Abstract
Objectives
Inflammatory/hemostatic biomarkers are associated with coronary heart disease (CHD) events, but relationships in asymptomatic midlife women are uncertain. We evaluated separately whether high sensitivity C-reactive protein (hsCRP), fibrinogen, plasminogen-activator inhibitor 1, tissue plasminogen activator antigen, and circulating factor VII (factor VIIc) were associated with coronary artery calcification (CAC) in healthy midlife women.
Methods
A cross-sectional study was performed of participants from the Study of Women’s Health Across the Nation (SWAN). Logistic and tobit regression were used to assess associations between log-transformed biomarkers and CAC presence (CAC >0) and extent. Effect modification by race/ethnicity was evaluated.
Results
The study included 372 women (mean age 51.3 years; 35.2% African-American). All biomarkers were positively associated with CAC presence and extent (p<0.001 for all), adjusting for Framingham risk score, site, race/ethnicity, menopausal status, income, and education. Additional adjustment for BMI explained all associations except for factor VIIc, which remained associated with CAC extent only (p=0.02). Final adjustment for insulin resistance, family history of cardiovascular (CV) disease, and CV medication use produced similar results. Associations between hsCRP and CAC presence and extent were modified by race/ethnicity (p<0.05). Log(hsCRP) was positively associated with CAC presence (OR, 3.25; 95% CI, 1.53–6.90; p=0.002; per 1 log unit increase) and CAC extent (β, 19.66; SE 7.67; p=0.01; per 1 log unit increase) in African-Americans only.
Conclusion
Inflammatory/hemostatic biomarkers were associated with CAC through obesity, except for factor VIIc. Among African-American women only, hsCRP was independently associated with CAC suggesting that hsCRP may have a role in CHD prevention in African-American midlife women.
Keywords: coronary artery calcification, hemostasis, inflammation, race/ethnicity, risk factor
INTRODUCTION
The importance of sex-specific study of coronary heart disease (CHD) is well recognized (1). CHD deaths dramatically increase after menopause, leading to a reduction in the ratio of male-to-female CHD deaths (2). Despite the significance of this period in the natural history of CHD, there is much that is still unknown. In one study, >10% of women aged 46–55 years with CHD events did not have any traditional risk factors and >35% had one risk factor (3). This suggests other unknown mechanisms to explain women’s risk.
Biomarkers associated with inflammation and hemostasis, such as high sensitivity C-reactive protein (hsCRP) and fibrinogen, have been associated with subsequent CHD events in asymptomatic individuals (4). The relationships of these biomarkers and others to coronary artery calcification (CAC) in women undergoing the menopausal transition are less certain. CAC is a well-recognized measure of subclinical cardiovascular disease (CVD) (5). It enhances prediction of CHD events beyond traditional risk factors (6). It has also been used as a surrogate end point in research in lieu of hard CHD events, particularly in lower risk populations.
In a systematic review of inflammation and CAC, only 5 studies included midlife, apparently healthy women (7). The study populations, however, were drawn from a wide age range, as much as six decades, and analyses did not take into account menopausal status. A more recent analysis from the Dallas Heart Study studied a group of 1,600 multi-racial/ethnic women in midlife with a mean age of 45 ± 9.9 years (8). HsCRP was positively associated with CAC prevalence among normal weight, but not overweight or obese women. Adjustment was not made for other CHD risk factors or menopausal status, and subgroup analysis by race/ethnicity was not performed. Racial/ethnic differences in hsCRP levels are well-documented (9). Our goal was to examine cross-sectional associations of five inflammatory/hemostatic biomarkers and CAC in women undergoing the menopausal transition who were free of known CVD. We hypothesized that hsCRP, fibrinogen, plasminogen-activator inhibitor 1 (PAI-1), tissue plasminogen activator antigen (tPA-ag), and circulating factor VII (factor VIIc) were positively associated with CAC, independent of traditional CHD risk factors, and that these associations varied by race/ethnicity.
METHODS
Study Population
The Study of Women’s Health Across the Nation (SWAN) is a multi-center, multi-racial/ethnic prospective cohort study in the United States designed to examine the menopausal transition (10). Seven clinical sites recruited women who were White and one additional pre-determined race/ethnicity (African-Americans in Boston, Chicago, Detroit, Pittsburgh; Chinese in Oakland; Hispanic in New Jersey; Japanese in Los Angeles). Eligibility criteria for the SWAN parent study included age 42–52 years, an intact uterus, menstruating within the prior 3 months, and not using reproductive hormones. Those with a prior hysterectomy or bilateral oophorectomy were excluded. Study protocols were approved by the institutional review board at each site and participants provided written informed consent.
Two sites, Pittsburgh and Chicago, participated in the SWAN Heart ancillary study, which was designed to evaluate the changes in subclinical measures of atherosclerosis during the menopausal transition. These two sites, by design, enrolled only self-identified White and African-American women. This present study utilized data from the baseline SWAN Heart study visits. These occurred during SWAN parent study annual visits 4 through 7 (2001–2005), within 3 months after the corresponding annual core SWAN assessment (11).
The SWAN Heart cohort originally consisted of 608 women. For the current study, women were excluded if they did not have baseline CAC measurements (n=47); reported myocardial infarction, angina or stroke at the baseline visit (n=11); and did not have baseline levels of hsCRP, fibrinogen, PAI-1, tPA-ag, and factor VIIc (n=178). The final sample size for those with both CAC measurements and novel risk factors of interest was 372 women. The women in the final sample, when compared to the 236 excluded women, were older (51.3 ± 2.8 years vs. 50.1 ± 2.9 years; p<0.0001), had a lower systolic blood pressure (SBP) (118.4 ± 17.1 mmHg vs. 121.4 ± 15.7 mmHg; p=0.004), and were less likely to be current smokers (13.4% vs. 21.6%; p=0.008). They were similar with respect to racial/ethnic distribution, body mass index (BMI), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), diabetes, and family history of CVD.
