Abstract
Programmed cell death protein 1 (PD-1), a costimulatory molecule of the CD28 family, has 2 ligands, PD-L1 and PD-L2. Our previous studies showed that the expression of PD-1 and PD-L1 is up-regulated during viral infection in pigs. Extensive studies have shown that blockade of the PD-1/PD-L1 pathways by anti-PD-L1 antibody or soluble PD-1 restores exhausted T-cells in humans and mice. In the present study the extracellular domains of PD-1 and PD-L1 were used to evaluate the binding of PD-1 and PD-L1 with peripheral blood mononuclear cells (PBMCs). We amplified the cDNA encoding the extracellular domains of PD-1 and PD-L1 to construct recombinant expression plasmids and obtain soluble recombinant proteins, which were then labeled with fluorescein isothiocyanate (FITC). The His-ExPD-1 and His-ExPD-L1 recombinant proteins were expressed in the form of inclusion bodies with a relative molecular weight of 33.0 and 45.0 kDa, respectively. We then prepared polyclonal antibodies against the proteins with a multi-antiserum titer of 1:102 400. Binding of the proteins with PBMCs was evaluated by flow cytometry. The fluorescence signals of His-ExPD-1-FITC and His-ExPD-L1-FITC were greater than those for the FITC control. These results suggest that the soluble recombinant proteins may be used to prepare monoclonal antibodies to block the PD-1/PD-L1 pathway.
Résumé
La protéine de la mort cellulaire programmée (PD-1), une molécule co-stimulatrice de la famille de CD28, a deux ligands, PD-L1 et PD-L2. Nos études antérieures ont montré que l’expression de PD-1 et PD-L1 est régulée à la hausse lors d’une infection virale chez des porcs. Des études exhaustives ont montré chez l’humain et la souris qu’un blocage de la voie PD-1/PD-L1 par des anticorps anti PD-L1 ou du PD-1 soluble permet la régénération des cellules T épuisées. Dans la présente étude les domaines extracellulaires de PD-1 et PD-L1 ont été utilisés afin d’évaluer l’attachement de PD-1 et PD-L1 avec des cellules mononucléaires du sang périphérique (CMSP). Nous avons amplifié l’ADNc codant pour les domaines extracellulaires de PD-1 et PD-L1 pour construire des plasmides d’expression recombinants et obtenir des protéines recombinants solubles, qui ont par la suite été marquées avec de l’isothiocyanate de fluorescéine (ITCF). Les protéines recombinantes His-ExPD-1 et His-ExPD-L1 étaient exprimées sous la forme de corps d’inclusion avec un poids moléculaire relatif de 33,0 et 45,0 kDa, respectivement. Nous avons par la suite préparé des anticorps polyclonaux contre ces protéines avec un antisérum titrant 1:102 400. L’attachement des protéines aux CMSP a été évalué par cytométrie en flux. Les signaux de fluorescence de His-ExPD-1-ITCF et His-ExPD-L1-ITCF étaient supérieurs à ceux pour le témoin ITCF. Ces résultats suggèrent que les protéines recombinantes solubles pourraient être utilisées afin de préparer des anticorps monoclonaux pour bloquer la voie PD-1/PD-L1.
(Traduit par Docteur Serge Messier)
Introduction
Optimal activation of T-cells for clonal expansion depends on 2 distinct signals from antigen-presenting cells. One is the delivery of specific antigen to the T-cell receptor by specific peptides in the context of major histocompatibility proteins on antigen-presenting cells; the other is triggered through a distinct T-cell surface molecule. The immunoglobulin (Ig) B7-CD28 superfamily, one of the best-characterized costimulatory receptor families, not only provides critical positive second signals to initiate and sustain the T-cell response but also contributes key negative second signals to down-regulate and terminate the T-cell response (1–4). The negative second signals include many cell surface molecules, especially negative receptors, such as cytolytic T-lymphocyte-associated Ag-4 (CTLA-4) (5), programmed cell death protein 1 (PD-1) (6), T-cell immunoglobulin and mucin-domain-containing molecule 3 (Tim-3) (7), lymphocyte-activation protein 3 (LAG-3) (8), and forkhead box P3 (FoxP3) (9).
