Abstract
Aims
Lipotoxicity, defined as cell death induced by excessive fatty acids, especially saturated fatty acids, is critically involved in the development of non-alcoholic steatohepatitis (NASH). Recent studies report that plasma cysteine concentrations is elevated in the subjects with either alcoholic steatohepatitis (ASH) or NASH than normal subjects. The present study was conducted to determine if elevation of cysteine could be a deleterious factor in palmitate-induced hepatocyte cell death.
Main methods
HepG2 and Hep3B cells were treated with palmitate with/without the inclusion of cysteine in the media for 24 hours. The effects of cysteine inclusion on palmitate induced cell death were determined by lactate dehydrogenase (LDH) release and MTT assay. Oxidative stress was evaluated by intracellular glutathione (GSH) level, malondialdehyde (MDA) formation, and DCFH-DA assay. Western blotting was performed to detect the changes of endoplasmic reticulum(ER) stress markers: C/EBP homologous transcription factor (CHOP), GRP-78, and phosphorylated c-jun N-terminal kinase (p-JNK).
Key findings
Elevated intracellular cysteine aggravates hepatocytes to palmitate-induced cell death. Enhancement of ER stress, specifically increased activation of JNK pathway, contributed to this cell death process.
Significance
Increase of plasma cysteine levels, as observed in both ASH and NASH patients, may play a pathological role in the development of the liver diseases. Manipulation of dietary amino acids supplementation could be a therapeutic choice.
Keywords: Cysteine, Palmitate, ER stress, HepG2, JNK
Introduction
Elevated plasma free fatty acids (FFA) levels play an etiologic role in the pathogenesis of non-alcoholic steatohepatitis (NASH) and are postulated to be a critical link among obesity, insulin resistance, and the risk of NASH. Whereas adipocytes have a unique capacity to store excess FFA in the form of triglyceride in lipid droplets, non-adipose tissues, such as liver, have a limited capacity for storage of lipids. It has been reported that sustained high levels of plasma saturated fatty acids, such as palmitate, induce cell death in many cell types, including pancreatic β-cells (Choi et al. 2007), cardiomyocytes (Miller et al. 2005), astrocytes (Pascual et al. 2003), and hepatocytes (Gu et al. 2010; Akazawa et al. 2010). Although the detailed mechanisms underlying FFA induced lipotoxicity are still inconclusive, it is generally accepted that oxidative stress and endoplasmic reticulum(ER) stress are the major intracellular pathways involved (Zhang et al. 2010; Kim et al. 2010; Pfaffenbach et al. 2010; Ibrahim et al.2010; Wei et al. 2006).
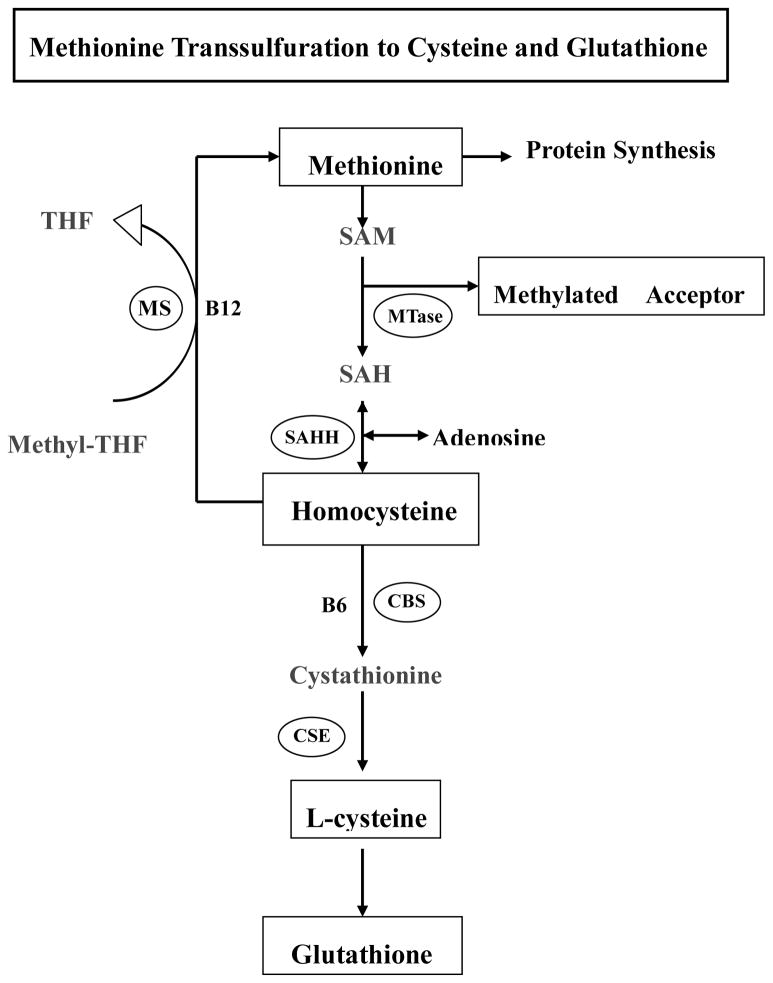
Cysteine is a sulfur-containing non-essential amino acid. In addition to the dietary sources, cysteine can be converted from intracellular methionine via transsulfuration pathway. As shown in Fig. 1, the first step of methionine metabolism is the formation of S-adenosylmethionine (SAM) in a reaction catalyzed by methionine adenosyltransferase. SAM is the principal biological methyl donor via transmethylation pathway. After its methyl group being transferred to a variety of methyl acceptors, SAM is eventually converted to homocysteine and then cysteine via the transsulfuration pathway. Cysteine is one of the precursor amino acids for glutathione synthesis, thereby conferring antioxidant function. Interestingly, a recent clinic investigation using data from 7038 Hordaland homocysteine study participants showed that plasma cysteine levels was positively associated with body mass index (BMI) (r = 0.28, p < 0.001), and fat mass (r = 0.25, p < 0.001), independent of diet, exercise, and plasma lipids (Elshorbagy et al. 2008). Furthermore, a couple of clinic studies recently reported that subjects with either alcoholic steatohepatitis (ASH) or NASH showed higher plasma cysteine concentrations compared to normal subjects (Cardin et al. 2003; Kalhan et al. 2010; Valentina ea al. 2010). These data altogether implied that increased plasma cysteine levels potentially involve in the pathogenesis of ASH or NASH. Indeed, a study by Noguchi et al. provided information that altered amino acids levels could modulate fatty acids induced lipotoxicity (Yasushi et al. 2009).
