°C for 15 s and 60°C for 45 s.
Personnel performing treatments, necropsies, and collecting samples were not blinded to the treatments. Blinding was not possible because a specific order was followed for all procedures starting with the negative control and ending with the positive control to minimize the risk of transmitting virus between treatment groups. Laboratory personnel who performed the PCR testing were blinded to treatment status of the pigs from which the samples were collected.
Study groups
Pigs (n = 28) were divided into 7 groups. Four treatment groups (n = 4, per group) representing combinations of fecal contamination (5 mL or 10 mL) and disinfectant concentration (1:16 or 1:32) were evaluated. In addition, a positive control group (n = 4) and negative control group (n = 4) were included without sham disinfection. The PEDV-negative feces were used to contaminate coupons in the negative control group. The PEDV-positive feces were used to contaminate coupons in the positive control group. The AHP disinfectant was applied as a foam. A good candidate for sham disinfection, to simulate the rinsing and diluting effects, would be a non-disinfecting foam similar to that produced with the AHP disinfectant; however, no such foam could be identified. Because any liquid used for a sham disinfection would have very different rinsing and dilution effects compared to the foam produced by the AHP disinfectant no sham disinfection was done. A transmission control group (n = 4) was included to validate that the animal housing and handling protocols used for the bioassay did not result in transmission of virus from one pig to another within the same treatment group. The study groups are summarized in Table I.
Table I.
Description of study groups
| Treatment group | Description of contamination and treatment |
|---|---|
| Negative control | 5 mL PEDV-negative feces, no treatment |
| Positive control | 5 mL PEDV-positive feces, no treatment |
| 5 mL-1:16 | 5 mL PEDV-positive feces, 1:16 concentration of AHP disinfectanta |
| 10 mL-1:16 | 10 mL PEDV-positive feces, 1:16 concentration of AHP disinfectanta |
| 5 mL-1:32 | 5 mL PEDV-positive feces, 1:32 concentration of AHP disinfectanta |
| 10 mL-1:32 | 10 mL PEDV-positive feces, 1:32 concentration of AHP disinfectanta |
| Transmission control | 1 of 4 pigs in the group was gavaged with 5 mL PEDV-positive feces; 3 of 4 were gavaged with 5 mL PEDV-negative feces, no treatment |
With 30 min of contact time at 20°C (room temperature).
PEDV — porcine epidemic diarrhea virus.
Contamination and disinfection procedures
The PEDV-positive feces were obtained from a separate experiment in which 3-week-old pigs were inoculated with PEDV isolate US/Iowa/18984/2013 (13). The feces were collected from the pigs at 7 d post-inoculation. The feces from individual pigs were placed on ice until they could be frozen at −80°C approximately 1 h later. On the day of the challenge, day 0, these samples were thawed and pooled into a single fecal homogenate to assure that the amount of virus and composition of the feces were uniform for each replicate. Samples from each replicate were tested at the ISU VDL by using real-time polymerase chain reaction (RT-PCR) and were positive for PEDV. The quantitative genomic copies/mL ranged from 109.61 to 1010.17 genomic copies/mL across all replicates. The PEDV-negative feces were collected from the negative control pigs in the same previous study. Negative feces were frozen at −80°C, stored, thawed on day 0 and homogenized into a single pool at that time. These fecal samples were confirmed PEDV-negative by using RT-PCR.
Prior to treatment, 5 mL (positive control, negative control, 5 mL-1:16, 5 mL-1:32, transmission control) or 10 mL (10 mL-1:16, 10 mL-1:32) of feces were applied to the aluminum coupons (Figure 1). The 5 and 10 mL of feces were chosen to represent the amount of organic matter that remains in a livestock trailer after the feces and bedding have been manually removed with a scraper. Feces that were positive for PEDV was applied to the coupons in the following groups: 5 mL-1:16, 10 mL-1:16, 5 mL-1:32, 10 mL-1:32, positive control, and 1 of the 4 pigs in the transmission control group. Feces that were negative for PEDV were applied to the coupons in the negative control study group and 3 of the 4 pigs in the transmission control group. The feces were spread in a thin (≤ 2 mm), even layer using a disposable spreader. The disposable spreader was a hard plastic spreader sold in hardware stores to spread adhesive on floors. A separate, new spreader was used for each coupon to avoid potential cross-contamination between replicates. Following application of feces, the coupons were individually swabbed using a commercial swab and transport system (StarSwab II; Starplex Scientific, Etobicoke, Ontario) that were submitted to the ISU VDL to be tested for the presence of viral RNA by RT-PCR.