Coronary Artery Calcification
A CT scanner (C-150 Ultrafast CT Scanner, GE Imatron, San Francisco, California) was used to quantify levels of calcification in the coronary arteries. The first pass allowed an evaluation of the participant’s anatomy so that the landmarks for the coronary scans were identified. The second pass was for the coronary arteries in which 30 to 40 contiguous 3-mm thick transverse images were obtained from the level of the aortic root to the apex of the heart. Images were obtained during a maximal breath hold, using electrocardiographic triggering so each 100-millisecond exposure was obtained during the same phase of the cardiac cycle (60%) of the R-R interval. All scan data were saved to an optical disk for central scoring, using a DICOM workstation and software by AcuImage, Inc (South San Francisco, California). This software program implements the Agatston scoring method (12). CAC was defined as a hyper-attenuating lesion >130 Hounsfield units with an area of ≥3 pixels. The Agatston unit score was calculated by multiplying the lesion area (mm2) by a density factor (between 1 and 4). The total calcium score was the sum of the individual scores for the four major epicardial coronary arteries. Under the supervision of a cardiologist, a technologist scored the scans. At the Pittsburgh site, this technique has been demonstrated to have an intraclass correlation coefficient = 0.99 (13).
Blood Assays
Phlebotomy was performed in the morning after an overnight fast within 2–5 days of a spontaneous menstrual cycle. If a timed sample could not be obtained because of irregular or cessation of menstrual cycles, a random fasting sample was taken within 90 days of the annual visit. Samples were maintained at 4° C until separated, frozen at −80° C, and shipped on dry ice to the central laboratory (Medical Research Laboratories, Highland Heights, KY), which is certified by the Centers for Disease Control Lipid Standardization Part III program (14). All lipid and lipoprotein fractions were analyzed on EDTA-treated plasma. Total cholesterol, HDL-C, insulin, and glucose were determined from a fasting blood sample with standard methods described previously (15). LDL-C was calculated using the Friedewald equation (16). The Homeostasis Model Assessment (HOMA) insulin resistance index was calculated using the following equation: [insulin × glucose]/22.5 (17). Glucose in mg/dL was converted to millimoles per liter by multiplying by 0.0555. HOMA represents a computer model of the glucose-insulin feedback system during a fasting state, specifically regarding the functions of the tissues and organs related to glucose regulation.
HsCRP was measured using ultrasensitive rate immunonephelometry (Dade-Behring, Marburg, Germany). The sensitivity of the assay was 0.03 mg/dL. The interassay coefficients of variations (CVs) at hsCRP concentrations of 0.05 and 2.2 mg/dL were 10% to 12% and 5% to 7%, respectively. Fibrinogen and factor VIIc were measured in frozen citrated plasma using a clot-based turbidometric detection system (MLA ELECTRA 1400C; Medical Laboratory Automation Inc., Mt. Vernon, NY). Fibrinogen monthly interassay CVs were 2.3% to 3.5% and 2.6% to 3.6% at mean concentrations of 250 and 140 mg/dL, respectively. Factor VIIc monthly interassay CVs were 7.8%, 5%, and 4% for mean activities of 8%, 45%, and 99%, respectively. PAI-1 was measured using a solid phased monoclonal antibody and a second enzyme-labeled goat antiserum for detection (IMUBIND plasma PAI-1 enzyme-linked immunosorbent assay; American Diagnostica, Greenwich, Connecticut). PAI-1 monthly interassay CVs were 5% to 9% and 4% to 9% at mean concentrations of 7 and 22.5 ng/dL, respectively. A double antibody in an enzyme-linked immunosorbent assay (American Diagnostica) measured t-PA-ag, with a human single chain t-PA-ag as a standard calibrated against an international standard (National Institute for Biological Standards and Control, Hertfordshire, United Kingdom). Monthly interassay CVs were 4.7% to 8.7% and 3.8% to 7.8% at mean concentrations of 5.6 and 11 ng/dL, respectively.
Other Study Variables
Blood pressure was measured twice with a minimum 2-minute rest period between measures and the average was used. Readings were taken on the right arm, with the respondent seated and feet flat on the floor for at least 5 minutes before the measurement. Respondents had not smoked or consumed any caffeinated beverage within 30 minutes of blood pressure measurement. Smoking was coded into current versus past or never smoker. BMI was derived from in-clinic measures of weight and height, and was calculated by [mass (kg)]/[height (m)]2. Framingham risk score (FRS) assessment was the summation of all Framingham risk-related points: age, LDL-C or total cholesterol, HDL-C, blood pressure, diabetes, and smoking (18).
Menopausal status, obtained annually from reported bleeding patterns in the year preceding the visit, was categorized as 1) pre-menopausal (bleeding in previous 3 months with no past year change in cycle predictability), 2) early perimenopausal (bleeding the previous 3 months with decrease in cycle predictability in the past year), 3) late perimenopausal (<12 to >3 months of amenorrhea), 4) natural post-menopausal (≥12 months of amenorrhea), 5) surgical post-menopausal (for those who had undergone hysterectomy with/without oophorectomy), and 6) indeterminate status (for women pre-/peri-menopausal who reported taking hormones in the past year because of the impact of hormone use, even if discontinued, on bleeding patterns). Post-menopausal women who reported taking hormones within the past month of the clinical visit were classified as hormone therapy users. For the current analyses, pre-menopausal and early perimenopausal women were combined into one group, as were late perimenopausal and post-menopausal women (19).
Income was initially reported in eight categories. These were compressed into three categories: <$50,000; $50,000–$150,000, and >$150,000. Education was reported in 5 categories. These were compressed into three categories: high school graduate or less, some college or college graduate, and graduate degree.
Race/ethnicity was determined in response to “How would you describe your primary racial or ethnic group?” CV medication use (Yes, if blood pressure and/or cholesterol medication use), and family history of CVD were self-reported. Diabetes was defined as the use of any medication for diabetes or a fasting blood glucose of >125 mg/dl.
Statistical Analysis
Summary statistics of the study variables were presented for total as well as by CAC presence status. Differences in study variables by CAC status were assessed by the Wilcoxon-Mann-Whitney test and the chi-square test as appropriate. hsCRP, fibrinogen, PAI-1, tPA-ag, and factor VIIc had skewed distributions and were therefore log transformed.