Figure 1. Methionine transsulfuration to cysteine and glutathione.
SAM, S-adenosyl methionine; SAH, S-adenosyl homocysteine; MTase, methionine adenosyltransferase; BHMT, betaine-homocysteine S-methyltransferase; SAHH, SAH hydrolase; MS, methionine synthase; CBS, cystathionine beta synthase.
In this study, we investigated (i) whether increased cysteine levels could sensitize hepatocytes to palmitate-induced cell death; and (ii) if it did, what was the underlying mechanism(s) involved?
Materials and methods
Chemicals
Palmitate, L-cysteine and N-Acetyl-Cysteine (NAC) were obtained from Sigma-Aldrich (USA). JNK inhibitor SP600125 was purchased from Calbiochem (La Jolla, CA). 2′, 7′-Dichlorohydrofluorescein diacetate (DCFH-DA) was purchased from Molecular Probes(Eugene, OR, USA).
Cells and culture conditions
HepG2 cells and Hep3B cells, two human hepatoma cell lines, were obtained from the American Type Culture Collection (Manassas, VA) and were cultured in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% (v/v) fetal bovine serum, 2 mM glutamine, 5 U/ml penicillin, and 50 ug/ml streptomycin at 37°C in a humidified O2/CO2 (19:1) atmosphere.
Preparation of palmitate-albumin complexes
Palmitate-albumin complex preparation was produced by a saponification technique according to Goldstein et al. using palmitate (C16:0) for complexing to bovine serum albumin (BSA), which is essentially fatty-acid free (Goldstein et al. 1983). Briefly, palmitate was dissolved in ethanol and saponified with sodium hydroxide. The sodium salt was dried, re-suspended in saline and heated at 80°C until it completely dissolved. While the solution was still warm, 20% (w/v) BSA was added and the mixture was stirred at 50°C for 4 hrs to allow palmitate to bind to albumin. Palmitate-BSA complex (6.4 mmol/L fatty acid: 0.9mmol/L BSA; molar ratio, 7:1) was then sterilized by filtering, and aliquoted for future use.
Lactate dehydrogenase (LDH) assay
Cell death was measured by the release of LDH into the culture medium. LDH activity was determined spectrophotometrically at 340nm by following the rate of NAD+ reduction in the presence of L-lactate.
MTT assay
Cells were seeded at a density of 2×104 cells/well on 96-well culture plates and incubated overnight. After the corresponding treatment, the medium was removed, and cell viability was evaluated by assaying for the ability of functional mitochondria to catalyze the reduction of thiazolyl blue tetrazolium bromide (MTT) to a formazan salt by mitochondrial dehydrogenases, as described previously (Caro and Cederbaum, 2001). Four independent samples were analyzed per experiment and each experiment was performed at least three times.
Hoechst staining
Hoechst 33342 is used for specifically staining the nuclei of living or fixed cells and tissues. Thus, it allows for the measurement of apoptosis within cells. Half an hour before the end of the incubation with the indicated treatment, Hoechst was added to each well of a 6-well plate at a final concentration of 1 μM. At the completion of the incubation, the cells were washed three times with ice-cold PBS, and then the fluorescence was measured by fluorescent microscope at an emission wavelength of 460 nm, using an excitation wavelength of 360 nm for the Hoechst fluorophore. All data are representative of at least three independent experiments.
Determination of intracellular glutathione (GSH) content
Intracellular GSH content was measured following the method as previously reported (Wang et al. 2006). Briefly, 2×105 cells were seeded on each well of 24-well culture plates and incubated for 24 hrs before corresponding treatment. At the end of the designated treatment, cells were washed with phosphate-buffered saline (PBS) twice and treated with 10% trichloroacetic acid for 30min at 4°C to extract cellular GSH. The mixture was centrifuged at 13,000g for 5 min to remove denatured proteins. The supernatants were assayed for GSH content using glutathione assay kit from Bioassay Systems according to manufacturer’s instruction. The data were expressed as nmol/mg protein.
Measurement of intracellular reactive oxygen species (ROS) production
DCFH-DA is a cell-permeable non-fluorescent compound that is cleaved into fluorescent products in the presence of H2O2 and other ROS molecules and esterase. Thus, it allows for the measurement of ROS production within cells. One hour before the end of the incubation with the indicated stimulus, the medium was removed and the cells were washed and placed in DMEM without serum. DCFH-DA was added to each well of a 24-well plate at a final concentration of 10μM. At the completion of the incubation, the cells were placed on ice, washed three times with ice-cold PBS, and then the fluorescence was measured by fluorescent microscope at an emission wavelength of 530nm, using an excitation wavelength of 485 nm for the DCF fluorophore. All data are representative of at least three independent experiments.
