Abstract
In order to study the ability of Lactobacillus casei to ameliorate murine enteritis, 18 mice were randomly divided into 3 groups: the enteritis group, intervention group, and control group. The interleukin (IL)-6 and transforming growth factor-β (TGF)-β content in mouse peripheral blood and duodenum was detected using an enzyme-linked immunosorbent assay (ELISA). The number of CD4+CD25+Foxp3+ T-regulatory cells (Tregs) and CD4+IL-17A+ Th17 cells in the mesenteric lymph nodes (MLN) and spleen were detected using flow cytometry, and quantitative reverse transcription polymerase chain reaction (PCR) and western blot analysis were used to measure Foxp3 and retinoid-related orphan receptor-γ (RORγt) mRNA and protein expression in the MLN. Histological changes in the duodenum were observed. Results indicate that in the intervention group, IL-6 content in mouse peripheral blood and duodenum was significantly lower than in the enteritis group (P < 0.05), while TGF-β content was significantly increased compared to the enteritis group (P < 0.05). For the intervention group, the percentages of CD4+CD25+Foxp3+ Tregs in spleen and MLN were increased (P < 0.05), while the percentages of CD4+IL-17A+ Th17 cells were decreased compared to the enteritis group (P < 0.05). The expression of Foxp3 mRNA and protein in the intervention group was higher than in the enteritis group, while RORγt mRNA and protein were significantly lower (P < 0.05). After mice in the enteritis group were treated with L. casei, duodenal inflammation was relieved. This study demonstrated that L. casei could have possible implications for the enterotoxigenic Escherichia coli (ETEC) induced intestinal inflammation by regulating the ratio imbalance of Th17/Treg cells.
Résumé
Afin d’étudier la capacité de Lactobacillus casei à soulager l’entérite murine, 18 souris ont été réparties de manière aléatoire en trois groupes : le groupe entérite, le groupe intervention, et le groupe témoin. Les quantités d’interleukine (IL)-6 et de facteur de croissance transformant β (FCT-β) dans le sang périphérique et le duodénum de souris furent détectées à l’aide d’une épreuve immunoenzymatique (ELISA). Les cellules T régulatrices (Tregs) CD4+CD25+Foxp3+ et les cellules CD4+IL-17A+ Th17 dans les noeuds lymphatiques mésentériques (NLM) et la rate ont été détectées et dénombrées par cytométrie en flux, et réaction d’amplification en chaîne quantitative avec la transcriptase réverse, et l’analyse par immunobuvardage fut utilisée afin de mesurer l’expression de l’ARNm et de la protéine Foxp3 et du récepteur orphelin γ apparenté au rétinoïde (RORγt) dans les NLM. Les changements histologiques dans le duodénum ont été observés. Les résultats indiquent que dans le groupe intervention, le contenu en IL-6 dans le sang périphérique et le duodénum était significativement moindre que dans le groupe entérite (P < 0,05), alors que le contenu en FCT-β était augmenté de manière significative comparativement au groupe entérite (P < 0,05). Pour le groupe intervention, les pourcentages de Tregs CD4+CD25+Foxp3+ dans la rate et le NLM étaient augmentés (P < 0,05), alors que les pourcentages de cellules CD4+IL-17A+ Th17 étaient diminués comparativement au groupe entérite (P < 0,05). L’expression de l’ARNm et de la protéine Foxp3 dans le groupe intervention était plus élevée que dans le groupe entérite, alors que l’expression de l’ARNm et de la protéine RORγt était significativement moindre (P < 0,05). Suite au traitement des souris du groupe entérite avec L. casei, l’inflammation duodénale était résorbée. La présente étude a démontré que L. casei pourrait avoir des implications possibles dans l’inflammation intestinale induite par Escherichia coli entérotoxinogène en régulant le débalancement du ratio de cellules Th17/Treg.
(Traduit par Docteur Serge Messier)
Introduction
Enterotoxigenic Escherichia coli (ETEC) strains are a major cause of diarrheal disease in humans and animals (1). Enterotoxigenic E. coli can produce heat-labile (LT) and heat-stable (ST) toxins, which stimulate fluid and electrolyte secretion from intestinal cells leading to diarrhea (2). Antibiotics have been widely applied in diarrhea prevention to improve animal health and benefit the animal industry economy. Due to the extensive veterinary use and potential to cause selective proliferation of antibiotic resistant bacteria, antibiotic use has recently raised increased concerns (3). As an alternative to antibiotics, probiotics have captured the public’s attention and considerable research is being conducted on these microorganisms. One such probiotic, Lactobacillus casei, can tolerate enzyme activity in the oral cavity, gastric acid, and intestinal bile acids and can thus survive in the intestinal tract, maintain intestinal flora balance, enhance animal immune capacity, and promote animal growth and development (4).