Figure 1.
Application of feces to the aluminum coupons. Feces were applied to the coupons using a disposable flat spreader to produce a thin even layer on the floor of the coupon.
A 4.25% concentrate of AHP was diluted with tap water from a municipal water source at a dilution rate of 1:16 for treatment groups 5 mL-1:16 and 10 mL-1:16 and at a dilution rate of 1:32 for treatment groups 5 mL-1:32 and 10 mL-1:32. The negative control, positive control, and transmission control groups were not sham disinfected. The AHP disinfectant was applied to all replicates (n = 4) of a treatment group simultaneously. Four was the minimum number of replicates required to demonstrate statistical significance (P < 0.05) for the bioassay between 0 of 4 and 4 of 4 pigs infected. A liquid volume of approximately 30 mL of diluted AHP was applied with a 5.7 L pump-up foamer (model #A8020A; Ogena Solutions, LLC, Stoney Creek, Ontario; Figure 2). Given the area of the coupons used in this study, it was calculated that 30 mL of diluted disinfectant was proportionally equivalent to the 189 L applied during a 10 min application to a typical full sized 15.8-meter double-decked livestock trailer using a proportioning foamer that attaches to the end of a hose with a flow rate of 18.9 L/min. With a series of timed applications prior to the start of the trial it was determined that 3 s per coupon would result in the desired liquid volume of 30 mL of diluted AHP at both concentrations.
Figure 2.
Application of accelerated hydrogen peroxide disinfectant to the aluminum coupons. Disinfectant was applied as a foam using a commercially available 5.7 L pump-up foamer.
The contact time with the AHP disinfectant for treatment groups 5 mL-1:16, 10 mL-1:16, 5 mL-1:32, and 10 mL-1:32 was 30 min. A minimum 30-minute contact time is attainable under nearly all circumstances encountered in the transport of swine. Contamination of the coupons, application of the AHP disinfectant and the 30-minute contact time occurred at 20°C. For the positive control, negative control, and transmission control groups, contamination occurred at 20°C, and the coupons were held at that temperature for 30 min before being collected for the bioassay.
Thirty minutes after contamination (positive control, negative control, and transmission control) or application of the AHP disinfectant (5 mL-1:16, 10 mL-1:16, 5 mL-1:32, and 10 mL-1:32), the coupons were swabbed using the same commercial swab and transport system used to swab the coupons immediately after contamination. The swabs were submitted to the ISU VDL to be tested for the presence of viral RNA by RT-PCR. The coupons were then tilted so that the remaining material in the coupon flowed away from the holes, and 10 mL of sterile 0.9% sodium chloride saline solution (Hospira, Lake Forest, Illinois, USA) was added to each coupon. A new toothbrush, one per coupon, was used to suspend the remaining feces and disinfectant for ease of re-collection. The liquid mixture of feces, remaining AHP disinfectant and saline was aspirated using a 20 mL syringe (Figure 3). The syringe was capped and labeled with the identification number of the single pig that was to receive the mixture. Nitrile gloves (VetOne; MWI Veterinary Supply Company, Boise, Idaho, USA) were worn and changed between each coupon during collection to prevent possible cross-contamination between plates.
Figure 3.
Collection of material from aluminum coupons used to inoculate pigs for the bioassay. A liquid mixture of feces, disinfectant, and saline was aspirated using a 20-mL syringe that was labeled with the pig number of the single pig to be inoculated with the contents from a single coupon.
