Abstract
Background
Salt intake was reported to be associated with increased clinical and MRI activity in adult patients with relapsing-remitting multiple sclerosis (MS).
Objective
To determine if salt intake is associated with time to relapse in patients with paediatric-onset MS.
Methods
Paediatric-onset MS and patients with clinically isolated syndrome (CIS) within 4 years of disease onset were recruited from 15 paediatric MS centres in the USA as part of a case–control study. Patients with available prospective relapse data subsequent to enrolment were included in this project. Dietary sodium intake was assessed by self-report questionnaire using the validated Block Kids Food Screener. Cox proportional-hazards regression models were employed to determine the association of sodium density, excess sodium intake and sodium density tertiles with time to relapse following study enrolment, adjusting for several confounders.
Results
174 relapsing-remitting MS/CIS patients were included in this analysis (mean age of 15.0 years, and 64.9% females). Median duration of follow-up was 1.8 years. In an unadjusted analysis, density of daily sodium intake was not associated with time to relapse, and patients with excess sodium intake had no decrease in time to relapse as compared with patients with non-excess sodium intake. The multivariable analysis demonstrated that patients in the medium and high tertile of sodium density had a HR of 0.69 (95% CI 0.37 to 1.30, p=0.25) and 1.37 (95% CI 0.74 to 2.51, p=0.32) compared with patients in the lowest tertile, respectively.
Conclusions
Higher salt intake was not associated with decreased time to relapse in patients with paediatric-onset MS.
INTRODUCTION
The course of multiple sclerosis (MS) can be highly variable. While some patients remain free of disease activity for relatively long periods of time, others experience frequent relapses from the outset. Genetic and environmental factors and their interactions may be important determinants of the course of MS.1 Vitamin D deficiency, for example, is an important risk factor for both developing MS and more active disease. This might also be true for Epstein-Barr virus (EBV) infection, parasitic infections and cigarette smoking.2–4 Sodium chloride may be a new addition to the growing list of exacerbating factors.
Increased sodium chloride concentration increases TH17 induction in mouse and human lymphocytes, and experimental autoimmune encephalomyelitis (EAE) in mice that are fed a high salt diet results in more severe disease.5 Sodium chloride also suppresses the activity of FoxP3 regulatory T cells.6 There is a report of an association between increased sodium excretion in random urine samples and clinical and radiological activity in adult patients with relapsing MS that has not yet been replicated.7
Paediatric MS patients, as compared to adults, may experience more frequent relapses which may increase the ability to detect an association between risk factors and relapse rate.8 Identifying the effects of a modifiable environmental factor such as dietary sodium intake could have important implications for the management of patients.
Using a multicentre cohort, we sought to determine if dietary salt intake, as measured by a validated food frequency questionnaire (FFQ), is associated with time to relapse in patients with paediatric-onset MS and clinically isolated syndrome (CIS).
METHODS
This exploratory prospective cohort study was carried out as part of a larger paediatric MS investigation between 15 paediatric MS centres in the USA. Prospective demographic, developmental and environmental data and medical history, including relapse rate, collected at each participating centre were entered into a collaborative database. Participants were recruited between November 2011 and April 2015. The institutional review board at each participating centre approved the study protocol. Assent and consent forms were signed by all the participants and one of their parents/legal guardians, as required by each centre. This study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Study participants
Patients with CIS, with at least two silent MRI lesions or relapsing-remitting MS,9 with onset of disease before 18 years of age, and disease duration of <4 years were included in this study. Case status was confirmed by a review panel of three paediatric MS specialists. Of the 15 centres participating in the case–control study, 13 collected prospectively relapse information into the US Paediatric MS Network database.10 Patients who had completed the FFQ and who had available prospective relapse data subsequent to enrolment in the study were included in this project.
Clinical and demographic information
Demographic data were prospectively collected at the time of enrolment in the study. Race and ethnicity were self-reported, according to NIH criteria.
Relapses were defined as new or recurrent neurological symptoms localising to the CNS, lasting for at least 24 hours after a remission of 30 days or more since the previous attack in the absence of an infection or fever.9 Pseudo-relapses were excluded. Only relapses that occurred after enrolling in the case–control study were used for the analyses.