Th17 cells are proinflammatory cells that secrete inflammatory factors such as interleukin (IL)-17A. Interleukin-17A is involved in various inflammatory diseases, autoimmune diseases, and acute transplant rejection reactions (5). Retinoid-related orphan receptor-γ (RORγt) is a specific transcriptional regulator required for Th17 cell differentiation (6). One of the important functions of regulatory T-cells (Tregs) is the immunosuppressive regulation of auto-reactive T-cells (7), and they play a critical role in immune self-tolerance. Forkhead transcription factor (Foxp3) is predominantly expressed in CD4+CD25+Treg cells and is a master regulator for the development and function of Treg cells (8). Regulatory T-cells are important in the development and outcome of various diseases, including cancer, infectious diseases, and transplant immunity (9,10). Regulatory T-cells can release anti-inflammatory cytokines IL-10 and transforming growth factor-β (TGF-β) to depress chronic inflammation. Th17 and Treg cells can differentiate in the thymus at the stage of CD4 single-positive thymocytes (11), and IL-6 and TGF-β are the key cytokines that determine their respective cell fates (12). Interleukin-6 promotes Th17 cells differentiation by inducing STAT-3-dependent activation of RORγt (13), while TGF-β signaling is required for the induction of Foxp3 expression (14). Their dynamic equilibrium can maintain the body’s immune response at appropriate levels. Therefore, setting the boundaries of mediation and regulation of inflammatory reactions, Treg/Th17 balance, distinct from the number and function of each subset alone, is an imperative checkpoint in immune homeostasis. In this study, we used L. casei to interfere with the ETEC-mediated mouse duodenal inflammation model that had previously been developed (15), the aim was to study the mechanism by which L. casei interferes with enteritis by analyzing the Treg/Th17 balance.
Materials and methods
Bacterial strain and animals
Lactobacillus casei ATCC393 and ETEC K88 C83912 were provided by the Institute of Microbiology, College of Animal Science and Technology, Jilin Agricultural University, China. Male specific pathogen free (SPF) BALB/c mice, 6 to 8 wk of age, were purchased (Beijing HFK Bioscience Company, Beijing, China). The mice were housed in polystyrene cages with stainless steel wire lids and given water and food ad libitum. The housing was maintained at a constant temperature (21°C to 22°C) with a 12-hour light-dark cycle. The protocol was approved by the Committee on the Ethics of Animal Experiments of the Jilin Agricultural University, China.
Experimental design
Eighteen mice were randomly divided into 3 groups: the enteritis group, intervention group, and control group. Six mice were assigned to each group, the experiments were repeated 3 times. Firstly, the intervention group was administered 0.2 mL of 2.0 × 108 colony-forming units (cfu)/mL L. casei for 7 d. Second, the mice in all 3 groups were pre-treated with 0.2 mL of 1% NaHCO3. After 30 min, the intervention and enteritis groups were administered 0.2 mL of 2.0 × 108 cfu/mL ETEC K88 daily for 3 d. The control group was administered the same dose of normal saline. Mice were euthanized on the 15th day.
Enzyme-linked immunosorbent assay (ELISA) analysis of IL-6 and TGF-β
On the 15th day, the mice were euthanized and peripheral blood, duodenum, and orbital venous blood samples from each mouse from each group were collected and serum was isolated. The duodenum was weighed and homogenized with cold saline and centrifuged at 7378 × g at 4°C for 10 min to get the supernatant. The levels of IL-6 and TGF-β in the serum and duodenum of each mouse were determined using an ELISA kit (Lengton Bioscience Company, Shanghai, China), according to the manufacturer’s instructions.
Flow cytometric analysis of CD4+CD25+Foxp3+ Tregs and CD4+IL-17A+ Th17 cell
The spleen and mesenteric lymph nodes (MLN) were aseptically isolated from each mouse, homogenized in 1 mL dulbecco’s modified eagle media (DMEM), and centrifuged 5 min (4°C, 295 × g). Cells in the spleen were split using red blood cell lysis buffer (Becton, Dickinson and Company, New Jersey, America). Cells were then washed twice and suspended in flow cytometry staining buffer. Viable cells were enumerated using a hemocytometer with trypan blue exclusion. Briefly, to measure the percentage of Tregs, prepared cells (1 × 106) were washed by centrifugation in flow cytometry staining buffer. Then, cells were immersed in the buffer for 30 min at 4°C. Next, cells were fixed and permeabilized in a fixation/permeabilization solution for 30 min and subsequently stained using 0.5 μg of phycoerythrin (PE) rat anti-mouse Foxp3. For analysis of Th17 cells, the cell suspension was stimulated using phorbol myristate acetate (PMA) 50 μL (25 ng/mL), ionomycin 20 μL (1 μg/mL), and breteldin A 0.7 μL (Sigma-Aldrich, St. Louis, Missouri, USA) in 24-well plates. After 4 h of culturing (37°C, 5% CO2), the cells were transferred to tubes and washed twice in phosphate-buffered saline (PBS). Then the cells were re-suspended with 1 × permeability buffer and incubated for 40 min at 4°C in the dark, and washed twice in wash buffer. Cells were stained using 10 μL of PE rat anti-mouse IL-17A at 4°C for 1 h. Finally, the cells were washed using 1 × wash buffer and analyzed by flow cytometry. At least 10 000 cells per sample were assayed using a flow cytometry apparatus (FACS Calibur flow cytometry apparatus; Becton Dickinson, California, USA) and analyzed using computer software (FlowJo7.6; Becton, Dickinson and Company, New Jersey, USA).