Measurement of intracellular lipid peroxidation
Intracellular lipid peroxides were measured according to Anal Biochem with slight modifications (Ohkawa et al.1979). Briefly, after corresponding treatment, cells in 24-well plate were trypsinized and resuspended in 25ul 1.15%KCl. And then the following reagents were added and incubated at 90°C for 70 min: 25ul 8.1% SDS solution, 190ul 20% acetate buffer (pH3.5), and 260ul 0.571% TBA solution (made fresh daily). After cooled on ice, 125ul H2O and 625ul butanol/pyridine mixture (15:1) was added and mixed for 2 min. Then the mixture was centrifuged at 3000rpm for 15 min at 4°C. Supernatant was assayed at 532nm for Malondialdehyde (MDA) content. The products were normalized by measuring the protein content of the cell lysates.
Measurement of intracellular cysteine level
Intracellular cysteine level was measured by a modification of the method of Gaitonde (Gaitonde 1967). Briefly, after corresponding treatment, cells were washed three times with 0.5ml ice-cold PBS, trypinsized and re-suspended in 150ul of 5% (wt/vol) sulfosalicyclic acid (SSA). After cells were centrifuged at 15,000g for 15min at 4°C, the protein pellet was saved for protein quantification to normalize the intracellular cysteine level. And 500mM dithiothreitol (DTT) was added into the supernatant to yield a final concentration of 5mM. Samples were reduced with DTT for 15min at room temperature. Following reduction, 100ul of sample were acidified with 100ul glacial acid and then reacted with 100ul acid ninhydrin reagent (250mg ninhydrin dissolved in a mixture of 6ml glacial acetic acid and 4ml pure HCl) for 10min at 100°C. After being heated, the sample mixture was cooled on ice for 3min and then was measured at 560nm quickly. Cysteine levels were quantified using cysteine hydrochloride standards (0–200uM) dissolved in 5% SSA and processed in the same manner as the samples.
Western blotting analysis
Cells were lysed in RIPA buffer and the isolated proteins were separated by SDS polyacrylamide gel electrophoresis and transferred to 0.45uM polyvinylidene difluoride (PVDF) membrane. After transferring, membranes were blocked in 5% BSA in PBS with 0.1% Tween-20 and probed with anti-GRP78 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-PARP, anti-phospho-JNK or anti-CHOP (Cell Signaling Technology, Danvers, MA) antibodies. Horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence substrate kit were used in detection of specific proteins.
Statistical analysis
All data were expressed as mean ± SD. Statistical analysis was performed using a one-way ANOVA and was analyzed further by Newman–Keuls test for statistical difference. Differences between treatments were considered to be statistically significant at p < 0.05.
Results
Palmitate induces cell death in HepG2 cells
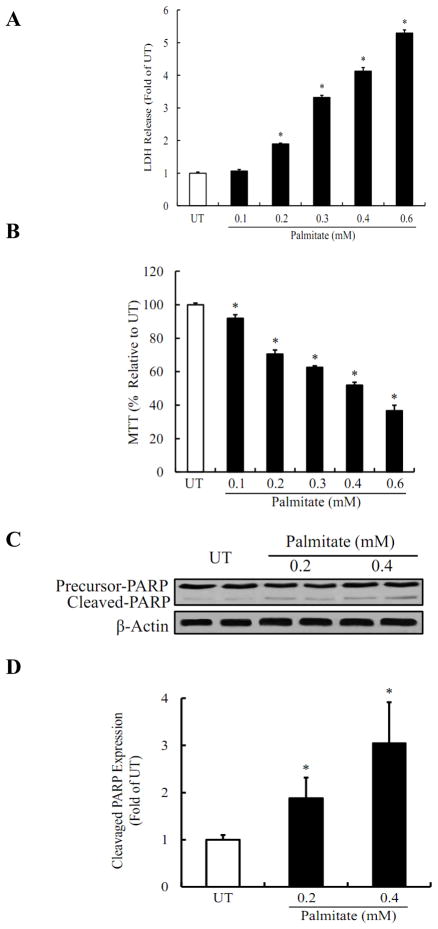
Palmitate-induced cell death in HepG2 cells was evaluated by measuring LDH release, MTT assay, and Western blotting for PARP-1 cleavage. As shown in Fig. 2A, inclusion of palmitate in the medium caused dose-dependent increases in LDH release. While palmitate at 0.2mM only caused a slight increase in LDH release, more than 5 times of LDH levels were observed when palmitate concentration was increased to 0.6mM. The similar results were observed by MTT assay (Fig. 2B). Palmitate-induced hepatoxicity was further confirmed by Western blot analysis for cleaved-form of PARP-1. As shown in Fig. 2C & D, incubation of HepG2 cells with palmitate for 16 hours caused a significant increase in PARP-1 cleavage.
Figure 2. Palmitate induces cell death in HepG2 cells.
(A) Palmitate caused a dose-dependent increase in LDH release. (B) Palmitate-induced a dose-dependent cell death measured by MTT assay. In A and B, HepG2 cells were incubated with increased concentration of palmitate (0, 0.1, 0.2, 0.3, 0.4, and 0.6mM) for 16 hrs. (C) Palmitate increased PARP-1 cleavage dose-dependently. (D) Quantitative analysis of the cleaved PARP-1 expression, normalized by corresponding β-actin expression level. HepG2 cells were incubated with increased concentration of palmitate (0, 0.2, and 0.4mM) for 16 hrs and cell lysates were collected for detecting PARP-1 cleavage. All values are denoted as means ± SD from three or more independent batches of cells, * p < 0.05 vs. UT.
