Abstract
Multi-walled carbon nanotubes (MWCNT) with their unique physico-chemical properties offer numerous technological advantages and are projected to drive the next generation of manufacturing growth. As MWCNT have already found utility in different industries including construction, engineering, energy production, space exploration and biomedicine, large quantities of MWCNT may reach the environment and inadvertently lead to human exposure. This necessitates the urgent assessment of their potential health effects in humans. The current study was carried out at NanotechCenter Ltd. Enterprise (Tambov, Russia) where large-scale manufacturing of MWCNT along with relatively high occupational exposure levels was reported. The goal of this small cross-sectional study was to evaluate potential biomarkers during occupational exposure to MWCNT. All air samples were collected at the workplaces from both specific areas and personal breathing zones using filter-based devices to quantitate elemental carbon and perform particle analysis by TEM. Biological fluids of nasal lavage, induced sputum and blood serum were obtained from MWCNT-exposed and non-exposed workers for assessment of inflammatory and fibrotic markers. It was found that exposure to MWCNTs caused significant increase in IL-1β, IL6, TNF-α, inflammatory cytokines and KL-6, a serological biomarker for interstitial lung disease in collected sputum samples. Moreover, the level of TGF-β1 was increased in serum obtained from young exposed workers. Overall, the results from this study revealed accumulation of inflammatory and fibrotic biomarkers in biofluids of workers manufacturing MWCNTs. Therefore, the biomarkers analyzed should be considered for the assessment of health effects of occupational exposure to MWCNT in cross-sectional epidemiological studies.
Keywords: MWCNT, Fibrosis, KL-6, TGF-beta, Osteopontin, Workers
1. Introduction
Engineered carbonaceous nanomaterials (CNMs) present tremendous opportunities for industrial growth and development and hold great promise through their applications in medicine, electronics, and numerous other areas. However, there are considerable gaps in our knowledge concerning the potential hazardous effects of CNMs on human health and the environment. The evidence for the potential adverse health effects associated with CNMs in humans comes from epidemiological studies of particulate matter (PM) arising from air pollution and other combustion processes (Delfino et al., 2005; Riley et al., 2005; Pope and Dockery, 2006; Brook, 2008a; Brook, 2008b; Craig et al., 2008; Simkhovich et al., 2008; Hamra et al., 2014). The U.S. EPA identified that PM represent serious public health problems in the United States causing premature mortality, aggravation of respiratory and cardiovascular disease, acute respiratory symptoms, chronic bronchitis and exacerbation of asthma. The International Agency for Research on Cancer (IARC) identified cancer concerns arising from core ultrafine particles with reactive chemicals adhered to their surfaces. The biological effects of CNMs can be comparable to or stronger than those of ambient PM. This emphasizes the urgent need for field studies focused on the assessment of exposure and health status in humans exposed to CNMs. Due to the lack of human data on CNMs exposure, only general non-specific precautionary measures can be taken. To establish effective regulatory standards, governments and regulating agencies around the world need precise and reliable data related to the potential adverse health effects associated with CNM exposures.
Multi-walled carbon nanotubes (MWCNTs) are the most promising CNMs that have been manufactured broadly during the last decade. MWCNTs are composed of layered graphene sheets, in the form of single-walled carbon nanotubes nested one inside the other. Their cylindrical nature, exceptional mechanical strength and intrinsic physico-chemical properties, render their feasibility for use in a number applications including electronics, optics, materials science, polymer chemistry and nanocomposites (Baughman et al., 2002; Wang et al., 2014). The large-scale manufacturing of MWCNTs in the past decade, and their expanded applications in new technologies, consumer products and biomedicine, raises the likelihood of human exposure to these materials, thus increasing concerns about their potential adverse health effects. In November 2014, the IARC classifiedMWCNT-7 (Mitsui ltd., Japan) to a Category 2B: as possibly carcinogenic to humans (Grosse et al., 2014).
The major health risks of exposure to MWCNT in workplaces are based on numerous in vivo animal studies that reported consistent noncancerous adverse effects (Poland et al., 2008; Ma-Hock et al., 2009; Mercer et al., 2010; Pauluhn, 2010; Porter et al., 2010; Mercer et al., 2011; Wang et al., 2011; Murphy et al., 2012; Huizar et al., 2013; Dong et al., 2015; Khaliullin et al., 2015; Snyder-Talkington et al., 2015;Wang et al., 2015). Acute pharyngeal aspiration or inhalation exposure of rodents to CNT causes dose-dependent pulmonary damage accompanied by a robust inflammatory response and severe oxidative stress leading to fibrosis and formation of granulomatous lesions (Porter et al., 2010; Mercer et al., 2011; Huizar et al., 2013; Sargent et al., 2014). Mechanistically, the histological features of fibrosis in animal lungs were associated with a significant dose and time-dependent increase in TGF-β and osteopontin (OPN) measured in the serum, bronchial alveolar lavage (BAL) fluids or lung tissue (Pauluhn, 2010; Porter et al., 2010; Mercer et al., 2011; Huizar et al., 2013; Dong et al., 2015; Khaliullin et al., 2015). Additionally, it was shown that exposure to MWCNT activated TGF-b/Smad signaling pathway in fibroblasts and myofibroblasts thus facilitating pulmonary fibrosis (Wang et al., 2015). Moreover, inhalation exposure to MWCNT promotes the growth and neoplastic progression of initiated lung cells in mice (Sargent et al., 2014; Snyder-Talkington et al., 2016). These effects were observed at a dose of 31.2 µg/mouse – that is achievable in human exposures in occupational settings (Erdely et al., 2013; Khaliullin et al., 2015).
Overall, the health effects associated with CNT exposures in rodents have not yet been confirmed in humans. In spite of the insufficiency of data on health effects of CNTs of exposed individuals, there are several documented cases showing CNT deposits found in human lungs and other organs after various exposures. One of them is the tragic event on 9/11 – the attack on the financial district of New York. This incident has caused a number of devastating diseases among rescue and recovery workers. Some of the first responders were presented with respiratory illness and have been diagnosed with pulmonary fibrosis, chronic bronchiolitis and granulomatous formation. The tubular carbon nanostructures, similar to CNTs - produced as a result of high combustion temperatures - were detected in the biopsy specimens of first responders as well as air samples collected at the crash site (Wu et al., 2010).