Quantitative real-time reverse transcription polymerase chain reaction (RT-PCR) analysis of Foxp3 and RORγt
Total RNA was isolated from mesenteric lymph nodes samples of 5 mice from each group with an RNA extraction kit (Bio-flux RNA Extraction Kit; BioFlux Company, Tokyo, Japan) and was subsequently reverse transcribed (PrimeScript 1 st Strand cDNA Synthesis Kit; BioFlux Company). The expression levels of FoxP3, RORγt, and GAPDH were determined using Synergy Brands Premix Ex TaqTM II (SYBR Premix Ex Taq II; TaKaRa, Dalian, China) based on the manufacturer’s instructions. The primer sequences are shown in Table I. Briefly, 2.0 μL of the cDNA template was added to a total volume of 25.0 μL containing 12.5 μL of SYBR Premix Ex Taq II, 0.2 mL of ROX reference dye (TaKaRa, Dalian, China), 8.5 μL of double distilled water (ddH2O) and 0.5 μL of the forward and reverse primers. The thermal cycling conditions were as follows: i) pre-denaturation (30 s at 95°C), and ii) amplification and quantification (40 cycles of 5 s at 95°C 30 s at 60°C). The relative expression was defined as the ratio of the target gene to the housekeeping gene using the formula 2−ΔΔt. Relative expression was normalized and expressed as a ratio to the expression in the control group.
Table I.
Quantitative reverse transcription polymerase chain reaction (qRT-PCR) primer sequences
| Primer name | Forward primer (5′-3′) | Reverse primer (5′-3′) | Fragment length (bp) |
|---|---|---|---|
| Foxp3 | 5′-TGCCTTCAGACGAGACTTGGA-3′ | 5′-GGCATTGGGTTCTTGTCAGAG-3′ | 86 |
| RORγt | 5′-CAGCAGTGTAATGTGGCCTACTCCT-3′ | 5′-TGTTGCAGTTGTTTCTGCACTTCT-3′ | 19 |
| GAPDH | 5′-CACTCACGGCAAATTCAACGGCAC-3′ | 5′-GACTCCACGACATACTCAGCAC-3′ | 14 |
bp — base pairs
Western blot analysis of Foxp3 and RORγt
Mesenteric lymph nodes were carefully isolated and crushed. Total protein was extracted using bicinchoninic acid (BCA) protein quantification kit (Keygen Biotech Company, Nanjing, China) according to the manufacturer’s instructions. Protein concentration was detected using the BCA protein detection kit (Biouniquer Technology Company, Nanjing, China), and 50 μg of protein lysate from each sample was used for further analyses. After sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), the protein was electro-transferred to a polyvinylidene fluoride (PVDF) membrane. Next, 5% skim milk powder was used to block non-specific antibody binding to the membrane for 1 h. Foxp3, RORγt, and lamin B primary antibodies (eBioscience, California, USA) were added, and samples were incubated overnight at 4°C. Samples were washed 3 times using tris-buffered saline and tween 20 (TBST) and horseradish peroxidise (HRP)-conjugated secondary antibodies were added. Samples were then incubated at room temperature for 1 h and then washed by TBST 3 times. Finally, the blots were visualized using enhanced chemiluminescent (ECL) kits (Amersham, Buckinghamshire, UK).
Histoloical analysis of the duodenum
For the histological examination, the entire duodenum was excised and cleaned using normal saline, one component (1 to 2 cm) was fixed in 10% neutral buffered formalin and embedded in paraffin. Four micrometer thick longitudinal sections were cut and stained with hematoxylin and eosin (H&E).
Statistical analyses
The results are presented as the mean ± SD. Statistical analysis was done using computer software (SPSS 18.0; International Business Machines Corporation, New York, America). Data were analyzed using 1-way analysis of variance (ANOVA) and Tukey’s multiple comparison test was used to determine significance levels between groups. The results were considered significant at a 95% confidence level (P < 0.05).
