Abstract
BACKGROUND & AIMS
Drug-induced liver injury (DILI) has features similar to those of other liver diseases including autoimmune hepatitis (AIH). We aimed to characterize the clinical and autoimmune features of liver injury caused by nitrofurantoin, minocycline, methyldopa, or hydralazine.
METHODS
We analyzed data from 88 cases of DILI attributed to nitrofurantoin, minocycline, methyldopa, or hydralazine included in the Drug-Induced Liver Injury Network prospective study from 2004 through 2014. Sera were collected from patients at baseline and follow-up examination and tested for levels of immunoglobulin G (IgG), antibodies to nuclear antigen (ANA), smooth muscle (SMA), and soluble liver antigen (SLA). An autoimmune score was derived on the basis of increases in levels of IgG, ANA, SMA, and SLA (assigned values of 0, 1+, or 2+). AIH-associated HLA DRB1*03:01 and DRB1*04:01 allele frequencies were compared with those of the general population (controls).
RESULTS
Of the 88 cases, 80 were women (91%), 74% had hepatocellular injury, and 25% had severe injury. At the onset of DILI, 39% of cases had increased levels of IgG, 72% had increased levels of ANA, 60% had increased levels of SMA, and none had increases in SLA. A phenotype of autoimmunity (autoimmune score ≥2) was observed in 82% of cases attributed to nitrofurantoin and 73% of cases attributed to minocycline (73%) but only 55% of cases attributed to methyldopa and 43% of cases attributed to hydralazine (P = .16 for nitrofurantoin and minocycline vs methyldopa and hydralazine). We observed a decrease in numbers of serum samples positive for ANA (P = .01) or SMA (P < .001) and in autoimmune scores (P < .001) between DILI onset and follow-up. Similar percentages of patients with DILI had HLA-DRB1*03:01 (15%) and HLA-DRB1*04:01 (9%) as controls (12% and 9%, respectively).
CONCLUSIONS
In analysis of data from the DILIN, we found that most cases of DILI attributed to nitrofurantoin or minocycline and about half of cases that were due to methyldopa and hydralazine have a phenotype of autoimmunity similar to AIH. These features decrease with recovery of the injury and are not associated with the typical HLA alleles found in patients with idiopathic AIH.
Keywords: Database Analysis, Toxicity, Hepatotoxicity, Immune Response, Immunoglobulin
Idiosyncratic drug-induced liver injury (DILI) affects an estimated 19 per 100,000 persons yearly and is a major cause of acute liver failure.1–3 Liver injury may occur as a result of direct toxicity but is more often idiosyncratic because of either metabolic or immunologic factors. Alternatively, liver cell damage may trigger a sensitization response to nuclear and actin autoantigens, resulting in B-cell mediated autoantibody production and cytotoxic T-cell responses that resemble what occurs in spontaneous, idiopathic autoimmune hepatitis (AIH). The presence of autoimmune features such as antinuclear antibodies (ANAs), smooth muscle antibodies (SMAs), and elevated immunoglobulin G (IgG) levels is not uncommon in DILI and may cause diagnostic confusion.4,5 Even liver histologic features of AIH such as presence of plasma cells, eosinophils, and hepatocyte rosettes may occur with drug-induced injury.6,7 For these reasons, differentiating DILI with concomitant autoimmune features from preexisting or new-onset AIH can be diagnostically challenging.8–10
Drugs best known to cause liver injury with autoimmune features include nitrofurantoin, minocycline, hydralazine, and methyldopa.7,11,12 Patients with these forms of DILI may have a rapid, beneficial response to corticosteroid therapy, but most can ultimately be withdrawn from treatment without relapse,12 whereas those with idiopathic AIH require long-term if not lifelong therapy.13 Although idiopathic AIH has been associated with genetic susceptibility loci, both inside and outside the major histocompatibility complex region,14–17 it has not been well-defined for drug-induced AIH. In this study we have analyzed the prevalence and the clinical relevance of the autoimmune phenotype in a cohort of patients with nitrofurantoin-, minocycline-, hydralazine-, and methyldopa-induced liver injury and have assessed the association of liver injury with or without autoimmune features with typical autoimmune HLA alleles.
Methods
Patients
The subjects in this study were participants in the Drug-Induced Liver Injury Network (DILIN) prospective study, the details of which have been described.18–21 Briefly, all patients with suspected DILI presenting at participating clinical centers (Supplementary Table 1) were asked to enroll in a prospective study. Eligibility requirements included age older than 2 years, enrollment within 6 months of onset, and predetermined degrees of serum alanine (ALT) or aspartate (AST) aminotransferase or alkaline phosphatase (Alk P) levels or a total bilirubin of 2.5 mg/dL or above or an international normalized ratio above 1.5. All cases were reviewed by the DILIN Causality Committee and assigned a causality score ranging from 1 to 5 in which 1 = definite (>95% likelihood), 2 = highly likely (75%–95%), 3 = probable (50%–74%), 4 = possible (25%–49%), and 5 = unlikely (<25%).19,21 Patients with known or suspected acetaminophen hepatotoxicity or a previous known history of AIH, primary sclerosing cholangitis, or bone marrow or liver transplant were excluded. A detailed medical and medication history was obtained at enrollment, and additional laboratory and radiologic testing was performed to exclude competing etiologies. All patients were asked to return 6 months after enrollment, and those with persistent abnormalities were asked to return at 12 and 24 months.
All patients were categorized for the pattern of liver injury by using the R ratio ([ALT/upper limit of the normal range (ULN)] ÷ [Alk P/ULN]): >5 = hepatocellular, <2 = cholestatic, and 2–5 = mixed hepatic injury.20 The severity of the DILI episode was categorized as mild (1), moderate (2), moderate-hospitalized (3), severe (4), or fatal (5; death or liver transplantation within 6 months of onset).20 For the current analysis, all patients in whom nitrofurantoin, minocycline, methyldopa, or hydralazine was considered as the definite, highly likely, or probable cause were included. The protocol for this observational study was approved by the Institutional Review Boards at each clinical site and the data coordinating center. All enrolled subjects provided written informed consent.