Among these negative receptors, PD-1 plays an important role in reversible immune dysfunction (10). It is a 55.0-kDa type I transmembrane glycoprotein of the CD28 superfamily. Its single extracellular Ig variable (V)-like domain (11–13) is expressed on activated T-cells, B-cells, and monocytes (14–16). Porcine PD-1 has 63% and 54% identity with the human and murine PD-1, respectively, and a similar structure, with 2 highly hydrophobic amino acid fragments constituting the signal peptide (amino acids 1 to 20) and the transmembrane domain (amino acids 168 to 194), as well as an extracellular domain and a cytoplasmic domain (17,18). The extracellular domain plays an important role in the binding of PD-1 to its 2 known ligands, PD-L1 (19,20) and PD-L2 (21,22). Many studies have demonstrated that a high level of PD-1 expression is closely related to infection in humans with viruses such as human immunodeficiency virus, hepatitis B virus, and hepatitis C virus (23–25). Binding of PD-1 with its 2 ligands inhibits the proliferation of T-cells and the production of cytokines, especially interleukin-2 and interferon gamma (20, 21). With blockade of the interaction between PD-1 and PD-L1 by antibodies or soluble proteins, impaired T-cells can regain their ability to proliferate, secrete cytokines, and kill infected cells (6,26–34). Our previous research demonstrated that the expression of PD-1 and PD-L1 is up-regulated during viral infection in pigs (35). In the present study the extracellular domains of PD-1 and PD-L1 were used to evaluate the function of PD-1 and PD-L1 during viral infection in pigs. Recombinant protein obtained by high-level expression and purification interacted in vitro with peripheral blood mononuclear cells (PBMCs) from pigs infected with classical swine fever virus (CSFV).
Materials and methods
Cloning of extracellular domains
Primers were designed according to the porcine PD-1 and PD-L1 gene sequences (NM_001204379.1 and NM_001025221.1) published in GenBank (National Center for Biotechnology Information, Bethesda, Maryland, USA). Important PD-1 and PD-L1 gene sites were modified according to an analysis of codon bias of Escherichia coli (36), and the integrated genes were synthesized by Shanghai Bio-engineering Company, Shanghai, China. The regions encoding the extracellular domains were then amplified by polymerase chain reaction (PCR). Total RNA was extracted from PBMCs with the use of Trizol reagent (Invitrogen, Carlsbad, California, USA) according to the manufacturer’s protocol (37). The ExPD-1 and ExPD-L1 genes modified with EcoRI and XhoI restriction sites were cloned into the corresponding sites of pMD-18T vector (TaKaRa Biotechnology Company, Dalian, China) to form the recombinant cloning plasmids. Positive colonies were identified by PCR, double enzymatic digestion, and DNA sequencing and named pMD-ExPD-1 and pMD-ExPD-L1.
Construction of recombinant expression plasmids
The positive colonies were digested by restriction enzymes EcoRI and XhoI (TaKaRa) and the target segments cloned into pET-32a(+) (kept in our laboratory) after digestion with the same enzymes to subclone the ExPD-1 and ExPD-L1 genes. The recombinant expression plasmids were then transformed into E. coli Rosetta (DE3) cells. Subsequently the positive colonies were identified by PCR amplification and DNA sequencing, named pET-32a-ExPD-1 and pET-32a-ExPD-L1, and cultured for 16 to 18 h in 3 mL of lysogeny broth containing ampicillin (Invitrogen), 100 μg/mL, to screen for resistant transformants. Genomic DNA was extracted from the cultured positive colonies by means of the E.Z.N.A. Plasmid Mini Kit I (Omega Bio-Tek, Norcross, Georgia, USA), according to the manufacturer’s instructions. Genomic DNA of the Rosetta cells was extracted as the negative control. Next, PCR was done with ExPD-1 and ExPD-L1 primers. A positive clone was cultured at 37°C in 2 × yeast–tryptone broth (1.6% tryptone, 1% yeast extract, and 0.5% NaCl; pH 7.0) to an optical density at 600 nm (OD600) of 0.5 to 1.0, induced with 0.5 mmol/L of isopropylthiogalactosidase (IPTG) for 4 h, and centrifuged to collect cell supernatant and cellular pellets for further analyses.
Identification of His-ExPD-1 and His-ExPD-L1 recombinant proteins
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE) and Western blot testing were done according to the manufacturers’ protocols (38). Briefly, 100 μL of cell supernatant or cellular pellets were resuspended in equal volumes of SDS-loading buffer. After electrophoresis the gel was stained with Coomassie Brilliant Blue R-250 (Sigma-Aldrich, St. Louis, Missouri, USA). Nitrocellulose transmembrane (Bio-Rad Laboratories, Hercules, California, USA) for the Western blot testing was blocked overnight with Tris-buffered saline (TBS; 20 mM Tris-HCl, pH 7.4, and 150 mM NaCl) containing 5% (w/v) nonfat dried milk, rinsed 3 times, and then immersed overnight in 20 mL of TBS containing 1% (w/v) nonfat dried milk, 1 μL of 6 × His-tag mouse monoclonal antibodies (PBL Biomedical Laboratories, Piscataway, New Jersey, USA), and 1 μL of mouse anti-human PD-1 monoclonal antibodies (Abcam, Cambridge, Massachusetts, USA) as the primary antibodies. Subsequently the membrane was rinsed 3 times and then immersed overnight in 20 mL of TBS containing 1% (w/v) nonfat dried milk and 1 μL of goat IgG monoclonal antibodies labeled with horseradish peroxidase (PBL) against mouse IgG as the secondary antibody. Then the membrane was rinsed 3 times and stained with diaminobenzidine (Sigma-Aldrich) to visualize peroxidase activity.