There are also several epidemiological attempts to assess the effects of NM on exposed workers (Liao et al., 2014a; Liao et al., 2014b; Vermeulen et al., 2014; Lee et al., 2015; Liou et al., 2015). In these studies, the results of exposure to NM other than MWCNTs were analyzed; one common limitation of these studies was inconsistent assessments or low/short-interval exposures which has been discussed in detail in a recent review (Liou et al., 2015). Our recent pilot cross-sectional study (Shvedova et al., 2016) revealed changes in mRNA expression profiles of whole blood samples derived from workers exposed to MWCNT aerosol. Expression analysis revealed augmented levels of several fibrotic bio-markers including TGF-β, KL-6 in whole blood samples of workers exposed to MWCNTs.
In spite of all the concerning data, to date the unambiguous identification and characterization of accurate and reliable markers of pulmonary injury associated with CNT exposure in humans have not been developed. This is due, at least in part, to the complexity of studies evaluating the adverse effects of CNTs in humans including the essential requirement of non-invasiveness of methods involving human subjects. The goal of the current work was to identify potential biomarkers in nasal lavage, sputum and serum of workers as a prognostic tool for assessment of occupational exposures to MWCNT.
2. Materials and methods
2.1. Study details
The study was conducted at a company producing MWCNTs: Nanotechcenter Ltd. (Tambov, Russia). Carbon nanotubes are synthesized using the catalytic vapor deposition method (CVD). MWCNT characteristics were as follows: external diameter − 8–15 nm, internal diameter − 4–8 nm, length 2 µm and more, catalysts content (Ni and Co) − < 5%.
Based on both workers' and supervisors' structured interviews and examination of workstations, potential workers in contact with the MWCNT aerosol for more than 1 year were selected and included as part of the exposure group (n = 10). These workers were represented by foremen, engineers, technical operators, and scientists. The personnel not exposed to the MWCNT aerosol, but who worked at the same enterprises as either engineers, scientists, or technical staff, were included as part of the control group (n = 12). In total, 22 workers of both genders (18 males, 4 females) aged 19–63 years participated in the study. Five of the exposed workers and 7 workers from the control group were under 30 years of age. None of them had a history of any diagnosed chronic respiratory diseases, including chronic obstructive pulmonary disease (COPD) and bronchial asthma, before they started working with CNTs. Six of 22 workers employed in the study were current smokers. Workers with acute respiratory symptoms, as a result of seasonal flu/cold, at the time of blood and sputum sampling were excluded from the study. The Institutional Review Board of Kazan State Medical University, Kazan, Russia, approved this study under the IRB protocol No. 14 dated 26.12.2011.
2.2. Exposure assessment
Exposure to MWCNT was monitored by transmission electron microscopy (TEM) and elemental carbon (EC) analyses. The EC analysis gave a quantitative measure of MWCNT exposure, while TEM confirmed the size and types of nanotube structures present. Air sampling was conducted in the employee's personal breathing zone (PBZ) simultaneously on mixed cellulose ester (MCE) filters (SKC Inc.) for TEM analysis and on ultraclean quartz-fiber filters (PallflexTissuquartz®) for EC analysis. At each sampling point, at least 400 liters (L), at a flow rate of 7–16 L/min, were sampled on the MCE filter; each set of samples was accompanied by at least 2 blank filters. For the EC analysis, sampling of both the inhalable and respirable aerosol fraction on quartz filters was conducted for 90min in the breathing zone at a rate of 3 L/min (270 L total). TEM coupled with energy dispersive x-ray spectroscopy was performed using modified method NMAM 7402 (NIOSH, 2006). The MCE filters were analyzed with a JEOL 2100F transmission electron microscope. The EC content (quartz filters) was determined by thermaloptical analysis, using a modified method NIOSH 5040 (Birch et al., 2011; NIOSH, 2013). The 8-hour time-weighted average (8-h TWA) EC concentrations were calculated for each worker taking into account the person's duties and time spent at different workstations, as described in detail in Shvedova et al. (2016).
2.3. Collection of blood and induced sputum samples
Blood sampling was drawn by venipuncture by a qualified technician aseptically from the cubital vein using a BD Vacutainer® Safety-lok ™ catheter (BD Diagnostic, Franklin Lakes, NJ; USA) into sterilized siliconed glass Vacu tubes (7ML LVDR 100EA/BX 10BX/CS; K3 EDTA 15%; BD Diagnostics, Franklin Lakes, NJ). Clotting was obtained at room temperature. Following the centrifugation (3000 rpm × 10 min) the serum was separated and stored at −20 °C until use.
Airway sampling is important for exposure study, diagnosis, and assessment of airway inflammation. However, sampling techniques such as bronchoscopy are invasive, time-consuming and costly procedures requiring sedation that could exacerbate lower respiratory tract inflammation. Sputum induction is less invasive, and suitable techniques were used to obtain fluid from the lower respiratory tract. Sputum samples from normal and healthy individuals were collected after several subsequent inhalations of aerosolized hypertonic saline (increased concentrations of NaCl: 3%, 4% and 5% at 10 min. Intervals) facilitating a bronchial secretion (Paggiaro et al., 2002). Collected sputum samples were centrifuged (3000 g × 15 min) then aliquoted and stored (at −80 °C) until use. Serum and sputum samples were analyzed using commercial ELISA kits for TGF-β1 (Invitrogen, Grand Island, NY), KL-6 (BioVendor LLC, Asheville, NC), and osteopontin (OPN) (R&D System, Minneapolis, MN). Measurement of the cytokines in seru mand sputum samples was carried out using a Th1/2 Multiplex (eBioscience Inc., San Diego, CA) employing the BD Canto Flow Cytometer (Beckton Dickenson, USA).
2.4. Statistical analysis
Initial statistical analysis was carried out using student's t-test. Results are presented as the mean ± SEM. A p value < 0.05was considered statistically significant. In addition, generalized linear models including both main effects (first variable - “exposure to MWCNTs: no/yes”, second - “age” (in years) or “sex: female/male” or “smoking: no/yes”) and pairwise interactions were performed using R statistical package. The confidence intervals of regression coefficients derived from a small sample were refined by bootstrap analysis (Moore et al., 2009). Bootstrap analysis included repeated sampling with replacement from the original sample, which represents the sampling distribution of the statistic. The bootstrap bias-corrected accelerated (BCa) intervals were constructed based on those for which we could make conclusions about the statistical significance of the relationship.
