Abstract
Nanomaterials, including nanoparticles and nanoobjects, are being incorporated into everyday products at an increasing rate. These products include consumer products of interest to toxicologists such as pharmaceuticals, cosmetics, food, food packaging, household products, and so on. The manufacturing of products containing or utilizing nanomaterials in their composition may also present potential toxicologic concerns in the workplace. The molecular complexity and composition of these nanomaterials are ever increasing, and the means and methods being applied to characterize and perform useful toxicologic assessments are rapidly advancing. This article includes presentations by experienced toxicologists in the nanotoxicology community who are focused on the applied aspect of the discipline toward supporting state of the art toxicologic assessments for food products and packaging, pharmaceuticals and medical devices, inhaled nanoparticle and gastrointestinal exposures, and addressing occupational safety and health issues and concerns. This symposium overview article summarizes 5 talks that were presented at the 35th Annual meeting of the American College of Toxicology on the subject of “Applied Nanotechnology.”
Keywords: nanotoxicology, nanomaterial toxicology, nanotechnology, nanoparticle, nanosafety, nanomaterial
Introduction to Applied Nanomaterial Toxicology and Applied Nanotoxicology for Pharmaceuticals and Medical Devices
Dave Hobson, LoneStar PharmTox LLC, Bergheim, TX, USA
In the universe, particles come in a wide range of sizes, shapes, and compositions. Life on earth involves and, in fact, requires particles of many types including nanoparticles. The atmosphere of our planet is so laden with particles, including nanoparticles, that even on the clearest of days at the right vantage point, we can easily observe the light scattering effects of these particles. (see Figure 1 showing a view over and across the atmosphere from 10,000 m).
Figure 1.
View across earth’s atmosphere observed over Meteor Crater, AZ, from 10 000 meters. Photo by D.W. Hobson
Without light being scattered by nanoparticles, our sunrises and sunsets would not be as beautiful. So living with nanoparticles is not exactly new. The nanoparticles that are ever present in our atmosphere such as ocean spray, volcanic ash, airborne soil sediment, and even man-made emissions such as industrial smoke, dust and vapor have been around us for a very long time and, in some cases, even since life began on our planet. In fact, when we examine biologic systems closely enough, we find that structures and particles with nanometer dimensions are essential for life.
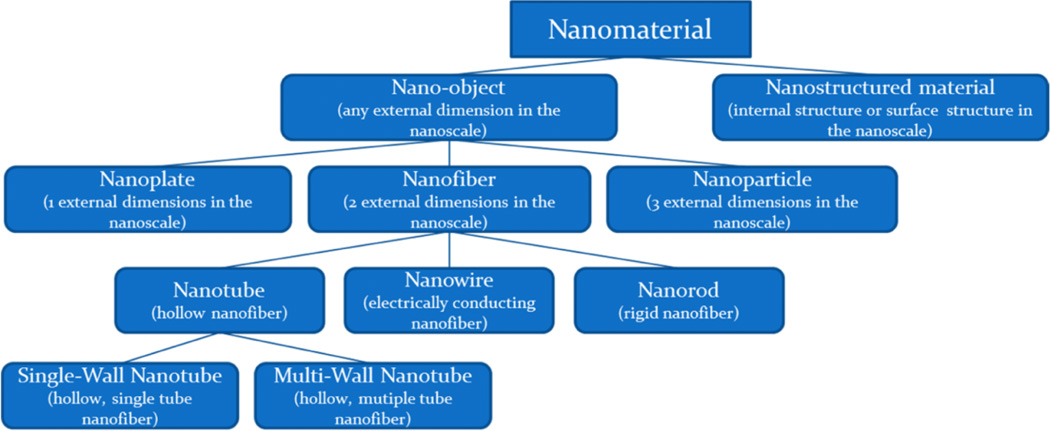
Our ability to manufacture and engineer nanomaterials or in other words “control” matter at the nanoscale at dimensions between approximately 1 and 100 nanometers is termed “nanotechnology” and is relatively new. In this range, unique phenomena that enable the novel applications occur and have become an exciting and promising new dimension in our ability to design and develop new products in essentially every area of human endeavor. Nanotechnology encompasses nanoscale science and nanoengineering that involves imaging, measuring, modeling, and manipulating matter at the nanoscale.1 Figure 2 provides and example classification and descriptors for nanomaterials based on nanoscale dimensions.
Figure 2.
Useful descriptors for nanomaterials.
With the development of current and emerging technology to manipulate matter in the nanoscale and even atomic range, we have arrived at a point where essentially any feasible molecular structure to include a vast number of nuclides can be engineered and constructed at will.
The rapidly growing number of engineered nanoproducts in development and commerce includes products that are of more concern for potential toxicologic effects than others. Those of greatest concern typically require contact with biologic systems and include uses in pharmaceuticals, medical devices, cosmetics, etc. and so on. While items such as electronics, use in polymers and coatings, glass, and plastics where the nanomaterials are permanently bound in a larger molecular structure or are otherwise not generally available for biologic contact would be of less concern.
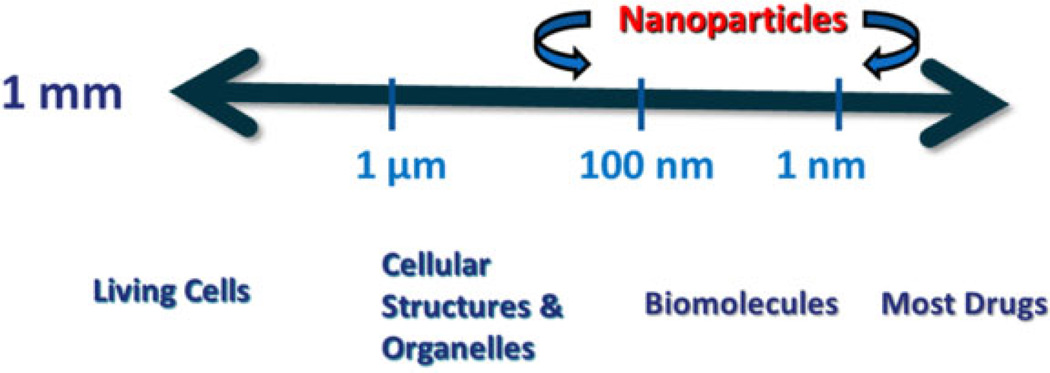
A size oriented, toxicologic perspective of nanomaterial exposure to biologic systems is shown in Figure 3 are within the size range where cellular structures and biomolecules occur. So, in addition to their chemical features, some nanomaterials also have structural features that could lead to a potential for adverse biologic effects.
Figure 3.
A size-oriented toxicologic perspective of nanoparticles.
Nanosize facilitates:
inhalation and gastrointestinal absorption;
uptake into cells and transcytosis across cells;
distribution into the blood and lymph circulation to reach potentially sensitive target sites such as bone marrow, lymph nodes, spleen, liver, kidneys, and heart;
brain entry via nasal nerves (eg, polio virus);
recognition and processing by the immune system;
entry into the cell nucleus;
… and other toxicologically significant processes.
Because the study of biology does in fact require that we develop an understanding of the practical aspects of exposure to various naturally occurring particles including smoke, ash, aerosols, and even viruses (which can be viewed as quite elegant nanoparticles in many cases), we do already possess a fundamental concept of the hazard range that nanoparticles could affect. Natural aerosols such as ocean spray, dust, and smoke at natural and atmospheric levels would be generally be considered to be low hazard, whereas exposure to the most pathogenic viruses at naturally occurring levels might be considered to be high hazard. So, what we don’t know is where the nanomaterials that we engineer occur on the scale of hazard within this range.
The developing area of toxicology termed “nanotoxicology” is addressing the issues and developing the tools necessary to evaluate the toxicologic characteristics and safety of engineered nanomaterials (ENMs).2,3 One of the fundamental questions that this area of toxicology, that this subdiscipline seeks to answer is “why might nanoparticles be inherently more toxic than the substances of which they are composed?”