Source of animals and housing for bioassay
The experimental protocol was approved by the Iowa State University Institutional Animal Care and Use Committee (Log Number: 6-14-7812-S) and the Iowa State University Institutional Biosafety Committee (Log Number: 14-I-0022-A) prior to initiation of any experimental activity. The study was carried out in strict adherence to IACUC guidelines regarding humane use of animals. Twenty-eight, 3-week-old, clinically healthy barrows were sourced from a private commercial producer in Iowa. On day −1 of the study, 72 h after arrival, blood was collected from each pig via jugular venipuncture using a 12-mL syringe with a 38 mm 18-gauge needle (Monoject; Covidien, Mansfield, Massachusetts, USA), then transferred to an 8.5 mL plastic serum separator tube (BD Vacutainer, 8.5 mL draw; Becton, Dickinson and Company, Franklin Lakes, New Jersey, USA). The blood was centrifuged at 2100 × g for 10 min. The serum was aliquoted into two 5-mL snap cap tubes (BD Falcon polypropylene round-bottom tube; Becton, Dickinson and Company). One aliquot was frozen and stored at −80°C and the other was submitted to ISU VDL for diagnostic testing. Rectal swabs were collected using a commercially available swab and transport system (Starswabs II) and submitted to the ISU VDL for diagnostic testing. Rectal swabs were tested for PEDV (13), porcine deltacoronavirus (PDCoV) (14), and transmissible gastroenteritis virus (TGEV) (10) by virus-specific RT-PCR. Serum samples were tested for PRRSV using a commercial RT-PCR (VetMax NA; Waltham, Massachusetts, USA) and European (EU) PRRSV RT-PCR (Thermo Fisher Scientific) following the manufacturer’s instructions. Serum samples were tested for antibodies to PEDV by immunofluorescence assay (IFA) following previously described procedures (12) and antibodies for TGEV using a commercial enzyme-linked immunosorbent assay (ELISA) (SVANOVIR TGEV/PRCV-Ab differential ELISA; Svanova Biotech AB, Uppsala, Sweden).
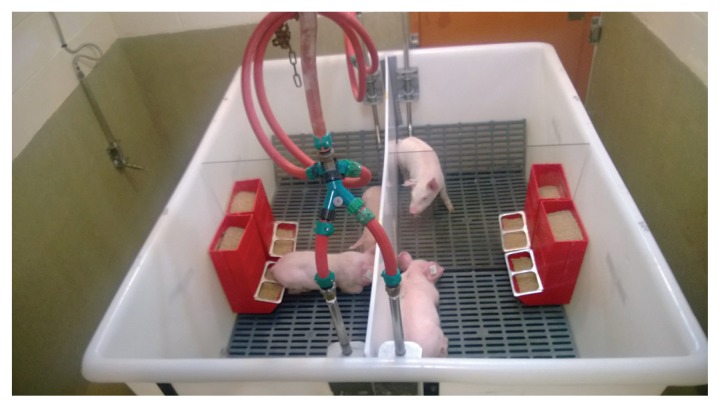
On arrival, each pig was identified with a unique plastic ear tag (Allflex USA, Dallas, Texas, USA) and weighed. Pigs were blocked by weight and randomly assigned to 1 of 7 groups (n = 4) using computer software (RAND function in Microsoft Excel, version 2010; Microsoft Corporation, Redmond, Washington, USA). Each group was housed in a separate room in the Iowa State University Veterinary Medical Research Institute for the duration of the study. The 4 pigs within each group were housed individually in elevated tubs constructed for a previous study involving a swine bioassay (11). The tubs had solid dividers completely separating each of the 4 pigs from one another. Each divided portion of the tub had dedicated water and feed sources (Figure 4). Pigs were fed an age-appropriate corn and soybean meal based diet ad libitum that was free of medications and any ingredients of porcine origin. Feces fell through the plastic, slatted flooring of the tub into a common collection area below the pigs, where it drained into a holding container to minimize the potential for environmental contamination.
Figure 4.
Elevated tubs used to house pigs for duration of the study. Tubs were divided into 4 quadrants, each with dedicated water and feed. All 4 pigs in each group were housed in the same tub, 1 pig per quadrant.
Inoculation of pigs for bioassay
The collected mixture of inoculum was then immediately used to inoculate the pigs for the bioassay. This was considered day 0 of the study. Personnel performing the inoculation wore disposable coveralls (Tyvek coveralls; DuPont, Wilmington, Delaware, USA) and a respirator (N95; 3M, St. Paul, Minnesota, USA) that were changed between groups. Additionally, personnel wore arm-length disposable obstetrical sleeves (Agri-Pro Enterprises, Iowa Falls, Iowa, USA), and nitrile gloves that were changed between each pig to prevent cross-contamination. Following the inoculation of each pig and discarding of the obstetrical sleeves and gloves, the coveralls were examined for possible contamination. If any contamination was observed, the coveralls were removed, discarded, and a new pair was donned. Inoculation was performed via gastrogavage, as previously described (11) using a 14 French rubber catheter (Kendall Covidien, Mansfield, Massachusetts, USA).