3. Results
3.1. TEM and EC analyses of PBZ samples
In planning the exposure assessment, we identified critical tasks where the workers could have been exposed to aerosolized MWCNTs, which included: i) unloading and collection of produced MWCNTs from the reactor; ii) mechanical disintegration of MWCNTs by electric mills; iii) packaging of final products; and iv) laboratory handling of MWCNTs. The TEM images confirmed the presence of MWCNTs agglomerates ranging from0.5–10 µmat each of site, but individual nanotubes were not found. The 8-h, TWA EC concentrations (respirable size fraction) were measured at different workstations and were in the range of 0.7–2.8 µg/m3. The 8-h TWA EC inhalable and respirable fractions are shown in Table 1.
Table 1.
The 8-h TWA EC concentrations calculated for the different occupations in the MWCNT manufacturing facility.
| Occupation | Workstations | 8 h EC inhalable conc., µg/m3 | 8 h EC respirable conc., µg/m3 |
|---|---|---|---|
| Technical operator (3 workers) | Reactor cleanout | 17.1 | 2.8 |
| Mechanical grinding | |||
| Packaging site | |||
| Foreman (1 worker) | Reactor cleanout | 10.4 | 1.8 |
| Mechanical grinding | |||
| Packaging site | |||
| Engineer (3 workers) | Reactor cleanout | 3.5 | 0.7 |
| Researcher (3 workers) | Reactor cleanout | 4.4 | 1.3 |
| Lab handling |
3.2. Inflammatory cytokines in the serum and sputum samples of MWCNT-exposed workers
The levels of inflammatory cytokines, TGF-β1, KL-6 and OPN, in the workers' serum and sputum samples are presented in Table 2. All profibrotic inflammatory mediators, such as IL-1β, IL-4, IL-5, IL-6, IL-8 and TNF-α, were significantly higher in the sputum samples collected from MWCNT-exposed group as compared to controls. In the serum samples, levels of IL-1β, IL-4, and TNF-αwere also significantly elevated in the MWCNT exposed group. Of all measured anti-fibrotic inflammatory mediators, only IL-10 was significantly augmented in the serum samples recovered from MWCNT-exposed workers compared to controls.
Table 2.
The content of the cytokines/proteins in serum and sputum samples in exposure and control groups.
| Cytokines/proteins, pg/ml | Sputum | Serum | ||
|---|---|---|---|---|
| Control | Exposed group | Control | Exposed group | |
| IL-1β | 646.90 ± 188.18 | 1988.70 ± 205.71* | 36.61 ± 3.86 | 90.08 ± 23.29* |
| IL-4 | 299.33 ± 108.77 | 818.86 ± 57.34* | 19.46 ± 8.30 | 47.38 ± 6.30* |
| Il-5 | 436.43 ± 173.40 | 1764.37 ± 424.12* | 15.24 ± 2.83 | 22.02 ± 3.77 |
| Il-6 | 47.98 ± 40.79 | 262.75 ± 22.34* | ||
| IL-8 | 863.84 ± 222.37 | 1753.51 ± 201.91* | 769.87 ± 214.95 | 1217.98 ± 237.00 |
| TNF-α | 321.41 ± 156.34 | 1512.67 ± 271.88* | 20.98 ± 3.66 | 52.47 ± 9.19* |
| IL-10 | 8.40 ± 3.24 | 18.05 ± 0.41* | ||
| Il-2 | 374.16 ± 128.07 | 887.59 ± 354.36 | 80.62 ± 12.73 | 88.70 ± 3.92 |
| IFN-γ | 992.15 ± 510.59 | 2635.29 ± 958.51 | 19.47 ± 5.12 | 29.15 ± 1.47 |
| IL-12 | 11.14 ± 2.26 | 17.37 ± 0.72 | ||
| KL-6 | 12.20 ± 8.69 | 220.50 ± 95.06* | 248.35 ± 37.81 | 313.52 ± 29.46 |
| TGF-β1 | 4.90 ± 0.34 | 5.0 ± 0.95 | 20.06 ± 0.31 | 21.82 ± 1.23 |
| Osteopontin | 59.19 ± 5.37 | 68.98 ± 7.20 | ||
p < 0.05, t-test.
To account for confounders such as age, sex and smoking status, the generalized linear models for three fibrosis biomarkers (KL-6, TGF-β1 and OPN) including both main effects (exposure to MWCNTs and one of the following variables – age or gender or smoking) and their pairwise interactions were created. The best-fitting regression model for KL-6 in sputum included “exposure to MWCNTs” and “age” variables. The KL-6 levels were higher in exposed workers compared with controls: β = 235.9 (95% BCa = 21.2–482), while the serum KL-6 levels in the exposed and control groups were not significantly different. The regression model for TGF-β1 in serum included a positive β-coefficient for exposure (β = 10.47, 95% BCa = 1.18–51.75) and negative β-coefficient for exposure-age interaction term (β = −0.4, 95% BCa = −2.02–−0.08). Thus, our data showed that increase in TGF-β1 in serum was clustered only in younger workers (<30 year old). Smoking and gender did not affect the content of the protein. The model for TGF-β1 in sputum showed the same trend, but BCa confidence intervals for regression coefficients included zero (data not shown). The best model for OPN in serum included a significant positive regression coefficient for the combined effect of age and exposure, but the bootstrap analysis did not confirm the findings (data not shown).
4. Discussion
With the exponential growth of the manufacturing and applications of MWCNTs in major industrial fields, research and biomedicine, concerns are growing with regards to their potential adverse effects on human health. This is particularly relevant to MWCNT exposures at workplaces with the highly elevated risk of occupational injury extrapolated from numerous previous in vivo animal studies showing consistent adverse lung effects including pulmonary inflammation, interstitial fibrosis and granulomatous lesions. Several lines of evidence indicate possible asbestiform pathogenicity of MWCNTs due to similarities in structure, which means carcinogenic and profibrotic risk similar to asbestos fibers. However, to date the potential markers of MWCNT exposure have not yet been explored in humans. In this study, we evaluated and compared levels of several potential fibrotic markers in the serum and sputum of MWCNT-exposed workers.
Exposure assessment employing TEM analysis documented agglomerated MWCNTs structures collected from the production site. The highest concentration of EC was found during the product harvesting from the reactor. In the absence of national standards, the time-weighted average (TWA) concentration was compared with the recommended exposure level (REL) proposed by the National Institute for Occupational Safety and Health (NIOSH, USA): 1 µg/m3 as EC (NIOSH, 2013). In our case, the individual 8-h TWA concentration of respirable EC reached 2.8 µg/m3. This elevated airborne MWCNTs concentration, nearly 3 times the NIOSH REL, is indicative of potential health risk, particularly during extended occupational exposures.