Histopathology
Liver biopsy was not a required part of the DILIN prospective study, but if a biopsy was done as part of clinical care, requests were made for unstained slides from the biopsy to be sent to a central repository where stains were made and biopsies read by a hepatopathologist (D.E.K.) who was not given any clinical information. Biopsies were read in a formulaic manner for individual features and overall pattern of injury as previously described.22
Autoantibodies and Immunoglobulin Levels
To standardize for differences in autoantibody and IgG tests between different clinical centers, stored serum samples drawn within 6 months of onset of DILI were used to determine baseline autoantibody and IgG status. If available, samples from 6-, 12-, and 24-month follow-up visits were also tested. The presence of ANA, SMA, and anti–soluble liver antigen (SLA) was tested by enzyme-linked immunosorbent assay (ELISA) (Quanta Lite; Inova Diagnostics, San Diego, CA), and results were categorized as negative (0), low-positive (+1), or high-positive (+2), according to the manufacturer’s instructions. Serum IgG levels were measured by using a standard immunoturbidimetric assay (Roche/Hitachi Cobas C system; Roche Diagnostics USA, Indianapolis, IN) and scored as 0 (≤1600 mg/dL: <ULN), +1 (1600–1760 mg/dL: 1.0–1.1 times ULN), or +2 (greater than 1760 mg/dL: >1.1 times ULN). A total autoimmune score was the sum of the ANA, SMA, SLA, and IgG scores (range, 0–8). Patients were considered to have an autoimmune phenotype if the total score was 2 or above. The auto-antibody and IgG results were compared with a reference group of patients with other common causes of DILI: amoxicillin-clavulanate, isoniazid, and diclofenac (Supplementary Table 2). The presence of rash (+1), fever (+1), facial edema (+1), lymphadenopathy (+1), and eosinophilia >500/μL (+1) was recorded at baseline in all patients. Patients with a score of 2 or greater were considered to have an immunoallergic phenotype.
HLA Testing
Genetic testing for major histocompatibility complex alleles HLA-DRB1*03:01 and HLA-DRB1*04:01 was performed by imputation from genotype data obtained by using the Illumina Human Exome BeadChip with HIBAG software (Illumina, San Diego, CA) for patients with available DNA samples at the Duke Center for Human Genome Variation.23 Frequencies were compared with allele frequencies from 1,242,890 European whites included in the National Marrow Donor Program, previously described by Gregart et al24 and publicly available at http://allelefrequencies.net.25
Results
Between September 2004 and February 2014, 1322 patients with suspected DILI were enrolled into the DILIN prospective study. Of the total, 94 (8%) were adjudicated as definitely, highly likely, or probably due to nitrofurantoin, minocycline, methyldopa, or hydralazine. Six of the cases were excluded because other drugs were judged to be possibly implicated (n = 5) or because of preexisting liver disease (n = 1, hepatitis C). Thus, the cohort for analysis included 88 cases, 42 due to nitrofurantoin, 28 minocycline, 11 methyldopa, and 7 hydralazine.
Clinical Features at Onset
The demographic, clinical, and biochemical features of the 88 cases are shown by drug in Table 1. Patients with nitrofurantoin and hydralazine injury were typically elderly (median ages, 65 and 60 years, respectively,) whereas those with minocycline and methyldopa injury were younger (median ages, 19 and 29 years, respectively), reflecting the different conditions for which these agents are prescribed. All 4 agents were marked by a female preponderance. The racial distribution also varied by agent; the majority of cases of minocycline and nitrofurantoin occurred in non-Hispanic whites, but blacks accounted for a high proportion of methyldopa (55%) or hydralazine (43%) cases. Seven patients with methyldopa-induced liver injury were treated with this agent for hypertension during pregnancy. At the time of onset of liver injury, 3 patients (27%) were pregnant, and 4 (36%) had recently delivered but were continued on methyldopa.
Table 1.