CAC was evaluated as presence of calcification if CAC Agatston score >0, and extent of calcification using CAC Agatston score. Logistic regression for CAC presence and tobit regression for CAC extent were used for statistical analyses. Tobit regression was chosen given a right-skewed distribution due to many zero scores (20). Tobit combines a logistic regression of CAC presence (CAC >0 vs. CAC = 0) with a linear regression in order to produce a single estimate. This modeling has been used before with log-transformed CAC (21,22). We chose to use the raw CAC score for ease of interpretation. Sensitivity analysis was performed for log(CAC+1) to assure consistency of results. The results were similar (not presented).
Covariates that were known to be associated with CAC were forced into the adjusted models as well as those associated at p<0.25 in the univariable analysis. Models were adjusted for the aggregate FRS, study site, race/ethnicity, menopausal status, education, and income. Second, models were additionally adjusted for BMI. Final models were further adjusted for HOMA insulin resistance index, family history of CVD, and CV medication use. To avoid multicollinearity, covariates that were components of the calculated FRS were not included in multivariable modeling. Multicollinearity was checked for BMI and HOMA insulin resistance index using variance inflation factor given correlation with the independent variables; none were significant. Interactions were assessed for race/ethnicity, menopausal status, and BMI and stratified analyses were performed if significant. Interactions with menopausal status or BMI were not significant. Due to few diabetics, sensitivity analyses were performed in the non-diabetic women only. A 2-tailed value of p<0.05 was considered significant for all analyses. Goodness-of-fit was assessed with the p-value from the Hosmer-Lemeshow Test for logistic regression and the Akaike information criterion for tobit regression. SAS version 9.4 (Cary, North Carolina) was used for statistical analyses.
RESULTS
The characteristics of the study population by presence of CAC at baseline are listed in Table 1. CAC was present in 184 (49.5%) participants. The majority of the study participants (97%) of the study participants had a CAC score between 0–100 U. For those with CAC (CAC>0), the median Agatston score was 8.6 U with a range from 1.0 U to 598.6 U. Participants with CAC had a worse CHD risk profile when compared to participants without CAC. They were older; more likely to be African-American; had a higher SBP, BMI, LDL-C, and FRS; and had lower HDL-C. All inflammatory/hemostatic biomarkers were significantly higher in those with baseline CAC present. There was no significant difference between the two groups in relation to menopausal status, hormone therapy, income, and education.
Table 1.
Baseline Characteristics.
| Characteristics | Total | CAC = 0 | CAC >0 | |
|---|---|---|---|---|
| n = 372 | n = 188 | n = 184 | p-value* | |
| Age at baseline CAC, mean (SD), y | 51.3 (2.8) | 50.9 (2.8) | 51.6 (2.7) | 0.005 |
| Site, n (%) | 0.5 | |||
| Chicago | 160 (43.0) | 78 (41.5) | 82 (44.6) | |
| Pittsburgh | 212 (57.0) | 110 (58.5) | 102 (55.4) | |
| Race, n (%) | 0.004 | |||
| African-American | 131 (35.2) | 53 (28.2) | 78 (42.3) | |
| White | 241 (64.8) | 135 (71.8) | 106 (57.6) | |
| Menopausal status, n (%) | 0.7 | |||
| Pre- and early perimenopausal | 200 (53.8) | 106 (56.4) | 94 (51.1) | |
| Late peri- and post-menopausal | 141 (37.9) | 66 (35.1) | 75 (40.8) | |
| Surgical menopause | 12 (3.2) | 7 (3.7) | 5 (2.7) | |
| Indeterminate | 19 (5.1) | 9 (4.8) | 10 (5.4) | |
| Income, n (%) | 0.1 | |||
| <$50,000 | 121 (32.7) | 55 (29.3) | 66 (36.3) | |
| $50,000–$100,000 | 154 (41.6) | 77 (41.0) | 77 (42.3) | |
| >$100,000 | 95 (25.7) | 56 (29.8) | 39 (21.4) | |
| Education, n (%) | 0.4 | |||
| High school or less | 58 (15.6) | 25 (13.3) | 33 (17.9) | |
| Some college or college | 192 (51.6) | 102 (54.3) | 90 (48.9) | |
| Graduate | 122 (32.8) | 61 (32.5) | 61 (33.2) | |
| SBP, mean (SD), mmHg | 118.4 (17.1) | 113.3 (14.7) | 123.5 (18.0) | <0.0001 |
| BMI, mean (SD), kg/m2 | 29.4 (6.4) | 25.7 (3.6) | 33.0 (6.5) | <0.0001 |
| HDL-C, mean (SD), mg/dL | 57.4 (14.3) | 60.4 (14.6) | 54.5 (13.4) | <0.0001 |
| LDL-C, mean (SD), mg/dL | 119.9 (33.2) | 116.0 (29.8) | 123.9 (36.0) | 0.03 |
| Hormone therapy, n (%) | 55 (14.8) | 31 (16.5) | 24 (13.0) | 0.3 |
| CV medication use, n (%) | 81 (21.8) | 24 (12.8) | 57 (31.0) | <0.0001 |
| Diabetes, n (%) | 18 (4.8) | 6 (3.2) | 12 (6.5) | 0.1 |
| Family history of CV disease, n (%) | 251 (67.5) | 118 (62.8) | 133 (72.3) | 0.05 |
| Current smoking, n (%) | 50 (13.4) | 29 (15.4) | 21 (11.4) | 0.3 |
| HOMA, median (Q1, Q3) | 2.0 (1.5, 3.1) | 1.6 (1.3, 2.2) | 2.8 (1.8, 4.7) | <0.0001 |
| FRS, mean (SD) | 10.3 (3.5) | 9.5 (3.3) | 11.1 (3.5) | <0.0001 |
| hsCRP, median (Q1, Q3), mg/L | 2.1 (0.8, 5.6) | 1.3 (0.6, 2.9) | 3.9 (1.3, 8.0) | <0.0001 |
| Fibrinogen, median (Q1, Q3), mg/dL | 279 (242, 316) | 262 (230, 300) | 279 (256, 316) | 0.0005 |
| PAI-1, median (Q1, Q3), ng/mL | 13.2 (7.8, 24.7) | 10.6 (6.8, 19.2) | 18.2 (9.2, 32.4) | <0.0001 |
| tPA-ag, median (Q1, Q3), ng/mL | 6.8 (5.2, 9.3) | 6.3 (4.7, 7.6) | 8.1 (6.0, 10.4) | <0.0001 |
| Factor VIIc (%), median (Q1, Q3) | 118 (102, 138) | 113 (99, 131) | 123 (106, 143) | 0.002 |
p-value for CAC groups - Wilcoxon rank-sum test for continuous variables; χ2 test for categorical variables
BMI = body mass index; CAC = coronary artery calcification; CV = cardiovascular; FRS = Framingham risk score;
HDL-C = high density lipoprotein cholesterol; HOMA = homeostasis assessment model; hsCRP = high sensitivity
C-reactive protein; LDL-C = low density lipoprotein cholesterol; PAI-1 = plasminogen-activator inhibitor 1;
SBP = systolic blood pressure; tPA-ag = tissue plasminogen activator antigen
Table 2 provides the results of the univariable logistic regression analyses for presence of baseline CAC, and univariable tobit regression analyses for extent of baseline CAC. The presence and extent of baseline level of CAC were both significantly associated with age, SBP, HDL-C, LDL-C, BMI, HOMA, CV medication use, FRS, log(hsCRP), log(fibrinogen), log(PAI-1), log(tPA-ag), and factor VIIc.