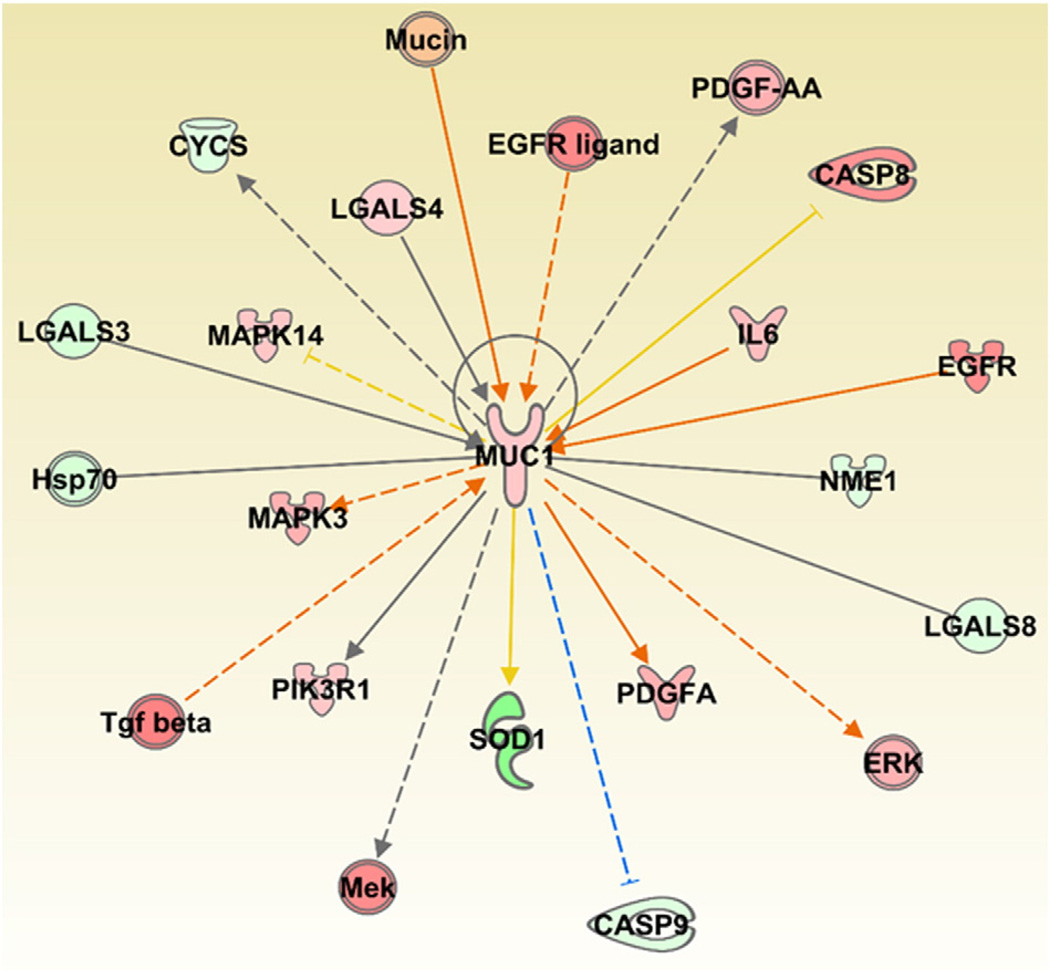
Our data suggest that exposure to MWCNTs at the workplace could change the content of some markers of fibrosis in serum and induced sputum samples. In particular, the KL-6 levels in induced sputum were significantly elevated with increased exposure to MWCNTs. In contrast, the serum KL-6 contents in the exposure and control groups were not significantly different. However, the analysis of whole blood mRNA expression profiles from humans exposed to MWCNTs, published recently (Shvedova et al., 2016), indicated a significant increase in the expression of KL-6 orMUC1 (Fig. 1). The upregulation of KL-6 in these samples was further supported by consistent expression of several genes that were either upstream (e.g., IL6, EGFR, TGFβ) or downstream (e.g., ERK, PDGFA, CASP8) of MUC1. Some of these genes, IL6 and EGFR - known to directly modulate MUC1 expression - also play an essential role in pulmonary inflammation, fibrosis, lung cancer development and progression. KL-6 is often used to distinguish between interstitial lung diseases and other pulmonary disease outcomes (Kondo et al., 2011). Exposure to MWCNT (e.g., inhalation, instillation or aspiration) causes various pulmonary effects in rodents including lung inflammation, sustained interstitial fibrosis, and granuloma formation (Poland et al., 2008; Ma-Hock et al., 2009; Pauluhn, 2010; Porter et al., 2010; Mercer et al., 2011; Wang et al., 2011; Reddy et al., 2012). Taken together these results indicate that the expression levels of KL-6 upon exposure to MWCNTs in whole blood samples is highly correlated with its expression in bio-fluids of pulmonary origin thus suggesting its potential to serve as a prognostic marker of exposure to CNTs in humans.
Fig. 1.
Gene centric network analysis of the KL-6/MUC1 and its associated genes differentially expressed in whole blood samples of MWCNT exposed workers. The dataset corresponding to the whole blood gene expression profiles of MWCNT-exposed workers was downloaded from NCBI's Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/) using the accession number: GSE73830. Red: Up regulated. Green: Down regulated. Orange and blue arrows represent activation and deactivation mechanisms, respectively, that are consistent with the expression of KL-6/MUC1. The upstream and/or downstream effects of activation or inhibition of molecules with “known” expression in the network was performed using MAP pathway tool provided by the IPA software.