Baseline Characteristics and Outcome
| Nitrofurantoin (n = 42) | Minocycline (n = 28) | Methyldopa (n = 11) | Hydralazine (n = 7) | |
|---|---|---|---|---|
| Feature | ||||
| Age (y)a | 65 (36–84) | 19 (16–61) | 29 (18–43) | 60 (42–76) |
| Female sex | 42 (100%) | 22 (79%) | 11 (100%) | 5 (71%) |
| Race | ||||
| White | 38 (91%) | 22 (79%) | 4 (36%) | 4 (57%) |
| Black | 3 (7%) | 3 (11%) | 6 (55%) | 3 (43%) |
| Other | 1 (2%) | 3 (11%) | 1 (9%) | 0 |
| BMI (kg/m2)a | 27 (17–47) | 22 (18–30) | 36 (23–50) | 27 (24–45) |
| Latency (mo) | ||||
| <1 | 5 (12%) | 1 (4%) | 1 (9%) | 3 (43%) |
| 1–6 | 7 (17%) | 7 (25%) | 9 (82%) | 3 (43%) |
| 6–12 | 4 (9%) | 6 (21%) | 1 (9%) | 1 (14%) |
| >12 | 26 (62%) | 14 (50%) | 0 | 0 |
| Initial results | ||||
| ALT (U/L)a | 739 (106–1700) | 938 (74–2942) | 1414 (206–3319) | 558 (132–2018) |
| AST (U/L)a | 643 (106–2221) | 765 (80–2504) | 1601 (282–2077) | 1046 (184–1410) |
| Alk P (U/L)a | 190 (80–688) | 153 (74–783) | 125 (83–346) | 260 (188–607) |
| R ratioa,b | 7.1 (1.0–56.9) | 16.6 (0.4–50.7) | 21.4 (11.0–59.2) | 2.1 (1.1–28.9) |
| Bilirubin (mg/dL) | 4.7 (0.3–27.5) | 3.4 (0.2–16.7) | 7.3 (0.5–17.6) | 2.1 (0.3–29.7) |
| Pattern of injuryb | ||||
| Hepatocellular | 28 (67%) | 23 (82%) | 11 (100%) | 3 (43%) |
| Mixed | 11 (26%) | 2 (7%) | 0 | 2 (29%) |
| Cholestatic | 3 (7%) | 3 (11%) | 0 | 2 (29%) |
| Symptoms, signs | ||||
| Jaundice | 26 (62%) | 14 (50%) | 9 (82%) | 3 (43%) |
| Itching | 18 (43%) | 14 (50%) | 6 (55%) | 3 (43%) |
| Rash | 10 (24%) | 5 (18%) | 3 (27%) | 1 (14%) |
| Fever | 11 (26%) | 7 (25%) | 3 (27%) | 1 (14%) |
| Eosinophilia | 1 (2%) | 2 (7%) | 0 | 1 (17%) |
| Phenotype | ||||
| Immunoallergic | 8 (19%) | 3 (11%) | 3 (27%) | 1 (14%) |
| Autoimmune | 24/29 (83%) | 14/19 (74%) | 6/10 (60%) | 3/7 (43%) |
| Course and outcome | ||||
| Severity scorec | ||||
| Mild (1+) | 17 (41%) | 12 (43%) | 2 (18%) | 2 (29%) |
| Moderate (2+, 3+) | 9 (21%) | 14 (50%) | 7 (64%) | 3 (43%) |
| Severe (4+, 5+) | 16 (38%) | 2 (7%) | 2 (18%) | 2 (29%) |
| Severity scorea | 3 (1–5) | 2 (1–4) | 3 (1–4) | 3 (1–5) |
| Liver transplant | 3 (8%) | 0 | 0 | 1 (14%) |
| Death | 2 (5%) | 0 | 0 | 0 |
| Corticosteroid therapy | 20 (48%) | 14 (50%) | 3 (27%) | 4 (57%) |
| Chronicity (6 mo)d | 6/33 (18%) | 6/24 (25%) | 2/10 (20%) | 1/7 (17%) |
| Chronicity (last value)d | 3/33 (9%) | 2/24 (8%) | 0/10 (0%) | 1/7 (17%) |
BMI, body mass index.
Median (range).
Definitions: R ratio = (ALT/ULN) ÷ (Alk P/ULN). Hepatocellular, R ratio >5; Mixed, R ratio 2–5; Cholestatic, R ratio <2.
Severity scores: Mild, serum enzyme elevations without jaundice; Moderate, serum enzyme elevations and jaundice (bilirubin >2.5 mg/dL); Severe, evidence of hepatic failure, death, or liver transplantation within 6 months of onset.
Chronicity = abnormal ALT, Alk P, or bilirubin values at 6 months or thereafter.
The latency to onset of injury varied widely from 4 days to more than 5 years. Nitrofurantoin and minocycline were marked by a majority of cases with latency above 6 months (71% each) and many above a year (62% and 50%, respectively). In contrast, latencies beyond 6 months were uncommon with methyldopa (9%) and hydralazine (14%), and all were within 1 year. The pattern of liver injury was predominantly hepatocellular (74% overall; 65%–100%) and rarely cholestatic (9% overall; 0%–11%).
Course and Outcome
Severity scores were mild (absence of jaundice) in 33 (38%), moderate in 33 (38%), and severe in 22 (25%) (Table 1). The anicteric cases often had a long latency to onset. Sixteen of the 22 severe cases were due to nitrofurantoin, which accounted for 5 of the 6 fatal instances (3 liver transplants and 2 deaths). The fatality rate was 12% with nitrofurantoin injury, 14% with hydralazine, but 0% with methyldopa and minocycline. A total of 41 patients (47%) received corticosteroid therapy for the liver injury, but the use of corticosteroids, initial dose, and subsequent dose modifications were at the discretion of the local treating physician.
The median follow-up after onset was 8 months (range, 0.1–35 months). Seventy-four patients survived the initial injury and were followed for at least 6 months. Fifteen patients (20%) still had liver test abnormalities at 6 months, and the chronicity rate was similar among the 4 drugs (17%–25%; Table 1). The residual liver injury at 6 months was generally mild; no patient had jaundice, and serum enzymes were only modestly or moderately elevated. The persistent abnormalities decreased over time and often resolved. At the time of the last visit (7 months–3 years after onset), 6 patients had abnormal liver test results; ALT values were raised in 3 (42, 58, and 610 U/L) and Alk P in 5 (126–222 U/L), but bilirubin values were normal in all (0.2–0.7 mg/dL). Four patients were suspected of having cirrhosis on the basis of imaging studies, and 3 were still on low doses of corticosteroids (n = 1) or azathioprine alone (n = 2). There were no late deaths from end-stage liver disease or liver cancer.
Autoimmune Features
Serum samples taken within 6 months of onset were available from 65 patients, 47 of which (72%) tested positive for ANA, 39 (60%) for SMA, but none for SLA. Serum IgG levels were elevated in 25 patients (39%) and were greater than 1.1 times ULN in 16 (25%). The autoimmune phenotype was present in 47 patients (72%) including 24 cases of nitrofurantoin (83%), 14 minocycline (74%), 6 methyldopa (60%), and 3 hydralazine (43%) injury (Table 1, Figure 1). In the control group of 67 selected subjects with liver injury attributed to amoxicillin/clavulanate (n = 33), isoniazid (n = 24), or diclofenac (n = 10), 15 (22%) were positive for ANA and 9 (13%) for SMA, 6 (9%) had elevated IgG levels, and 11 (16%) had an autoimmune phenotype (Supplementary Table 2, Figure 2). Autoimmune features were most common among the subjects with diclofenac injury; 50% had ANA, 30% had SMA, and 30% had elevated IgG levels, with 50% having the autoimmune phenotype.