Results
Interleukin-6 and TGF-β content
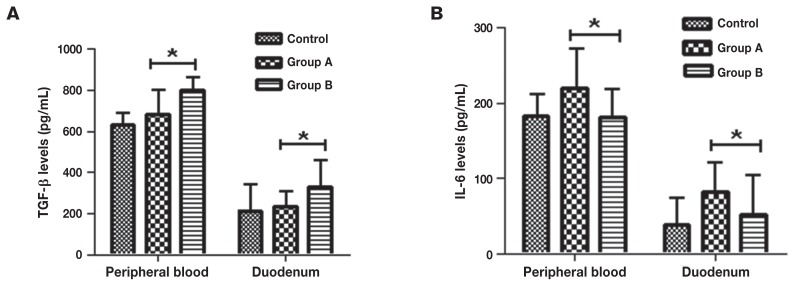
All T-cells depend on cytokines for their growth and survival. Interleukin-6 and TGF-β are the key cytokines required to induce Th17 and Tregs phenotypes, respectively. In order to study the differentiation of the native T-cells, the levels of IL-6 and TGF-β in mouse peripheral blood and duodenum from different groups were detected, the results showed that IL-6 content in mouse peripheral blood and duodenum for the L. casei intervention group was significantly lower than in the enteritis group (P < 0.05), while TGF-β content was significantly increased (P < 0.05) (Figure 1).
Figure 1.
The levels of interleukin (IL)-6 and transforming growth factor-β (TGF-β) in different groups of mice. A — TGF-β content in mouse peripheral blood and duodenum was determined by ELISA from the different groups; B — IL-6 content in mouse peripheral blood and duodenum was determined by ELISA from the different groups. Control: control group, the mice were administered normal saline. Group A: enteritis group, the mice were administered 0.2 mL 2.0 × 108 cfu/mL ETEC K88 daily for 3 d. Group B: intervention group, the mice were administered 2.0 × 108 cfu/mL L. casei for 7 d, then 2.0 × 108 cfu/mL ETEC K88 for 3 d. Results are expressed as means ± SD of the 6 mice in each group.
Changes in CD4+CD25+Foxp3+ Tregs and CD4+IL-17A+ Th17 cell percentages after L. casei intervention
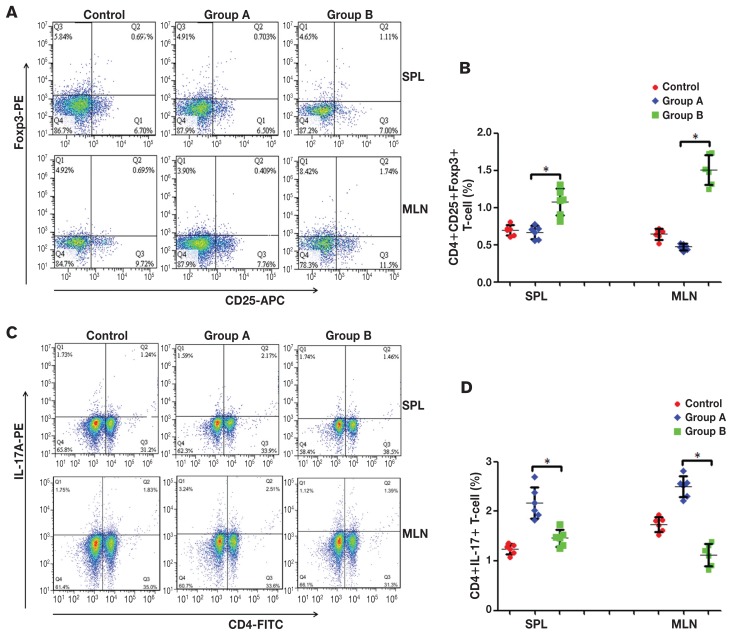
The Tregs and Th17 cells have opposite effects on autoimmunity and inflammation. The percent difference between Tregs and Th17 cells in the spleen and MLN from different groups was studied. The results showed that the percentages of CD4+CD25+Foxp3+ Tregs and CD4+IL-17A+ Th17 cells in the spleen lymphocytic population for the control group were 0.696 ± 0.071% and 1.243 ± 0.104%, respectively, and 0.644 ± 0.0747% and 1.7383 ± 0.149% in MLN. For the enteritis group, the percentages of CD4+CD25+Foxp3+ Tregs and CD4+IL-17A+ Th17 cells were 0.667 ± 0.0861% and 2.170 ± 0.319%, respectively, in spleen lymphocytic population, and 0.473 ± 0.0436% and 2.496 ± 0.213% in MLN. For the intervention group, the percentages of CD4+CD25+Foxp3+ Tregs and CD4+IL-17A+ Th17 cells was 1.0767 ± 0.1831% and 1.461 ± 0.168%, respectively, in spleen lymphocytes, and 1.507 ± 0.203% and 1.123 ± 0.224%, respectively, in MLN (Figures 2A, 2C). There were significant differences in the percentages of CD4+CD25+Foxp3+ Tregs and CD4+IL-17A+ Th17 cells when comparing the intervention and enteritis groups (P < 0.05) (Figures 2B, 2D).