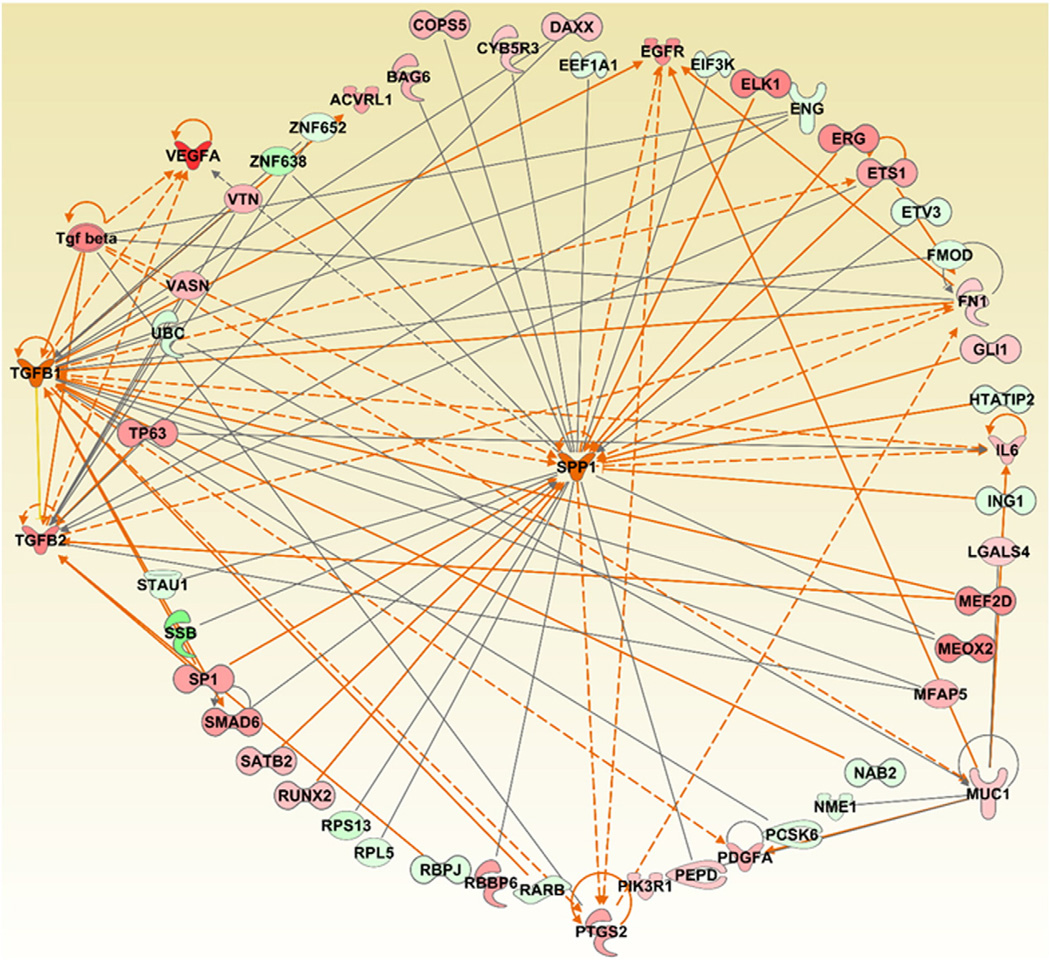
Previous in vivo studies using rodent models clearly revealed a profibrotic potential of MWCNTs (Poland et al., 2008; Ma-Hock et al., 2009; Pauluhn, 2010; Porter et al., 2010; Mercer et al., 2011; Wang et al., 2011; Reddy et al., 2012). A significant dose- and time-dependent increase in TGF-β and OPN (gene: SPP1) was found in the serum, broncho-alveolar lavage fluid (BAL) and pulmonary tissues of rodents exposed to MWCNTs (Huizar et al., 2013; Dong et al., 2015; Khaliullin et al., 2015). This result corroborates our findings in workers where we were able to detect an exposure-related increase in the serum TGF-β1 levels, albeit in young workers. However, no significant OPN changes were found in the serum of exposed workers. This could be due to many factors. One being the latency period required to trigger such responses in rodents and humans, and another related to the gender specific differences in responses exhibited upon exposure to MWCNTs. It should be emphasized that the MWCNT exposed workers investigated as a part of this study are all males, have various exposure regimes and have only spent about ~1–3 years working at the facility. In fact, the upregulation of TGF-β1 and OPN upon exposure to MWCNTs was not always consistent and depended on several experimental factors including CNT characteristics, animal gender, concentration, exposure type and time points employed in each study (Porter et al., 2010; Dymacek et al., 2015; Khaliullin et al., 2015; Poulsen et al., 2015; Snyder-Talkington et al., 2015; Snyder-Talkington et al., 2016). A larger cohort of workers exposed to MWCNTs during life-time employment would provide a more accurate validation of realistic markers by simultaneously comparing nasal lavage, sputum and serum. This is further supported by the differential regulation of several genes involved in TGF-β signaling in the whole blood samples of workers exposed to MWCNTs (SMAD6, CDC42, JNK, ERK, p38 MAPK and TAB1). These genes have been linked to the development of fibrosis. Based on gene expression profiles in workers exposed to MWCNTs, we predict that OPN can be activated according to its up- and down-stream mediators, similar to TGF-β1. Moreover, a set of genes (SP1, SMAD6, ETS1, PTGS2, EGFR and FN1) was also found to be commonly correlated to the predicted upregulated changes in TGF-β1 and SPP1 (Fig. 2). Even though OPN (gene: SPP1) was not as an informative human biomarker in this study, based on its predicted activation from gene expression studies and the up-regulation of other fibrotic markers, we still recommend it to be included in the test battery for future research.
Fig. 2.
Gene centric network generation and analysis of the three pro-fibrotic markers (KL-6/MUC1, TGF-β1, SPP1), and their associated genes differentially expressed in whole blood samples of MWCNT exposed workers. The dataset corresponding to the whole blood gene expression profiles of MWCNT-exposed workers was downloaded from NCBI's Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/) using the accession number: GSE73830. Red: Up regulated. Green: Down regulated. Orange and blue arrows represent activation and deactivation mechanisms that are consistent with the expression and/or predicted activation of the three pro-fibrotic markers. The Grow feature of the IPA pathway tool was used to construct and expand the network based on the various relationships between the selected molecules and other molecules in the given dataset based on their expression. Following this, the MAP pathway tool of IPA was used to determine and predict the upstream and/or downstream effects of activation or inhibition of the molecules in a generated network.