Already some significant issues that require careful consideration of nanotoxicology findings can be observed. Doak et al4 notes that “nanomaterials cannot be treated in the same manner as chemical compounds with regards to their safety assessment, as their unique physico-chemical properties are also responsible for unexpected interactions with experimental components that generate misleading data-sets.” Most nanotoxicity studies (>70%) have been and are currently being done in vitro, using a wide variety of models, many of which have not been validated to in vivo observed toxicologic effects. There are very few “control” or “standard” reference nanomaterials available for use in the conduct of nanotoxicologic studies, and the degree and methods used in test article characterization for many published nanotoxicology studies are substantially variable to the extent that in many cases, the studies could not be duplicated as state-of-the-art toxicologic science would demand.
This is a fast emerging and developing area of technology, and at the rate at which some new nanomaterials are being developed, collecting essential toxicological information presents a road block, and high throughput screening techniques are being proposed and developed for toxicologic assessment with significant focus on the following aspects:
absorption, distribution, toxicokinetics, and metabolism in vivo still must be understood,
targets and mechanism(s) of action must be identified in vivo and evaluated, and
routes and rates of elimination must be identified and characterized.
The observation that, in biologic systems, nanoparticles often are not best characterized by their fundamental material characteristics of size, shape, surface features, and composition but rather by the protein “corona” that coats them following exposure to the biologic systems appears to be of fundamental significance in understanding how nanoparticles and nanomaterials interact with these systems.5–7 The “corona” that coats nanoparticles is a natural process that covers the particle with a combination of “hard,” nanoparticle-bound protein that is itself then covered with “soft” proteins that are weakly bound to the hard protein surfaces. The formation of the corona on a nanoparticle appears to be pharmacologically and toxicologically very significant to its biologic activity. The corona proteins may be nanoparticle unique, and thousands of different types of proteins may be involved. Immune system recognition, cellular processing, biodistribution, kinetics, elimination, and so on may be affected individually or in combination.
The use of nanotechnology in medical pharmaceuticals and medical devices may involve one or more of the following characteristics:
improvement/modification of solubility and/or absorption/distribution/metabolism,
improvement of formulation stability,
increase excipient solubility and reduce excipient use,
patent extension/new patents (new active pharmaceutical ingredient [API] forms/formulations),
nanotechnology targeted new molecular/biologic entity forms that reduce exposure and toxicity,
modification of device surface properties of medical devices, and
enhancement of device function (ie, molecular targeting, nanobrachytherapy, and so on).
Regulatory agencies such as the US Food and Drug Administration (FDA) and the European Medicines Agency already have some familiarity with the incorporation of nanotechnology in pharmaceutical and medical device products. They are continuously learning and improving their ability to understand the unique and potentially beneficial characteristics that the use of nanotechnology may provide to their regulated products. The evaluation of product safety for each nanomaterial containing product is taken on a case-by-case basis, and it is generally thought that current regulations for medicines and medical devices are sufficiently stringent and comprehensive in scope to cover the theoretical risks associated with nanomaterials. Guidance is in place that requires nanomaterials used in pharmaceutical products be reported and that pharmaceuticals containing nanotechnology be closely evaluated for safety using state of the science risk/benefit principles. The US FDA has currently approved over 20 products that use nanotechnology.
Some examples of nanotechnology-enabled, medical products in development include cancer therapeutics utilizing nanoparticles to improve tumor targeting and localization; radioisotopic nanoparticles (Au, Fe, In, etc); and active pharmaceutical carrying liposomal, polymeric, solid-lipid nanoparticles (SLNs), and so on.
Pharmacokinetics of a nanoparticle-incorporated drug substance can be modified by changing the surface characteristics of polymeric (eg, polyethylene–polyethylene glycol [PEG]) SLNs by using amphiphilic solvation enhancers.8 This type of strategy opens up a wide variety of nanoparticle—API— strategies that can now be developed and tried. Antiretroviral HIV/AIDS drug delivery with macrophage targeted peptide-PEG nanocarriers, for example, increases cellular uptake and increases accumulation in macrophages of liver, kidney, and spleen compared with those which are nontargeted.9 This type of nanocarrier targeting increases antiretroviral concentrations where needed, with longer persistence and can result in decreased systemic toxicity.
Nanoliposomal therapeutics are being developed that exhibit low toxicity, are predominately metabolized and/or are excreted via the lymphatics, and can be prepared with a variety of APIs. Diagnostic radiolabels can be incorporated and used to image and monitor API delivery to target tissues as well as observed therapeutic efficacy.10
Targeted nanotherapeutics typically have 4 components in their design consisting of:
a particle matrix composed of a polymer, lipid, solid, carbon, or other type of nanostructure (eg, dendrimers),
a pharmaceutical active that is a small molecule or biopharmaceutical,
a surface functionalization component, and
a targeting ligand.
Doxil, for example, is a nanoliposomal drug that is “passively targeted” to tumors and releases doxorubicin to the tumor tissue after targeting. Other nanoenabled drug products in development include products that use target-specific targeting such as oral parathyroid hormone for bone loss and oral clotting factors for hemophilia (both currently in preclinical development), a glycolipodepsipeptide antibiotic for grampositive bacteria (currently in phase 2), and a number of cancer therapeutics and theragnostic combinations of API and imaging agent in early stage discovery and development. Recent reviews of targeted nanotherapeutics provide more information with respect to this area of development.12,13
Examples of FDA-cleared pharmaceuticals listed by general nanocarrier type and therapeutic indications include the following:
- Liposomals
- Liposomal amphotericin B—mycotic infection
- Liposomal daunorubicin (DaunoXome)—Kaposi sarcoma
- Cytarabine liposome injection (Depocyt)—lymphomatous meningitis
- Collagran MMP inhibiting—wound dressings
- “Stealth” liposome doxorubicin—Kaposi sarcoma
- Doxil/Caelyx—ovarian/breast cancer
- Verteporfin liposomal (Visudyne)—Wet macular degeneration
- Solid polymeric
- Carmustine (Gliadel)—Glioblastoma multiforme
- Abraxane (nanoparticles of paclitaxel-taxol)— Mammary cancer (metastitic)
- TrivCor (nanoparticulate form)—high cholesterol treatment
- PEGylated
- PEG-succinimidyl-L-asparaginase—lymphoblastic leukemia
- PEG-adenosine deaminase—serius immunodeficiency
- PEG-interferon −2α (Pegasyls)—Hepatitis C
- Nanocrystal
- Emend nanocrystals—nausea prevention in chemotherapy
- Rapamune nanocrystal—rejection prevention
Over the past few years, a number of lessons have been learned regarding the conduct drug safety studies and the interpretation of data from these studies. Some of these include the following:
Dose expression needs to include particulate properties (dimension, shape, surface characteristics, charge, aggregation/agglomeration state, and so on) as well as chemical composition.
Determination of biodistribution and kinetics while difficult is very important. TEM (Transmission electron microscopy) is still the “gold standard” but new techniques (eg, hyperspectral imaging) are emerging.
The immunological recognition and protein coronal affinity properties of nanoparticles can be significant and must not be overlooked.
Whole animal perspective must be maintained even when using in vitro methods to isolate and evaluate specific processes as necessary.
When a nondigestable nanoparticle becomes bound and sequestered in the liver reticuloendothelial system (or some other tissue sites), there may be reason for concern that it might not have a route of elimination other than cremation.
Therefore, for these and other reasons, studies with nanomaterials intended for therapeutic use need to carefully characterize and evaluate nanoparticles used in the delivery system or pharmaceutical ingredient itself as they enter, distribute, and are eliminated by the body.