Table 2.
Univariable Logistic and Tobit Regression Analyses for the Presence and Extent of CAC.
| CAC Presence
|
CAC Extent
|
|||
|---|---|---|---|---|
| OR (95% CI) | p-value | β (SE) | p-value | |
| Age, y | 1.10 (1.03–1.19) | 0.009 | 5.469 (1.639) | 0.0008 |
| Site | ||||
| Chicago | Reference | Reference | ||
| Pittsburgh | 0.88 (0.59–1.33) | 0.5 | −1.119 (9.218) | 0.9 |
| Race | ||||
| African-American | Reference | Reference | ||
| White | 0.53 (0.35–0.82) | 0.004 | −10.250 (9.498) | 0.3 |
| Menopausal status | ||||
| Pre- and early perimenopausal | Reference | Reference | ||
| Late peri- and post-menopausal | 1.28 (0.83–1.97) | 0.3 | 5.971 (20.879) | 0.8 |
| Surgical menopause | 0.81 (0.25–2.62) | 0.7 | 31.491 (25.869) | 0.2 |
| Indeterminate | 1.25 (0.49–3.22) | 0.6 | 13.625 (9.593) | 0.2 |
| Income | ||||
| <$50,000 | Reference | Reference | ||
| $50,000–$100,000 | 0.83 (0.52–1.34) | 0.5 | −14.546 (12.341) | 0.2 |
| >$100,000 | 0.58 (0.34–1.00) | 0.05 | −1.452 (10.646) | 0.9 |
| Education | ||||
| High school or less | Reference | Reference | ||
| Some college or college | 0.67 (0.37–1.21) | 0.2 | −23.810 (13.629) | 0.08 |
| Graduate | 0.76 (0.40–1.42) | 0.4 | −25.012 (12.799) | 0.05 |
| SBP, mmHg | 1.04 (1.03–1.06) | <0.0001 | 1.220 (0.262) | <0.0001 |
| BMI, kg/m2 | 1.32 (1.24–1.34) | <0.0001 | 4.978 (0.743) | <0.0001 |
| HDL-C, mg/dL | 0.97 (0.95–0.99) | 0.0001 | −1.421 (0.335) | <0.0001 |
| LDL-C, mg/dL | 1.01 (1.00–1.01) | 0.02 | 0.291 (0.134) | 0.03 |
| Hormone therapy | 0.76 (0.43–1.35) | 0.4 | −9.465 (13.152) | 0.5 |
| CV medication use | 3.07 (1.81–5.21) | <0.0001 | 20.320 (10.732) | 0.06 |
| Diabetes | 2.12 (0.78–5.76) | 0.1 | 51.591 (19.684) | 0.009 |
| Family history of CV disease | 1.55 (1.00–2.40) | 0.05 | 10.464 (9.932) | 0.3 |
| Current smoking | 0.71 (0.39–1.29) | 0.3 | −0.368 (13.643) | 1.0 |
| HOMA* | 5.52 (3.41–8.94) | <0.0001 | 39.468 (7.661) | <0.0001 |
| FRS | 1.15 (1.08–1.23) | <0.0001 | 6.815 (1.364) | <0.0001 |
| hsCRP*, mg/L | 1.91 (1.58–2.32) | <0.0001 | 17.765 (3.839) | <0.0001 |
| Fibrinogen*, mg/dL | 8.19 (2.61–25.72) | 0.0003 | 83.724 (24.253) | 0.0006 |
| PAI-1*, ng/mL | 1.67 (1.31–2.12) | <0.0001 | 19.277 (4.900) | <0.0001 |
| tPA-ag*, ng/mL | 3.67 (2.16–6.25) | <0.0001 | 37.072 (8.889) | <0.0001 |
| Factor VIIc*, % | 4.73 (1.83–12.23) | 0.001 | 66.521 (17.671) | 0.0002 |
Log transformed
BMI = body mass index; CAC = coronary artery calcification; CV = cardiovascular; FRS = Framingham risk score;
HDL-C = high density lipoprotein cholester; HOMA = homeostasis assessment model; hsCRP = high sensitivity
C-reactive protein; LDL-C = low density lipoprotein cholesterol; OR = odds ratio; PAI-1 = plasminogen-activator inhibitor 1; SE = standard error; SBP = systolic blood pressure; tPA-ag = tissue plasminogen activator antigen
All inflammatory/hemostatic biomarkers were positively associated with CAC presence and extent (p<0.0001 for all), adjusting for FRS, site, race/ethnicity, menopausal status, income, and education (Table 3). Additional adjustment for BMI explained all associations except for factor VIIc, which remained associated with CAC extent only (p=0.02), but not presence. Final adjustment for insulin resistance, family history of CVD, and CV medication use produced similar results.
Table 3.