Of interest is also the significant overexpression of proinflammatory cytokines IL-1β and TNF-α in both induced sputum and serum samples of exposed workers (Table 2). These potent proinflammatory cytokines secreted by alveolar macrophages play crucial roles in host defense during infection and injury by recruiting various immune cells (Dinarello, 1996; Dinarello, 2011). Furthermore, the secretion of IL-1β has been implicated to mediate diverse inflammatory responses, autoimmune disorders and fibrosis (Maynard et al., 2006; Chen et al., 2007; Cassel et al., 2008; Rock et al., 2010; Kono et al., 2012). In fact, the expression of IL-1β, IL6 and TNF-α was reported in several in vivo and in vitro studies upon exposure to CNTs (Palomaki et al., 2011; Meunier et al., 2012; Murphy et al., 2012; Sun et al., 2015). Although, IL6 was not detected in the serum samples, its levels in the whole blood mRNA expression profiles and in the sputum samples of MWCNT-exposed workers were significantly elevated (Table 2). Taken together these findings indicate that, if used in conjunction with pulmonary function testing (PFT) and biochemical biomarkers (e.g., IL6, TNF-α, VEGFA, EGFR – common mediators involved), serum and/or sputum KL-6, TGF-β1, SPP1, as well as IL-1β levels, it could be useful for health surveillance of workers potentially exposed to carbonaceous particles in occupational settings (but not for predicting/diagnosing pulmonary disease outcomes in these populations). More studies involving a greater number of workers and a longer latency period, however, are required to further corroborate our findings.
There are several limitations of this study including cross-sectional design and small sample size. However, organizational issues in this type of research are challenging given the current relatively small number of potentially exposed workers and a small workforce compared to other industries. There are also specific difficulties associated with conducting such studies in the nano-industry at this stage of nanotechnology development. Nevertheless, such studies are an important step in planning large-scale studies aimed at more accurate and reliable risk assessment. Previous epidemiological studies conducted by other research groups also reported some health outcomes suggesting broad biomarker panels (Liao et al., 2014a; Liao et al., 2014b; Vermeulen et al., 2014; Lee et al., 2015; Liou et al., 2015). While they have provided exposure assessment related parameters, the results involving human samples were not reported at the time of this writing.
In summary, the findings of this study are indicative of possible health risks associated with the monitored occupational exposures to MWCNTs. Based on the general principle of reasonable precautions, enterprises producing and handling MWCNTs should implement measures to strictly control MWCNT exposure in the working area. It is also important to create a repository of biological samples (blood, urine, induced sputum) for future studies.
Acknowledgments
This work was supported by Grant of the Government of Tatarstan Republic “Algarysh” for prospective area “Nanotechnology” (2008– 2009) and by EC-FP-7-NANOSOLUTIONS, NORA 939051G and NTRC 939011K.
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health. Mention of trade names or commercial products do not constitute endorsement or recommendation for use.
Conflict of interest statement
The authors declare that they do not have any conflict of interest to disclose.
Transparency document
The Transparency document associated with this article can be found, in online version.
References
- Baughman RH, Zakhidov AA, de Heer WA. Carbon nanotubes–the route toward applications. Science. 2002;297:787–792. doi: 10.1126/science.1060928. [DOI] [PubMed] [Google Scholar]
- Birch ME, Ku BK, Evans DE, Ruda-Eberenz TA. Exposure and emissions monitoring during carbon nanofiber production–part; I elemental carbon and iron-soot aerosols. Ann. Occup. Hyg. 2011;55:1016–1036. doi: 10.1093/annhyg/mer073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD. Cardiovascular effects of air pollution. Clin. Sci. (Lond.) 2008a;115:175–187. doi: 10.1042/CS20070444. [DOI] [PubMed] [Google Scholar]
- Brook RD. Potential health risks of air pollution beyond triggering acute cardiopulmonary events. JAMA. 2008b;299:2194–2196. doi: 10.1001/jama.299.18.2194. [DOI] [PubMed] [Google Scholar]
- Cassel SL, Eisenbarth SC, Iyer SS, Sadler JJ, Colegio OR, Tephly LA, Carter AB, Rothman PB, Flavell RA, Sutterwala FS. The Nalp3 inflammasome is essential for the development of silicosis. Proc. Natl. Acad. Sci. U. S. A. 2008;105:9035–9040. doi: 10.1073/pnas.0803933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat. Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- Craig L, Brook JR, Chiotti Q, Croes B, Gower S, Hedley A, Krewski D, Krupnick A, Krzyzanowski M, Moran MD, Pennell W, Samet JM, Schneider J, Shortreed J, Williams M. Air pollution and public health: a guidance document for risk managers. J. Toxic. Environ. Health A. 2008;71:588–698. doi: 10.1080/15287390801997732. [DOI] [PubMed] [Google Scholar]
- Delfino RJ, Sioutas C, Malik S. Potential role of ultrafine particles in associations between airborne particle mass and cardiovascular health. Environ. Health Perspect. 2005;113:934–946. doi: 10.1289/ehp.7938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- Dinarello CA. A clinical perspective of IL-1beta as the gatekeeper of inflammation. Eur. J. Immunol. 2011;41:1203–1217. doi: 10.1002/eji.201141550. [DOI] [PubMed] [Google Scholar]
- Dong J, Porter DW, Batteli LA, Wolfarth MG, Richardson DL, Ma Q. Pathologic and molecular profiling of rapid-onset fibrosis and inflammation induced by multi-walled carbon nanotubes. Arch. Toxicol. 2015;89:621–633. doi: 10.1007/s00204-014-1428-y. [DOI] [PubMed] [Google Scholar]