When used in medical devices, the primary toxicologic concern is for exposure to “unbound” nanomaterials. Many devices that employ nanotechnology may not require extensive safety evaluation if it can be established that by design and relevant data the nanomaterial is not bioavailable. In vitro diagnostic tests that employ nanotechnology generally would not require in vivo safety or biocompatibility evaluation. In all cases, the manufacturer is responsible for demonstrating that there is a lack of potential exposure to nanomaterials used in the device. The FDA Draft Guidance for Industry (April 2013) entitled “Use of International Standard ISO-10993, Biological Evaluation of Medical Devices Part 1: Evaluation and Testing” specifically addresses submicron or nanotechnology medical device components.14 This guidance clearly indicates that considerations for dose characterization and the design and conduct of safety studies will have to take into consideration the unique properties of these materials.
In conclusion, life depends as well as has adapted to the presence of naturally occurring nanostructures and nanomaterials. Modern science and engineering have now developed the ability to make and manufacture nanomaterials for an expanding variety of purposes, including many that involve direct exposure to biologic systems. Nanotoxicology has emerged as a subdiscipline of toxicology needed to support the discovery and development of safe nanotechnology products. Therefore, “applied nanotoxicology” incorporates an ongoing and comprehensive understanding of the state of the science of both nanotechnology and nanotoxicology toward development of practices and products that safely employ and utilize nanotechnology where exposure to biologic systems is possible.
Uptake and Distribution of Ingested Nanomaterials
Stephen M. Roberts and Georgia K. Hinkley, Univ. of Florida, Gainesville, FL, USA
There are several challenges in conducting studies on the biological fate and effects of nanomaterials. These challenges include (1) inconsistency in nanomaterials available for study, for example, lot-to-lot variations in important characteristics such as size, shape, and surface properties, and deviations from the labeled description; (2) limitations in quantities available for study; (3) uncertainty as to the proper dose metric (eg, mass vs surface area vs particle concentration); (4) difficulty detecting and quantifying nanomaterials in tissues; and (5) changing chemical and physical properties of nanomaterials with time, handling, and in biological environments.
To these challenges, several others are added when studying ingested nanomaterials due to the inherent complexity of the gastrointestinal tract (GIT). As ingested nanomaterials transit the GIT, their immediate environment changes in several ways that can affect nanomaterial properties thought to influence biological activity, including size, shape, and surface properties such as charge, catalytic properties, and adsorbed materials (ie, their “corona”). Examples of changes in the dynamic environment of the GIT include pH, ionic strength and composition of GIT fluids, and microflora and digested food matrices with which nanoparticles can interact. Absorptive surfaces also change, as nanomaterials pass through the GIT in terms of surface area, absorption mechanisms in play, and thickness of the protective mucin layer.
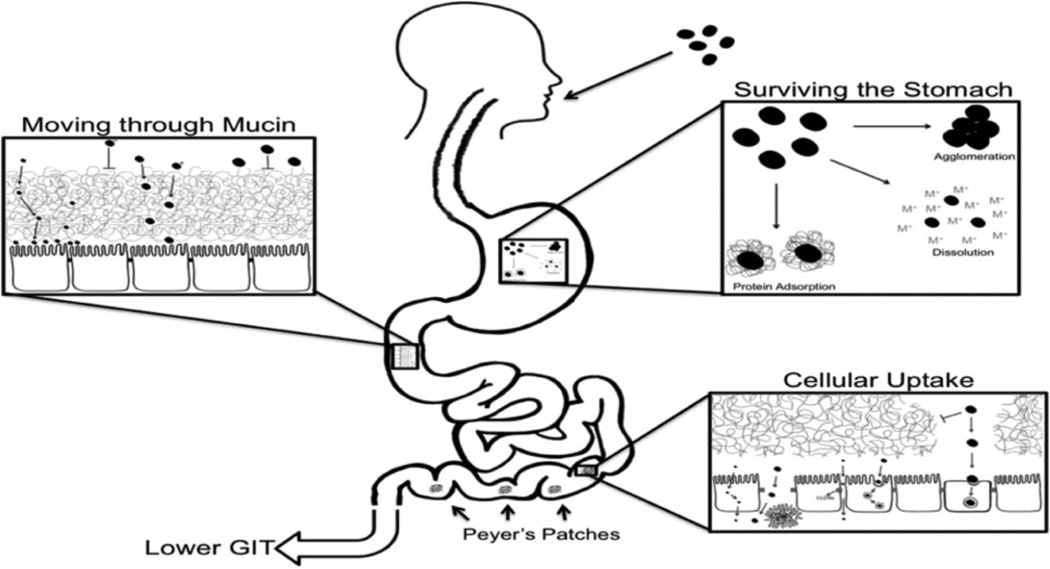
In order for ingested nanomaterials to be absorbed from the GIT, they must overcome several barriers (Figure 4). They must survive the low pH, high ionic environment of the stomach, where they may be dissolved, extensively agglomerated, or coated with macromolecules, altering their surface properties. In the intestine, they must be able to penetrate the mucin layer (Figure 5).15 Previous studies have shown that, in general, smaller size favors movement through the mucin layer,16 and neutral particles move the fastest, followed by positively charged particles, with negatively charged particles moving the slowest.17 Coatings such as chitosan, latex, and PEG can increase the rate of movement of nanoparticles through mucin.16,18
Figure 4.
Overview of barriers to gastrointestinal absorption of small particles.
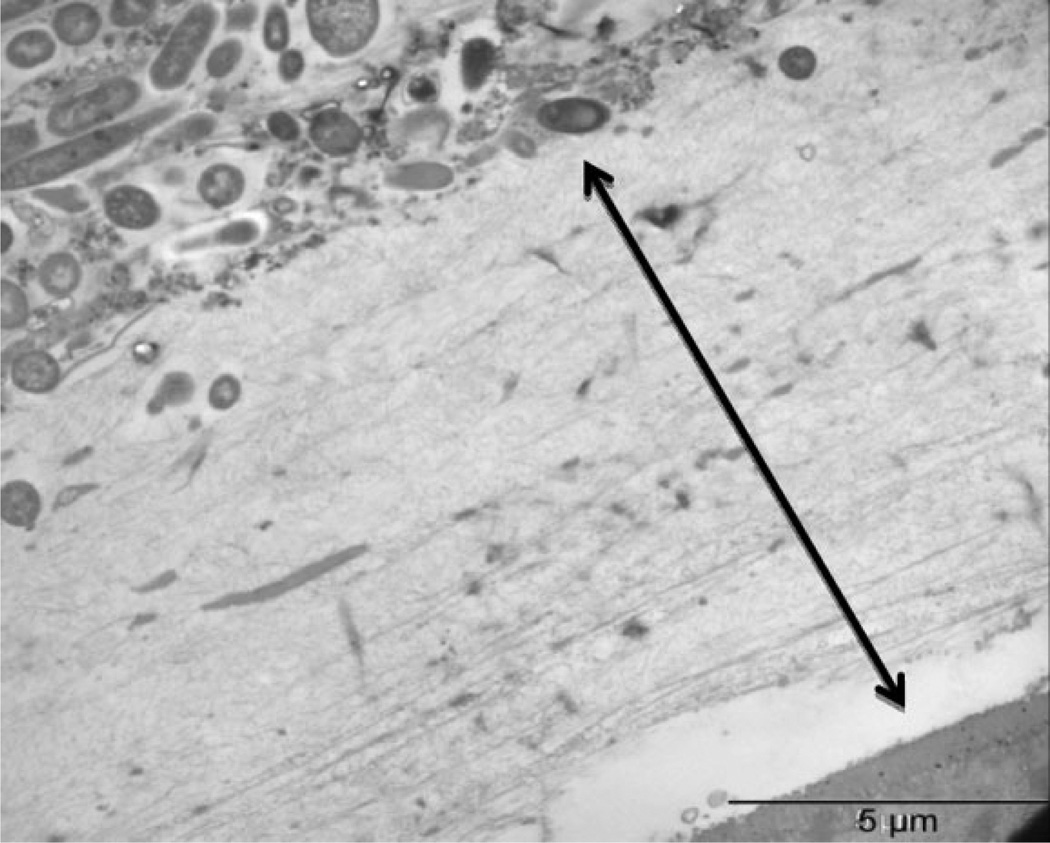
Figure 5.