Figure 2.
Percentages of CD4+CD25+Foxp3+ Tregs and CD4+IL-17A+ Th17 cells in CD4+ T-cells. A — Representative flow cytometric analysis results of CD4+CD25+Foxp3+ Tregs in mouse spleen and lymph nodes. B — The frequency of CD4+CD25+Foxp3+ Treg in each group. a P < 0.05. C — Representative flow cytometric analysis results of CD4+IL-17A+ Th17 cells in mouse spleen and lymph nodes. D — The frequency of CD4+IL-17A+ Th17 cells in each group. a P < 0.05. Control: control group, the mice were administered normal saline. Group A: enteritis group, the mice were administered 0.2 mL 2.0 × 108 cfu/mL ETEC K88 daily for 3 d. Group B: intervention group, the mice were administered 2.0 × 108 cfu/mL L. casei for 7 d, then 2.0 × 108 cfu/mL ETEC K88 for 3 d. Results are expressed as mean ± SD of 6 mice from each group.
Quantitative PCR and western blot analysis of Foxp3 and RORγt
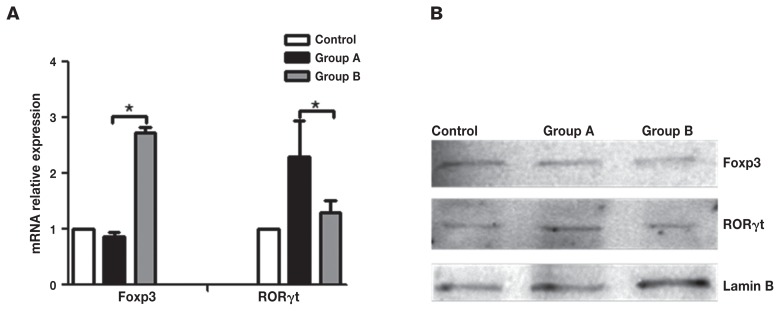
The RORγt is an important transcription factor for the differentiation of Th17 cells, whereas Foxp3 is the master transcription factor for Tregs. The relative mRNA and protein expression of Foxp3 and RORγt in the MLN was analyzed. The results showed that the expression of Foxp3 mRNA and protein for the intervention group was significantly higher than in the enteritis group (P < 0.05), while the expression of RORγt mRNA and protein was lower than the enteritis group (P < 0.05) (Figure 3).
Figure 3.
Foxp3 and RORγt relative mRNA and protein expression content. A — mRNA content of Foxp3 and RORγt in MLN was determined using quantitative PCR; B — Foxp3 and RORγt protein expression in MLN was determined by western blot analysis, Lamin B was detected as a normalization control. Control: control group, the mice were administered normal saline. Group A: enteritis group, the mice were administered 0.2 mL 2.0 × 108 cfu/mL ETEC K88 daily for 3 d. Group B: intervention group, the mice were administered 2.0 × 108 cfu/mL L. casei for 7 d, then 2.0 × 108 cfu/mL ETEC K88 for 3 d. Results are expressed as mean ± SD of the 6 mice in each group. a Results are statistically significant at P < 0.05.