- Dymacek J, Snyder-Talkington BN, Porter DW, Mercer RR, Wolfarth MG, Castranova V, Qian Y, Guo NL. mRNA and miRNA regulatory networks reflective of multi-walled carbon nanotube-induced lung inflammatory and fibrotic pathologies in mice. Toxicol. Sci. 2015;144:51–64. doi: 10.1093/toxsci/kfu262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdely A, Dahm M, Chen BT, Zeidler-Erdely PC, Fernback JE, Birch ME, Evans DE, Kashon ML, Deddens JA, Hulderman T, Bilgesu SA, Battelli L, Schwegler-Berry D, Leonard HD, McKinney W, Frazer DG, Antonini JM, Porter DW, Castranova V, Schubauer-Berigan MK. Carbon nanotube dosimetry: from workplace exposure assessment to inhalation toxicology. Part Fibre Toxicol. 2013;10:53. doi: 10.1186/1743-8977-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse Y, Loomis D, Guyton KZ, Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Scoccianti C, Mattock H, Straif K International Agency for Research on Cancer Monograph Working, G. Carcinogenicity of fluoro-edenite, silicon carbide fibres and whiskers, and carbon nanotubes. Lancet Oncol. 2014;15:1427–1428. doi: 10.1016/S1470-2045(14)71109-X. [DOI] [PubMed] [Google Scholar]
- Hamra GB, Guha N, Cohen A, Laden F, Raaschou-Nielsen O, Samet JM, Vineis P, Forastiere F, Saldiva P, Yorifuji T, Loomis D. Outdoor particulate matter exposure and lung cancer: a systematic review and meta-analysis. Environ. Health Perspect. 2014;122:906–911. doi: 10.1289/ehp/1408092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizar I, Malur A, Patel J, McPeek M, Dobbs L, Wingard C, Barna BP, Thomassen MJ. The role of PPARgamma in carbon nanotube-elicited granulomatous lung inflammation. Respir. Res. 2013;14:7. doi: 10.1186/1465-9921-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaliullin TO, Shvedova AA, Kisin ER, Zalyalov RR, Fatkhutdinova LM. Evaluation of fibrogenic potential of industrial multi-walled carbon nanotubes in acute aspiration experiment. Bull. Exp. Biol. Med. 2015;158:684–687. doi: 10.1007/s10517-015-2835-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Hattori N, Ishikawa N, Murai H, Haruta Y, Hirohashi N, Tanigawa K, Kohno N. KL-6 concentration in pulmonary epithelial lining fluid is a useful prognostic indicator in patients with acute respiratory distress syndrome. Respir. Res. 2011;12:32. doi: 10.1186/1465-9921-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono H, Orlowski GM, Patel Z, Rock KL. The IL-1-dependent sterile inflammatory response has a substantial caspase-1-independent component that requires cathepsin C. J. Immunol. 2012;189:3734–3740. doi: 10.4049/jimmunol.1200136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Choi YC, Shin JH, Lee JH, Lee Y, Park SY, Baek JE, Park JD, Ahn K, Yu IJ. Health surveillance study of workers who manufacture multi-walled carbon nanotubes. Nanotoxicology. 2015;9:802–811. doi: 10.3109/17435390.2014.978404. [DOI] [PubMed] [Google Scholar]
- Liao HY, Chung YT, Lai CH, Lin MH, Liou SH. Sneezing and allergic dermatitis were increased in engineered nanomaterial handling workers. Ind. Health. 2014a;52:199–215. doi: 10.2486/indhealth.2013-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao HY, Chung YT, Lai CH, Wang SL, Chiang HC, Li LA, Tsou TC, Li WF, Lee HL, Wu WT, Lin MH, Hsu JH, Ho JJ, Chen CJ, Shih TS, Lin CC, Liou SH. Six-month follow-up study of health markers of nanomaterials among workers handling engineered nanomaterials. Nanotoxicology. 2014b;8(Suppl. 1):100–110. doi: 10.3109/17435390.2013.858793. [DOI] [PubMed] [Google Scholar]
- Liou SH, Tsai CS, Pelclova D, Schubauer-Berigan MK, Schulte PA. Assessing the first wave of epidemiological studies of nanomaterial workers. J. Nanoparticle Res. 2015;17:413. doi: 10.1007/s11051-015-3219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma-Hock L, Treumann S, Strauss V, Brill S, Luizi F, Mertler M, Wiench K, Gamer AO, van Ravenzwaay B, Landsiedel R. Inhalation toxicity of multiwall carbon nanotubes in rats exposed for 3 months. Toxicol. Sci. 2009;112:468–481. doi: 10.1093/toxsci/kfp146. [DOI] [PubMed] [Google Scholar]
- Maynard AD, Aitken RJ, Butz T, Colvin V, Donaldson K, Oberdorster G, Philbert MA, Ryan J, Seaton A, Stone V, Tinkle SS, Tran L, Walker NJ, Warheit DB. Safe handling of nanotechnology. Nature. 2006;444:267–269. doi: 10.1038/444267a. [DOI] [PubMed] [Google Scholar]
- Mercer RR, Hubbs AF, Scabilloni JF, Wang L, Battelli LA, Schwegler-Berry D, Castranova V, Porter DW. Distribution and persistence of pleural penetrations by multi-walled carbon nanotubes. Part Fibre Toxicol. 2010;7:28. doi: 10.1186/1743-8977-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer RR, Hubbs AF, Scabilloni JF, Wang L, Battelli LA, Friend S, Castranova V, Porter DW. Pulmonary fibrotic response to aspiration of multi-walled carbon nanotubes. Part Fibre Toxicol. 2011;8:21. doi: 10.1186/1743-8977-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier E, Coste A, Olagnier D, Authier H, Lefevre L, Dardenne C, Bernad J, Beraud M, Flahaut E, Pipy B. Double-walled carbon nanotubes trigger IL-1beta release in human monocytes through Nlrp3 inflammasome activation. Nanomedicine. 2012;8:987–995. doi: 10.1016/j.nano.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Moore DS, McCabe GP, Craig BA. Introduction to the Practice of Statistics. New York: W.H. Freeman; 2009. [Google Scholar]
- Murphy FA, Schinwald A, Poland CA, Donaldson K. The mechanism of pleural inflammation by long carbon nanotubes: interaction of long fibres with macrophages stimulates them to amplify pro-inflammatory responses in mesothelial cells. Part Fibre Toxicol. 2012;9:8. doi: 10.1186/1743-8977-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIOSH. Method 7402 asbestos by TEM (modified for carbon nanotubes) In: Schlecht PC, O′Connor PF, DHHS, CN, editors. NIOSH method of analytical methods. Cincinnati OH: 2006. Publication 94–113. [Google Scholar]
- NIOSH. Current intelligence bulletin 65: Occupational exposure to carbon nanotubes and nanofibers. 2013 [Google Scholar]
- Paggiaro PL, Chanez P, Holz O, Ind PW, Djukanovic R, Maestrelli P, Sterk PJ. Sputum induction. Eur. Respir. J. 2002;(Suppl. 37):3s–8s. doi: 10.1183/09031936.02.00000302. [DOI] [PubMed] [Google Scholar]