Mucin layer in mouse colon (approximately 10 µm). The thick, unstirred mucin layer is constantly being regenerated. In order to gain access to the absorptive surfaces in the gastrointestinal tract (GIT), particles must transit the mucin layer in a 4- to 6-hour recycling window.
Finally, nanoparticles must be able to pass through the epithelial lining of the GIT. Uptake can be passive, through transcellular, paracellular, or cell turnover processes, or active (eg, clathrin mediated and caveolar mediated). Uptake through the lymphatics can occur in specialized cells in Peyer’s patches.
Unfortunately, most studies of nanoparticle uptake treat the GIT as a “black box.” Nanomaterials are characterized before dosing, and tissues are analyzed over time for the presence of nanomaterials. Because these studies do not follow events within the GIT, no insight is provided as to how the nanoparticles change in the dynamic GIT environment, and most importantly, the characteristics of the particles when they reach the absorptive surfaces of the gut. This greatly limits our ability to gain understanding of how and why nanoparticle characteristics affect their uptake into the body.
Among the few studies that have attempted to measure the bioavailability of orally administered nanomaterials, the results have been uniformly low. For example, Schleh et al19 measured the bioavailability of native gold particles in sizes ranging from 1.4 to 200 nm, some with surface modifications to produce positive or negative charges. The bioavailabilities of all of the nanoparticles were less than 1%. Explaining findings such as these is difficult because the extent of agglomeration that took place within the GIT, and therefore the actual size of the gold particles when they reached the intestinal epithelium, is unknown. If nearly all of the primary particles were extensively agglomerated into particles too large to penetrate the mucin lining of the GIT or be taken up by any of the particle uptake mechanisms, then the uniformly low bioavailabilities are not surprising.
We conducted a study in which the oral bioavailability of uncoated and PEG-coated 20 nm gold nanoparticles was compared in mice.20 The agglomeration state of the gold nanoparticles in each treatment group was followed within the GIT using transmission electron microscopy. Uncoated gold particles agglomerated extensively in the stomach and remained as large agglomerates throughout the GIT. Polyethylene glycolcoated gold nanoparticles remained as primary particles, and in contrast to the large agglomerates in the uncoated gold-treated animals, were able to penetrate the mucin barrier. Mice treated with PEG-coated gold nanoparticles had significantly higher nanoparticle concentrations in some tissues, but the differences were not striking and the oral bioavailabilities of both the uncoated and PEG-gold were low (<1%). These findings suggest that agglomeration state can influence uptake but that even with well-dispersed particles, bioavailability of nanoparticles is low.
Are there circumstances in which the oral uptake of solid nanoparticles is sufficient that they might serve as effective drug delivery vehicles, or for nontherapeutic particles, as a significant health risk? To answer this question, a new generation of studies will be needed in which the characteristics (eg, size, surface charge, and adsorbed materials) of orally administered nanoparticles are examined in situ, within the GIT, and critical determinants of uptake are identified.
Nanoparticles as an Emerging Environmental and Occupational Hazard—Toxicology Prospective
Anna A. Shvedova, CDC-National Institute for Occupational Safety and Health (NIOSH) and WVU, Morgantown, WV, USA
Innovations in the nanotechnology field, with a wide-range of different products, raise the issue of potential adverse health effects particularly in occupational and environmental settings. Carbonaceous nanomaterials, specifically nanotubes, are among the most widely studied and utilized.21 Carbon nanotubes (CNTs) may be single walled (SW) or multiwalled (MW); in this presentation, CNT refers to both types while SWCNT refers to SW nanotubes only. The production of CNT and their composites are increasing globally.22 The CNT products have a wide scope of applications and are used in textiles, cosmetics, biomedicine, and polymer chemistry in manufacturing of motor vehicles and sports equipment and integrated circuits for electronic modules. Human exposure to CNTs is primarily through inhalation and dermal contact, especially during the manufacturing and handling processes.23 However, the lung is the major portal of unintended CNT entry into the human body, potentially leading to pulmonary damage, inflammation, oxidative stress, fibrosis, and cancer.24,25
Studies of respiratory toxicity of CNTs following aspiration in C57BL/6 mice revealed that these nanomaterials caused a dose-dependent augmentation of biomarkers of cellular injury, pulmonary inflammation, lung damage, and increased oxidative stress. This is evidenced by increase in oxidation of protein sulfhydryls, reduced level of glutathione, and total antioxidant reserve along with the accumulation of lipid peroxidation products found in bronchoalveolar lavage fluid and in the lung following pharyngeal aspiration and inhalation exposures.26 Moreover, markers of pulmonary cytotoxicity were associated with early development of acute inflammation, collagen accumulation, and progressive fibrosis and formation of granulomas. A subsequent study using C57BL/6 mice that were maintained on vitamin E-sufficient or vitamin E-deficient diets further emphasized the importance of oxidative stress and anti-oxidant depletion in the overall inflammatory response insofar as the toxicity of SWCNTs and the fibrotic responses (enhanced collagen deposition) were significantly higher in the latter group of mice, lacking the major lipid-soluble antioxidant, vitamin E.27 Overall, pharyngeal aspiration of SWCNTs elicits a robust acute inflammatory response, with early onset of progressive pulmonary fibrosis whose expression and severity are associated with the intensity of oxidative stress in the lung of the exposed C57BL/6 mice.
The most commonly used technologies in the manufacturing of CNTs rely heavily on the use of catalytically active metals, either iron or nickel. As a result, the final material could potentially contain significant amounts (up to 30–40 wt%) of these metals that may act as catalysts of oxidative stress. Indeed, many in vitro studies of CNTs appear to describe adverse effects of these contaminants and not of the nanotubes themselves.28–30 A number of other reports have indicated elevated oxidative stress in response to CNT employing cell-free models and in vitro cell exposures; however, in many cases, these studies could have false outcomes. By employing in vivo electron spin resonance (ESR) spin-trapping technology to directly record free radical formation along with redox assessments of antioxidant balance, we were able to reveal that CNT—in doses relevant to potential occupational exposures—exerts their toxic effects in the lung and distant organs (heart and liver) of exposed animals. We reported that exposure to partially purified CNTs (HiPco, CNI, Inc, Texas) resulted in the augmentation of oxidative stress as evidenced by ESR detection of a-(4-pyridyl-1-oxide)-N-tert-butylnitrone spin-trapped carbon-centered lipid-derived radicals recorded shortly after the treatment. This was accompanied by a significant depletion of antioxidants and elevated biomarkers of inflammation presented by recruitment of inflammatory cells and an increase in proinflammatory cytokines in the lungs as well as development of multifocal granulomatous pneumonia, interstitial fibrosis, and suppressed pulmonary function. Moreover, pulmonary exposure to SWCNTs also caused the formation of carbon-centered, lipid-derived radicals in the heart and liver at later time points (day 7 postexposures). Additionally, CNTs induced a significant accumulation of oxidatively modified proteins, an increase in lipid peroxidation products, depletion of antioxidants, and an inflammatory response in both the heart and the liver. Furthermore, the iron chelator deferoxamine noticeably reduced lung inflammation and oxidative stress, indicating an important role of metal-catalyzed species in lung injury caused by CNTs. Overall, this study for the first time provided direct evidence that lipid-derived free radicals are a critical contributor to tissue damage induced by CNTs not only in the lungs but in distant organs.31
Realistic exposures to CNT may occur in conjunction with other pathogenic impacts (microbial infections) and trigger enhanced responses. Sequential exposure to CNT and Listeria monocytogenes (LM) amplified lung inflammation and collagen formation. Notably, despite the robust inflammatory response, CNT pre-exposure significantly decreased the pulmonary clearance of LM-exposed mice measured 3 to 7 days after microbial infection versus vehicle/LM-treated mice. We established that decreased bacterial clearance in CNT-preexposed mice was associated with suppressed phagocytosis of bacteria by macrophages and a decrease in nitric oxide production by the phagocytes. Preincubation of primarily murine alveolar macrophages with SWCNT in vitro also resulted in reduced nitric oxide generation and suppressed phagocytizing activity toward LM. Failure of SWCNT-exposed mice to clear LM led to a continued elevation in nearly all major chemokines and acute-phase cytokines into the later course of infection. Overall, these data indicate that enhanced acute inflammation and pulmonary injury with delayed bacterial clearance after CNT exposure may lead to increased susceptibility to lung infection in exposed populations.32
Multidisciplinary studies combining proteomics analysis with assessments of changes in pulmonary morphology (including collagen) in the lung samples recovered from mice exposed to CNT, carbon black, and asbestos in doses relevant to potential occupational exposures are of particular value. Global proteomic analysis revealed that 69% and 93% of proteins affected by asbestos and carbon black were also affected by CNT exposure, demonstrating that the asbestos and carbon responses can be considered a subset of the proteins and biological pathways affected by CNT. The CNT treatment caused the greatest changes in abundance of identified lung tissue proteins. The trend in number of proteins affected (CNT [376] > asbestos [231] > UFCB [184]) was similar to inflammatory cytokine responses seen in bronchiolar lavage and lung tissue.33 Overall, a significantly smaller number of proteins were affected by asbestos and carbon black treatment than for CNT.