Protein purification and measurement
The greatest expression of the recombinant proteins was induced by IPTG under optimized conditions. Cells were harvested, the supernatant was discarded, and the inclusion body pellet was resuspended in phosphate buffer and centrifuged for 20 min. The cell pellets were then solubilized in 10 mL of elution buffer containing urea (0.1 mol/L of NaH2PO4·H2O, 0.01 mol/L of Tris base, 8 mol/L of urea, and 20 mmol/L of imidazole, adjusted to a pH of 8.0 with concentrated HCl). The precipitate was resuspended by centrifugation. The supernatant was collected and filtered with a 0.4-μm membrane and then loaded on a manually packed column containing 2 mL of nickel–nitrilotriacetic acid–agarose resin according to the instructions of the manufacturer (Qiagen, Valencia, California, USA).
Bovine serum albumin was diluted into 8, 4, 2, 1, 0.5, 0.25, and 0.125 mg/mL solutions and the OD280 of the different concentrations determined to establish a standard curve. With the same method the OD280 of His-ExPD-1 and His-ExPD-L1 was also determined and calculated according to the standard curve.
Preparation of polyclonal antibodies to the recombinant proteins
Four New Zealand female specific-pathogen-free rabbits (body mass 2 to 3 kg) were purchased from the Laboratory Animal Center, Henan Academy of Agricultural Sciences, Zhengzhou, Henan Province, China, and maintained under conventional conditions with food and water provided ad libitum. All experimental procedures were conducted according to institutional guidelines for animal ethics. On day 0, negative-control blood samples were collected and the rabbits vaccinated as previously described (39). Briefly, the rabbits were divided into 2 groups and injected intramuscularly with either His-ExPD-1 or His-ExPD-L1, 500 μg emulsified in 500 pL of phosphate-buffered saline (PBS)/Freund’s complete adjuvant. Boosters were given on days 15, 29, and 44 with the same dose and by the same route of antigen in Freund’s incomplete adjuvant. On day 54, blood samples were collected to be tested by Western blot or enzyme-linked immunosorbent assay (ELISA) for antibody activity. The ELISA results were expressed as OD630 and OD450 for each sample. Mean values for the positive (mouse anti-Human PD-1 and mouse anti-6×His monoclonal antibodies) and negative controls were obtained as the average value for 2 wells. The sample/positive (S/P) value was calculated with the following formula: S/P = [OD450 + OD630 of sample]/[OD450 + OD630 of negative control]. Samples with an S/P value of less than 3 were classified as negative and samples with an S/P value of 3 or greater as positive. When the antibody levels peaked, the rabbits were killed to collect blood. Cell-free serum was decanted gently into a clean test tube and stored at −20°C for further study.
Protein labeling
The amino acid residues imidazole, carbonyl, and cheese ammonia acyl groups of the His-ExPD-1 and His-ExPD-L1 proteins were labeled with fluorescein isothiocyanate (FITC) by means of an antibody labeling kit (Applied Biosystems, Foster City, California, USA) according to the manufacturer’s protocol, as follows. First, 40 μL of the borate buffer (0.67 M) was added to 0.5 mL of protein in PBS (2 mg/mL). Then 0.5 mL of the prepared protein was added to the vial of FITC reagent (30 μL) and pipetted up and down 10 times until all the dye was dissolved. The vial was briefly centrifuged to collect the sample in the bottom of the tube. The reaction mixture was incubated for 60 min at room temperature, protected from light. Second, 2 spin columns were placed in separate microcentrifuge collection tubes. The purification resin was mixed to ensure uniform suspension, and 400 μL of the suspension was added to both spin columns. The stored solution was centrifuged for 30 to 45 s at about 1000 × g to remove the storage solution. The used collection tubes were discarded and the columns placed in new collection tubes. Third, 250 to 270 μL of the labeled reaction mixture was added to each spin column and mixed with the resin by pipetting up and down or briefly vortexing. The columns were centrifuged for 30 to 45 s at about 1000 × g to collect the purified proteins. Alternatively, labeled proteins were stored in single-use aliquots at −20°C.
Identification of protein binding with PBMCs in vitro
We isolated PBMCs from 45-day-old pigs weighing approximately 15 kg that were provided by pig breeding center of Henan province, (Zhengzhou, Henan Province, China) for our previous study (35). All experimental procedures were conducted according to institutional guidelines for animal ethics. In the previous study we had found the expression levels of PD-1 and PD-L1 mRNA to be significantly up-regulated in the pigs experimentally infected with CSFV compared with the control pigs at 3 d (P < 0.05) and 7 d (P < 0.01) after infection. The PBMCs were diluted with PBS to 1 × 106 cells/mL and divided into 2 experimental groups and a control group. They were then resuspended in 100 μL of phosphate buffer and incubated for 30 min at 4°C in a mixed solution of 10 μg/mL of His-ExPD-1-FITC, His-ExPD-L1-FITC, or FITC as the control. Washing buffer (PBS, 1 mL) was added to collect the PBMCs, which were then resuspended in 1 mL of phosphate buffer and filtered through a 200-μm mesh screen. Flow cytometry was used to recognize PD-1 and PD-L1 on the surface of the PBMCs.