Multivariable Logistic and Tobit Regression Analyses for the Presence and Extent of CAC.
| CAC Presence
|
CAC Extent
|
|||
|---|---|---|---|---|
| OR (95% CI) | p-Value | β (SE) | p-Value | |
| Model 1: FRS, Site, Race, Menopausal status, Income, Education | ||||
| hsCRP, mg/L | 1.86 (1.52–2.31) | <0.0001 | 16.232 (4.155) | <0.0001 |
| Fibrinogen, mg/dL | 7.07 (2.01–24.94) | 0.002 | 74.200 (25.917) | 0.004 |
| PAI-1, ng/mL | 1.72 (1.32–2.25) | <0.0001 | 19.683 (5.240) | 0.0002 |
| tPA-ag, ng/mL | 3.74 (2.08–6.74) | <0.0001 | 31.910 (9.330) | 0.0006 |
| Factor VIIc, % | 4.14 (1.41–12.11) | 0.01 | 53.611 (19.689) | 0.007 |
| Model 2: Model 1 + BMI | ||||
| hsCRP, mg/L | 1.19 (0.92–1.54) | 0.2 | 7.107 (4.580) | 0.1 |
| Fibrinogen, mg/dL | 1.60 (0.34–7.52) | 0.6 | 49.139 (26.803) | 0.07 |
| PAI-1, ng/mL | 1.04 (0.75–1.44) | 0.8 | 10.719 (5.644) | 0.06 |
| tPA-ag, ng/mL | 1.36 (0.70–2.61) | 0.4 | 15.933 (9.942) | 0.1 |
| Factor VIIc, % | 2.57 (0.77–8.61) | 0.1 | 47.284 (19.930) | 0.02 |
| Model 3: Model 2 + Log(HOMA), Family history, CV medication use | ||||
| hsCRP, mg/L | 1.16 (0.88–1.53) | 0.3 | 5.769 (4.958) | 0.2 |
| Fibrinogen, mg/dL | 2.22 (0.40–12.28) | 0.4 | 51.812 (28.639) | 0.07 |
| PAI-1, ng/mL | 0.83 (0.57–1.22) | 0.3 | 7.474 (6.514) | 0.3 |
| tPA-ag, ng/mL | 0.88 (0.43–1.79) | 0.7 | 7.754 (10.917) | 0.5 |
| Factor VIIc, % | 2.78 (0.72–10.71) | 0.1 | 46.721 (21.183) | 0.03 |
All inflammatory/hemostatic biomarkers were log transformed.
BMI = body mass index; CAC = coronary artery calcification; CV = cardiovascular; FRS = Framingham risk score; hsCRP = high sensitivity C-reactive proteins; OR = odds ratio; PAI-1 = plasminogen-activator inhibitor 1; tPA-ag = tissue plasminogen activator antigen
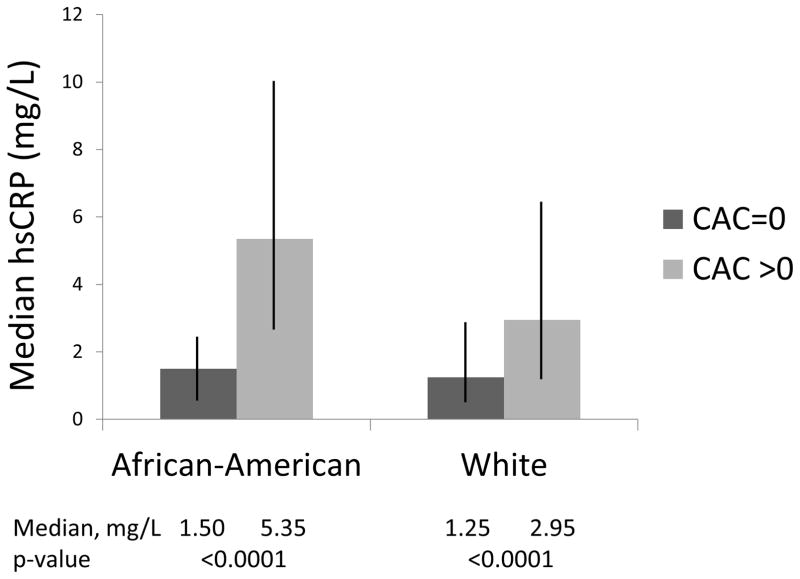
In univariable and final multivariable models, associations between hsCRP and CAC presence and extent were significantly modified by race/ethnicity (p<0.05). Compared to those with no CAC, women with CAC had higher levels of hsCRP and this was significant in both African-Americans (5.35 mg/L vs. 1.50 mg/L, p<0.0001) and Whites (2.95 mg/L vs. 1.25 mg/L, p<0.0001), respectively (Figure). In participants with CAC >0, the median hsCRP level was significantly higher in African-American women when compared to White women (p=0.002). Stratified analyses were performed on the relationship between log(hsCRP) and CAC by race/ethnicity (Table 4). Log(hsCRP) was positively associated with CAC presence (OR, 3.25; 95% CI, 1.53–6.90; p=0.002; per 1 log unit increase) and CAC extent (β, 19.664; SE, 7.671; p=0.01; per 1 log unit increase) in final models for African-Americans only. Associations in Whites were not significant.
Figure. Median hsCRP Levels By Race/Ethnicity And CAC Status.
African-American without CAC, 1.50 mg/L; African-American with CAC, 5.35 mg/L; White without CAC, 1.25 mg/L; White with CAC, 2.95 mg/L. HsCRP levels were significantly higher in participants with CAC in both African-Americans and Whites (p<0.0001). HsCRP levels were similar among African-Americans and Whites when CAC = 0 (p=0.5). HsCRP levels were significantly higher in African-Americans compared to Whites when CAC >0 (p=0.002). Vertical bars show the interquartile range (Q1, Q3). CAC = coronary artery calcification. HsCRP = high sensitivity C-reactive protein.
Table 4.
Race/Ethnicity-Stratified Multivariable Logistic and Tobit Regression Analyses for the Presence and Extent of CAC for hsCRP.
| CAC Presence
|
CAC Extent
|
|||
|---|---|---|---|---|
| OR (95% CI) | p-value | β (SE) | p-value | |
| Unadjusted | ||||
| hsCRP, mg/L - African-American | 2.41 (1.68–3.47) | <0.0001 | 24.282 (6.055) | <0.0001 |
| hsCRP, mg/L - White | 1.65 (1.31–2.08) | <0.0001 | 13.892 (5.146) | 0.007 |
| Model 1: FRS, Site, Menopausal status, Income, Education | ||||
| hsCRP, mg/L - African-American | 2.79 (1.79–4.35) | <0.0001 | 26.719 (6.882) | 0.0001 |
| hsCRP, mg/L - White | 1.61 (1.26–2.06) | 0.0002 | 10.726 (5.219) | 0.04 |
| Model 2: Model 1 + BMI | ||||
| hsCRP, mg/L - African-American | 2.33 (1.32–4.14) | 0.004 | 21.117 (7.099) | 0.003 |
| hsCRP, mg/L - White | 0.94 (0.68–1.30) | 0.7 | −1.152 (6.125) | 0.9 |
| Model 3: Model 2 + Log(HOMA), Family history, CV medication use | ||||
| hsCRP, mg/L - African-American | 3.25 (1.53–6.90) | 0.002 | 19.664 (7.671) | 0.01 |
| hsCRP, mg/L - White | 0.87 (0.62–1.23) | 0.4 | −2.756 (6.599) | 0.7 |
hsCRP was log transformed.