Following inoculation, clinical signs, including diarrhea, were assessed daily. On days 3 and 7 post-challenge, samples of feces from each pig were collected with rectal swabs (Starswabs II) and tested for PEDV by RT-PCR. The same biosecurity procedures using coveralls, masks, gloves, and obstetrical sleeves used when pigs were intragastrically inoculated, were used when sampling pigs. During sampling, pigs were not removed from their individual pens to avoid cross-contamination between individuals. Bioassays were considered to be positive if the fecal samples were positive for PEDV by RT-PCR on days 3 and 7. A Ct value less than 35 was considered positive.
Following collection of rectal swabs on study day 7, all animals were humanely euthanized using penetrating captive bolt and necropsied. Gross evaluation was performed on all organ systems and any gross pathology noted. From each pig, fresh cecal and spiral colon contents, sections of fresh and 10% formalin-fixed ileum, and fresh and formalin-fixed mesenteric lymph nodes were collected. Fresh samples were immediately frozen at −80°C, and all samples were held in the event further testing might be required to confirm the results obtained on rectal swabs by RT-PCR.
Statistical analysis was done using computer software (SAS version 9.3; SAS Institute, Cary, North Carolina, USA), with P < 0.05 considered statistically significant. Pre-treatment Ct values were compared between groups using analyses of variance (ANOVA). Difference in Ct values between pre- and post-treatment were assessed for each group using linear mixed models and compared between groups using an F-test. Analysis of the bioassay results was done using Fisher’s exact test to evaluate differences in proportions of pigs positive for the bioassay between groups.
Results
One of the 4 pigs in the negative control group died on study day −1 after the pigs were assigned to the study groups but before being inoculated on day 0. The pig was submitted to the ISU VDL for a full necropsy and diagnostic workup from which it was concluded that the cause of death was not linked to any study procedures. No other animals were removed from the study. The diagnostic results from samples taken on study day −1 confirmed that the pigs were negative for PEDV, PDCoV, PRRSV, and TGEV by RT-PCR. All of the pigs were negative for antibodies to PEDV by IFA and for antibodies to TGEV by differential ELISA.
The summary of PEDV RT-PCR results for swabs of contaminated coupons before and after treatment is presented in Table II. The Ct values and the quantitative genomic copies/mL are both reported. Swabs taken immediately after contamination (pre-treatment) from all of the coupons contaminated with PEDV-negative feces in the negative control group and the 3 coupons in the transmission control group that were designated as negative (3 of 4) tested negative for PEDV by RT-PCR. Swabs taken immediately after contamination from all of the coupons contaminated with PEDV-positive feces in the positive control group (4 of 4) and the 1 coupon in the transmission control group that was designated as positive (1 of 4) tested positive for PEDV by RT-PCR with the quantitative results ranging from 109.61 to 109.91 genomic copies/mL. Swabs from coupons contaminated with PEDV-positive feces in the treatment groups (5 mL-1:16, 10 mL-1:16, 5 mL-1:32, and 10 mL-1:32) collected immediately after contamination and before treatment with AHP were all positive for PEDV by RT-PCR with the quantitative results ranging from 109.61 to 1010.17 genomic copies/mL. Pre-treatment Ct values for the groups contaminated with PEDV-positive feces (positive control, 5 mL-1:16, 10 mL-1:16, 5 mL-1:32, and 10 mL-1:32) were not significantly different (P = 0.07). Swabs from coupons contaminated with PEDV-positive feces in the treatment groups using the 1:16 concentration of AHP (5 mL-1:16, 10 mL-1:16) collected after exposure to AHP for 30 min of contact time were all either negative (Ct ≥ 40) or suspect (35 ≤ Ct < 40) for PEDV by RT-PCR. The highest concentration was 103.61 genomic copies/mL. Treatment groups with the 1:32 concentration of AHP (5 mL-1:32 and 10 mL-1:32) each had one positive (Ct < 35) and one suspect swab collected after 30 min of contact time with the less concentrated AHP for 30 min and the highest concentration was 104.43 genomic copies/mL. However, only half of the swabs from trays treated with the 1:32 concentration of AHP (5 mL-1:32 and 10 mL-1:32) were positive or suspect compared to 75% or 100% of the swabs in the trays treated with the higher concentration of AHP (5 mL-1:16, 10 mL-1:16). The increase in Ct values between pre- and post-treatment were significantly different from zero for all treatment groups (5 mL-1:16, 10 mL-1:16, 5 mL-1:32, and 10 mL-1:32); however, the changes in Ct values after treatment were not statistically significant between treatment groups (P = 0.73).