Results
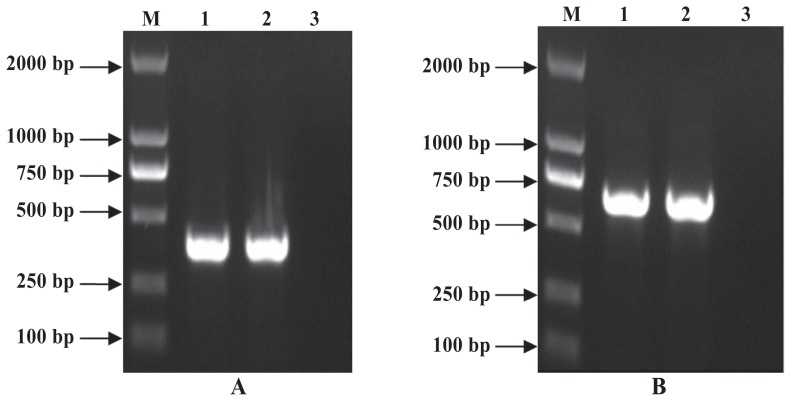
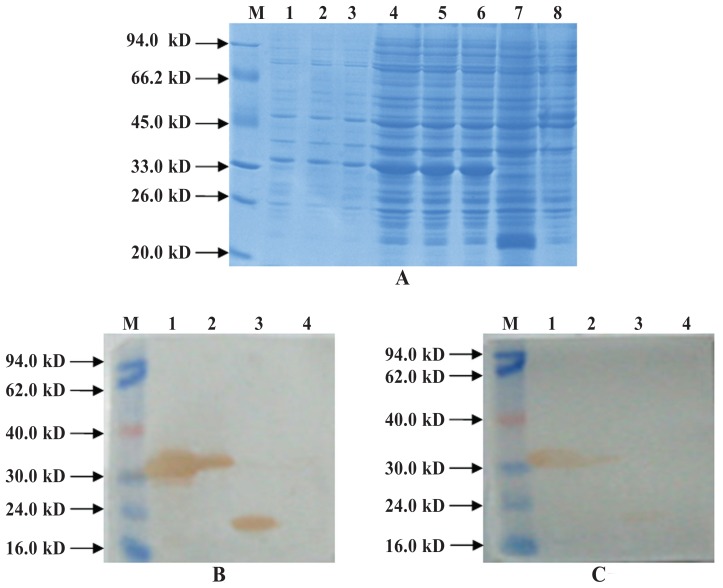
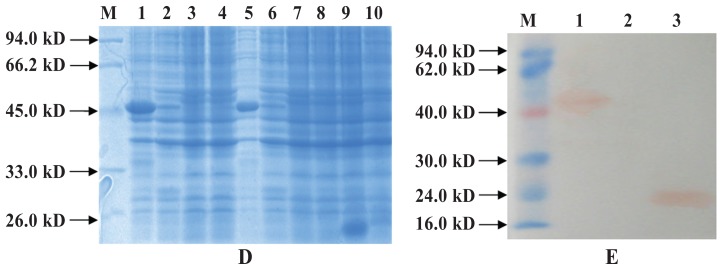
After cloning of the ExPD-1 and ExPD-L1 gene regions encoding the extracellular domains of PD-1 and PD-L1, DNA fragments of 366 base pairs (bp) and 684 bp were obtained by PCR (Figure 1), double enzymatic digestion, and DNA sequencing. High-level production of recombinant protein in bacteria was induced by IPTG under optimized conditions: these proteins, His-ExPD-1 and His-ExPD-L1, were in the form of inclusion bodies with relative molecular weights of 33.0 and 45.0 kDa, which agreed with the predicted values from the gene sequences (Figures 2A and 3A). Western blot testing indicated that the recombinant proteins were recognized by 6 × His-tag mouse monoclonal antibodies (Figures 2B and 3B); His-ExPD-1 was also recognized by PD-1 mouse monoclonal antibodies against human antigen (Figure 2C), which showed that human PD-1 and porcine His-ExPD-1 recombinant protein have high protein homology, not only high sequence homology. The SDS-PAGE and Western blot results suggested that the recombinant expression plasmid could effectively express His-ExPD-1 and His-ExPD-L1 as inclusion bodies.
Figure 1.
Polymerase chain reaction (PCR) products of genomic DNA from recombinant plasmids pET-32a-ExPD-1/Rosetta (A) and pET-32a-ExPD-L1/ Rosetta (B) amplified by primers. Lane M — DL2000 DNA marker; lanes 1 and 2 — PCR products; lane 3 — negative control. bp — base pairs.
Figure 2.