BMI = body mass index; CAC = coronary artery calcification; CV = cardiovascular; FRS = Framingham risk score;
hsCRP = high sensitivity C-reactive protein; OR = odds ratio
Sensitivity analyses were performed on the 352 non-diabetic participants after exclusion of the 18 diabetic participants and the results were similar to those reported in this manuscript (data not shown).
DISCUSSION
To the best of our knowledge, this is the first study to evaluate the relationships of hsCRP, fibrinogen, PAI-1, tPA-ag, and factor VIIc to CAC in women undergoing the menopausal transition. This period is recognized as a critical time for the change in CHD risk in women (2). Our investigation yielded three important observations. First, hsCRP, fibrinogen, PAI-1, tPA-ag, and factor VIIc were all positively associated with the presence and extent of CAC in midlife women. These relationships were all attenuated by BMI in the multivariable models, with the exception of factor VIIc. Second, a significant interaction was noted for race/ethnicity on the relationship between hsCRP and baseline CAC. hsCRP was associated with CAC in African-American women, but not White women. Third, factor VIIc was associated with CAC extent, but not CAC presence, even after adjustment for multiple CHD risk factors and BMI.
In generally healthy women, evidence suggests that there is no relationship between hsCRP and CAC, but that a relationship between fibrinogen and CAC does exist. Jenny et al. evaluated hsCRP and fibrinogen, divided in quartiles, in over 6,783 participants from the Multi-Ethnic Study of Atherosclerosis (MESA) (23). The study reported a significant independent association between CAC presence and fibrinogen quartiles, but not hsCRP quartiles. The results were adjusted for age, race/ethnicity, sex, smoking, diabetes, SBP, BMI, and dyslipidemia. The authors reported sex- and race/ethnicity-stratified analyses and these results were similar to the results from the overall cohort. Differences between results from MESA and those from our current study may be due to differences in the baseline characteristics of the study population and/or analyses. The mean age of the MESA population was 62 years, thus the vast majority was post-menopausal, and participants had higher rates of traditional risk factors.
Findings from the Coronary Artery Calcification in Type 1 Diabetes demonstrated a positive association between PAI-1 and CAC in young participants with type 1 diabetes, but not healthy control (24). Approximately half the population was comprised of women. he mean age of the non-diabetics was 39 years. Our findings for association between PAI-1 and CAC were in agreement with the results from the above study among healthy controls, although our population was mainly women of older age. Associations of tPA-ag and factor VIIc with CAC are not clear. We are not aware of any studies that have evaluated these associations in any population. Our current study fills not only needed information on the relationships of PAI-1, tPA-ag, and factor VIIc to CAC in a healthy population, but also in an important age group in women that is not well-represented in current literature.
The finding from our study that the relationships between hemostatic/inflammatory biomarkers and CAC were attenuated after adjusting for BMI suggests that these relationships may be largely mediated through the metabolic changes that are linked with increasing BMI. Higher BMI levels were found to be independently associated with higher levels of hsCRP, fibrinogen, and PAI-1 in women, but not men, in a cross-sectional, community-based study of 166 healthy men and women with a mean age of 52.1 years (26–80 years) (25). Prior investigators have suggested that the association between adipose tissue and hsCRP, mediated through interleukin-6, may diminish the predictive value of hsCRP for CHD in obese individuals (8). The hsCRP signal from adipose tissue may overwhelm that from subclinical atherosclerosis. A similar mechanism may explain the correlation between PAI-1 and BMI (26). Thus, increasing adipose tissue may be the primary reason for increasing CRP, fibrinogen, PAI-1, and tPA-ag in midlife women. Our findings support recommendations of weight maintenance or reduction to achieve an appropriate body weight (1).
The situation may not be the same among African-American midlife women. Our study showed significant relationship between CRP and CAC presence and extent independent of BMI only among African-American women. Findings from a SWAN parent study comprised of 3,154 women without known CV disease demonstrated significant racial/ethnic differences in hsCRP concentrations (27). African-American women had greater odds of hsCRP levels >3 mg/L when compared with White women, even after adjusting for age, socio-economic status, BMI, and other CV risk factors. Despite similar median hsCRP values in women without CAC, African-American women with CAC in our study had a median hsCRP almost twice as much as their White counterparts (Figure). In human umbilical vein endothelial cells from African-Americans and White, cells from African-Americans had higher nitric oxide levels, higher interleukin-6 levels, and lower superoxide dismutase activity (28). It strengthens the theory that genetic differences in oxidative stress and inflammation are involved in racial/ethnic differences in clinical CHD rates.
The 2010 American College of Cardiology Foundation/American Heart Association (AHA) Guidelines for Assessment of CV Risk in Asymptomatic Adults does not recommend selective race/ethnic-based risk assessment approaches despite recognition of disparities between racial and ethnic groups with regards to traditional risk factors, CHD incidence, and outcomes (29). Current literature calls for more attention particularly to African-American women, who were shown to have the poorest 15-year adjusted survival rate of all four age-racial/ethnic groups in a Duke University Medical Center study of greater than 22,000 African-American and White women and men with established CHD (30).
Pharmacotherapy guideline recommendations from the AHA for primary prevention in non-diabetic women without high-risk features for CVD focus on control of blood pressure and dyslipidemia, as well as aspirin (1). Statin recommendations for primary prevention are largely dependent upon CHD risk classification. Statins, but not aspirin, are associated with lower hsCRP levels in participants with stable CHD (31). This suggests that inflammatory mechanisms that promote atherogenesis may be particularly responsive to statins (32). Whether this extends to primary prevention scenarios requires further investigation.