Oxidative stress is not involved in palmitate-induced cell death
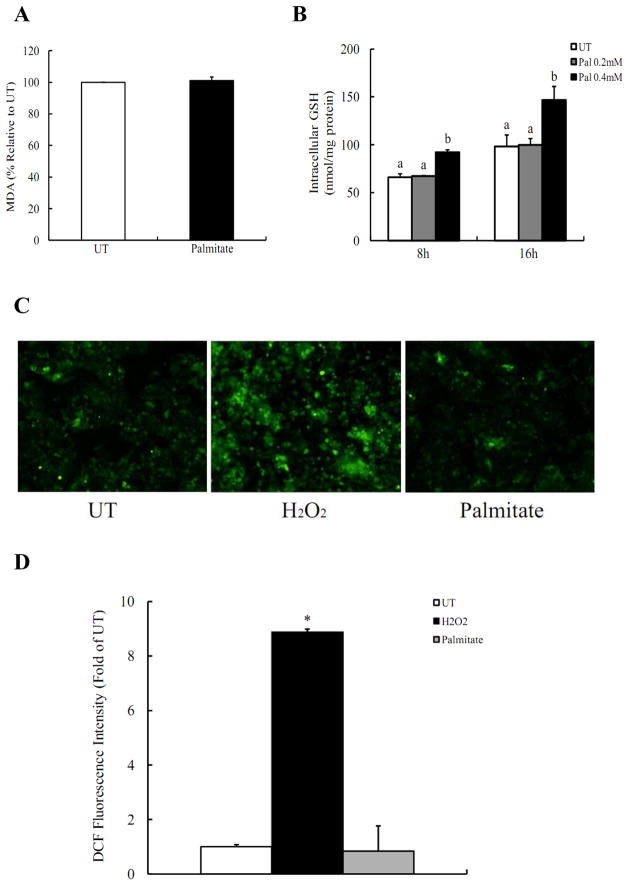
The contribution of oxidative stress in palmitate-induced cell death was investigated by detecting lipid peroxidation, intracellular GSH levels, and reactive oxygen species (ROS) production. As shown in Fig. 3A, culturing HepG2 cells with 0.2mM palmitate for 16 hrs did not cause significant increases in intracellular MDA levels in comparison with untreated cells. In contrast, intracellular GSH levels were unexpectedly increased by palmitate after 8 hrs and 16 hrs with a dose-dependent manner (Fig. 3B). To further verify our results, intracellular ROS production was directly detected in HepG2 cells using DCFH-DA, a membrane-permeable dye which can enter cells and produce a fluorescent signal after intracellular oxidation by ROS such as hydrogen peroxide and hydroxyl radical. As shown in Fig. 3C & D, incubation of HepG2 cells with 0.2mM palmitate for 2 hrs did not lead to increased oxidation of DCFH-DA, while incubation with 1mM H2O2 resulted in a significant increase in the fluorescence in comparison with control cells, suggesting that in our cell culture system, Palmitate-induced cell death is independent on the induction of intracellular oxidative stress.
Figure 3. Oxidative stress is not involved in palmitate-induced cell death.
(A) Intracellular malondialdehyde (MDA) levels were not affected by 0.2mM palmitate exposure. HepG2 cells were incubated with 0, 0.2mM palmitate for 16 hrs. (B) Palmitate caused a dose- and time-dependent intracellular glutathione (GSH) content increase. HepG2 cells were incubated with 0, 0.2, 0.4mM palmitate for 8 hrs and 16 hrs, respectively. (C) Palmitate had no effect on ROS production. (D) Quantitative analysis of the DCF fluorescence density. HepG2 cells were incubated for 2 hrs with palmitate (0.2mM) or H2O2 (1mM), an ROS-generating agent as a positive control. All values are denoted as means ± SD from three or more independent batches of cells. Bars with different letters differ significantly (p < 0.05).
Cysteine sensitizes HepG2 cells to palmitate-induced cell death
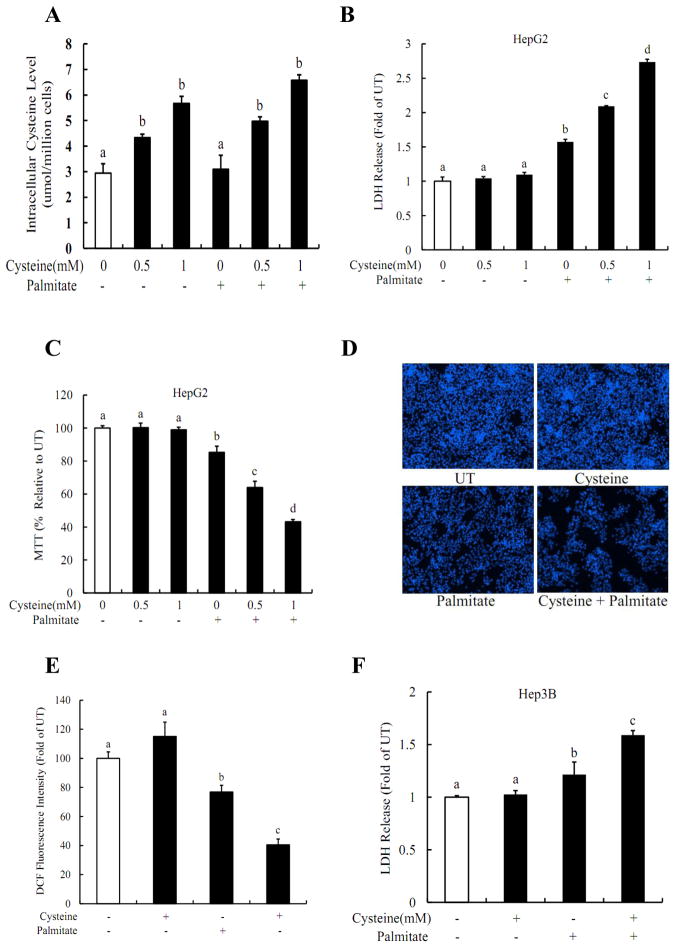
The effect of increased intracellular cysteine levels on palmitate-induced cell death in both HepG2 and Hep3B cells was examined. As shown in Fig. 4A, exogenous L-cysteine treatments increased intracellular cysteine levels in a dose-dependent manner, which were not affected by the inclusion of palmitate in the medium. Although supplementation of exogenous L-cysteine alone (0.5mM and 1mM) did not cause obvious cytotoxic effects, pre-treatment of HepG2 cells with L-cysteine for 2 hrs sensitized HepG2 cells to palmitate-induced cell death (Fig. 4B & C). Cysteine-induced sensitization to palmitate cytotoxicity was further confirmed by fluorescence microscopic examination of Hoechst 33342 staining. As shown in Fig. 4D & E, incubation of HepG2 cells with cysteine/palmitate for 16 hours resulted in significant decreases in cell population. The similar results were observed in Hep3B cells (Fig. 4F) via measuring LDH release.
