Supplemental Digital Content is available in the text
Keywords: all-cause mortality, cardiovascular disease, coronary heart disease, diabetes, stroke, whole grains
Abstract
Background:
To investigate the correlation between consumption of whole grains and the risk of all-cause, cardiovascular disease (CVD), and diabetes-specific mortality according to a dose–response meta-analysis of prospective cohort studies.
Methods:
Observational cohort studies, which reported associations between whole grains and the risk of death outcomes, were identified by searching articles in the MEDLINE, EMBASE, and the reference lists of relevant articles. The search was up to November 30, 2015. Data extraction was performed by 2 independent investigators, and a consensus was reached with involvement of a third.
Results:
Ten prospective cohort studies (9 publications) were eligible in this meta-analysis. During follow-up periods ranging from 5.5 to 26 years, there were 92,647 deaths among 782,751 participants. Overall, a diet containing greater amounts of whole grains may be associated with a lower risk of all-cause, CVD-, and coronary heart disease (CHD)-specific mortality. The summary relative risks (RRs) were 0.93 (95% confidence intervals [CIs]: 0.91–0.95; Pheterogeneity < 0.001) for all-cause mortality, 0.95 (95% CIs: 0.92–0.98; Pheterogeneity < 0.001) for CVD-specific mortality, and 0.92 (95% CIs: 0.88–0.97; Pheterogeneity < 0.001) for CHD-specific mortality for an increment of 1 serving (30 g) a day of whole grain intake. The combined estimates were robust across subgroup and sensitivity analyses. Higher consumption of whole grains was not appreciably associated with risk of mortality from stroke and diabetes.
Conclusion:
Evidence from observational cohort studies indicates inverse associations of intake of whole grains with risk of mortality from all-cause, CVD, and CHD. However, no associations with risk of deaths from stroke and diabetes were observed.
1. Introduction
Worldly, whole grain foods have been widely recommended for the general population in numerous dietary guidelines. For the term “whole grain,” all the available definitions emphasize the presence of 3 parts: the bran, the germ, and the endosperm. Compared with refined grain products that comprise mainly the endosperm, whole grain foods are rich in a wide variety of nutrients in the outer bran and germ layers: several vitamins, dietary fiber, minerals, and phytochemicals.[1] Many a prospective cohort studies and meta-analyses have linked a higher intake of whole grains and a decreased risk of cardiovascular disease (CVD),[2–4] type 2 diabetes,[5,6] and some types of cancers.[7,8] Human clinical trials have showed that consumption of whole grain foods have beneficial roles in total cholesterol and LDL-cholesterol,[9,10] glucose metabolism,[11] endothelial function,[12] and inflammatory biomarkers.[13]
Although whole grains are proposed to be responsible for health-promoting effects,[14,15] data regarding the association between consumption of whole grains and deaths were not entirely consistent. For example, higher intake of whole grains was reported to be associated with a decreased risk of mortality in the Atherosclerosis Risk in Communities (ARIC) study,[16] the Iowa Women's Health Study (IWHS),[17] the Nurses’ Health Study (NHS), and the Health Professionals Follow-Up Study (HPFS),[18] whereas null associations were found among a healthy elderly population[19] or diabetes patients.[20] To our knowledge, no comprehensive quantitative assessment of the associations between whole grains consumption and the risk of mortality from all-cause, CVD, and diabetes has been carried out. The objective of the current study was to evaluate these associations based on prospective cohort studies in apparently healthy adults.
2. Methods
We preformed the meta-analysis following the criteria set out by the Preferred reporting items for systematic reviews and meta-analysis guidelines.[21] There are no ethical issues involved in our study since our data were based on published studies.
2.1. Literature search
Two authors (LBL and JGX) searched the bibliographical databases of MEDLINE and EMBASE for studies published in journals from January 1, 1966 to November 30, 2015. We conducted searches in each database using the following search algorithm (as formatted for Ovid): (cereals OR bread OR oat OR grains OR wheat) AND (fatal OR coronary heart disease OR cardiovascular disease OR death OR mortality OR stroke OR diabetes). The search was limited to articles published in English. A manual search was also conducted, using references of key articles.
2.2. Study selection
Two authors (LBL and JGX) independently read titles, abstracts, and key words of identified articles. To be included, studies had to meet the criteria as follows: had a prospective cohort study design; reporting relative risks (RRs) and corresponding 95% confidence intervals (CIs) for at least 3 quantitative categories of whole grains consumption and mortality; mortality type included all-cause, CVD (coronary heart disease [CHD] and stroke), or diabetes. For each cohort study, we selected the publication with the longest follow-up time. Studies were excluded if they reported animal models and populations defined by preexisting disease or participants younger than 16 years. Studies selected for detailed analysis by these 2 investigators had an agreement value (κ) of 95%; disagreements were resolved by a 3rd investigator (ZK).