Disease duration was calculated from the date of first demyelinating event to the end of available follow-up. Disease-modifying treatment (DMT) use was categorised in four groups: (1) patient on no DMT; (2) patients receiving interferon β, glatiramer acetate, azathioprine, mycophenolate mofetil or IVIg; (3) patients receiving fingolimod, dimethyl fumarate or daclizumab and (4) patients receiving natalizumab, rituximab, alemtuzumab, cyclophosphamide or mitoxantrone. Patients who switched treatment during the follow-up period were counted in the treatment group with the longest follow-up time.
Sodium intake assessment
Dietary sodium intake was estimated using Block Kids Food Screener (BKFS) (NutritionQuest, 2007). BKFS is a validated FFQ designed for children aged between 2 years and 17 years, and is available in English and Spanish. Patients or their caregivers completed the questionnaire after getting instructions from a research staff. This questionnaire consists of 41 questions evaluating the frequency and portions of food and beverages consumed during the past week. NutritionQuest provided dietary estimates based on National Health and Nutrition Examination Survey 24 h recall data (NutritionQuest). Six age-sex categories were used to provide dietary estimates: males and females 2–3 years old, males/females 4–8 years old, males 9–13 years old, males 14–17 years old, females 9–15 years old and females 16–17 years old. Subjects were excluded if >15 answers were missing to questions, or if reported estimates reflected biologically implausible daily energy intakes (<500 or >5000 kcal/day) based on NutritionQuest food screener analysis. The BKFS has been validated against three, 24 h dietary recalls in children aged 10–17 years.11
Sodium density is defined as the ratio of sodium intake (mg/day) to energy intake (thousands of kcal/day), and it represents energy-adjusted sodium intake.12
Statistical analysis
The outcome of the study was the time to the next relapse, calculated from the time of enrolment into the case–control study. Descriptive statistics for patient characteristics were presented either as counts and percentages (%) or mean and SD.
In univariate Cox proportional-hazards models, sodium was included as a continuous variable and as a categorical variable based on excess sodium intake. Excess sodium was defined by the adequate intake, the recommended daily average intake level of sodium for each age group (1000 mg/day 1–3 years old, 1200 mg/day 4–8 years old, 1500 mg/day 9–19 years old). In the multivariate Cox proportional hazard regression model, adjusting for age, gender, race/ethnicity, disease duration, body mass index (BMI) and DMT, sodium was included as a categorical variable based on sodium density tertiles for the entire sample of patients in the study. Model checking, including testing and visual inspection for proportional hazard assumption were performed. All statistical analyses were performed using R language and environment V.3.1.1 (R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, 2013. http://www.R-project.org/).
RESULTS
Clinical and demographic characteristics of the participants
Clinical and demographic characteristics of the patients who participated in this study are summarised in table 1. Of the 275 relapsing-remitting MS patients (MS/CIS) enrolled in the case–control study at the time of the data lock, 174 were included in this analysis as they had documented follow-up information (mean age of 15.0, 64.9% females). Characteristics were similar between those with follow-up and those without. Median duration of follow-up after enrolment was 1.8 years (IQR 0.9–2.6). Median time to the next relapse was 2.7 years.
Table 1.
Baseline characteristics of the cohort
| Patients with available relapse data |
Patients without available relapse data |
p Value |
|
|---|---|---|---|
| N | 174 | 101 | |
| Mean age in years (SD) | 15 (3.3) | 15.3 (3.4) | 0.32 |
| Mean body mass index (SD) |
25.0 (6.9) | 25.3 (6.7) | 0.49 |
| Female (%) | 113 (64.9) | 64 (64.0) | 0.88 |
| Race | 0.46 | ||
| White (%) | 101 (58.0) | 68 (67.3) | |
| African-American (%) | 34 (19.5) | 12 (11.9) | |
| Asian (%) | 7 (4.0) | 4 (4.0) | |
| Other (%) | 20 (11.5) | 9 (8.9) | |
| Unknown (%) | 12 (6.9) | 8 (7.9) | |
| Hispanic or Latino (%) | 54 (31.2) | 28 (28) | 0.82 |
| On disease-modifying treatment (%) |
94 (54) | 45 (44.6) | 0.13 |
Estimated daily sodium intake
The estimated mean daily sodium intake was 1975 mg (SD 1088). Of the 174 cases included in the analysis, 104 (59.8%) had sodium in excess of recommended levels. The mean sodium density was 1.51 mg/kcal (SD 0.28), and the tertiles of sodium density were 1.40 and 1.64 mg/kcal per day.