Figure 4. Cysteine sensitizes hepatocytes to palmitate-induced cell death.
(A) Inclusion of exogenous cysteine elevated the intracellular cysteine levels in a dose-dependent manner in HepG2 cells and sensitized HepG2 cells to palmitate induced hepatotoxicity, evidenced by LDH release (B), MTT assay (C), and Hoechst staining (D, E). (F) Cysteine increased palmitate-induced LDH release from Hep3B cells. HepG2 or Hep3B cells were pre-treated with different dose of cysteine for 2 hrs, and then incubated with 0.2mM palmitate for 16 hrs. All values are denoted as means ± SD from three or more independent batches of cells. Bars with different letters differ significantly (p < 0.05).
The sensitization to palmitate-induced cell death is independent on oxidative stress
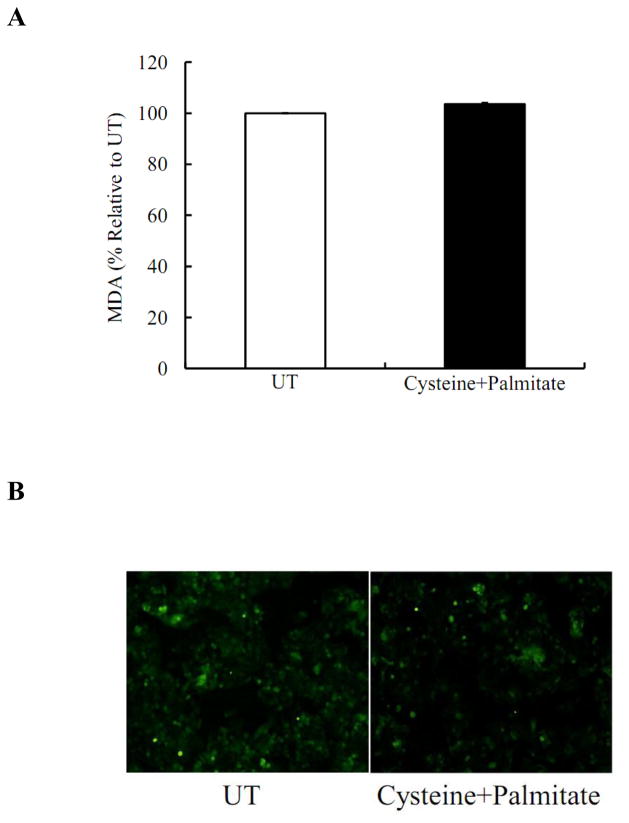
To determine if oxidative stress is inovled in the sensitization to palmitate-induced cell death. We first measured intracellular concentration of MDA, a lipid peroxidation product, after cysteine and palmitate treatment for 16 hrs. As shown in Fig. 5A, the combination had no significant effects on intracellular MDA levels in comparison with untreated cells. We then measured intracellular H2O2 production by DCFH-DA staining after 2- hour treatment with cysteine and palmitate. As shown in Fig 5B, there was no difference in H2O2 production between two groups.
Figure 5. Cysteine/palmitate-induced cell death is independent on oxidative stress.
HepG2 cells were incubated with and without cysteine (0.5mM)/palmitate (0.2mM) palmitate for 16 hrs. Intracellular MDA levels were measured. (A) Cysteine/palmitate treatment did not affect intracellular MDA levels. HepG2 cells were incubated with and without cysteine (0.5mM)/palmitate (0.2mM) palmitate for 2 hrs. Intracellular H2O2 productions were determined. (B) Cysteine/palmitate treatment did not affect intracellular H2O2 productions.