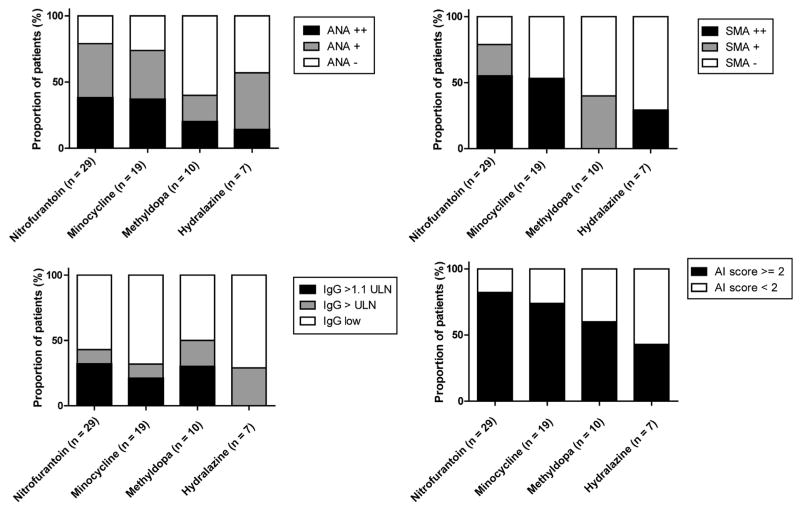
Figure 1.
Frequencies of ANA, SMA, and IgG positivity and autoimmune phenotype at baseline. Frequencies of ANA, SMA positivity, IgG levels, and autoimmune (AI) score in 65 DILI cases due to nitrofurantoin- (n = 29), minocycline- (n = 19), methyldopa- (n = 10), and hydralazine- (n = 7) induced liver injury with available stored serum samples.
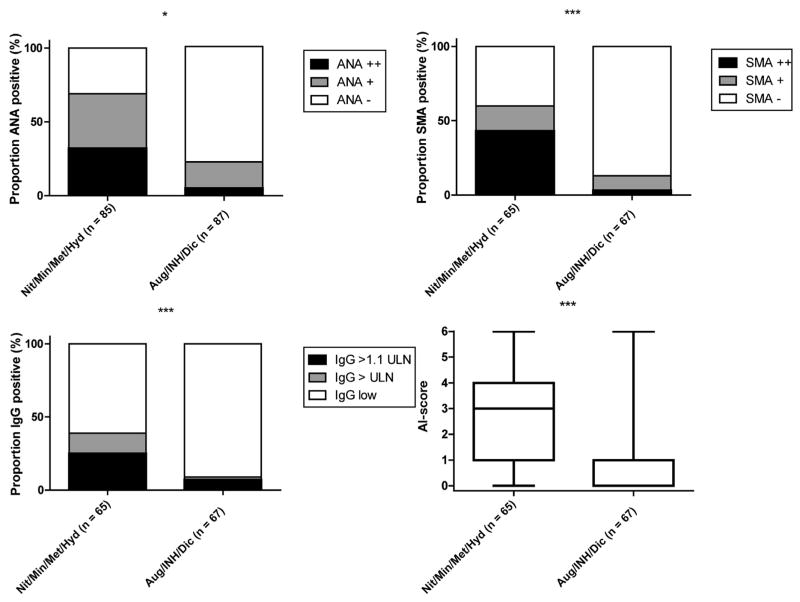
Figure 2.
Comparison of autoimmune features with reference cohort. Comparison of ANA, SMA positivity, IgG levels, and autoimmune (AI) score between 65 cases due to nitrofurantoin (n = 29), minocycline (n = 19), methyldopa (n = 10), and hydralazine (n = 7) (Mit/Myn/Met/Hyd) and 67 reference cases due to amoxicillin/clavulanate (n = 33), isoniazid (n = 24), and diclofenac (n = 10) (Aug/INH/Dic).
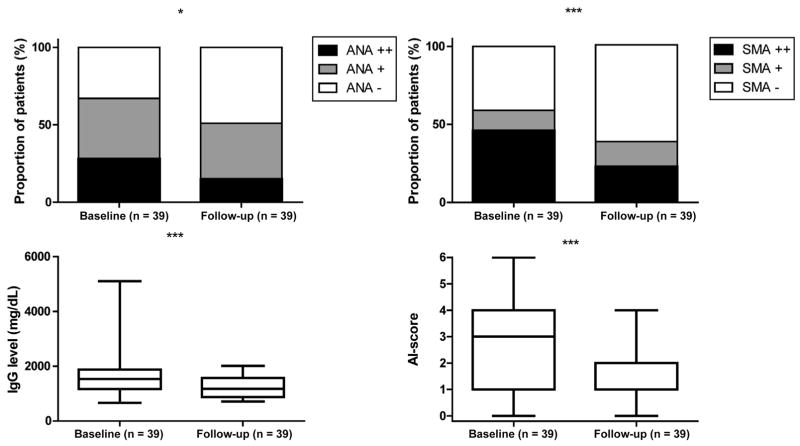
Follow-up serum samples were available from 39 patients (61%), which showed a decline in autoantibody prevalence and both antibody and IgG levels (Figure 3). Eight of 27 patients (30%) who were initially ANA positive were negative on follow-up, as were 8 of 23 (35%) who were initially SMA positive. Mean IgG levels fell during follow-up from 1536 mg/dL (range, 668–5105) to 1179 mg/dL (range, 718–2017) (P < .001). Neither the decrease in IgG levels nor the loss of autoimmune phenotype status was associated with corticosteroid treatment (P = .60) or the presence of abnormalities at 6 months (chronicity: P = .10) (data not shown).