Table II.
Porcine epidemic diarrhea virus (PEDV) real-time reverse transcription polymerase chain reaction (RT-PCR) results for swabs of contaminated coupons before and after treatment
| Treatment groupa | Pre-treatmentb Ct value and (genomic copies/mL) | Percentage positive or suspect for PEDV | Post-treatmentc Ct value and (genomic copies/mL) | Percentage positive or suspect for PEDVd |
|---|---|---|---|---|
| Negative controle | > 40 (0) | 0% (0 of 3) | N/A | N/A |
| > 40 (0) | ||||
| > 40 (0) | ||||
| Positive control | 14.2 (109.73) | 100% (4 of 4) | N/A | N/A |
| 14.2 (109.73) | ||||
| 13.6 (109.91) | ||||
| 14.6 (109.61) | ||||
| 5 mL-1:16 | 14.1 (109.76) | 100% (4 of 4) | 36.1 (103.28) | 75% (3 of 4) |
| 12.7 (1010.17) | > 40 (0) | |||
| 13.4 (109.97) | 36.4 (103.19) | |||
| 13.5 (109.94) | 38.5 (102.57) | |||
| 10 mL-1:16 | 14.3 (109.70) | 100% (4 of 4) | 38.7 (102.52) | 100% (4 of 4) |
| 13.9 (109.82) | 37.4 (102.90) | |||
| 13.9 (109.82) | 35.5 (103.46) | |||
| 13.2 (1010.02) | 35.0 (103.61) | |||
| 5 mL-1:32 | 14.3 (109.70) | 100% (4 of 4) | 37.6 (102.84) | 50% (2 of 4) |
| 14.1 (109.76) | 32.2 (104.43) | |||
| 14.2 (109.73) | > 40 (0) | |||
| 14.6 (109.61) | > 40 (0) | |||
| 10 mL-1:32 | 13.8 (109.85) | 100% (4 of 4) | 34.3 (103.81) | 50% (2 of 4) |
| 13.7 (109.88) | > 40 (0) | |||
| 13.6 (109.91) | 37.6 (102.84) | |||
| 14.2 (109.73) | > 40 (0) | |||
| Transmission control | 14.4 (109.67) | 25% (1 of 4) | N/A | N/A |
| > 40 (0) | ||||
| > 40 (0) | ||||
| > 40 (0) |
Treatment groups are summarized in Table I.
Results for swabs of coupons following contamination with feces before exposure to AHP disinfectant for 30 min of contact time. Genomic copies/mL in parenthesis.
Results for swabs of coupons following treatment with AHP disinfectant for 30 min of contact time. Negative control, positive control, and transmission control groups were not sham disinfected and no “post-treatment” swabs were collected for these groups.
Cycle threshold (Ct), genomic copies and percentage positive or suspect reported. Positive, Ct < 35; Suspect, 35 ≤ Ct < 40; Negative, Ct ≥ 40.
One pig in the negative control group died prior to initiation of the bioassay (day 0).
N/A — Not available.
The summary of PEDV RT-PCR results for rectal swabs collected from pigs on days 3 and 7 post-inoculation for the swine bioassay are reported in Table III. Rectal swabs collected from all pigs in the negative control group, the 3 pigs designated as negative (3 of 4) in the transmission control group and all pigs in the 4 treatment groups (5 mL-1:16, 10 mL-1:16, 5 mL-1:32, and 10 mL-1:32) were negative for PEDV by RT-PCR on days 3 and 7 post-inoculation. Rectal swabs collected from all pigs in the positive control group and from the 1 pig designated as positive (1 of 4) in the transmission control group were all positive for PEDV by RT-PCR on days 3 and 7 post-inoculation. No rectal swabs in any group had Ct values within the suspect range (35 ≤ Ct < 40).
Table III.