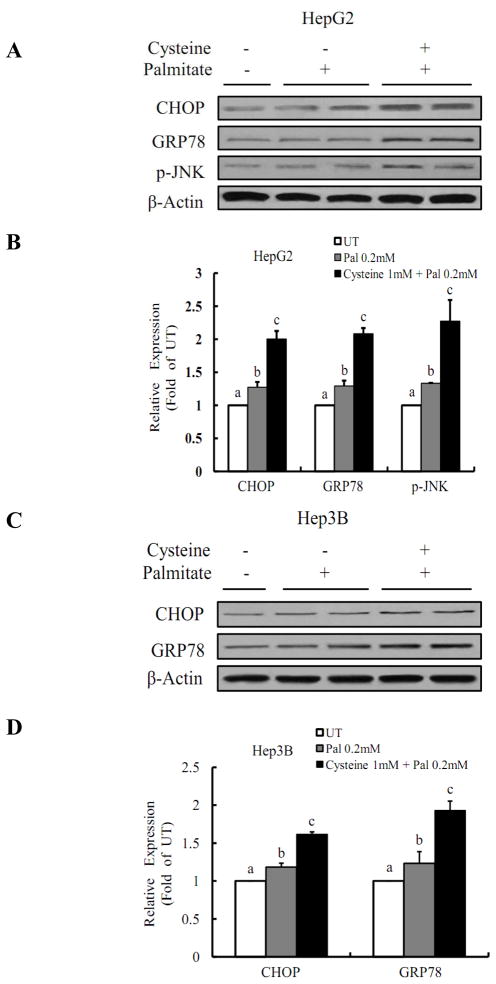
Cysteine aggravates palmitate-induced ER stress
We next examined if the sensitization of HepG2 and Hep3B cells to palmitate-induced cell death by cysteine was resulted from enhanced ER stress. Protein markers for ER stress were measured by Western blotting and the results were shown in Fig.6. Although palmitate at 0.2mM did not induce significant increases in protein abundance of three ER stress markers: C/EBP homologous transcription factor (CHOP), GRP-78, and p-JNK, they were all significantly elevated by the pretreatment with cysteine.
Figure 6. Cysteine aggravates palmitate-induced ER stress.
Pretreatment with cysteine enhanced palmitate-induced Chop, GRP78, and p-JNK protein abundances in both HepG2 (A & B) and Hep3B cells (C & D). HepG2 or Hep3B cells were incubated with 0.2mM palmitate for 16 hrs with or without 1 mM cysteine pretreatment for 2 hrs and cell lysates were collected. All values are denoted as means ± SD from three or more independent batches of cells. Bars with different letters differ significantly (p < 0.05).
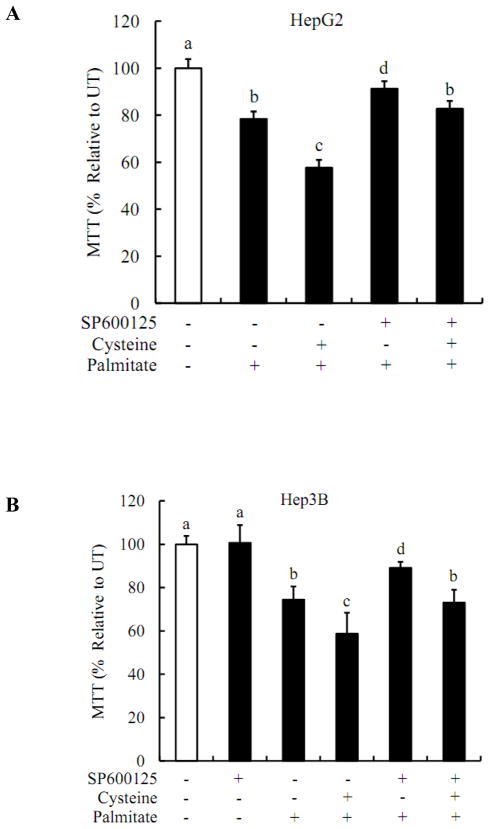
Inhibition of JNK pathway prevents cytotoxicity
The involvement of JNK pathway in observed cytotoxic effects was examined via pre-treating HepG2 or Hep3B cells with SP600125, a specific inhibitor of JNK pathway. As shown in Fig.7, SP600125 at 20uM markedly improved cell viability in cysteine/palmitate-treated hepatocytes, suggesting that JNK activation plays an important role in the sensitization of cysteine to palmitate-induced cell death.
Figure 7. Inhibition of JNK pathway prevents cytotoxicity.
HepG2 or Hep3B cells were pretreated with 20uM JNK inhibitor SP600125 for 2 hrs prior to cysteine treatment, followed with or without 0.2mM palmitate treatment. Cell viabilities were detected by MTT assay and were expressed as a percent of the untreated cells. All values are denoted as means ± SD from three or more independent batches of cells. Bars with different letters differ significantly (p < 0.05).
Discussion
Lipotoxicity is implicated in the pathogenesis of NASH, and FFA appears to be important contributors of lipotoxicity. Much evidence supports that saturated long-chain fatty acids are the major contributor to lipotoxicity (Alkhouri et al. 2009). Palmitate is the most abundant saturated fatty acid in human blood. In consistent with previous studies (Gu et al. 2010; Zhang et al. 2010), we showed that inclusion of palmitate in the cell culture medium induced cell death in hepatocytes. Interestingly, we found that exogenous supplementation of cysteine, a rate-limiting precursor for intracellular glutathione synthesis, sensitized hepatocytes to palmitate-induced cell death. Mechanistic investigations revealed that pretreatment of hepatocytes with cysteine enhanced palmitate-induced unfolded protein response (UPR), evidenced by increased intracellular protein abundance of ER stress markers, including CHOP, GRP-78, and p-JNK. Furthermore, our results showed that inhibition of JNK pathway conferred protective effects.