Figure 3.
Comparison of autoimmune features at baseline and 6 months of follow-up. Frequencies of ANA, SMA positivity, IgG levels, and autoimmune (AI) score at baseline and 6 months of follow-up of 39 cases due to nitrofurantoin-, minocycline-, hydralazine-, and methyldopa-induced with available samples.
Clinical Features Associated With the Autoimmune Phenotype
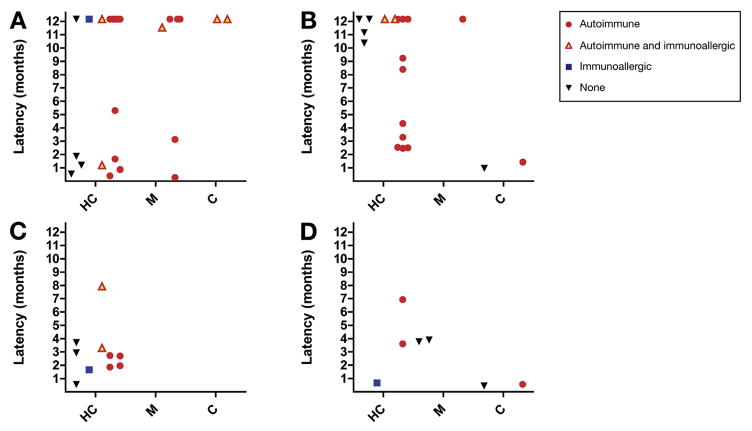
There were no significant differences in age, sex, race, body mass index, presence and types of clinical symptoms, pattern of enzyme elevations, bilirubin elevations, disease severity, chronic laboratory abnormalities, use of corticosteroids, and time to recovery between those with or without the autoimmune phenotype (Table 2). However, all 5 patients with available serum samples in the most severe group (5+) had autoimmune features, consisting of 3 patients who underwent liver transplantation and 2 who died. Furthermore, the latency to onset was longer in patients with the autoimmune phenotype than in those without (median, 277 days, range, 8–7032 vs 100 days, range, 13–1572, respectively; P = .03), particularly in nitrofurantoin and minocycline associated injury. The latency and pattern of serum enzyme elevations for each patient with depiction of whether there were autoimmune or immunoallergic features are shown in Figure 4.
Table 2.
Clinical Characteristics of Cases With and Without Autoimmune Features
| Autoimmune (n = 47) | Non-autoimmune (n = 18) | P value | |
|---|---|---|---|
| Features | |||
| Age (y)a | 53.1 (16–84) | 43.3 (18–77) | .4 |
| Female sex | 43 (92%) | 15 (83%) | .4 |
| Race | |||
| White | 33 (70%) | 13 (72%) | .4 |
| Black | 11 (23%) | 4 (22%) | |
| Other | 3 (6%) | 1 (5%) | |
| BMI (kg/m2) | 26.8 (18.2–49.9) | 24.6 (17.7–49.5) | .5 |
| Latency | |||
| Latency (days)a | 277 (8–7032) | 100 (13–1572) | .03 |
| <1 mo | 4 (9%) | 5 (28%) | |
| 1–6 mo | 16 (34%) | 7 (39%) | .1 |
| 6–12 mo | 5 (11%) | 2 (11%) | |
| >12 mo | 22 (47%) | 4 (22%) | |
| Initial results | |||
| ALT (U/L)a | 954 (74–3700) | 1237 (108–3242) | .5 |
| AST (U/L)a | 867 (172–2504) | 923 (97–2221) | .9 |
| Alk P (U/L)a | 176 (74–783) | 188 (83–607) | .9 |
| R ratioa,b | 14.4 (0.4–82.4) | 14.0 (1.1–59.2) | .8 |
| Bilirubin (mg/dL)a | 6.3 (0.3–27.5) | 4.7 (0.3–29.7) | .4 |
| Pattern of injuryb | |||
| Hepatocellular | 35 (74%) | 14 (78%) | .8 |
| Mixed | 8 (17%) | 2 (11%) | |
| Cholestatic | 4 (9%) | 2 (11%) | |
| Symptoms, signs | |||
| Jaundice | 32 (68%) | 10 (56%) | .4 |
| Itching | 25 (53%) | 8 (44%) | .6 |
| Rash | 9 (19%) | 4 (22%) | .7 |
| Fever | 15 (32%) | 3 (17%) | .4 |
| Eosinophilia | 1 (2%) | 3 (19%) | .05 |
| Phenotype | |||
| Immunoallergic | 9 (19%) | 3 (17%) | 1.0 |
| Autoimmune | 47 (100%) | 0 (0%) | |
| Course and outcome | |||
| Severity scorec | |||
| Mild (1+) | 15 (32%) | 5 (28%) | .4 |
| Moderate (2+, 3+) | 18 (38%) | 10 (56%) | |
| Severe (4+, 5+) | 14 (30%) | 3 (17%) | |
| Severity scored | 2.6 (1–5) | 2.6 (1–4) | .8 |
| Liver transplant | 3 (6%) | 0 | .6 |
| Death | 2 (4%) | 0 | 1.0 |
| Corticosteroid therapy | 20 (43%) | 11 (61%) | .3 |
| Chronicity (6 mo)e | 7/41 (17%) | 3/14 (21%) | .70 |
| Histologic features | (n = 13) | (n = 5) | |
| Interface hepatitis | 13 (100%) | 5 (100%) | |
| Plasma cells | 9 (69%) | 2 (40%) | |
| Hepatocyte rosettes | 10 (77%) | 4 (80%) | |
| Acute hepatitic pattern | 7 (54%) | 2 (40%) | |
| Chronic hepatitic pattern | 0 | 1 (20%) | |
BMI, body mass index.