Association between sodium intake and time to next relapse
In an unadjusted analysis (table 2), sodium density (energy-adjusted sodium intake) was not associated with time to relapse (HR per 1 mg/10 kcal 0.98, 95% CI 0.91 to 1.06, p=0.67). Similarly, patients with excess sodium intake had no decrease in time to relapse (HR 0.85, 95% CI 0.54 to 1.35, p=0.49) as compared with patients with non-excess sodium intake. In the multivariable analysis adjusted for age, gender, race/ethnicity, disease duration, BMI and use of DMT (table 2), patients in the medium and high tertile of sodium density had a HR of 0.69 (95% CI 0.37 to 1.30, p=0.25) and 1.37 (95% CI 0.74 to 2.51, p=0.32) compared with patients in the lowest tertile, respectively (figure 1). There was no differential effect of Hispanic ethnicity (versus non-Hispanic), pre-enrolment relapse rate, disease duration before enrolment, and latitude of living place on the association between salt intake and time to relapse.
Table 2.
Univariable and multivariable association between daily sodium intake and relapse rate
| HR | 95% CI | p Value | |
|---|---|---|---|
| Sodium density (per 1mg/10kcal) (univariable) | 0.98 | 0.91 to 1.06 | 0.67 |
| Excess sodium (univariable) | 0.85 | 0.54 to 1.35 | 0.49 |
| Sodium tertiles (Multivariable) | 0.31 | ||
| 2nd | 0.69 | 0.37 to 1.30 | |
| 3rd | 1.37 | 0.74 to 2.51 | |
| Age (1-year increment) | 1.06 | 0.97 to 1.16 | 0.72 |
| Gender (male) | 0.84 | 0.49 to 1.45 | 0.57 |
| BMI | 1.00 | 0.96 to 1.04 | 0.63 |
| Race (non-Caucasian) | 0.73 | ||
| Asian | 2.00 | 0.53 to 7.59 | |
| African-American | 1.37 | 0.72 to 2.63 | |
| Other | 1.03 | 0.46 to 2.31 | |
| Unknown or not reported | 0.97 | 0.38 to 2.53 | |
| Ethnicity | 0.06 | ||
| Hispanic | 1.68 | 0.97 to 2.90 | |
| Unknown or not reported | 0.28 | 0.03 to 2.20 | |
| Disease duration (1-year increment) | 1.03 | 0.72 to 1.46 | 0.47 |
| Disease modifying treatment | 0.004 | ||
| IFN, GA, AZTH, MMF, IVIg | 0.66 | 0.37 to 1.18 | |
| DMF, Fingolimod, daclizumab | 0.21 | 0.06 to 0.75 | |
| NTZ, RTX, AMZ, CTX, MTX | 0.15 | 0.03 to 0.66 |
AMZ, alemtuzumab; BMI, body mass index; CTX, cyclophosphamide; DMF, dimethyl fumarate; GA, glatiramer acetate; IFN, interferons; IVIg, intravenous immunoglobulin; MMF, mycophenolate mofetil; MTX, mitoxantrone; NTZ, natalizumab; RTX, rituximab.
Figure 1.
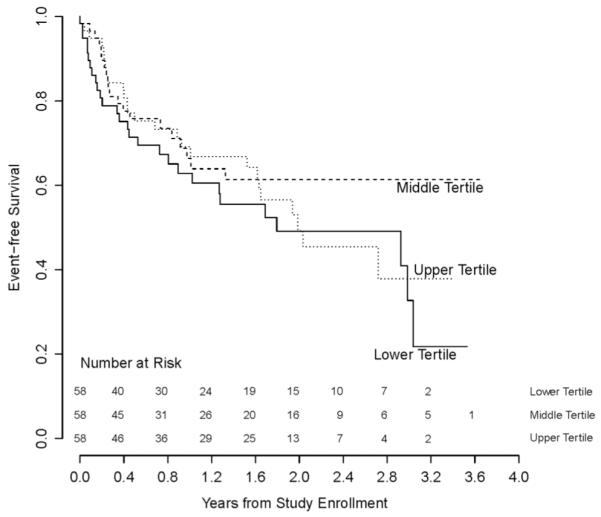
Kaplan–Meier survival curves depicting time to the first relapse of patients in different dietary sodium intake tertiles.