Although the underlying mechanisms are still elusive, the oxidative stress has been considered to play a critical role in palmitate-induced cell death in a variety of cell types, including hepatocytes (Kim et al. 2010; Cacicedo et al. 2005; Barreyro et al. 2007). In this context, it is predictable that cysteine, a precursor amino acid for the synthesis of glutathione, the most important intracellular antioxidant, should confer protective effect. Unexpectedly, our data demonstrated that both L-cysteine and its precursor, N-acetyl-cysteine (data not shown), aggravated palmitate-induced cell death. Furthermore, using palmitate at non-toxic concentration or a concentration inducing only minor cytotoxicity, we found that pretreatment with cysteine sensitized hepatocytes to palmitate-induced cell death. Based on these observations, we therefore examined if palmitate exposure induced oxidative stress in our cell culture system using standard DMEM medium. Data obtained from the present study conclude that oxidative stress is not involved in palmitate-induced cell death in HepG2 cells in our cell culture condition based on the following results: (i) palmitate treatment did not cause lipid peroxidation as demonstrated by unchanged intracellular malondialdehyde levels in comparison with untreated cells, (ii) palmitate incubation did not lower intracellular GSH content, in contrast, elevated GSH levels were indeed observed with palmitate treatment; (iii) most importantly, there was no any increase of the observed DCF fluorescence induced by intracellular oxidation in palmitate treated cells compared to untreated cells. This conclusion seemed to contradict with a recent study by Yasushi et al., who reported that ROS generation played a central role in apoptotic process induced by palmitate exposure in H4IIEC3 cells, a rat hepatoma cell line (Yasushi et al. 2009). The major discrepancy may originate from different cell culture condition. In their study, a customized cell culture medium was used to mimic the composition of physiological fluids. The cell culture medium used in their study contained lower concentrations of most amino acids, including cysteine, in comparison to standard DMEM medium. Therefore, our study in fact further supports the notion that mechanisms involved in palmitate’s cytotoxic effect are multifactorial and the nutritional status may play a critical role in this process. While the increase in cysteine levels may prevent palmitate-induced oxidative stress in hepatocytes, dysregulated methionine/cysteine metabolism, such as these reported in NASH and ASH patients, may enhance lipotoxicity via exaggerate ER stress.
In addition to oxidative stress, many recent studies also support that ER stress is another important molecular mechanism involved in palmitate-induced cell death (Pfaffenbach et al. 2010; Ibrahim et al. 2010; Wei et al. 2006). ER stress is originated from the excessive accumulation of unfolded or misfolded proteins in the ER lumen, which stimulates the activation of several ER proteins, leading to either inhibition of translation or induction of gene expression. In response to ER stress, unfolded proteins bind to the ER chaperone immunoglobulin heavy-chain binding protein (BiP/Grp78) to inhibit protein translation, thereby protecting ER from stress-induced dysfunction. However, when the protective response is insufficient, a set of responses leads to cell malfunction. Prolonged ER stress leads to transcriptional induction and activation of a number of genes and proteins critically involved in the process of apoptosis; among which CHOP/GADD153 and JNK are two critical proteins in cytotoxicity pathway. To investigate the potential involvement of ER stress in cell death pathway observed in our study, we examined the protein abundance of three ER stress markers by western blot analysis: CHOP, GRP-78, and p-JNK. As a result, we found that cysteine pretreatment significantly increased the protein abundance of three ER stress markers, even when palmitate was added in a concentration that had no effects on these markers by itself, suggesting that the involvement of ER stress in the sensitization to palmitate-induced cell death by cysteine. Furthermore, we found that inhibition of JNK signaling pathway by SP600125 almost completely blocked cysteine and palmitate-induced cell death, confirming that JNK activation plays a crucial role in the sensitization process.
Cysteine is a sulfur-containing non-essential amino acid and it can be converted from methionine via transsulfuration pathway besides the dietary sources (Fig. 1). Cysteine is a constituent amino acid of GSH, an important endogenous intracellular antioxidant. Nevertheless, our findings indicate that under certain circumstances, elevated cysteine levels can be detrimental to the liver via aggravating palmitate-induced lipotoxicity in hepatocytes. Considering several recent clinic studies showing that plasma cysteine levels are increased in the patients either with ASH or NASH (Elshorbagy et al. 2008; Cardin et al. 2003; Kalhan et al. 2010; Valentina ea al. 2010), our findings imply that increased cysteine levels may play a pathological role in the development of certain types of fatty liver diseases and manipulation of dietary amino acids supplementation could be a therapeutic choice.