Histological analysis
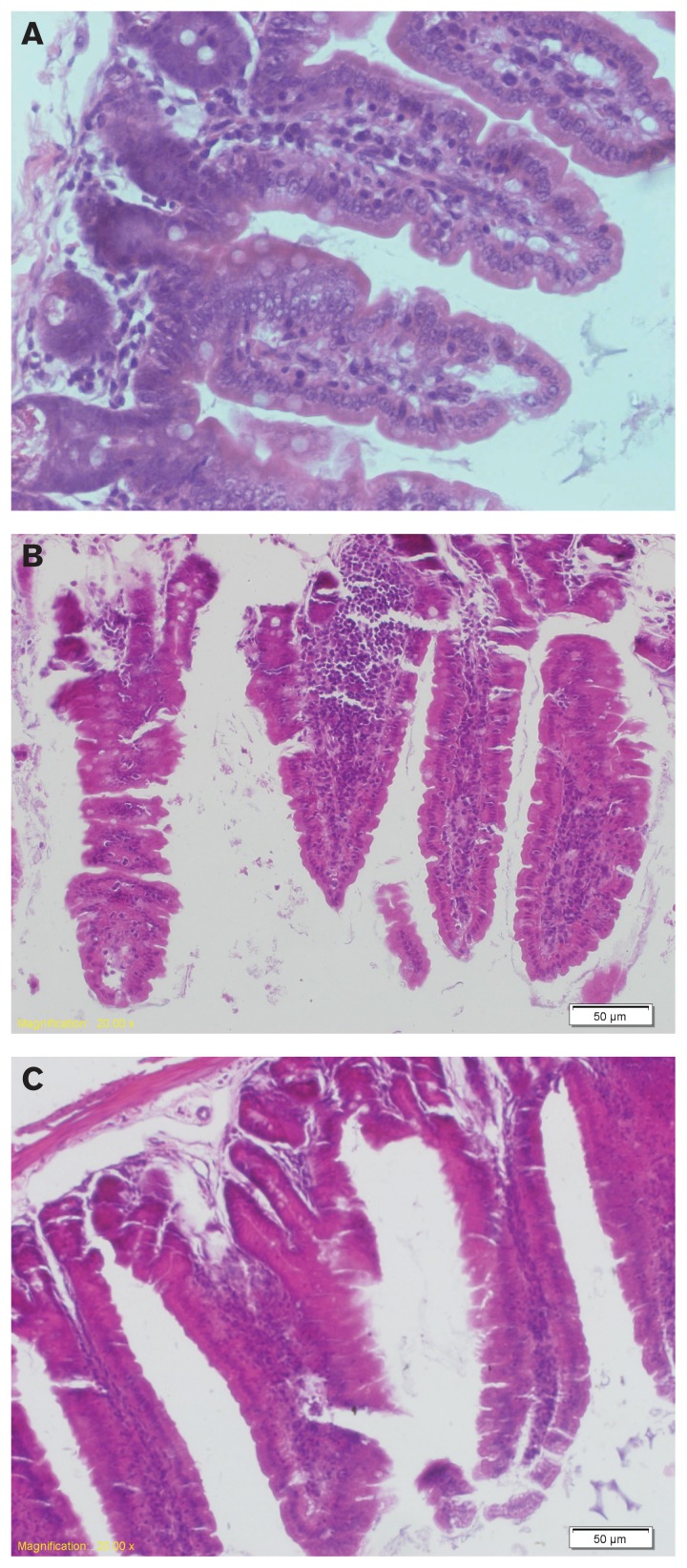
Compared to the control group (Figure 4A), intestinal villi disintegrated and fractured in the enteritis group. There were increased numbers of goblet cells in the mucosal layer, the intestinal lumen was filled with inflammatory secretions, and the gut gland gap was widened. Fluid had leaked from the gap and resulted in edema (Figure 4B). For the intervention group, inflammation was relieved and the numbers of goblet and inflammation cells decreased (Figure 4C).
Figure 4.
Mouse intestinal mucosa morphological changes observed by light microscopy [hematoxylin and eosin (H&E), 200×]. Histopathology of the duodenum from 1 of 2 independent experiments (n = 6 per group). This section was stained with H&E (original magnification, 200×). Control: control group, the mice were administered normal saline. Group A: enteritis group, the mice were administered 0.2 mL 2.0 × 108 cfu/mL ETEC K88 daily for 3 d. Group B: intervention group, the mice were administered 2.0 × 108 cfu/mL L. casei for 7 d, then 2.0 × 108 cfu/mL ETEC K88 for 3 d.
Discussion
The Th17 cells promote inflammatory reactions by secreting IL-17. The Treg cells are an important component of immune regulation and immunosuppression, and CD4+CD25+ Tregs are the Treg population that have been most widely studied. These cells play an important role in inflammatory diseases, allergies, and induction of transplantation tolerance (16). “Probiotics” is the general term for live microorganisms that are beneficial to host organisms and can improve resistance to and prevent disease. At present, it has been shown that probiotics can be used to treat antibiotics induced diarrhea, inflammatory bowel disease (IBD), irritable bowel syndrome, and other bowel diseases (17). Probiotics can prevent toxins from entering the circulation, suppress translocation of pathogenic bacteria to the intestinal mucosa, and prevent inflammation (18,19). Probiotics are increasingly widely applied in the management of enteritis, but its mechanism of action is not clear to date. Thus, this experiment aimed to study effects of L. casei on Th17 and Treg cells.
The Th cell precursors differentiate into naive T-cells under antigenic stimulation, and naive T-cells can differentiate into various auxiliary T-cell subsets under the influence of various cytokines, antigens, and antigen-presenting cells. Naive T-cells will preferentially express the transcription factor, RORγt, under the influence of IL-6 and TGF-β to become Th17 cells (20), or will preferentially express the transcription factor FoxP3 under the influence of IL-10 and TGF-β to become Tregs (12). Through in vivo experiments, this study showed that IL-6 expression in the intervention group was lower than in the enteritis group, indicating that L. casei induced naive T-cells to differentiate toward Treg cells.