Median (range).
R ratio = (ALT/ULN) ÷ (Alk P/ULN). Hepatocellular, R ratio >5; Mixed, R ratio 2–5; Cholestatic, R ratio <2.
Severity score: Mild = serum enzyme elevations without jaundice; Moderate = serum enzyme elevations and jaundice (bilirubin >2.5 mg/dL); Severe = jaundice and evidence of hepatic failure, death, or liver transplantation within 6 months of onset.
Chronicity = abnormal ALT, Alk P, or bilirubin values at 6 months.
Mean (range).
Figure 4.
Latency and pattern of injury. Time of onset to DILI in days (capped at 12 months) in nitrofurantoin (A), minocycline (B), methyldopa (C), and hydralazine (D) cases with available serum samples, stratified according to drug, pattern of injury, and autoimmune and/or immunoallergic phenotype. C, cholestatic; HC, hepatocellular; M, mixed.
Genetics
Fifty-one white patients with DILI that was due to nitrofurantoin (n = 30), minocycline (n = 16), methyldopa (n = 2), or hydralazine (n = 3) had been genotyped by using Illumina Human Exome BeadChip.23 The allele frequencies of the idiopathic AIH risk alleles HLA-DRB1*03:01 and DRB1*04:01 among patients were similar to those of population controls from the National Marrow Donor Program (DRB1*03:01: 15% vs 12%, respectively, P = .4; DRB1*04:01: 9% vs 9%, respectively, P = 1.0).24,25 Among the drug-induced cases, none were homozygotes or compound heterozygotes for these alleles. In addition, in those patients with available serum samples (n = 36), there was no association between the presence or absence of the autoimmune phenotype and either DRB1*03:01 (13% vs 17%, P = .7) or DRB1*04:01 (11% vs 11%, P = 1.0).
Histology
Baseline liver biopsies, performed within 28 days of DILI onset, were available in 21 patients. Virtually all patients had interface and lobular hepatitis, which was usually marked or severe (Table 2). Thirteen of 18 patients (72%) with both an available biopsy and a serum sample had the autoimmune phenotype, and five (28%) did not. Plasma cells were increased in 9 cases (69%) with vs 3 cases (60%) without the autoimmune phenotype, whereas hepatocyte rosettes were seen in 10 cases (77%) with vs 4 cases (80%) without this phenotype (Table 2). In regard to overall histologic pattern, most cases (72%) were classified as having an acute hepatitic (n = 9) or combined hepatitic/cholestatic (n = 6) pattern. Two cases (1 nitrofurantoin and 1 minocycline) displayed a chronic hepatitic pattern, both of whom had mild liver injury with serum aminotransferase elevations but no jaundice and presented more than a year after starting the medication. The 2 fatal cases (1 nitrofurantoin and 1 hydralazine) with available liver histology displayed a pattern of massive necrosis. One severe case due to hydralazine showed marked inflammation and bridging necrosis that were confluent in zone 3, and a more moderate case due to methyldopa showed a mixed or unclassifiable pattern. No patient had purely cholestatic hepatitis or bland cholestasis.
Discussion
Nitrofurantoin, minocycline, methyldopa, and hydralazine are well-known causes of DILI with autoimmune features.7,11,12 In this study, the proportion of cases of liver injury that were due to these drugs that displayed autoimmune features was found to be 70% for nitrofurantoin and minocycline compared with 40%–50% for hydralazine and methyldopa. Although autoimmune features were present in these cases, they were not overly prominent; IgG was only modestly elevated, and autoantibody reactivity was mild to moderate during the acute illness and improved with recovery. In addition, autoimmune markers were more frequent with severe hepatic injury and were most frequent in patients with a prolonged latency, especially with nitrofurantoin and minocycline. As noted in previous studies, these 4 agents were infrequently associated with short latency liver injury (ie, <1 month) (n = 10, 11%), but latencies of 1–6 months were not uncommon (n = 26, 30%), although they were somewhat less likely to be associated with autoimmune features.12,26–32
Although this study represents the largest cohort of investigated autoimmune-like DILI cases that were due to these 4 drugs, no statistically significant associations were found between the autoimmune phenotype and demographic or clinical features or with outcome and prognosis. However, the follow-up in this study was only 2 years and was incomplete in a proportion of cases, which makes it impossible to provide a complete overview and assessment of the long-term prognosis of patients with the autoimmune phenotype of DILI. However, the clinical and histologic similarity of cases with and without autoimmune features suggests that the presence of autoantibodies merely reflects the variability in clinical and biochemical expression of the injury, and perhaps all cases are immunologically mediated.