Summary of porcine epidemic diarrhea virus (PEDV) real-time reverse transcription polymerase chain reaction (RT-PCR) results for rectal swabs collected on days 3 and 7 post-inoculation for the swine bioassay
| Treatment groupa | Day 3 post-inoculation; percentage positive for PEDV (positive/tested) | Day 7 post-inoculation; percentage positive for PEDV (positive/tested) | Bioassay result; percentage positive for PEDV (positive/tested)b |
|---|---|---|---|
| Negative control | 0% (0/3) | 0% (0/3) | 0% (0/3)a |
| Positive control | 100% (4/4) | 100% (4/4) | 100% (4/4)b |
| 5 mL-1:16 | 0% (0/4) | 0% (0/4) | 0% (0/4)a |
| 10 mL-1:16 | 0% (0/4) | 0% (0/4) | 0% (0/4)a |
| 5 mL-1:32 | 0% (0/4) | 0% (0/4) | 0% (0/4)a |
| 10 mL-1:32 | 0% (0/4) | 0% (0/4) | 0% (0/4)a |
| Transmission control | 25% (1/4) | 25% (1/4) | 25% (1/4) |
Treatment groups are summarized in Table I.
Values with different letters were significantly different (P < 0.05) by Fishers exact test. The transmission control group was included to validate the animal housing and handling protocols used for the bioassay. Values for transmission control group were not compared to those of the other groups.
A Fisher’s exact test was performed to evaluate differences between groups in the proportion of pigs positive for the bioassay. Each pig was considered positive for the bioassay if the fecal samples from the pig were positive for PEDV by real-time RT-PCR on days 3 and 7. The proportion of pigs that were positive for the bioassay in the negative control and all of the treatment groups (5 mL-1:16, 10 mL-1:16, 5 mL-1:32, and 10 mL-1:32) were significantly different than the positive control (P < 0.05).
Discussion
An AHP disinfectant inactivated PEDV in the presence of significant feces on metal surfaces like those found in livestock trailers, at room temperature with 30 min of contact time. The PEDV was inactivated by both concentrations of the AHP disinfectant evaluated, 1:16 and 1:32, and when 5 mL and 10 mL of feces were present. Wood shavings are frequently used as bedding in livestock trailers that haul swine. Inclusion of wood shavings was considered; however, the type of wood and size of the shavings used as bedding varies considerably and some types of wood have been shown to have virucidal properties (15). Therefore, feces alone without wood shavings was used to contaminate the coupons in the current study to avoid the potentially confounding effect of the choice of shavings.
Because disinfection, natural drying, or TADD systems are more effective when applied to a clean trailer with little or no remaining organic matter, the industry standard for sanitation and decontamination of livestock trailers includes trailer washing, disinfection, and natural drying or TADD systems. When a complete wash, disinfection, and drying cannot be done, due to lack of resources or other logistical constraints, the results of this study suggest that scraping livestock trailers to remove as much organic material as possible followed by disinfection with an AHP, may be used as an alternative to doing nothing to reduce the risk of PEDV transmission associated with livestock trailers.
Limitations of current viral isolation methods in cell culture make it difficult to culture PEDV outside of an animal model. Currently swine bioassay remains the best means to determine if infectious PEDV is present in a sample. The use of a bioassay also eliminates the possible negative impact, such as cytotoxicity, of feces and disinfectant present in a sample on virus isolation outcomes in cell culture. In the current study, a 3-week-old pig bioassay model was used to assess the effectiveness of an AHP disinfectant on inactivating PEDV. Three-week-old pigs were used because PEDV does not typically cause mortality in this age of pig but they are still highly susceptible to infection. Thomas et al (12) reported that 100% of 21-day-old pigs inoculated with 10 mL of a virulent PEDV prototype isolate with titers of 560–5.6 TCID50/mL were infected while 10 mL of inoculum with titers 0.56–0.0056 TCID50/mL failed to infect the 21-day-old pigs.
The PCR results for the swabs collected from the coupons after treatment with the AHP suggest that, in most cases, the disinfectant degraded the genetic material of the virus to the extent that the amount of intact RNA was close to or below the limit of detection of the RT-PCR assay used in this study. Of the 16 replicates in the 4 treatment groups with AHP (5 mL-1:16, 10 mL-1:16, 5 mL-1:32, and 10 mL-1:32), only 2 (5 mL-1:32 and 10 mL-1:32) had replicates that remained positive (Ct < 35) after treatment, but every group had at least 1 suspect (35 ≤ Ct < 40) swab that remained negative on the bioassay. Because PCR tests for PEDV are readily available at the major veterinary diagnostic laboratories, and because bioassays are expensive and difficult to perform, it is tempting to use PCR results as endpoints for disinfection studies. However, there are multiple mechanisms that result in viral inactivation including deterioration of genetic material but also membrane disruption or protein denaturation (16). The results of this study suggest that a positive result for PEDV by RT-PCR on an environmental sample following application of AHP does not necessarily indicate that an infectious dose of live virus remains. This is consistent with results from another study that evaluated a phenol, quaternary ammonium compound, sodium hypochlorite, oxidizing agent, and quaternary ammonium/glutaraldehyde combination, for their ability to inactivate PEDV and reduce the amount of viral RNA detectable by RT-PCR (17).