DISCUSSION
This is the first study to evaluate the effects of dietary sodium intake on disease activity in patients with paediatric-onset MS. Our findings do not support an association between dietary sodium intake as measured by the BKFS questionnaire and relapse rate in paediatric MS. Our results are in contrast with a recent report of increased risk of clinical and radiological activity in adult patients with relapsing-remitting MS who had higher sodium intake, calculated based on random samples of urinary sodium excretion.7 Possible explanations for these opposing findings include: dissimilar pathophysiological mechanisms related to the age and duration of the disease, and the use of different tools for estimating sodium intake in these studies. Neither FFQs nor spot urine sampling are perfectly valid and reproducible methods of estimating daily sodium intake. FFQs often underestimate sodium intake due to underreporting and difficulty in quantifying sodium in recipes. In addition, there are no specific FFQs validated for sodium intake in children. Spot urine testing is affordable and practical although its use for estimating sodium intake has remained controversial.13 Using the same cohort of patients and appropriate controls, we did not find any association of sodium intake and risk of developing paediatric MS.14 Our study had 80% power to detect a modest increase of 60% in the hazard of relapse in patients with excessive sodium intake (HR of 1.6). Farez et al7 reported incidence rate ratios of more than 2.5 and 3.3 for patients with medium and high sodium intake compared to those with low sodium intake, respectively. Our study had high power of detecting as much (or even less) difference between the groups.
It has been shown that feeding mice a high-salt diet results in more severe EAE. Increased salt intake promotes development of Th17 cells, thought to be important effectors cells in several autoimmune conditions, including MS.15 However, Freund’s adjuvant used in the induction of EAE increases brain blood vessel permeability.16 This might influence the effect of high sodium diet on disease severity.
However, at this time, the evidence that salt plays a role in susceptibility or disease activity in human autoimmune disorders is scant, and this hypothesis is open to question.
Study strengths include prospective capture of relapses with a large catchment area across the USA, diverse patient population and stringent case and relapse ascertainment, well-controlled analyses including use of DMT. This study has several limitations. BKFS is not specifically designed to assess sodium intake, and FFQs, in general, have relatively low correlation with actual sodium intake. The dietary sodium estimates were obtained at the time of enrolment. There is a possibility that some patients changed their dietary habits after being diagnosed with MS or after enrolment in the study, although this is unlikely, as there are currently no recommendations for salt intake in MS. We also cannot exclude the effects of known but unmeasured confounders (such as serum vitamin D level) and unknown confounders.
In summary, we did not find an association between dietary salt intake and relapse rate in a cohort of patients with paediatric-onset MS. Despite several limitations, our study was well designed and our questionnaire was sufficient to pick up a major and clinically important effect on the association of salt intake and MS disease activity in children. Future studies of longitudinal dietary sodium measurements using salt-specific food questionnaires or 24 h urinary sodium excretion may provide stronger evidence of the effect of salt intake in patients with MS.
Acknowledgments
Funding National Institutes of Health (grant number: R01NS071463-04).
Competing interests BN is a grantee of the National MS Society. This research was conducted while BN was an American Brain Foundation and a Biogen Idec Postdoctoral Fellow. JG was supported by the Foundation for Consortium of Multiple Sclerosis Centers and the NIH Bridging Interdisciplinary Research Careers in Women’s Health programmes during this work. She has been a one-time consultant for EMD-Serono. TCC has been supported by the National MS Society and the NIH (R01NS071463). He is an ad-hoc consultant for Biovest International, Inc. EWaubant is funded by the National MS Society, the NIH and the Race to Erase MS. She has received honorarium from Teva Neuroscience and Genzyme for two educational lectures, and is an ad-hoc consultant for Actelion, Sanofi-Aventis and Roche. She is on an advisory board for a clinical trial of Novartis. LK is supported by the National MS Society, NIH, Robert and Lisa Lourie Foundation, Department of Defense. She has received honoraria, consulting payments, grant support or royalties from Biogen, Medimmune, Novartis, Teva Neuroscience, Sanofi-Aventis, and EMD Serono. BW-G received honoraria for serving in advisory boards and educational programmes from Teva Pharmaceuticals, Biogen Idec, Novartis, Accorda EMD Serono, Novartis, Genzyme and Sanofi. She also received support for research activities from the National Institutes of Health, National Multiple Sclerosis Society, National Science Foundation, Department of Defense, EMD Serono, Biogen Idec, Teva Neuroscience, Novartis, Acorda, Genzyme and the Jog for the Jake Foundation. TC has served as a consultant for Biogen-Idec, Teva Neurosciences, Novartis, Sanofi-Aventis, and has received grant support from NIH, National MS Society, Guthy-Jackson Charitable Foundation, CMSC and Merck-Serono and Novartis. JR has research funding from Teva Neuroscience and Biogen. He is a member of the Medical Advisory Board for the DECIDE trial which is funded by Biogen and AbbiV.