The only randomized placebo-controlled study to demonstrate significant reduction in total or CHD mortality for women was the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin that included 6,801 women (33). Inclusion criteria for women were age ≥60 years, LDL-C <130 mg/dL, and hsCRP of ≥2.0 mg/L. Elevated hsCRP levels may therefore identify women who stand to benefit the most from statin medication. Routine screening for hsCRP, however, is currently not recommended (1).
The study of hsCRP in African-Americans has been limited (34). A goal of risk prediction is reclassification of individuals based on low-, intermediate-, and high-risk estimation (35). For non-diabetics with LDL-C levels <190 mg/dL, the current threshold for recommending primary prevention statin therapy is an estimated 10-year atherosclerotic CVD risk ≥7.5% (36). Additional factors that may influence prescribing statins are hsCRP ≥2 mg/L and the presence of any CAC (36,37). One recommendation for statin consideration is the presence of CAC score ≥75th percentile which, in African-American women between the ages of 45–54 years, is any CAC (36,37). In those with elevated hsCRP, the presence of any CAC may dramatically reduce the number needed to treat for statins on CHD events (38). Our data suggests hsCRP screening may be particularly useful in midlife African-American women. Ultimately, a prospective trial on the utility of hsCRP and CAC screening is warranted in midlife African-American women.
Factor VIIc is a procoagulant that has had variable association with subclinical atherosclerosis in past studies (39,40). Our finding of a positive association between CAC extent, but not CAC presence, should be further investigated.
Limitations and Strengths
The cross-sectional design limits the ability to assess temporal relationships and causality. We only had women of African-American and White race, so the results may not be generalizable to other racial/ethnic groups. The majority of the participants with CAC had low CAC scores so these results may not be generalized to women with higher CAC scores. We do not expect that the excluded women would have significant impact on our results. The excluded and included women were similar in most characteristics. Excluded women were older, but also healthier in that they had lower SBP and were less likely to be current smokers. Finally, due to our limited sample size we were not able to adjust our results to many other potential covariates such as alcohol consumption and stress. Although the analysis was cross-sectional, the data collection was performed in a systematic and prospective manner. An additional strength was our analysis of both presence and extent of CAC.
CONCLUSIONS
Our results suggest that the associations of inflammation and hemostasis with CAC presence are virtually all mediated through BMI, except for factor VIIc, in women undergoing the menopausal transition. The association of hsCRP with CAC in African-American women, however, was significant and independent of traditional CV risk factors and socio-economic factors. Future studies should assess whether longitudinal changes in inflammatory/hemostatic markers are association with calcification progression in a larger sample of women at midlife and adjusting for important potential confounders.
Acknowledgments
Funding: SWAN has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). SWAN Heart was supported by grants from the NHLBI (HL065581, HL065591). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, NIA, NINR, ORWH, or the NHLBI.
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011 – present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Winifred Rossi 2012 - present; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012 - present; Kim Sutton-Tyrrell, PI 2001 – 2012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair
Chris Gallagher, Former Chair
We thank the study staff at each site and all the women who participated in SWAN.
Footnotes
Disclosures: The authors report no relationships relevant to the contents of this paper.
References
- 1.Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women-2011 update: a guideline from the American Heart Association. Circulation. 2011;123:1243–62. doi: 10.1161/CIR.0b013e31820faaf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tunstall-Pedoe H. Myth and paradox of coronary risk and the menopause. Lancet. 1998;351:1425–27. doi: 10.1016/S0140-6736(97)11321-6. [DOI] [PubMed] [Google Scholar]
- 3.Khot UN, Khot MB, Bajzer CT, et al. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA. 2003;290:898–904. doi: 10.1001/jama.290.7.898. [DOI] [PubMed] [Google Scholar]
- 4.The Emerging Risk Factors Collaboration. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012;367:1310–20. doi: 10.1056/NEJMoa1107477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peters SAE, den Ruijter HM, Bots ML, Moons KGM. Improvements in risk stratification for the occurrence of cardiovascular disease by imaging subclinical atherosclerosis: a systematic review. Heart. 2012;98:177–84. doi: 10.1136/heartjnl-2011-300747. [DOI] [PubMed] [Google Scholar]
- 6.Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for prediction in asymptomatic individuals. JAMA. 2004;291:210–5. doi: 10.1001/jama.291.2.210. [DOI] [PubMed] [Google Scholar]
- 7.Hamirani YS, Pandey S, Rivera JJ, et al. Markers of inflammation and coronary artery calcification: a systematic review. Atherosclerosis. 2008;201:1–7. doi: 10.1016/j.atherosclerosis.2008.04.045. [DOI] [PubMed] [Google Scholar]
- 8.Gupta NK, de Lemos JA, Ayers CR, et al. The relationship between C-reactive protein and atherosclerosis differs on the basis of body mass index: the Dallas Heart Study. J Am Coll Cardiol. 2012;60:1148–55. doi: 10.1016/j.jacc.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 9.Woloshin S, Schwartz LM. Distribution of C-reactive protein values in the United States. N Engl J Med. 2005;352:1611–3. doi: 10.1056/NEJM200504143521525. [DOI] [PubMed] [Google Scholar]
- 10.Sowers M, Crawford S, Sternfeld B, et al. SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo FA, Kelsey J, Marcus R, editors. Menopause: Biology and Pathology. New York, NY: Academic Press; 2000. pp. 175–188. [Google Scholar]