Rather than perform the experiment on full size livestock trailers or small-scale models of trailers, 15.24 cm × 15.24 cm × 2.54 cm smooth aluminum coupons were used as a model in the present study. The ease with which the coupons can be handled made it possible to contaminate the coupons, perform the treatments, collect the inoculum, and inoculate pigs for the bioassay for all study groups in < 1 d. The model also enabled the investigators to stagger the start time for each treatment group so that the pigs could be inoculated immediately after the inoculum was collected, thereby eliminating the need to attempt to neutralize the AHP disinfectant after the 30-minute contact time. However, livestock trailers used to haul pigs have many different types of surfaces and it is not possible to represent all with a single type of coupon. The sidewalls and gates are generally smooth with varying angles that would be represented by the 90° angles where the sidewall meets the bottom surface of the coupons used in this study. The floor of livestock trailers used to haul swine frequently have a raised diamond plate pattern. There are also typically corners and crevices, and surfaces that are perpendicular to the ground where the AHP foam would be drawn away by gravity more quickly. The inability of the coupons to represent all of the surfaces of livestock trailers used to haul swine is a limitation of this study.
The experimental unit for the bioassay was the individual pig. Elevated tubs with solid dividers completely separating each of the 4 pigs from one another were used to house the pigs. The tubs were designed to prevent any contact with neighboring pigs or with feces from neighboring pigs. Strict biosecurity procedures were followed, but because all of the pigs in a single treatment group were housed in the same room there was a perceived risk that pigs not infected by PEDV in the inoculum could become infected by lateral transmission from one pig in a room to another by study personnel or aerosol transmission. Recent research demonstrated that aerosol transmission did not occur from pigs experimentally infected with PEDV to sentinel pigs housed in close proximity and in the same air space (18). However, in another study, it was shown that PEDV can become airborne and remain infectious while suspended in air (19). To evaluate this risk, a transmission control group was added to this study. One pig in the tub was inoculated with feces that were positive for PEDV while the other 3 were inoculated with feces that were negative for PEDV. During sampling procedures, animals were handled in alternating orders, so that the negative animals were handled after handling the positive animal within the group. In this way, the biosecurity practices were also tested. The pig inoculated with the positive feces became infected, while the other 3 remained negative to PEDV for the 7-day duration of the study. The results suggest that the housing system and animal handling procedures were effective at preventing lateral transmission between pigs by direct contact, humans, or aerosol. The results help demonstrate the validity of the housing model and associated biosecurity practices for this study and future PEDV studies using a swine bioassay.
The formulation of Accel used in this study was registered for use in the United States, contains 4.25% AHP and was labeled against multiple viruses at dilutions of 1:16 to 1:64 with 5 min of contact time. Evaluation of the efficacy of AHP for inactivating PEDV in the presence of feces was conducted at lower dilution rates of AHP (1:16 and 1:32), at room temperature and with coupons on a flat surface during contamination and treatment to mimic the surfaces of a livestock trailer. Further study of the efficacy of AHP under less favorable conditions, including higher dilution rates, colder temperatures, drier and thicker fecal contamination, and with coupons representative of more challenging surfaces of livestock trailers is merited.
Acknowledgments
Funding for this study was provided by Virox Technologies Inc., Oakville, Ontario and Ogena Solutions, Stoney Creek, Ontario. The authors thank AMVC Management Services for in-kind support and students and staff of the Swine Medicine Education Center at Iowa State University for assistance with live animal work.