The strengths of this study included the large number of patients, the use of standardized assays for autoantibodies, and the ability to compare cases of AIH-like injury that were due to several drugs (and control agents). The weaknesses of the study were that the cases were managed by many different physicians and were not evaluated and treated in a standardized manner. For instance, decisions regarding liver biopsy and use of corticosteroids were at the discretion of the local physician and were not a part of the prospective DILIN protocol. Furthermore, the standardized ELISAs used have not been as well-characterized for sensitivity and specificity as conventional immunofluorescence tests.33 Nevertheless, these assays allowed for uniform testing of all specimens and did show differences between cases and controls as well as before and after recovery. The sensitivity and specificity of the ANA and SMA assays have been reported in the literature.33,34
In general, nitrofurantoin-associated liver injury tended to be more severe and more likely to persist than that associated with the other 3 agents. Indeed, all except 1 of the fatalities was attributed to nitrofurantoin. Thus, nitrofurantoin fell into the conundrum described by Professor Hyman Zimmerman: drugs that cause hepatocellular injury with jaundice generally have a high fatality rate, in excess of 10% (referred to colloquially as “Hy’s Law”).35 Of interest, despite causing acute icteric hepatocellular injury, neither minocycline nor methyldopa had any associated mortality and rarely caused persistent and long-lasting chronic injury. In support of these findings in regard to minocycline, large case series of fatal DILI invariably list nitrofurantoin and often include hydralazine and methyldopa as causes but do not mention minocycline.1,3,18
This study also sought to assess the role of the idiopathic AIH risk alleles HLA-DRB1*03:01 and -DRB1*04:01 in drug-induced AIH-like injury by using a candidate gene approach.14 The analysis and the absence of homozygotes and compound heterozygotes showed that neither alleles were overrepresented in this cohort when compared with population controls. These findings suggest that HLA-DRB1*03:01 and -DRB1*04:01 alleles do not represent risk factors for nitrofurantoin-, minocycline-, methyldopa-, and hydralazine-induced liver injury and the associated autoimmune phenotype. It should be noted that although this is the largest series of its kind, this study had a low power to detect associations and that analyses were restricted to white cases and controls because the original association in sporadic AIH was identified in whites.
Conclusions
An autoimmune-like hepatitis occurs in most but not all patients with nitrofurantoin- and minocycline-induced liver injury and in at least half of those with methyldopa and hydralazine injury. The autoimmune phenotype is not associated with clinical outcome and may slowly resolve with discontinuation of the drug and recovery from the acute hepatocellular liver injury. Furthermore, the presence of autoimmune features is not associated with the typical HLA alleles found in idiopathic AIH, indicating that it does not represent drug-induced injury occurring in patients with a predisposition to AIH.
Supplementary Material
Acknowledgments
The authors thank Elisavet Serti for help with the ELISA testing for autoantibodies and Rita Lapointe and Jacqueline Danna for help with the IgG assays. Members of the DILIN Network are listed in Supplementary Table 1.
Funding
The Drug Induced Liver Injury Network (DILIN) is structured as a U01 cooperative agreement supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and the National Institutes of Health (NIH) with funds provided by the following grants: U01DK065211 (Indiana University [Purdue]), U01DK065184 (University of Michigan [Ann Arbor]), U01DK065201 (University of North Carolina [Chapel Hill], Asheville, Wake Forest Baptist Medical Center), U01DK083020 (University of Southern California, University of California-Los Angeles [Pfleger Liver Institute]), U01DK083027 (Albert Einstein Medical Center), U01DK100928 (Icahn School of Medicine at Mount Sinai), and U01DK065176 (Duke Clinical Research Institute). Additional support was provided by the Intramural Division of the National Cancer Institute (NCI), NIH. Partial salary support (Y.D.B.) and funding for reagents for autoantibody testing were provided by the Foundation for the National Institutes of Health. A complete listing of participants in DILIN is provided in the Supplementary Material.
Abbreviations used in this paper
- AIH
autoimmune hepatitis
- Alk P
alkaline phosphatase
- ALT
alanine aminotransferase
- ANA
antinuclear antibody
- AST
aspartate aminotransferase
- DILI
drug-induced liver injury
- DILIN
Drug-Induced Liver Injury Network
- ELISA
enzyme-linked immunosorbent assay
- IgG
immunoglobulin G
- SLA
soluble liver antigen
- SMA
smooth muscle antibody
- ULN
upper limit of the normal range
Footnotes
Conflicts of interest
This author discloses the following: Naga Chalasani serves as a consultant to and receives research grants from several pharmaceutical companies, but none are directly relevant for this manuscript. The remaining authors disclose no conflicts.
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at http://dx.doi.org/10.1016/j.cgh.2016.05.043.
References
- 1.Wei G, Bergquist A, Broomé U, et al. Acute liver failure in Sweden: etiology and outcome. J Intern Med. 2007;262:393–401. doi: 10.1111/j.1365-2796.2007.01818.x. [DOI] [PubMed] [Google Scholar]
- 2.Björnsson ES, Bergmann OM, Björnsson HK, et al. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–1425. doi: 10.1053/j.gastro.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Reuben A, Koch DG, Lee WM, et al. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–2076. doi: 10.1002/hep.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarez F, Berg PA, Bianchi FB, et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929–938. doi: 10.1016/s0168-8278(99)80297-9. [DOI] [PubMed] [Google Scholar]