- 11.Thurston RC, Sutton-Tyrrell K, Everson-Rose SA, Hess R, Matthews KA. Hot flashes and subclinical cardiovascular disease: findings from the Study of Women’s Health Across the Nation Heart Study. Circulation. 2008;118:1234–40. doi: 10.1161/CIRCULATIONAHA.108.776823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 13.Sutton-Tyrrell K, Kuller LH, Edmundowicz D, Feldman A, Holubkov R, Givens L, Matthews KA. Usefulness of electron bean tomography to detect progression of coronary and aortic calcium in middle-aged women. Am J Cardiol. 2001;87:560–4. doi: 10.1016/s0002-9149(00)01431-4. [DOI] [PubMed] [Google Scholar]
- 14.Myers GL, Cooper GR, Winn CL, Smith SJ. The Centers for Disease Control – National Heart, Lung and Blood Institute Lipid Standardization Program: an approach to accurate and precise lipid measurements. Clin Lab Med. 1989;9:105–35. [PubMed] [Google Scholar]
- 15.Matthews KA, Santoro N, Lasley B, et al. Relation of cardiovascular risk factors in women approaching menopause to menstrual cycle characteristics and reproductive hormones in the follicular and luteal phases. J Clin Endocrinol Metab. 2006;91:1789–96. doi: 10.1210/jc.2005-1057. [DOI] [PubMed] [Google Scholar]
- 16.Friedewald WT, Levy RI, Frederickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparation ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 17.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 18.Wilson PWF, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 19.El Khoudary SR, Shields KJ, Chen H, Matthews KA. Menopause, complement, and hemostatic markers in women at midlife: The Study of Women’s Health Across the Nation. Atherosclerosis. 2013;231:54–8. doi: 10.1016/j.atherosclerosis.2013.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tobin J. Estimation of relationships for limited dependent variables. Econometrica. 1958;26:24–36. [Google Scholar]
- 21.Reilly MP, Wolfe ML, Localio R, Rader DJ. C-reactive protein and coronary artery calcification: The Study of Inherited Risk of Coronary Atherosclerosis (SIRCA) Arterioscler Thromb Vasc Biol. 2003;23:1851–6. doi: 10.1161/01.ATV.0000092327.60858.4A. [DOI] [PubMed] [Google Scholar]
- 22.Qasim AN, Budharaju V, Mehta NN, et al. Gender differences in the association of C-reactive protein with coronary artery calcium in type-2 diabetes. Clin Endocrinol. 2011;74:44–50. doi: 10.1111/j.1365-2265.2010.03879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jenny NS, Brown ER, Detrano R, et al. Associations of inflammatory markers with coronary artery calcification: results from the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2010;209:226–9. doi: 10.1016/j.atherosclerosis.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pratte KA, Barón AE, Ogden LG, et al. Plasminogen activator inhibitor-1 is associated with coronary artery calcium in type 1 diabetes. J Diabetes Complications. 2009;23:387–93. doi: 10.1016/j.jdiacomp.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Bowles LK, Cooper JA, Howarth DJ, Miller GJ, MacCallum PK. Associations of haemostatic variables with body mass index: a community-based study. Blood Coagul Fibrinolysis. 2003;14:569–73. doi: 10.1097/00001721-200309000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Rega G, Kaun C, Weiss TW, et al. Inflammatory cytokines interleukin-6 and oncostatin M induce plasminogen activator inhibitor-1 in human adipose tissue. Circulation. 2005;111:1938–45. doi: 10.1161/01.CIR.0000161823.55935.BE. [DOI] [PubMed] [Google Scholar]
- 27.Kelley-Hedgepeth A, Lloyd-Jones DM, Colvin A, et al. Ethnic differences in C-reactive protein concentrations. Clin Chem. 2008;54:1027–37. doi: 10.1373/clinchem.2007.098996. [DOI] [PubMed] [Google Scholar]
- 28.Feairheller DL, Park J, Sturgeon KM, et al. Racial differences in oxidative stress and inflammation: in vitro and in vivo. Clin Trans Sci. 2011;4:32–7. doi: 10.1111/j.1752-8062.2011.00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:e50–e103. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Thomas KL, Honeycutt E, Shaw LK, Peterson ED. Racial differences in long-term survival among patients with coronary artery disease. Am Heart J. 2010;160:744–51. doi: 10.1016/j.ahj.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 31.Takeda T, Hoshida S, Nishino M, Tanouchi J, Otsu K, Hori M. Relationship between effects of statin, aspirin and angiotensin II modulators on high-sensitive C-reactive protein levels. Atherosclerosis. 2003;169:155–8. doi: 10.1016/s0021-9150(03)00158-8. [DOI] [PubMed] [Google Scholar]
- 32.Tousoulis D, Psarros C, Demosthenous M, Patel R, Antoniades C, Stefanadis C. Innate and adaptive inflammation as a therapeutic target in vascular disease: the emerging role of statins. J Am Coll Cardiol. 2014;63:2491–502. doi: 10.1016/j.jacc.2014.01.054. [DOI] [PubMed] [Google Scholar]
- 33.Ridker PM, Danielson E, Fonseca FAH, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 34.Buckley DI, Fu R, Freeman M, Rogers K, Helfand M. C-reactive protein as a risk factor for coronary heart disease: a systematic review and meta-analyses for the U.S. Preventative Services Task Force. Ann Intern Med. 2009;151:483–95. doi: 10.7326/0003-4819-151-7-200910060-00009. [DOI] [PubMed] [Google Scholar]
- 35.Lloyd-Jones D. Cardiovascular risk prediction: basic concepts, current status, and future directions. Circulation. 2010;121:1768–77. doi: 10.1161/CIRCULATIONAHA.109.849166. [DOI] [PubMed] [Google Scholar]
- 36.Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. J Am Coll Cardiol. 2013 doi: 10.1016/j.jacc.2013.11.002. [DOI] [Google Scholar]
- 37.McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: results of the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2006;113:30–7. doi: 10.1161/CIRCULATIONAHA.105.580696. [DOI] [PubMed] [Google Scholar]
- 38.Blaha MJ, Budoff MJ, DeFilippos AP, et al. Association between C-reactive protein, coronary artery calcium, and cardiovascular events: implications for the JUPITER population from MESA, a population-based cohort study. Lancet. 2011;378:684–92. doi: 10.1016/S0140-6736(11)60784-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghaddar HM, Folsom AR, Aleksic N, et al. Correlation of factor VIIa values with factor VII gene polymorphism, fasting and postprandial triglyceride levels, and subclinical carotid atherosclerosis. Circulation. 1998;98:2815–21. doi: 10.1161/01.cir.98.25.2815. [DOI] [PubMed] [Google Scholar]
- 40.Green D, Foiles N, Chan C, Kang J, Schreiner PJ, Liu K. An association between clotting factor VII and carotid intima-media thickness. Stroke. 2010;41:1417–22. doi: 10.1161/STROKEAHA.110.580100. [DOI] [PMC free article] [PubMed] [Google Scholar]