Footnotes
Contributors BN drafted and edited the manuscript and contributed to the conception and design of the study. TCC performed the statistical analyses, edited the manuscript and contributed to the conception and design of the study. JG, SL, AW, AB, BG, BW-G, GA, J-MT, JH, JN, JR, LK, MG, LB, MR, TC, JR, LB, EW acquired the data, edited and revised the manuscript and contributed to the conception and design of the study. All authors approved the final version of the manuscript.
Ethics approval Institutional Review Board.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Frohman EM, Racke MK, Raine CS. Multiple sclerosis--the plaque and its pathogenesis. N Engl J Med. 2006;354:942–55. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- 2.Lucas RM, Hughes AM, Lay ML, et al. Epstein-Barr virus and multiple sclerosis. J Neurol Neurosurg Psychiatry. 2011;82:1142–8. doi: 10.1136/jnnp-2011-300174. [DOI] [PubMed] [Google Scholar]
- 3.Weston M, Constantinescu CS. What role does tobacco smoking play in multiple sclerosis disability and mortality? A review of the evidence. Neurodegener Dis Manag. 2015;5:19–25. doi: 10.2217/nmt.14.45. [DOI] [PubMed] [Google Scholar]
- 4.Correale J, Gaitán MI. Multiple sclerosis and environmental factors: the role of vitamin D, parasites, and Epstein-Barr virus infection. Acta Neurol Scand. 2015;132:46–55. doi: 10.1111/ane.12431. [DOI] [PubMed] [Google Scholar]
- 5.Kleinewietfeld M, Manzel A, Titze J, et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496:518–22. doi: 10.1038/nature11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernandez AL, Kitz A, Wu C, et al. Sodium chloride inhibits the suppressive function of FOXP3+ regulatory T cells. J Clin Invest. 2015;125:4212–22. doi: 10.1172/JCI81151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farez MF, Fiol MP, Gaitán MI, et al. Sodium intake is associated with increased disease activity in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2015;86:26–31. doi: 10.1136/jnnp-2014-307928. [DOI] [PubMed] [Google Scholar]
- 8.Gorman MP, Healy BC, Polgar-Turcsanyi M, et al. Increased relapse rate in pediatric-onset compared with adult-onset multiple sclerosis. Arch Neurol. 2009;66:54–9. doi: 10.1001/archneurol.2008.505. [DOI] [PubMed] [Google Scholar]
- 9.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casper TC, Rose JW, Roalstad S, et al. The US network of pediatric multiple sclerosis centers: development, progress, and next steps. J Child Neurol. 2015;30:1381–7. doi: 10.1177/0883073814550656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunsberger M, O’Malley J, Block T, et al. Relative validation of Block Kids Food Screener for dietary assessment in children and adolescents. Matern Child Nutr. 2015;11:260–70. doi: 10.1111/j.1740-8709.2012.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willett W. Nutritional epidemiology. 3rd edn Oxford University Press; Oxford; New York: 2012. [Google Scholar]
- 13.Ji C, Dary O, Campbell NR, et al. Spot and overnight urine are inappropriate to assess population sodium intake. Rev Panam Salud Pública Pan Am J Public Health. 2013;34:283. [PubMed] [Google Scholar]
- 14.McDonald J, Graves J, Waldman A, et al. A case-control study of dietary salt intake in pediatric-onset multiple sclerosis. Mult Scler Relat Disord. 2016;6:87–92. doi: 10.1016/j.msard.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sie C, Korn T, Mitsdoerffer M. Th17 cells in central nervous system autoimmunity. Exp Neurol. 2014;262(Pt A):18–27. doi: 10.1016/j.expneurol.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Rabchevsky AG, Degos JD, Dreyfus PA. Peripheral injections of Freund’s adjuvant in mice provoke leakage of serum proteins through the blood-brain barrier without inducing reactive gliosis. Brain Res. 1999;832:84–96. doi: 10.1016/s0006-8993(99)01479-1. [DOI] [PubMed] [Google Scholar]