Results of His-ExPD-1 analysis by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE) (A) and Western blot testing with, as primary antibodies, 6 × His-tag mouse monoclonal antibodies (B) and mouse PD-1 monoclonal antibodies against human antigen (C). A, lane M — low molecular weight standard prestained marker (Fermentas, Waltham, Massachusetts, USA); lanes 1 to 3 — recombinant Rosetta (DE3) cells transformed with pET-32a-PD-1, uninduced; lanes 4 to 6 — recombinant Rosetta (DE3) cells transformed with pET-32a-PD-1 and induced by isopropylthiogalactosidase (IPTG); lane 7 — recombinant Rosetta (DE3) cells electrotransformed with pET-32a(+) and induced by IPTG; lane 8 — Rosetta (DE3) cells induced by IPTG as a negative control. B and C, lane M — low molecular weight standard prestained marker; lane 1 — recombinant Rosetta (DE3) cells transformed with pET-32a-PD-1 and induced by IPTG; lane 2 — recombinant Rosetta (DE3) cells transformed with pET-32a-PD-1, uninduced; lane 3 — recombinant Rosetta (DE3) cells electrotransformed with pET-32a(+) and induced by IPTG; lane 4 — Rosetta (DE3) cells induced by IPTG as a negative control. A gel band revealed the expressed protein to be about 33.0 kDa, and Western blot testing with goat monoclonal antibodies to mouse IgG labeled with horseradish peroxidase as the secondary antibody confirmed that the 33.0-kDa band was the His-ExPD-1 protein (B and C).
Figure 3.
Results of His-ExPD-L1 analysis by SDS-PAGE (A) and Western blot testing (B). A, lane M — low molecular weight standard prestained marker; lanes 1, 3, 5, and 7 — recombinant Rosetta (DE3) cells transformed with pET-32a-PD-L1 and induced by IPTG; lanes 2, 4, 6, and 8 — recombinant Rosetta (DE3) cells electrotransformed with pET-32a-PD-L1, uninduced; lane 9 — recombinant Rosetta (DE3) cells electrotransformed with pET-32a(+) and induced by IPTG; lane 10 — Rosetta (DE3) cells induced by IPTG as a negative control. B, lane M — low molecular weight standard prestained marker; lane 1 — recombinant Rosetta (DE3) cells transformed with pET-32a-PD-L1 and induced by IPTG; lane 2 — Rosetta (DE3) cells induced by IPTG as a negative control; lane 3 — recombinant Rosetta (DE3) cells electrotransformed with pET-32a(+) and induced by IPTG. A gel band revealed the expressed protein to be about 45.0 kDa, and Western blot testing confirmed that the 45.0-kDa band was the His-ExPD-L1 protein.
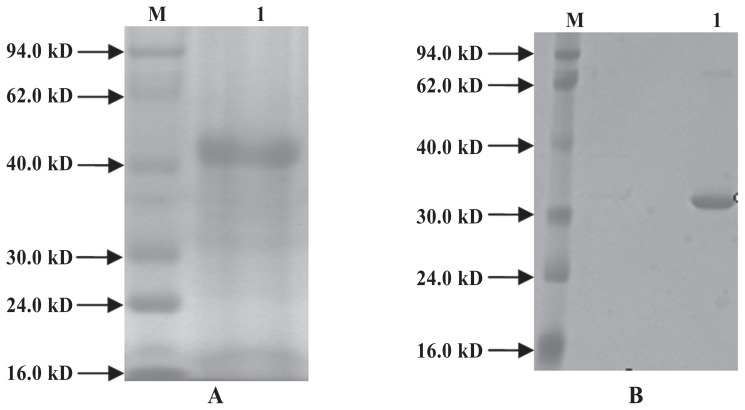
Under optimum inducement conditions the recombinant proteins were abundantly expressed, constituting as much as 95% of the total cell protein (Figure 4). The levels of His-ExPD-1 and His-ExPD-L1 were 0.9 and 1.5 mg/mL, respectively, as determined by protein scanning and ultraviolet absorbance.
Figure 4.
Results of analysis of the purity of the purified recombinant His-ExPD-1 (A) and His-ExPD-L1 (B) proteins. A, lane M — low molecular weight standard prestained marker; lane 1 — purified His-ExPD-1. B, lane M — low molecular weight standard prestained marker; lane 1 — purified His-ExPD-L1.
The Western blot results suggested that polyclonal antibodies against His-ExPD-1 and His-ExPD-L1 recombinant proteins could react with PD-1 and PD-L1 recombinant proteins. The multi-antiserum titers of His-ExPD-1 and His-ExPD-L1 were both 1:102 400.