2.3. Data extraction
Two investigators (LBL and JGX) extracted data as follows: author, year of publication, country, the study name, follow-up time, number of participants, age, and gender, dietary assessment method (type, number of food items, and whether it had been validated), number of cases, adjustment for potential confounders, and RR estimates and the corresponding 95% confidence interval (CI) for the highest compared with the lowest grain intake. If separate risk estimates for men and women were available in 1 study, we treated it as separate studies. We emailed the authors of studies with appropriate data but with specific missing information, but none responded.
2.4. Quality assessment
According to the Newcastle–Ottawa quality assessment scale (NOS),[22] we conducted the quality assessment to determine the likely risk of bias associated with each of the included studies. This scale uses 8 items, categorized into the 3 domains: selection (4 points), comparability (2 points), and the ascertainment of outcomes of interest (3 points). As such, NOS ranges between 0 and 9 points; and a total score of 7 or greater was used to indicate high-quality studies and a total score of 6 or smaller indicated low-quality studies.
2.5. Statistical methods
We used the statistical program STATA, version 11.0 (STATA, College Station, TX) and R package (version 2.11.0 beta) statistical software for all dose–response analyses. Significance was set at P < 0.05. To take into account within- and between-study heterogeneity, we employed random effects modeling to meta-analyses the data.[23]
The dose–response results in the forest plots are presented in a 1 serving/day increment, which was according to the method of generalized least-squares trend estimation developed by Orsini and Greenland.[24,25] This analysis is performed on the basis of the data for categories of whole grains intake levels on median dose, number of cases and participants, and adjusted logarithm of the RR with its SE. Lack of this information led us to estimate the dose–response slopes using variance-weighted least squares regression analysis.[24,25] This analysis was restricted to the studies reporting 3 or more exposure levels. In the absence of such median intake value, we used the midpoint of each category. For an open-ended upper category of intake, we assumed the size of the interval to be the same as the previous category. In studies that reported the intakes in grams, we used 30 g as a serving size for recalculation of the intakes to a common scale (serving).
We employed the Q statistic for homogeneity between studies. P value of <0.10 represents statistically significant heterogeneity. Q statistic tests whether the between-study variability in effect sizes exceeds that expected from corresponding within-study variability. We also examined I2 statistic, which measures the percentage of the total variation across studies that is due to heterogeneity, rather than chance.[26] We explored heterogeneity between studies using 3 strategies. First, subgroup analyses were performed to assess the potential modifying effects of the following variables on outcomes: sex, continent, duration of follow-up, and degree of adjustment for confounding including a history of hypertension, deslipidemia, type 2 diabetes, and dietary fiber intake. Second, we carried out stepwise meta-regression analyses. Third, we reran the meta-analysis removing studies once at a time to determine whether a particular study accounted for the heterogeneity.
According to fractional polynomial models,[27] we evaluated a potential nonlinear relationship between whole grains consumption and risk of all-cause and CVD mortality. A likelihood ratio test was used to assess the difference between the nonlinear and linear models to test for nonlinearity.[27]
To detect publication biases, we explored heterogeneity in funnel plots and the degree of asymmetry by using Begg test (rank correlation) and Egger test (linear regression).[28,29] Significant publication bias was defined as a P value < 0.1. We also conducted the trim and fill computation to evaluate the effect of publication bias.[30]
3. Results
3.1. Search results
Figure 1 showed the study selection process and results from the literature search. We identified 4655 articles from the MEDLINE database and 4629 articles from the EMBASE database. Additional 28 articles were included from the reference reviews. After exclusion of duplicates and papers that did not meet the inclusion criteria, 85 of which had potential value and were available as full-text articles. In the review of these 85 articles, 76 were subsequently excluded from the meta-analysis for various reasons. Therefore, a total of 9 articles with 10 prospective cohort studies (1 article[18] contained 2 large independent cohort studies: the NHS and the HPFS, respectively) were included in the meta-analysis.
Figure 1.