- Palomaki J, Valimaki E, Sund J, Vippola M, Clausen PA, Jensen KA, Savolainen K, Matikainen S, Alenius H. Long, needle-like carbon nanotubes and asbestos activate the NLRP3 inflammasome through a similar mechanism. ACS Nano. 2011;5:6861–6870. doi: 10.1021/nn200595c. [DOI] [PubMed] [Google Scholar]
- Pauluhn J. Subchronic 13-week inhalation exposure of rats to multiwalled carbon nanotubes: toxic effects are determined by density of agglomerate structures, not fibrillar structures. Toxicol. Sci. 2010;113:226–242. doi: 10.1093/toxsci/kfp247. [DOI] [PubMed] [Google Scholar]
- Poland CA, Duffin R, Kinloch I, Maynard A, Wallace WA, Seaton A, Stone V, Brown S, Macnee W, Donaldson K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat. Nanotechnol. 2008;3:423–428. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- Pope CA, 3rd, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J. Air Waste Manage. Assoc. 2006;56:709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- Porter DW, Hubbs AF, Mercer RR, Wu N, Wolfarth MG, Sriram K, Leonard S, Battelli L, Schwegler-Berry D, Friend S, Andrew M, Chen BT, Tsuruoka S, Endo M, Castranova V. Mouse pulmonary dose- and time course-responses induced by exposure to multi-walled carbon nanotubes. Toxicology. 2010;269:136–147. doi: 10.1016/j.tox.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Poulsen SS, Saber AT, Mortensen A, Szarek J, Wu D, Williams A, Andersen O, Jacobsen NR, Yauk CL, Wallin H, Halappanavar S, Vogel U. Changes in cholesterol homeostasis and acute phase response link pulmonary exposure to multiwalled carbon nanotubes to risk of cardiovascular disease. Toxicol. Appl. Pharmacol. 2015;283:210–222. doi: 10.1016/j.taap.2015.01.011. [DOI] [PubMed] [Google Scholar]
- Reddy AR, Reddy YN, Krishna DR, Himabindu V. Pulmonary toxicity assessment of multiwalled carbon nanotubes in rats following intratracheal instillation. Environ. Toxicol. 2012;27:211–219. doi: 10.1002/tox.20632. [DOI] [PubMed] [Google Scholar]
- Riley MR, Boesewetter DE, Turner RA, Kim AM, Collier JM, Hamilton A. Comparison of the sensitivity of three lung derived cell lines to metals from combustion derived particulate matter. Toxicol. in Vitro. 2005;19:411–419. doi: 10.1016/j.tiv.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Rock KL, Latz E, Ontiveros F, Kono H. The sterile inflammatory response. Annu. Rev. Immunol. 2010;28:321–342. doi: 10.1146/annurev-immunol-030409-101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent LM, Porter DW, Staska LM, Hubbs AF, Lowry DT, Battelli L, Siegrist KJ, Kashon ML, Mercer RR, Bauer AK, Chen BT, Salisbury JL, Frazer D, McKinney W, Andrew M, Tsuruoka S, Endo M, Fluharty KL, Castranova V, Reynolds SH. Promotion of lung adenocarcinoma following inhalation exposure to multi-walled carbon nanotubes. Part Fibre Toxicol. 2014;11:3. doi: 10.1186/1743-8977-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvedova AA, Yanamala N, Kisin ER, Khailullin TO, Birch MEF, L M. Integrated Analysis of Dysregulated ncRNA and mRNA Expression Profiles in Humans Exposed to Carbon Nanotubes. PLOS ONE. 2016 doi: 10.1371/journal.pone.0150628. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkhovich BZ, Kleinman MT, Kloner RA. Air pollution and cardiovascular injury epidemiology, toxicology, and mechanisms. J. Am. Coll. Cardiol. 2008;52:719–726. doi: 10.1016/j.jacc.2008.05.029. [DOI] [PubMed] [Google Scholar]
- Snyder-Talkington BN, Dong C, Zhao X, Dymacek J, Porter DW, Wolfarth MG, Castranova V, Qian Y, Guo NL. Multi-walled carbon nanotube-induced gene expression in vitro: concordance with in vivo studies. Toxicology. 2015;328:66–74. doi: 10.1016/j.tox.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder-Talkington BN, Dong C, Sargent LM, Porter DW, Staska LM, Hubbs AF, Raese R, McKinney W, Chen BT, Battelli L, Lowry DT, Reynolds SH, Castranova V, Qian Y, Guo NL. mRNAs and miRNAs in whole blood associated with lung hyperplasia, fibrosis, and bronchiolo-alveolar adenoma and adenocarcinoma after multi-walled carbon nanotube inhalation exposure in mice. J. Appl. Toxicol. 2016;36:161–174. doi: 10.1002/jat.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Wang X, Ji Z, Wang M, Liao YP, Chang CH, Li R, Zhang H, Nel AE, Xia T. NADPH oxidase-dependent NLRP3 inflammasome activation and its important role in lung fibrosis by multiwalled carbon nanotubes. Small. 2015;11:2087–2097. doi: 10.1002/smll.201402859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen R, Pronk A, Vlaanderen J, Hosgood D, Rothman N, Hildesheim A, Silverman D, Melis A, Spaan S, Voogd E, Hoet P, Godderis L, Lan Q. 0282 A cross-sectional study of markers of early immunological and cardiovascular health effects among a population exposed to carbon nanotubes: the CANTES study0282 A cross-sectional study of markers of early immunological and cardiovascular health effects among a population exposed to carbon nanotubes: the CANTES study. Occup. Environ. Med. 2014;71:A35. [Google Scholar]
- Wang X, Katwa P, Podila R, Chen P, Ke PC, Rao AM, Walters DM, Wingard CJ, Brown JM. Multi-walled carbon nanotube instillation impairs pulmonary function in C57BL/6 mice. Part Fibre Toxicol. 2011;8:24. doi: 10.1186/1743-8977-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Zhu Y, Liao S, Li J. Carbon nanotubes reinforced composites for biomedical applications. BioMed Research International. 2014;2014:518609. doi: 10.1155/2014/518609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Wang Y, Nie X, Braini C, Bai R, Chen C. Multiwall carbon nanotubes directly promote fibroblast-myofibroblast and epithelial-mesenchymal transitions through the activation of the TGF-beta/Smad signaling pathway. Small. 2015;11:446–455. doi: 10.1002/smll.201303588. [DOI] [PubMed] [Google Scholar]
- Wu M, Gordon RE, Herbert R, Padilla M, Moline J, Mendelson D, Litle V, Travis WD, Gil J. Case report: lung disease in World Trade Center responders exposed to dust and smoke: carbon nanotubes found in the lungs of World Trade Center patients and dust samples. Environ. Health Perspect. 2010;118:499–504. doi: 10.1289/ehp.0901159. [DOI] [PMC free article] [PubMed] [Google Scholar]