Acknowledgments
This project was supported by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) grants K01 AA015344 and R01 AA017442 (Z Song).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alkhouri N, Dixon LJ, Feldstein AE. Lipotoxicity in nonalcoholic fatty liver disease: not all lipids are created equal. Expert Rev Gastroenterol Hepatol. 2009;3:445–51. doi: 10.1586/egh.09.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akazawa Y, Cazanave S, Mott JL, Elmi N, Bronk SF, Kohno S, et al. Palmitoleate attenuates palmitate-induced Bim and PUMA up-regulation and hepatocyte lipoapoptosis. J Hepatol. 2010;52:586–93. doi: 10.1016/j.jhep.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreyro FJ, Kobayashi S, Bronk SF, Werneburg NW, Malhi H, Gores GJ. Transcriptional Regulation of Bim by FoxO3A Mediates Hepatocyte Lipoapoptosis. J Biol Chem. 2007;282:27141–54. doi: 10.1074/jbc.M704391200. [DOI] [PubMed] [Google Scholar]
- Cacicedo JM, Benjachareowong S, Chou E, Ruderman NB, Ido Y. Palmitate-induced apoptosis in cultured bovine retinal pericytes: roles of NAD(P)H oxidase, oxidant stress, and ceramide. Diabetes. 2005;54:1838–45. doi: 10.2337/diabetes.54.6.1838. [DOI] [PubMed] [Google Scholar]
- Choi SE, Kim HE, Shin HC, Jang HJ, Lee KW, Kim YS, et al. Involvement of Ca2+-mediated apoptotic signals in palmitate-induced MIN6N8a beta cell death. Mol Cell Endocrinol. 2007;272:50–62. doi: 10.1016/j.mce.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Cardin R, D'Errico A, Fiorentino M, Cecchetto A, Naccarato R, Farinati F. Hepatocyte proliferation and apoptosis in relation to oxidative damage in alcohol-related liver disease. Alcohol Alcohol. 2002;37:43–8. doi: 10.1093/alcalc/37.1.43. [DOI] [PubMed] [Google Scholar]
- Caro AA, Cederbaum AI. Synergistic Toxicity of Iron and Arachidonic Acid in HepG2 Cells Overexpressing CYP2E1. Mol Pharmacol. 2001;60:742–52. [PubMed] [Google Scholar]
- Elshorbagy AK, Nurk E, Gjesdal CG, Tell GS, Ueland PM, Nygård O, et al. Homocysteine, cysteine, and body composition in the Hordaland Homocysteine Study: does cysteine link amino acid and lipid metabolism? Am J Clin Nutr. 2008;88:738–46. doi: 10.1093/ajcn/88.3.738. [DOI] [PubMed] [Google Scholar]
- Gaitonde MK. A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. J Biochem. 1967;104:627–33. doi: 10.1042/bj1040627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JL, Basu SK, Brown MS. Receptor-mediated endocytosis of low-density lipoproteins in cultured cells. Meth Enzymol. 1983;98:241–60. doi: 10.1016/0076-6879(83)98152-1. [DOI] [PubMed] [Google Scholar]
- Gu X, Li K, Laybutt DR, He ML, Zhao HL, Chan JC, et al. Bip overexpression, but not CHOP inhibition, attenuates fatty-acid-induced endoplasmic reticulum stress and apoptosis in HepG2 liver cells. Life Sci. 2010;87:724–32. doi: 10.1016/j.lfs.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Ibrahim SH, Akazawa Y, Cazanave SC, Bronk SF, Elmi NA, Werneburg NW, et al. Glycogen synthase kinase-3 (GSK-3) inhibition attenuates hepatocyte lipoapoptosis. J Hepatol. 2011;54:765–72. doi: 10.1016/j.jhep.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalhan SC, Guo L, Edmison J, Dasarathy S, McCullough AJ, Hanson RW, et al. Plasma metabolomic profile in nonalcoholic fatty liver disease. Metabolism. 2011;60:404–13. doi: 10.1016/j.metabol.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JE, Song SE, Kim YW, Kim JY, Park SC, Park YK, et al. Adiponectin inhibits palmitate-induced apoptosis through suppression of reactive oxygen species in endothelial cells: involvement of cAMP/protein kinase A and AMP-activated protein kinase. J Endocrinol. 2010;207:35–44. doi: 10.1677/JOE-10-0093. [DOI] [PubMed] [Google Scholar]
- Miller TA, LeBrasseur NK, Cote GM, Trucillo MP, Pimentel DR, Ido Y, et al. Oleate prevents palmitate-induced cytotoxic stress in cardiac myocytes. Biochem Biophys Res Commun. 2005;336:309–15. doi: 10.1016/j.bbrc.2005.08.088. [DOI] [PubMed] [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Pascual M, Valles SL, Renau-Piqueras J, Guerri C. Ceramide pathways modulate ethanol- induced cell death in astrocytes. J Neurochem. 2003;87:1535–45. doi: 10.1046/j.1471-4159.2003.02130.x. [DOI] [PubMed] [Google Scholar]
- Pfaffenbach KT, Gentile CL, Nivala AM, Wang D, Wei Y, Pagliassotti MJ. Linking endoplasmic reticulum stress to cell death in hepatocytes: roles of C/EBP homologous protein and chemical chaperones in palmitate-mediated cell death. Am J Physiol Endocrinol Metab. 2010;298:1027–35. doi: 10.1152/ajpendo.00642.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiniakos DG, Vos MB, Brunt EM. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu Rev Pathol. 2010;5:145–71. doi: 10.1146/annurev-pathol-121808-102132. [DOI] [PubMed] [Google Scholar]
- Valentina M, Janet MP, Sally PS, Samuel WF, Jesse FG, Maria CV, et al. Impaired homocysteine transsulfuration is an indicator of alcoholic liver disease. J Hepatol. 2010;53:551–7. doi: 10.1016/j.jhep.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YM, Peng SQ, Zhou Q, Wang MWi, Yan CH, Yang HY, et al. Depletion of intracellular glutathione mediates butenolide induced cytotoxicity in HepG2 cells. Toxicol Lett. 2006;164:231–8. doi: 10.1016/j.toxlet.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol Endocrinol Metab. 2006;291:275–81. doi: 10.1152/ajpendo.00644.2005. [DOI] [PubMed] [Google Scholar]
- Yasushi N, Jamey DY, Jose OA, Michael EH, Joanne KK, Gregory S. Effect of Anaplerotic Fluxes and Amino Acid Availability on Hepatic Lipoapoptosis. J Biol Chem. 2009;284:33425–36. doi: 10.1074/jbc.M109.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Seitz LC, Abramczyk AM, Chan C. Exp Synergistic effect of cAMP and palmitate in promoting altered mitochondrial function and cell death in HepG2 cells. Cell Res. 2010;316:716–27. doi: 10.1016/j.yexcr.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]