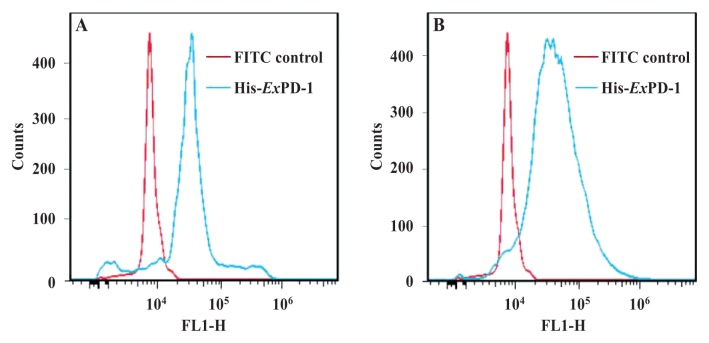
The fluorescence signals of His-ExPD-1-FITC and His-ExPD-L1-FITC were greater than those of the FITC control (Figure 5). The flow cytometry results showed that His-ExPD-1-FITC and His-ExPD-L1-FITC could bind with PBMCs in vitro. These results suggest that these recombinant proteins have the biologic activity of the natural porcine PD-1 and PD-L1 proteins.
Figure 5.
Flow cytometry results for the binding of His-ExPD-1-FITC (A), His-ExPD-L1-FITC (B), and FITC as a control with peripheral blood mononuclear cells (PBMCs) from pigs 7 d after experimental infection with Classical swine fever virus. The fluorescence signal of the protein binding was greater than the signal of the control binding for both proteins. In addition, PD-L1 on the surface of the PBMCs from the infected pigs was recognized by His-ExPD-1-FITC but not by FITC, and PD-1 on the surface of the PBMCs from the infected pigs was recognized by His- ExPD-L1-FITC but not by FITC. FL1-H — fluorescence height or intensity (relative units).
Discussion
Porcine PD-1 and its ligand PD-L1 are type I transmembrane glycoproteins of the B7-CD28 Ig receptor superfamily with a single IgV-like extracellular domain (11–13,40). The crystal structure of the PD1/PD-L2 complex suggested that PD-L1 and PD-L2 may form similar assemblies with PD-1 (41). The binding interfaces were formed by the front β-sheets of both the PD-1 IgV domain (labeled ABED and A′GFCC′ C″) and the PD-L2 IgV domain (labeled AGFCC′C″ and BED). Side chains of residues on β-strands (CC′FG) of PD-1 and on β-strands (GFCC′) of PD-L1 made PD-1/PD-L1 contacts interface (40). Residues from the GFCC′ strands and the CC′, CC′, and FG loops of PD-1 contributed to the binding interface and packed against the AGFC strands and the FG loop of the PD-L2 IgV domain, as well as the AGFC strands and the CC′ loop of the PD-L1 IgV domain (40). These reports suggest that the extracellular domains of PD-1 and its ligands play an important role in their interactions. Therefore, antibodies and soluble proteins of the extracellular domain are potent inhibitors of PD-1 and PD-L1.
Previous studies showed that producing biologically active recombinant protein in bacteria was feasible (4). In this study, the extracellular domains of porcine PD-1 and PD-L1 were the object of study to obtain soluble protein. Residues of the PD-L1 V domain that bind to PD-1 are conserved across species (40). The residues (Ala-121, Asp-122, Tyr-123, and Lys-124 in the G strand of PD-L1) make intimate contacts with PD-1 and are conserved in all available PD-L1 sequences from mammals and birds (40). The extracellular domains of murine PD-1 and of human PD-L1 were from Leu-25 to Ser-157 and from Ala-18 to Thr-239, respectively (40). According to the published porcine PD-1 and PD-L1 gene sequences (NM_001204379.1 and NM_001025221.1) in GenBank, expression constructs encoding the extracellular domains of porcine PD-1 and PD-L1 were from Leu-24 to Leu-166 and from Val-23 to Thr-237, respectively.
The His-ExPD-1 and His-ExPD-L1 recombinant proteins were generated with use of the well-known polyhistidine tag (6 × His) fusion system, with the pET-32a(+) expression vector, which provided high-level expression and easy purification (42). The recombinant proteins were almost all in the form of inclusion bodies and soluble protein. Soluble active production can be induced by altering expression conditions (temperature, induction time, IPTG concentration, cell density aeration, or pH of culture). Unfortunately, all methods were unsuccessful at inducing soluble active products. Therefore, soluble biologically active protein was obtained by denaturing and refolding the inclusion bodies. Finally, high-quality protein with good bioactivity was obtained by optimizing the refolding conditions. Protein scanning and ultraviolet absorbance were used to elevate the purity, to more than 95%. Thus, this method can be used to obtain these proteins in high amounts and high purity for preparing polyclonal antibodies, monoclonal antibodies, and FITC-labeled proteins for further study.
Murine PD-1 can bind with human PD-L1 (40), which can interact with porcine PD-1 (17,43,44), and human PD-1 can bind with porcine PD-L1 (43). The conformations of PD-1 from different species are highly conserved. Thus, mouse PD-1 against human antigen was used herein as the primary antibody for Western blot testing with porcine His-ExPD-1 recombinant protein. The results hint that porcine PD-1 may play a negative role in immune regulation similar to that of human and murine PD-1. We will evaluate this hypothesis in future experiments.