References
- 1.Oldham J. Epidemic Diarrhea — How it all began [letter to the editor] Pig Farming. 1972;(October Supplement):72–73. [Google Scholar]
- 2.Chasey D, Cartwright SF. Virus-like particles associated with porcine epidemic diarrhea. Res Vet Sci. 1978;25:255–256. doi: 10.1016/S0034-5288(18)32994-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pensaert MB, de Bouck P. A new coronavirus-like particle associated with diarrhea in swine. Arch Virol. 1978;58:243–247. doi: 10.1007/BF01317606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevenson GW, Hoang H, Schwartz KJ, et al. Emergence of porcine epidemic diarrhea virus in the United States: Clinical signs, lesions, and viral genomic sequences. J Vet Diagn Invest. 2013;25:649–654. doi: 10.1177/1040638713501675. [DOI] [PubMed] [Google Scholar]
- 5.Lowe J, Gauger P, Harmon K, et al. Role of transportation in spread of porcine epidemic diarrhea virus infection, United States. Emerg Infect Dis. 2014;20:872–874. doi: 10.3201/eid2005.131628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dee SA, Deen J, Otake S, Pijoan C. An experimental model to evaluate the role of transport vehicles as a source of transmission of porcine reproductive and respiratory syndrome virus to susceptible pigs. Can J Vet Res. 2004;68:128–133. [PMC free article] [PubMed] [Google Scholar]
- 7.Dee S, Deen J, Burns D, Douthit G, Pijoan C. An assessment of sanitation protocols for commercial transport vehicles contaminated with porcine reproductive and respiratory syndrome virus. Can J Vet Res. 2004;68:208–214. [PMC free article] [PubMed] [Google Scholar]
- 8.Dee S, Torremorell M, Thompson B, Deen J, Pijoan C. An evaluation of thermo-assisted drying and decontamination for the elimination of porcine reproductive and respiratory syndrome virus from contaminated livestock transport vehicles. Can J Vet Res. 2005;69:58–63. [PMC free article] [PubMed] [Google Scholar]
- 9.Dee S, Burns D, Douthit G, Pijoan C. An evaluation of disinfectants for the sanitation of porcine reproductive and respiratory syndrome virus-contaminated transport vehicles at cold temperatures. Can J Vet Res. 2005;69:64–70. [PMC free article] [PubMed] [Google Scholar]
- 10.Schneider PT, Zhang J, Ramirez A, Holtkamp DJ. An evaluation of disinfection protocols to reduce virus transmission via livestock transport vehicles. J Swine Health Prod. 2015;23:306–316. [Google Scholar]
- 11.Thomas P, Karriker L, Ramirez A, et al. Evaluation of time and temperature sufficient to inactivate PEDV in swine feces on metal surfaces. J Swine Health Prod. 2015;23:84–90. [Google Scholar]
- 12.Thomas JT, Chen Q, Gauger PC, et al. Effect of porcine epidemic diarrhea virus infectious doses on infection outcomes in naïve conventional neonatal and weaned pigs. PLoS ONE. 2015;10:e0139266. doi: 10.1371/journal.pone.0139266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madson DM, Magstadt DR, Arruda PHE, et al. Pathogenesis of porcine epidemic diarrhea virus isolate (US/Iowa/18984/2013) in 3-week-old weaned pigs. Vet Microbiol. 2014;174:60–68. doi: 10.1016/j.vetmic.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Chen Q, Gauger P, Stafne M, et al. Pathogenicity and pathogenesis of a United States porcine deltacoronavirus cell culture isolate in 5-day-old neonatal piglets. Virology. 2015;482:51–59. doi: 10.1016/j.virol.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greatorex JS, Digard P, Curran MD, et al. Survival of influenza A(H1N1) on materials found in households: Implications for infection control. PLoS ONE. 2011;6:e27932. doi: 10.1371/journal.pone.0027932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laude H. Thermal inactivation studies of a coronavirus, transmissible gastroenteritis virus. J Gen Virol. 1981;56:235–240. doi: 10.1099/0022-1317-56-2-235. [DOI] [PubMed] [Google Scholar]
- 17.Bowman AS, Nolting JM, Nelson SW, et al. Effects of disinfection on the molecular detection of porcine epidemic diarrhea virus. Vet Microbiol. 2015;179:213–218. doi: 10.1016/j.vetmic.2015.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hesse D, Suddith A, Breazeale B, et al. Oral/nasal inoculation of four-week-old pigs with PEDV: Tissue tropism, shedding, carriage, antibody response and aerosol transmission. Proc 23rd Intl Pig Vet Soc Congr; 2014. p. O.162. [Google Scholar]
- 19.Alonso C, Goede DP, Morrison RB, et al. Evidence of infectivity of airborne porcine epidemic diarrhea virus and detection of airborne viral RNA at long distances from infected herds. Vet Res. 2014;45:73. doi: 10.1186/s13567-014-0073-z. [DOI] [PMC free article] [PubMed] [Google Scholar]