Exposure to engineered carbonaceous nanomaterials, including CNT and nanofibers, is considered a potential health hazard based on their physical similarities with asbestos. Carbon nanotubes may also be as pathogenic as asbestiform fibers because of their shape, dimensions, and high aspect ratio, biopersistence, and capacity to generate reactive oxygen species, oxidative stress, and genotoxicity.34–38 The resemblance of the fibrous needle-like shape to asbestos has raised further concerns regarding the potential carcinogenicity of CNT or their effects on tumor–hosts relationships and growth of tumors in the body. Chronic exposure to CNT was shown to induce DNA damage and increase mutation frequency in mouse embryonic cells and human epithelial cells.39,40 Carbon nanotubes induce mitotic abnormality with 1 rather than 2 mitotic spindle poles, a potential mechanism that impaired cell division.41 Many studies have reported that CNT can induce apoptosis, DNA damage, and activation of major regulatory pathways—MAPKs (Mitogen-Activated Protein Kinase), AP-1 (Activator Protein Factor-1), NF-κB (Nuclear Factor kappa B), and Akt (Protein Kinase B)— all of which recapitulate key molecular events involved in asbestos-induced lung cancer.41,42 Recently, we have demonstrated that a single exposure to inhalable CNT accelerated metastatic growth in lung of mice.43 In particular, we observed that acute exposure to CNT facilitated accumulation of lung-associated, myeloid-derived suppressor cells (MDSC) and promoted lung tumor growth. This was achieved by alterations in the tissue microenvironment that enabled early recruitment of MDSC to the lungs, as depletion of MDSC abrogates protumor effects of acute exposure to CNT.43
In conclusion, the growing use of nanomaterials for consumer products, food packaging, and biomedicine imply their increasing levels of manufacturing. Carbon nanotubes hold great potential as a promising material for a number of applications in various electronic, chemical, and bioengineering fields; however, major knowledge gaps still exist with respect to the effects of CNT on humans.23,25,44 The use of CNTs has increased substantially in the last decade and is expected to grow in the near future.45 Considering the potential health implications and increasing, widespread use of CNTs, comprehensive toxicology data and risk assessment—integrated with a life-cycle perspective—is necessary for the development of new safe-by-design nanomaterials and hence nanoapplications.
Consumer Product Example: Strategies for Setting Occupational Exposure Limits for Engineered Titanium Dioxide Nanomaterials
David B. Warheit, Chemours Company, Wilmington, DE, USA
Titanium dioxide (Tio2) particles can be found in nature but generally are synthesized from minerals such ilmenite, rutile, or anatase. Titanium dioxide particles have a variety of applications, although the most common forms are utilized in pigments and paints. This is due, primarily, to the physicochemical properties of these particle types, particularly optimized within a certain particle size range (~200–300 nm).46 This results in a high refractive index feature, providing the properties of brightness and natural white coloring. These characteristics are often referred to as “opacifier” properties which facilitate color optimization—essential properties necessary for use in applications such as paints, papers, plastics, coatings, and food items.
It is important to note that not all forms of Tio2 are alike— both compositionally and/or from a hazard standpoint. In this regard, physicochemical composition of Tio2 particles in various products is specified for the particular commercial application/function. For example, some common commercial applications (1) exist in the form of opacifiers (white pigments used in paints and cosmetics), (2) as ultraviolet ray scavengers (in sunscreens), or (3) in catalytic functions. The composition or various forms of Tio2 particles in these products can have different crystal structures (rutile or anatase or combinations of the 2 forms), different particle sizes, and corresponding surface area metrics as well as significant differences in particle surface characteristics (i.e., neutralized vs. naked surfaces); and different surface coatings versus uncoated surfaces. For instance, “catalytic forms” are very different from pigments” from a physicochemical characteristics standpoint, although both are identified as polymorphs of Tio2 particles. Catalytic and photocatalyst functions favor enhanced “surface reactivity” effects (ie, “naked” particle surfaces to facilitate/catalyze reactions).
Although TiO2 particles are generally known as low-toxicity materials, there can be some potency differences when assessing hazard effects following inhalation exposures.
The major routes of TiO2 particle and nanoparticle exposure occur via 3 entry portals. These are identified as (1) oral exposures—primarily via food consumption; (2) dermal exposures—often through cosmetic and sunscreen applications; and (3) inhalation exposures—primarily under occupational and manufacturing (workplace) conditions. The focus of the presentation concerned the implementation of occupational exposure levels in the workplace using pulmonary bridging or read across methodologies.
The development of occupational exposure limits (OELs) can be implemented by considering both the pulmonary toxicity and the epidemiological data available for the different forms of TiO2 particulates. For Tio2 particles in particular, physicochemical parameters to be evaluated should include crystal structure conformation, surface area, particle size distributions in relevant media, as well as composition/surface coatings, surface reactivity, method of nanomaterial synthesis method, and impurities. As an illustration of potential lung hazards, pulmonary bioassay studies comparing 3 different ultrafine TiO2 particle types resulted in development of hazard profiles which could then be bridged (or read-across) to the extensive database record of the pigment-grade particulate form of TiO2.47,48,49 Assessments of the toxicity data documented in studies, along with consideration of epidemiological findings in TiO2 occupational workers, concomitant with bridging (read-across) comparisons provided in estimated OEL values of 1 mg/m3, 2 mg/m3, and 5 mg/m3, respectively, for the high surface reactivity anatase-rutile uf-TiO2 form, low surface reactivity uf-TiO2 forms, as well as pigment-grade TiO2 particle types, respectively. The values obtained in this process differ from the NIOSH-recommended exposure levels for uf-TiO2. In this regard, NIOSH utilized a different methodology, which lacked a comprehensive evaluation of physicochemical characterization and species-related biological responses.