Our previous analysis by quantitative real-time PCR of the biologic activity of His-ExPD-1 and His-ExPD-L1 with PBMCs from pigs with CSFV infection showed that the expression of PD-1 and PD-L1 mRNA was significantly upregulated compared with that in the control groups (35). Therefore, in the present study natural PD-1 and PD-L1 on the surface of PBMCs were used to evaluate the bioactivity of His-ExPD-1 and His-ExPD-L1. We found that His-ExPD-1-FITC and His-ExPD-L1-FITC could recognize natural PD-1 and PD-L1 proteins on PBMCs. The results suggest that His-ExPD-1 and His-ExPD-L1 recombinant proteins can bind with natural PD-1 and PD-L1 on the surface of PBMCs from pigs and therefore could be used to develop monoclonal antibodies that can block the PD-1/PD-L1 pathway to restore immune status. Furthermore, with blockade of the interaction between PD-1 and PD-L1 by soluble proteins, impaired T-cells are able to regain their ability to proliferate, secrete cytokines, and kill infected cells (6,26–34). Therefore, His-ExPD-1 and His-ExPD-L1 recombinant proteins, or even their polyclonal antibodies, could be used to block the PD-1/PD-L1 pathway in pigs during the virus infection. Other biologic characteristics of His-ExPD-1 and His-ExPD-L1 and their possible roles during virus infection in pigs in vitro and in vivo are being investigated in our laboratory.
Acknowledgments
This work was supported by grants (nos. 31272539 and 31201877) from the National Natural Science Foundation of China.
References
- 1.Coyle AJ, Gutierrez-Ramos JC. The expanding B7 superfamily: Increasing complexity in costimulatory signals regulating T cell function. Nat Immunol. 2001;2:203–209. doi: 10.1038/85251. [DOI] [PubMed] [Google Scholar]
- 2.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 3.Carreno BM, Collins M. The B7 family of ligands and its receptors: New pathways for costimulation and inhibition of immune responses. Annu Rev Immunol. 2002;20:29–53. doi: 10.1146/annurev.immunol.20.091101.091806. [DOI] [PubMed] [Google Scholar]
- 4.Li DW, Yu JF, Chen YJ, et al. Refolding and characterization of recombinant human GST-PD-1 fusion protein expressed in Escherichia coli. Acta Biochim Biophys Sin (Shanghai) 2004;36:141–146. doi: 10.1093/abbs/36.2.141. [DOI] [PubMed] [Google Scholar]
- 5.Janeway CA, Jr, Travers P, Walport M, Shlomchik MJ. Immunobiology. 6th ed. New York and London: Garland Science; 2005. pp. 328–329. [Google Scholar]
- 6.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 7.Zhu C, Anderson AC, Schubart A, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 8.Huang CT, Workman CJ, Flies D, et al. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–513. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Paust S, Lu L, McCarty N, Cantor H. Engagement of B7 on effector T cells by regulatory T cells prevents autoimmune disease. Proc Natl Acad Sci U S A. 2004;101:10398–10403. doi: 10.1073/pnas.0403342101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu ZQ, Zhao WH. Critical role of PD-1/PD-L1 pathway in generation and function of follicular regulatory T cells. Cell Mol Immunol. 2013;10:286–288. doi: 10.1038/cmi.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shinohara T, Taniwaki M, Ishida Y, Kawaichi M, Honjo T. Structure and chromosomal localization of the human PD-1 gene (PDCD1) Genomics. 1994;23:704–706. doi: 10.1006/geno.1994.1562. [DOI] [PubMed] [Google Scholar]
- 13.Finger LR, Pu J, Wasserman R, et al. The human PD-1 gene: Complete cDNA, genomic organization, and developmentally regulated expression in B cell progenitors. Gene. 1997;197:177–187. doi: 10.1016/s0378-1119(97)00260-6. [DOI] [PubMed] [Google Scholar]
- 14.Agata Y, Kawasaki A, Nishimura H, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 15.Vibhakar R, Juan G, Traganos F, Darzynkiewicz Z, Finger LR. Activation-induced expression of human programmed death-1 gene in T-lymphocytes. Exp Cell Res. 1997;232:25–28. doi: 10.1006/excr.1997.3493. [DOI] [PubMed] [Google Scholar]
- 16.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng JM, Tian ZJ, Liu HG, et al. Cloning and identification of porcine programmed death 1. Vet Immunol Immunopathol. 2010;136:157–162. doi: 10.1016/j.vetimm.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, Schwartz JC, Guo X, et al. Structural and functional analysis of the costimulatory receptor programmed death-1. Immunity. 2004;20:337–347. doi: 10.1016/s1074-7613(04)00051-2. [DOI] [PubMed] [Google Scholar]