- 5.Hennes EM, Zeniya M, Czaja AJ, et al. Hepatology. 2008;48:169–176. doi: 10.1002/hep.22322. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki A, Brunt EM, Kleiner DE, et al. The use of liver biopsy evaluation in discrimination of idiopathic autoimmune hepatitis versus drug-induced liver injury. Hepatology. 2011;54:931–939. doi: 10.1002/hep.24481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Czaja AJ. Drug-induced autoimmune-like hepatitis. Dig Dis Sci. 2011;56:958–976. doi: 10.1007/s10620-011-1611-4. [DOI] [PubMed] [Google Scholar]
- 8.Bernal W, Ma Y, Smith HM, et al. The significance of autoantibodies and immunoglobulins in acute liver failure: a cohort study. J Hepatol. 2007;47:664–670. doi: 10.1016/j.jhep.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Weiler-Normann C, Schramm C. Drug induced liver injury and its relationship to autoimmune hepatitis. J Hepatol. 2011;55:747–749. doi: 10.1016/j.jhep.2011.02.024. [DOI] [PubMed] [Google Scholar]
- 10.Castiella A, Zapata E, Lucena MI, et al. Drug-induced autoimmune liver disease: a diagnostic dilemma of an increasingly reported disease. World J Hepatol. 2014;6:160–168. doi: 10.4254/wjh.v6.i4.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Licata A, Maida M, Cabibi D, et al. Clinical features and outcomes of patients with drug-induced autoimmune hepatitis: a retrospective cohort study. Dig Liver Dis. 2014;46:1116–1120. doi: 10.1016/j.dld.2014.08.040. [DOI] [PubMed] [Google Scholar]
- 12.Björnsson E, Talwalkar J, Treeprasertsuk S, et al. Drug-induced autoimmune hepatitis: clinical characteristics and prognosis. Hepatology. 2010;51:2040–2048. doi: 10.1002/hep.23588. [DOI] [PubMed] [Google Scholar]
- 13.van Gerven NM, Verwer BJ, Witte BI, et al. Relapse is almost universal after withdrawal of immunosuppressive medication in patients with autoimmune hepatitis in remission. J Hepatol. 2013;58:141–147. doi: 10.1016/j.jhep.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 14.de Boer YS, van Gerven NM, Zwiers A, et al. Genome-wide association study identifies variants associated with autoimmune hepatitis type 1. Gastroenterology. 2014;147:443–452. doi: 10.1053/j.gastro.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 15.van Gerven NM, de Boer YS, Zwiers A, et al. HLA-DRB1*03:01 and HLA-DRB1*04:01 modify the presentation and outcome in autoimmune hepatitis type-1. Genes Immun. 2015;16:247–252. doi: 10.1038/gene.2014.82. [DOI] [PubMed] [Google Scholar]
- 16.Donaldson PT, Doherty DG, Hayllar KM, et al. Susceptibility to autoimmune chronic active hepatitis: human leukocyte antigens DR4 and A1-B8-DR3 are independent risk factors. Hepatology. 1991;13:701–706. [PubMed] [Google Scholar]
- 17.Oliveira LC, Porta G, Marin ML, et al. Autoimmune hepatitis, HLA and extended haplotypes. Autoimmun Rev. 2011;10:189–193. doi: 10.1016/j.autrev.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 18.Chalasani N, Bonkovsky HL, Fontana R, et al. Features and outcomes of 899 patients with drug-induced liver injury: the DILIN prospective study. Gastroenterology. 2015;148:1340–1352. doi: 10.1053/j.gastro.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rockey DC, Seeff LB, Rochon J, et al. Causality assessment in drug-induced liver injury using a structured expert opinion process: comparison to the Roussel-Uclaf causality assessment method. Hepatology. 2010;51:2117–2126. doi: 10.1002/hep.23577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fontana RJ, Watkins PB, Bonkovsky HL, et al. Drug-Induced Liver Injury Network (DILIN) prospective study: rationale, design and conduct. Drug Saf. 2009;32:55–68. doi: 10.2165/00002018-200932010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayashi PH, Barnhart HX, Fontana RJ, et al. Reliability of causality assessment for drug, herbal and dietary supplement hepatotoxicity in the Drug-Induced Liver Injury Network (DILIN) Liver Int. 2015;35:1623–1632. doi: 10.1111/liv.12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleiner DE, Chalasani NP, Lee WM, et al. Hepatic histological findings in suspected drug-induced liver injury: systematic evaluation and clinical associations. Hepatology. 2014;59:661–670. doi: 10.1002/hep.26709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng X, Shen J, Cox C, et al. HIBAG–HLA genotype imputation with attribute bagging. Pharmacogenomics J. 2014;14:192–200. doi: 10.1038/tpj.2013.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gragert L, Madbouly A, Freeman J, et al. Six-locus high resolution HLA haplotype frequencies derived from mixed-resolution DNA typing for the entire US donor registry. Hum Immunol. 2013;74:1313–1320. doi: 10.1016/j.humimm.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 25. [Accessed January 21, 2016]; Available at: http://allelefrequencies.net/
- 26. [Accessed January 5, 2016]; Available at: http://livertox.nih.gov/index.html.
- 27.Stricker BH, Blok AP, Claas FH, et al. Hepatic injury associated with the use of nitrofurans: a clinicopathological study of 52 reported cases. Hepatology. 1988;8:599–606. doi: 10.1002/hep.1840080327. [DOI] [PubMed] [Google Scholar]
- 28.Malcolm A, Heap TR, Eckstein RP, et al. Minocycline-induced liver injury. Am J Gastroenterol. 1996;91:1641–1643. [PubMed] [Google Scholar]
- 29.Goldstein NS, Bayati N, Silverman AL, et al. Minocycline as a cause of drug-induced autoimmune hepatitis: report of four cases and comparison with autoimmune hepatitis. Am J Clin Pathol. 2000;114:591–598. doi: 10.1309/KV2J-VX6Q-L95V-VDE4. [DOI] [PubMed] [Google Scholar]
- 30.Toghill PJ, Smith PG, Benton P, et al. Methyldopa liver damage. Br Med J. 1974;3:545–548. doi: 10.1136/bmj.3.5930.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maddrey WC, Boitnott JK. Severe hepatitis from methyldopa. Gastroenterology. 1975;68:351–360. [PubMed] [Google Scholar]
- 32.Forster HS. Hepatitis from hydralazine. N Engl J Med. 1980;302:1362. [PubMed] [Google Scholar]
- 33.Meroni PL, Schur PH. ANA screening: an old test with new recommendations. Ann Rheum Dis. 2010;69:1420–1422. doi: 10.1136/ard.2009.127100. [DOI] [PubMed] [Google Scholar]
- 34.Frenzel C, Herkel J, Lüth S, et al. Evaluation of F-actin ELISA for the diagnosis of autoimmune hepatitis. Am J Gastroenterol. 2006;101:2731–2736. doi: 10.1111/j.1572-0241.2006.00830.x. [DOI] [PubMed] [Google Scholar]
- 35.Temple R. Hy’s law: predicting serious hepatotoxicity. Pharmacoepidemiol Drug Saf. 2006;15:241–243. doi: 10.1002/pds.1211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.