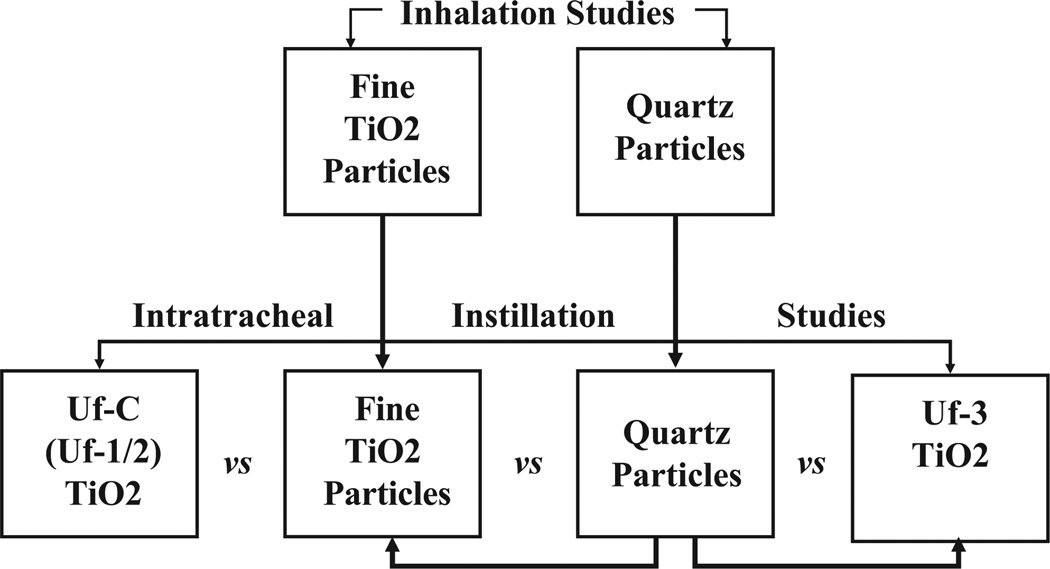
Recently, a workshop was conducted with the objective of comparing and evaluating different strategies for developing OELs for ENMs.50 A number of approaches were presented at this meeting for setting OELs for ENMs, since it will be impossible to generate experimental inhalation toxicity or epidemiological data on the various forms of particles or nanoparticles in commerce. The publication by Gordon and colleagues50 reviewed the workshop findings and identified areas for additional research. During this workshop, Warheit49 proposed a bridging approach to estimate OELs for 3 forms of nano-Tio2 particulates, based upon comparative results of pulmonary bioassay/intratracheal instillation toxicity studies and bridging or benchmarking the results to a rather complete subchronic and long-term, dose–response database of inhalation studies in rats with pigment-grade Tio2 particles. In addition, a rather extensive database of >4 epidemiological studies covering >23 000 Tio2-exposed workers were included in the analysis. This application of the data could provide a useful and practical methodology for estimating occupational exposure limits, in the absence of a complete toxicological data set on the various forms of nanoscale Tio2 particles. A schematic example of bridging studies to estimate occupational exposure levels using pulmonary bioassay studies to longer term inhalation studies is demonstrated in Figure 6.
Figure 6.
Schematic demonstrating the strategy for conducting pulmonary bioassay bridging studies. Bridging studies can have utility in providing an inexpensive preliminary safety screen when evaluating the hazards of new developmental compounds. The basic idea for the bridging concept is that the effects of the instilled materials serve as a control (known) material and then are “bridged” on the one hand to the inhalation toxicity data for that material and on the other hand to the new materials being tested.
Applied Considerations for Safety Assessment of Food Products and Food Packaging Containing Nanomaterials
Robin C. Guy, Robin Guy Consulting LLC, Lake Forest, IL, USA
Food Applications
Nanofood ingredients are present in many foods and food contact items. Foods have contained natural nanofood ingredients for hundreds of years. Mayonnaise and some dairy products, for example, are foods that may contain natural-occurring nanomaterials in emulsions. Engineered nanofood ingredients are now used in many food applications, mostly as food additives and dietary supplements. These ingredients may be used to alter or enhance a food’s appearance, color, flavor, sweet/sour perception, odor, or texture. Nanofood ingredients may also be used to decrease or increase absorption or act as a preservative.
Food contact items are materials that come in contact with food but are not intended to be consumed directly. Food contact products with nano-sized ingredients are being used in a wide variety of applications. These applications may include indicators of freshness and those to extend product shelf-life, detection of contamination or spoilage, UV barriers, increase of packaging strength, water resistant barriers, radio frequency identification, nanosized barcodes, trademark and fraud protection, and packaging for extreme hot, extreme cold, and aerospace conditions.
Food-Related Applications
Nano-sized materials may also be found in agriculture and food production. Applications may include controlled release of fertilizers and nutrients, specific targeted delivery of nutrients and pesticides to crops, treatment of microbial and chemical soil contaminants, nano-sized sensors used to monitor livestock and crops, and the use of encapsulated vaccines for livestock. Water may even be treated or come in contact with nano-sized particles during filtration processes. For example, procedures exist for the “nanofiltration” of water, including reverse-osmosis procedures for desalination, and to remove hardness, pesticides, metals, viruses, and bacteria.
Regulatory
The US FDA has Guidance Documents to assist with nanomaterial safety testing and approval considerations. The guidance51 Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology, issued June 24, 2014, describes FDA’s current thinking on determining whether FDA-regulated products involve the application of nanotechnology. This guidance identifies 2 points to consider that should be used to evaluate whether FDA-regulated products involve the application of nanotechnology. These points address both particle dimensions and dimension-dependent properties or phenomena.
The guidance52 Assessing the Effects of Significant Manufacturing Process Changes, Including Emerging Technologies, on the Safety and Regulatory Status of Food Ingredients and Food Contact Substances, Including Food Ingredients that Are Color Additives, issued June 24, 2014, discusses the safety assessment of a food substance including the following considerations: identity, technical effect, self-limiting levels of use, dietary exposure and safety studies, manufacturing process, and how manufacturing changes affect the safety and regulatory status.
The European Commission has a regulation that addresses manufacturing changes to an item on the community list.53 The regulation states that when a food additive is already included in a community list and there is a significant change in its production methods or in the starting materials used, or there is a change in particle size, for example, through nanotechnology, the food additive prepared by those new methods or materials shall be considered as a different additive and a new entry in the community lists or a change in the specifications shall be required before it can be placed on the market.
The European Union also regulates food contact ingredients.54 This regulation states that new technologies that engineer substances in particle size that exhibit chemical and physical properties that significantly differ from those at a larger scale, for example, nanoparticles, should be assessed on a case-by-case basis as regard their risk until more information is known about such new technology.
The European Food Safety Authority Scientific Committee developed a scientific opinion entitled, Guidance on the risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain.55
The US FDA has several options for getting products containing nanoingredients on the US market.
Generally Recognized as Safe (GRAS): Types of GRAS includes GRAS Determination, GRAS Self-Affirmation, and Flavor and Extract Manufacturer’s Association GRAS. GRAS, through scientific procedures, requires the same quantity and quality of scientific evidence as is required to obtain approval of the substance as a food additive and may be based upon published studies, which may also be supported by unpublished studies and other data and information. The lack of transparency with Self-Affirmations remains a safety concern. The Grocery Manufacturer’s Association launched an initiative to improve the GRAS process in 2014 to provide clear guidance on how to conduct transparent state of the art ingredient safety assessments.56 The FDA publishes GRAS Determination information on the FDA Web site.
New Dietary Ingredient Notification: FDA’s New Dietary Ingredient Notification Draft guidance57: NDI is defined by statute as a dietary ingredient that was not marketed in the United States before October 15, 1994. The submission should include a description of the identity and composition of the nanomaterial, a basis for the conclusion that the substance is an NDI, a description of the conditions of use, and an explanation of how the history of use or other evidence of safety justifies the conclusion that the dietary supplement containing the NDI will reasonably be expected to be safe.
Food Additive Petition58: Guidance for Industry and Other Stakeholders: Toxicological Principles for the Safety Assessment of Food Ingredients (Redbook 2000): The Redbook gives guidance on details on Food Additive Petition and discusses how to determine the need for toxicity studies; design, conduct, and report results of toxicity studies; conduct statistical analyses of data; and review histological data.