- 19.Mkrtichyan M, Najjar YG, Raulfs EC, et al. B7-DC-Ig enhances vaccine effect by a novel mechanism dependent on PD-1 expression level on T cell subsets. J Immunol. 2012;189:2338–2347. doi: 10.4049/jimmunol.1103085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 22.Tseng SY, Otsuji M, Gorski K, et al. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001;193:839–846. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saha A, Aoyama K, Taylor PA, et al. Host programmed death ligand 1 is dominant over programmed death ligand 2 expression in regulating graft-versus-host disease lethality. Blood. 2013;122:3062–3073. doi: 10.1182/blood-2013-05-500801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klenerman P, Hill A. T cells and viral persistence: Lessons from diverse infections. Nat Immunol. 2005;6:873–879. doi: 10.1038/ni1241. [DOI] [PubMed] [Google Scholar]
- 25.Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Curr Opin Immunol. 2007;19:408–415. doi: 10.1016/j.coi.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Velu V, Titanji K, Zhu B, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458:206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res. 2013;73:3591–3603. doi: 10.1158/0008-5472.CAN-12-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fourcade J, Sun Z, Benallaoua M, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDermott DF, Atkins MB. PD-1 as a potential target in cancer therapy. Cancer Med. 2013;2:662–673. doi: 10.1002/cam4.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dai B, Xiao L, Bryson PD, Fang J, Wang P. PD-1/PD-L1 blockade can enhance HIV-1 Gag-specific T cell immunity elicited by dendritic cell-directed lentiviral vaccines. Mol Ther. 2012;20:1800–1809. doi: 10.1038/mt.2012.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan XC, Li L, Mao JJ, et al. Synergistic effects of soluble PD-1 and IL-21 on antitumor immunity against H22 murine hepatocellular carcinoma. Oncol Lett. 2013;5:90–96. doi: 10.3892/ol.2012.966. Epub 2012 Oct 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao L, Wang D, Sun C, et al. Enhancement of SIV-specific cell mediated immune responses by co-administration of soluble PD-1 and Tim-3 as molecular adjuvants in mice. Hum Vaccin Immunother. 2014;10:724–733. doi: 10.4161/hv.27340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Porichis F, Hart MG, Zupkosky J, et al. Differential impact of PD-1 and/or interleukin-10 blockade on HIV-1-specific CD4 T cell and antigen-presenting cell functions. J Virol. 2014;88:2508–2518. doi: 10.1128/JVI.02034-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen LT, Ohashi PS. Clinical blockade of PD1 and LAG3 — potential mechanisms of action. Nat Rev Immunol. 2015;15:45–56. doi: 10.1038/nri3790. [DOI] [PubMed] [Google Scholar]
- 35.Yue F, Zhu YP, Zhang YF, et al. Up-regulated expression of PD-1 and its ligands during acute classical swine fever virus infection in swine. Res Vet Sci. 2014;97:251–256. doi: 10.1016/j.rvsc.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 36.Gurkan C, Ellar DJ. Recombinant production of bacterial toxins and their derivatives in the methylotrophic yeast Pichia pastoris. Microb Cell Fact. 2005;4:33. doi: 10.1186/1475-2859-4-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Felipe P, Hughes LE, Ryan MD, Brown JD. Co-translational, intraribosomal cleavage of polypeptides by the foot-and-mouth disease virus 2A peptide. J Biol Chem. 2003;278:11441–11448. doi: 10.1074/jbc.M211644200. [DOI] [PubMed] [Google Scholar]
- 38.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd ed. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 1989. pp. 880–897. [Google Scholar]
- 39.Santos-Argumedo L, Teixeira C, Preece G, Kirkham PA, Parkhouse RM. A B-lymphocyte surface molecule mediating activation and protection from apoptosis via calcium channels. J Immunol. 1993;151:3119–3130. [PubMed] [Google Scholar]
- 40.Lin DY, Tanaka Y, Iwasaki M, et al. The PD-1/PD-L1 complex resembles the antigen-binding Fv domains of antibodies and T cell receptors. Proc Natl Acad Sci U S A. 2008;105:3011–3016. doi: 10.1073/pnas.0712278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lázár-Molnár E, Yan Q, Cao E, Ramagopal U, Nathenson SG, Almo SC. Crystal structure of the complex between programmed death-1 (PD-1) and its ligand PD-L2. Proc Natl Acad Sci U S A. 2008;105:10483–10488. doi: 10.1073/pnas.0804453105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nallamsetty S, Waugh DS. A generic protocol for the expression and purification of recombinant proteins in Escherichia coli using a combinatorial His6-maltose binding protein fusion tag. Nat Protoc. 2007;2:383–391. doi: 10.1038/nprot.2007.50. [DOI] [PubMed] [Google Scholar]
- 43.Jeon DH, Oh K, Oh BC, et al. Porcine PD-L1: Cloning, characterization, and implications during xenotransplantation. Xenotransplantation. 2007;14:236–242. doi: 10.1111/j.1399-3089.2007.00403.x. [DOI] [PubMed] [Google Scholar]
- 44.Plege A, Borns K, Baars W, Schwinzer R. Suppression of human T-cell activation and expansion of regulatory T cells by pig cells overexpressing PD-ligands. Transplantation. 2009;87:975–982. doi: 10.1097/TP.0b013e31819c85e8. [DOI] [PubMed] [Google Scholar]