The Th17 and Treg cells are closely related in both differentiation and function. Under physiological conditions, inflammatory responses promoted by Th17 cells are in equilibrium with those suppressed by Tregs, while their imbalance is associated with infectious diseases (21,22), autoimmune diseases (23,24), and cancer (25,26). Pertinent studies have shown that RORγt/Foxp3 are key regulatory factors in the development and maturation of Th17 and Treg cells (27–30), and play an important role in maintaining their functions. Further, IL-17A/IL-10 are the main effector molecules of Th17/Treg cells (31–33). This study found that after mice with enteritis were administered L. casei, the number of CD4+CD25+Foxp3+ Tregs and expression of Foxp3 mRNA and protein were all significantly increased. Naive T-cells were induced to differentiate toward Treg cells. Through detection of morphological changes in intestinal histology, it was determined that intestinal inflammation was relieved. This study suggests that the dynamic expression of Th17 and Treg cells was closed associated with occurrence and outcome of intestinal inflammation.
The balance of Th17/Treg cells can regulate immune responses to both self and foreign antigens and can underlie the pathogenesis and pathophysiological changes of certain inflammatory and autoimmune diseases. This study showed that by correcting the ratio imbalance of Th17/Treg cells, L. casei could have possible implications for the ETEC induced intestinal inflammation. This study adds interesting information about the growing field of probiotics and commensal microbiota.
Acknowledgment
This work was supported by the China Natural Science Foundation (No. 31372413).
References
- 1.Zhu J, Yin XH, Yu H, Zhao LP, Sabour P, Gong J. Involvement of quorum sensing and heat-stable enterotoxin a in cell damage caused by a porcine enterotoxigenic E. coli strain. Infect Immun. 2011;79:1688–1695. doi: 10.1128/IAI.01281-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fairbrother JM, Nadeau E, Gyles CL. Escherichia coli in post-weaning diarrhea in pigs: An update on bacterial types, pathogenesis, and prevention strategies. Anim Health Res. 2005;6:17–39. doi: 10.1079/ahr2005105. [DOI] [PubMed] [Google Scholar]
- 3.Wilkinson JL, Hooda PS, Barker J, Barton S, Swinden J. Ecotoxic pharmaceuticals, personal care products, and other emerging contaminants: A review of environmental, receptor-mediated, developmental, and epigenetic toxicity with discussion of proposed toxicity to humans. Crit Rev Env Sci Tec. 2016;46:336–381. [Google Scholar]
- 4.Meng X, Francois GB, Marie-Josee D, Suha J. Encapsulation of Lactobacillus casei ATCC 393 cells and evaluation of their survival after freeze-drying, storage and under gastrointestinal conditions. J Food Eng. 2016;168:52–59. [Google Scholar]
- 5.Li T, Si ZZ, Qi HZ, He ZJ, Li YN. IL-17 in the early diagnosis of acute renal allograft rejection in mice. J Cent South Univ. 2011;36:1147–1152. doi: 10.3969/j.issn.1672-7347.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Rathore JS, Wang Y. Protective role of Th17 cells in pulmonary infection. Vaccine. 2016;34:1504–1514. doi: 10.1016/j.vaccine.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 7.Campbell DJ, Ziegler SF. Foxp3 modifies the phenotypic and functional properties of regulatory T cells. Nat Rev Immunol. 2007;7:305–310. doi: 10.1038/nri2061. [DOI] [PubMed] [Google Scholar]
- 8.Ruggieri S, Frassanito MA, Dammacco R, Guerriero S. Treg lymphocytes in autoimmune uveitis. Ocul Immunol Inflamm. 2012;20:255–261. doi: 10.3109/09273948.2012.681830. [DOI] [PubMed] [Google Scholar]
- 9.Maggio R, Viscomi C, Andreozzi P, et al. Normocaloric low cholesterol diet modulates Th17/Treg balance in patients with chronic hepatitis C virus infection. PLoS One. 2014;9:e112346. doi: 10.1371/journal.pone.0112346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waigh JD, Takai SJ, Marelli B, et al. Cutting edge: Epigenetic regulation of Foxp3 defines a stable population of CD4 regulatory T cells in tumors from mice and humans. J Immunol. 2015;194:878–882. doi: 10.4049/jimmunol.1402725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fontenot JD, Dooley JL, Farr AG, Rudensky AY. Developmental regulation of Foxp3 expression during ontogeny. J Exp Med. 2005b;202:901–906. doi: 10.1084/jem.20050784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimura A, Kishimoto T. IL-6: Regulator of Treg/Th17 balance. Eur J Immunol. 2010;40:1830–1835. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 13.Harris TJ, Grosso JF, Yen HR, et al. Cutting edge: An in vivo requirement for STAT3 signaling in Th17 development and TH17-dependent autoimmunity. J Immunol. 2007;179:4313–4317. doi: 10.4049/jimmunol.179.7.4313. [DOI] [PubMed] [Google Scholar]