CVM (Center for Veterinary Medicine) Feed (note: updated with final guidance)59: This final guidance identifies potential issues related to safety or regulatory status of food for animals containing nanomaterials or otherwise involving the application of nanotechnology.
EPA (Environmental Protection Agency): In general, producers of pesticide products must submit scientific and technical data for EPA review to ensure that the use of a pesticide will not generally cause unreasonable adverse effects on human health or the environment.
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr Shvedova’s portion of this manuscript was supported by NIOSH OH008282, NORA 0HELD015, and EC-FP-7-NANOSOLUTIONS.
Footnotes
Author Contributions
All authors, David W. Hobson, Stephen M. Roberts, Anna A. Shvedova, David B. Warheit, Georgia K. Hinkley, and Robin C. Guy, substantially contributed to conception and design; drafted the manuscript; critically revised the manuscript; gave final approval; and agree to be accountable for all aspects of the work.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
Declaration of Conflicting Interests
The authors declare no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
References
- 1.NNI, National Nanotechnology Initiative. [Accessed January 4, 2015];Definition of nanotechnology. Web site. http://www.nano.gov/
- 2.Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. J Environ Health Perspect. 2005;113(7):823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monteiro-Riviere NA, Tran Lang C, editors. Nanotoxicology: Progress Toward Nanomedicine. Second. Boca Raton, FL: CRC Press; 2014. p. 514. [Google Scholar]
- 4.Doak SH, Griffiths SM, Manshian B, et al. Confounding experimental considerations in nanogenotoxicology. Page 285 in Mutagenesis. 2009;24(4):285–293. doi: 10.1093/mutage/gep010. [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal P, Hall JB, McLeland CB, Dobrovolskaia MA, McNeil SE. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv Drug Deliv Rev. 2009;61(6):428–437. doi: 10.1016/j.addr.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karmali PP, Simberg D. Interactions of nanoparticles with plasma proteins: implication on clearance and toxicity of drug delivery systems. Expert Opin Drug Deliv. 2011;8(3):343–357. doi: 10.1517/17425247.2011.554818. [DOI] [PubMed] [Google Scholar]
- 7.Lundqvist M, Stigler J, Elia G, Lynch I, Cedervall T, Dawson KA. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc Natl Acad Sci U S A. 2008;105(38):14265–14270. doi: 10.1073/pnas.0805135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heiati H, Tawashi R, Phillips NC. Drug retention and stability of solid lipid nanoparticles containing azidothymidine palmitate after autoclaving, storage and lyophilization. J Microencapsul. 1998;15(2):173–184. doi: 10.3109/02652049809006847. [DOI] [PubMed] [Google Scholar]
- 9.Wan L, Pooyan S, Hu P, Leibowitz MJ, Stein S, Sinko PJ. Peritoneal macrophage uptake, pharmacokinetics and biodistribution of macrophage-targeted PEG-fMLF (N-formyl-methionyl-leucylphenylalanine) nanocarriers for improving HIV drug delivery. Pharm Res. 2007;24(11):2110–2119. doi: 10.1007/s11095-007-9402-5. 2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phillips WT, Goins BA, Bao A. Radioactive liposomes. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1(1):69–83. doi: 10.1002/wnan.3. [DOI] [PubMed] [Google Scholar]
- 11.Barenholz Y. Doxil®-the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160(2):117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Bazak R, Houri M, Achy SE, Hussein W, Refaat T. Passive targeting of nanoparticles to cancer: A comprehensive review of the literature. Mol Clin Oncol. 2014;2(6):904–908. doi: 10.3892/mco.2014.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Onoue S, Yamada S, Chan HK. Nanodrugs: pharmacokinetics and safety. Int J Nanomedicine. 2014;9:1025–1037. doi: 10.2147/IJN.S38378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.US Food and Drug Administration. Use of International Standard ISO-10993, “Biological Evaluation of Medical Devices Part 1: Evaluation and Testing. US Food and Drug Administration; 2013. Apr, [Google Scholar]
- 15.Chen MC, Sonaje K, Chen KJ, Sung HW. A review of the prospects for polymeric nanoparticles platforms in oral insulin delivery. Biomaterials. 2011;32(36):9826–9838. doi: 10.1016/j.biomaterials.2011.08.087. [DOI] [PubMed] [Google Scholar]
- 16.Szenturi L. Light microscopic observation on luminally administered dyes, dextrans, nanospheres and microspheres in the preepithelial mucus gel layer of the rat distal colon. J Control Release. 1997;46(3):233–242. [Google Scholar]
- 17.Crater JS, Carrier RL. Carrier properties of gastrointestinal mucus to nanoparticles transport. Macromol Biosci. 2010;10(12):1473–1483. doi: 10.1002/mabi.201000137. [DOI] [PubMed] [Google Scholar]
- 18.Diab R, Jaafar-Maalej, Fessi H, Mancent P. Engineered nanoparticulate drug delivery systems: the next frontier for oral administration. AAPS J. 2012;14(4):688–702. doi: 10.1208/s12248-012-9377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schleh C, Semmler-Behnke M, Lipka J, et al. Size and surface charge of gold nanoparticles determine absorption across intestinal barriers and accumulation in secondary target organs after oral administration. Nanotoxicology. 2012;6(1):36–46. doi: 10.3109/17435390.2011.552811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinkley GK, Carpinone P, Munson JW, Powers KW, Roberts SM. Oral absorption of PEG-coated versus uncoated gold nanospheres: does agglomeration matter? Part Fibre Toxicol. 2015;12(9):1–12. doi: 10.1186/s12989-015-0085-5. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maynard AD, Aitken RJ, Butz T, et al. Safe handling of nanotechnology. Nature. 2006;444(7117):267–269. doi: 10.1038/444267a. [DOI] [PubMed] [Google Scholar]
- 22.De Volder MFL, Tawfick SH, Ray H, Baughman RH, Hart AJ. Carbon nanotubes: present and future commercial applications. Science. 2013;339(6119):535–539. doi: 10.1126/science.1222453. [DOI] [PubMed] [Google Scholar]
- 23.Schulte PA, Geraci CL, Murashov V, et al. Occupational safety and health criteria for responsible development of nanotechnology. J Nanopart Res. 2014;16(1):2153. doi: 10.1007/s11051-013-2153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shvedova AA, Kisin ER, Porter D, et al. Mechanisms of pulmonary toxicity and medical applications of carbon nanotubes: two faces of Janus? Pharmacol Ther. 2009;121(2):192–204. doi: 10.1016/j.pharmthera.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Kagan VE, Shi J, Feng W, Shvedova AA, Fadeel B. Fantastic voyage and opportunities of engineered nanomaterials: what are the potential risks of occupational exposures? J Occup Environ Med. 2010;52(9):943–946. doi: 10.1097/JOM.0b013e3181dc6c52. [DOI] [PubMed] [Google Scholar]
- 26.Shvedova AA, Kisin E, Murray AR, Inhalation vs, et al. aspiration of single-walled carbon nanotubes in C57BL/6 mice: Inflammation, fibrosis, oxidative stress, and mutagenesis. Am J Physiol. 2008;295(4):L552–L565. doi: 10.1152/ajplung.90287.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shvedova AA, Kisin ER, Murray AR, et al. Vitamin E deficiency enhances pulmonary inflammatory response and oxidative stress induced by single-walled carbon nanotubes in C57BL/6 mice. Toxicol Appl Pharmacol. 2007;221(3):339–348. doi: 10.1016/j.taap.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fadeel B, Kagan V, Krug H, et al. There’s plenty of room at the forum: Potential risks and safety assessment of engineered nanomaterials. Nanotoxicology. 2007;1(2):73–84. [Google Scholar]