- 14.Liu YZ, Zhang P, Li J, Kulkarni AB, Perruche S, Chen WJ. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol. 2008;9:632–640. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- 15.Wang K, Qi Y, Yi SS, Pei ZH, Pan N, Hu GX. Mouse duodenum as a model of inflammation induced by enterotoxigenic Escherichia coli K88. J Vet Res. 2016;60:19–23. [Google Scholar]
- 16.Margarita MVL, Valerie LE, Nadine ML, et al. Induction of transplantation tolerance by allogeneic donor-derived CD4+CD25+Foxp3+ regulatory T cells. Transpl Immunol. 2008;19:127–135. doi: 10.1016/j.trim.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Marteau P, Seksik P, Jian R. Probiotics and intestinal health effects: A clinical perspective. Brit J Nutr. 2002;88:S51–57. doi: 10.1079/BJN2002629. [DOI] [PubMed] [Google Scholar]
- 18.Alexandre Y, Le-Blay G, Boisrame-Gastrin S, et al. Probiotics: A new way to fight bacterial pulmonary infections? Med Maladies Infect. 2014;44:9–17. doi: 10.1016/j.medmal.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Zareie M, Johnson-Henry K, Jury J, et al. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut. 2006;55:1553–1560. doi: 10.1136/gut.2005.080739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ziegler SF, Buckner JH. Foxp3 and the regulation of Treg/Th17 differentiation. Microbes Infect. 2009;11:594–598. doi: 10.1016/j.micinf.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang GD, Lim S, Kim DH. Oleanolic acid ameliorates dextran sodium sulfate-induced colitis in mice by restoring the balance of Th17/Treg cells and inhibiting NF-κB signaling pathway. Int Immunopharmacol. 2015;29:393–400. doi: 10.1016/j.intimp.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 22.Yuan XS, Tong B, Dou YN, Wu X, Wei ZF, Dai Y. Tetrandrine ameliorates collagen-induced arthritis in mice by restoring the balance between Th17 and Treg cells via the aryl hydrocarbon receptor. Biochem Pharmacol. 2015;27:396–403. doi: 10.1016/j.bcp.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 23.Marcin P, Sarah EJ, Joseph DP, et al. Renal allograft rejection: Examination of delayed differentiation of Treg and Th17 effector T cells. Immunobiology. 2013;21:303–310. doi: 10.1016/j.imbio.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Ryu JG, Lee J, Kim EK, et al. Treatment of IL-21R-Fc control autoimmune arthritis via suppression of STAT3 signal pathway mediated regulation of the Th17/Treg balance and plasma B cells. Immunol Lett. 2015;163:143–150. doi: 10.1016/j.imlet.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Domenico G, Marina DM, Annamaria T, et al. Peripheral depletion of NK cells and imbalance of the Treg/Th17 axis in idiopathic pulmonary fibrosis patients. Cytokine. 2014;66:119–126. doi: 10.1016/j.cyto.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Poonam G, Avadhesh KS, Nootan KS, Satya D. Inter-relation of Th1, Th2, Th17 and Treg cytokines in oral cancer patients and their clinical significance. Hum Immunology. 2014;75:330–337. doi: 10.1016/j.humimm.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 28.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor ROR-γt. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marson A, Kretschmer K, Frampton GM, et al. Foxp3 occupancy and regulation of key target genes during T-cell stimulation. Nature. 2007;445:931–935. doi: 10.1038/nature05478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michel ML, Mendes-da-Cruz D, Keller AC, et al. Critical role of ROR-γt in a new thymic pathway leading to IL-17-producing invariant NKT cell differentiation. P Natl Acad Sci USA. 2008;105:19 845–19 850. doi: 10.1073/pnas.0806472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deng B, Zhu JM, Wang Y, et al. Intratumor hypoxia promotes immune tolerance by inducing regulatory T cells via TGF-β1 in gastric cancer. PLoS One. 2013;8:e63777. doi: 10.1371/journal.pone.0063777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 33.Kullberg MC, Hay V, Cheever AW, et al. TGF-β1 production by CD4+CD25+ regulatory T cells is not essential for suppression of intestinal inflammation. Eur J Immunol. 2005;35:2886–2895. doi: 10.1002/eji.200526106. [DOI] [PubMed] [Google Scholar]