- 29.Kagan VE, Tyurina YY, Tyurin VA, et al. Direct and indirect effects of single walled carbon nanotubes on RAW 264.7 macrophages: Role of iron. Toxicol Lett. 2006;165(1):88–100. doi: 10.1016/j.toxlet.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Pulskamp K, Diabate S, Krug HF. Carbon nanotubes show no sign of acute toxicity but induce intracellular reactive oxygen species in dependence on contaminants. Toxicol Lett. 2007;168(1):58–74. doi: 10.1016/j.toxlet.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Shvedova AA, Kisin ER, Murray AR, et al. ESR evidence for in vivo formation of free radicals in tissue of mice exposed to single-walled carbon nanotubes. Free Radic Biol Med. 2014;73:154–165. doi: 10.1016/j.freeradbiomed.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Shvedova AA, Fabisiak JP, Kisin ER, et al. Sequential exposure to carbon nanotubes and bacteria enhances pulmonary inflammation and infectivity. Am J Respir Cell Mol Biol. 2008;38(5):579–590. doi: 10.1165/rcmb.2007-0255OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teeguarden JG, Webb-Robertson BJ, Waters KM, et al. Comparative proteomics and pulmonary toxicity of instilled single-walled carbon nanotubes, crocidolite asbestos, and ultrafine carbon black in mice. Toxicol Sci. 2011;120(1):123–135. doi: 10.1093/toxsci/kfq363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donaldson K, Aitken R, Tran L, et al. Carbon nanotubes: a review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol Sci. 2006;92(1):5–22. doi: 10.1093/toxsci/kfj130. [DOI] [PubMed] [Google Scholar]
- 35.Murray AR, Kisin ER, Tkach AV, et al. Factoring-in agglomeration of carbon nanotubes and nanofibers for better prediction of their toxicity versus asbestos. Particle Fibre Toxicol. 2012;9:10. doi: 10.1186/1743-8977-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shvedova AA, Yanamala N, Kisin ER, et al. Long-term effects of carbon containing engineered nanomaterials and asbestos in the lung: One year postexposure comparisons. Am J Physiol. 2014;306(2):172–182. doi: 10.1152/ajplung.00167.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kisin ER, Murray AR, Sargent L, et al. Genotoxicity of carbon nanofibers: Are they potentially more or less dangerous than carbon nanotubes or asbestos? Toxicol Appl Pharmacol. 2011;252(1):1–10. doi: 10.1016/j.taap.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poland CA, Rodger Duffin R, Ian Kinloch I, et al. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Immunol. 2008;3(7):423–428. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- 39.Singh N, Manshian B, Jenkins JS, et al. Nano-Genotoxicology: The DNA damaging potential of engineered nanomaterials. Biomaterials. 2009;30(23–24):3891–3914. doi: 10.1016/j.biomaterials.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 40.Lindberg HK, Falck GS-M, Suhonen S, et al. Genotoxicity of nanomaterials: DNA damage and micronuclei induced by carbon nanotubes and graphite nanofibres in human bronchial epithelial cells in vitro. Toxicol Lett. 2009;186(3):166–173. doi: 10.1016/j.toxlet.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 41.Sargent LM, Shvedova AA, Hubbs AF, et al. Induction of aneuploidy by single-walled carbon nanotubes. Environ Mol Mutagen. 2009;50(8):708–717. doi: 10.1002/em.20529. [DOI] [PubMed] [Google Scholar]
- 42.Pacurari M, Yin XJ, Zhao J, et al. Raw single-wall carbon nanotubes induce oxidative stress and activate MAPKs, AP-1, NF-b, and Akt in normal and malignant human mesothelial cells. Environ Health Perspect. 2008;116(9):1211–1217. doi: 10.1289/ehp.10924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shvedova AA, Tkach AV, Kisin ER, et al. Carbon nanotubes enhance metastatic growth of lung carcinoma via up-regulation of myeloid-derived suppressor cells. Small. 2013;9(9–10):1691–1695. doi: 10.1002/smll.201201470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oberdörster G, Stone V, Donaldson K. Toxicology of nanoparticles: a historical perspective. Nanotoxicology. 2009;1(1):2–25. [Google Scholar]
- 45.United States Congress Joint Economic Committee Study. Nanotechnology: The Future is Coming Sooner than you Think. Washington, DC: JEC; 2007. [Google Scholar]
- 46.Braun JH. Titanium dioxide—a review. J Coatings Technol. 1997;69:59–72. [Google Scholar]
- 47.Warheit DB, Hoke RA, Finlay C, Donner EM, Reed KL, Sayes CM. Development of a base set of toxicity tests using ultrafine TiO2 particles as a component of nanoparticle risk management. Toxicol Lett. 2007;171(3):99–110. doi: 10.1016/j.toxlet.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 48.Warheit DB, Webb TR, Reed KL, Frerichs S, Sayes CM. Pulmonary toxicity study in rats with three forms of ultrafine-TiO2 particles: differential responses related to surface properties. Toxicology. 2007;230(1):90–104. doi: 10.1016/j.tox.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 49.Warheit DB. How to measure hazards/risks following exposures to nanoscale or pigment-grade titanium dioxide particles. Toxicol Lett. 2013;220(2):193–204. doi: 10.1016/j.toxlet.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 50.Gordon SC, Butala JH, Carter JM, et al. Workshop report: strategies for setting occupational exposure limits for engineered nanomaterials. Regul Toxicol Pharmacol. 2014;68(3):305–311. doi: 10.1016/j.yrtph.2014.01.005. doi:10.1016/j.yrtph.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 51.Food and Drug Administration. Guidance for Industry. [Accessed November 16, 2015];“Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology Guidance for Industry”. 2014 Jun 24; Web site. http://www.fda.gov/downloads/RegulatoryInformation/Guidances/UCM401695.pdf.
- 52.Food and Drug Administration. Guidance for Industry. [Accessed November 16, 2015];“Assessing the Effects of Significant Manufacturing Process Changes, Including Emerging Technologies, on the Safety and Regulatory Status of Food Ingredients and Food Contact Substances, Including Food Ingredients that Are Color Additives”. 2014 Jun 24; Web site. http://www.fda.gov/downloads/Cosmetics/GuidanceRegulation/GuidanceDocuments/UCM300927.pdf.
- 53. [Accessed November 16, 2015];European Commission 2008 Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. Article 12 Changes in the production process or starting materials of a food additive already included in a Community list. Web site. http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:32008R1333.
- 54. [Accessed November 16, 2015];Commission Regulation (EC) No 450/2009 of 29 May 2009 on active and intelligent materials and articles intended to come into contact with food. Recital 14. Web site. http://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32009R0450.
- 55.European Food Safety Authority Scientific Committee; Scientific Opinion on Guidance on the risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain. [Accessed November 16, 2015];EFSA J. 2011 9(5):2140. doi: 10.2903/j.efsa.2018.5327. Web site. www.efsa.europa.eu/efsajournal.htm and also at http://www.efsa.europa.eu/sites/default/files/scientific_output/files/main_documents/2140.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grocery Manufacturer’s Association. [Accessed November 16, 2015];Grocery Manufacturer Association Announces Industry Initiative on Ingredients Added to Food. 2014 Aug 28; 2014. Web site. http://www.gmaonline.org/news-events/newsroom/grocery-manufacturer-association-announces-industry-initiative-on-ingredien/
- 57.Food and Drug Administration. [Accessed November 16, 2015];Draft Guidance for Industry: Dietary Supplements: New Dietary Ingredient Notifications and Related Issues. 2011 Web site. http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/ucm257563.htm.
- 58.Food and Drug Administration. [Accessed December 5, 2015];Guidance for Industry and Other Stakeholders: Toxicological Principles for the Safety Assessment of Food Ingredients (Redbook 2000) Web site. http://www.fda.gov/downloads/Food/GuidanceRegulation/UCM222779.pdf.
- 59.Food and Drug Administration. [Accessed November 16, 2015];Guidance for Industry: #220 Use of Nanomaterials in Food for Animals. 2015 Aug; Web site. http://www.fda.gov/downloads/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/UCM401508.pdf.