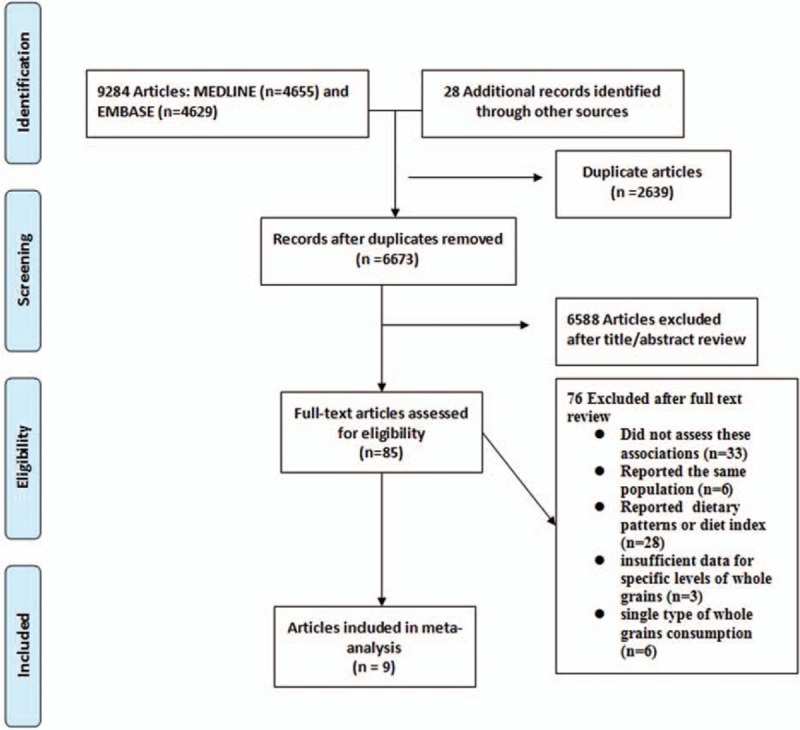
Flow diagram of systematic literature search.
3.2. Study characteristics
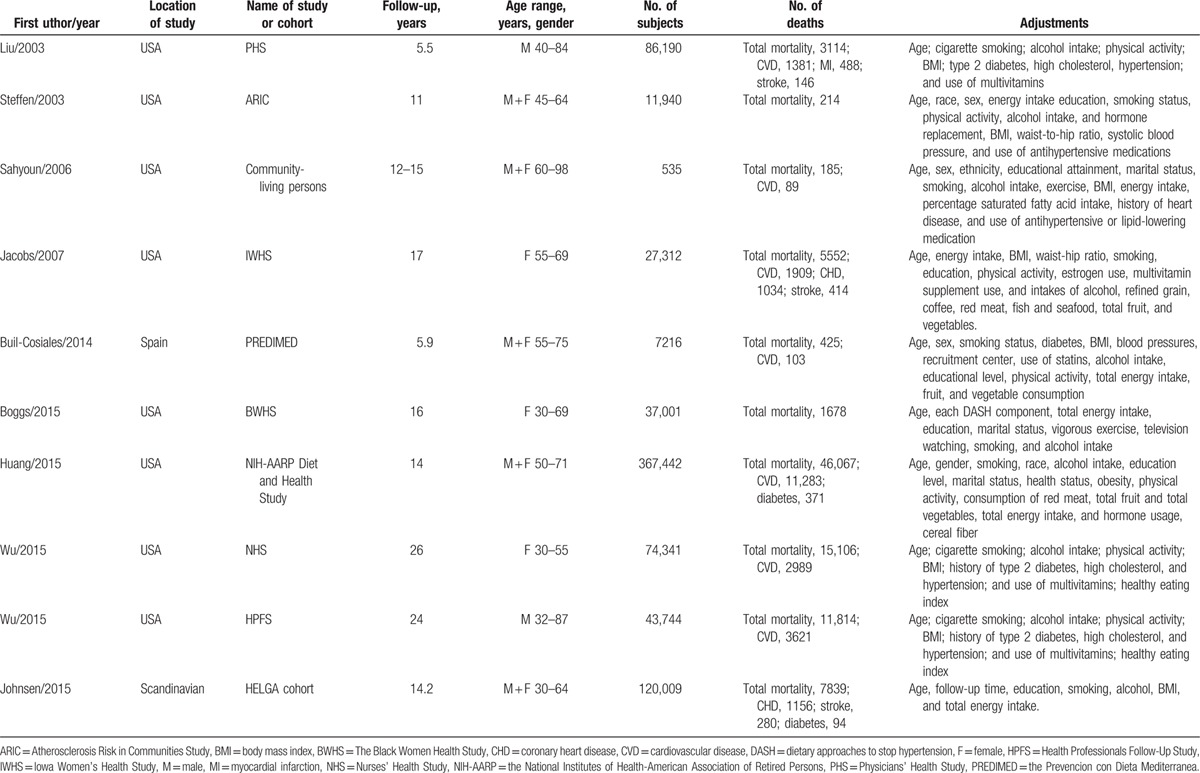
Table 1 and supplementary Table 1 showed the characteristics of the included studies, all of which had a prospective cohort design. The 10 studies contained a total of 782,751 subjects (ranged from 535 to 367,442) and 92,647 deaths from all-cause (ranged from 165 to 46,067). One study provided results specific for both genders.[31] Two cohorts consisted entirely of males[18,32] and 3 cohorts consisted entirely of females.[17,18,33] Four cohort studies included men and women. Eight cohort studies were conducted in the United States and 2 in Europe. One study measured consumption of whole grains by diet records,[19] and all other studies used validated Food Frequency Questionnaire (FFQ). All studies made adjustments for age, smoking, alcohol intake, body mass index (BMI), and exercise. Adjustments were conducted for history of hypertension or measured blood pressure (6 cohorts), diabetes (5 cohorts), and total energy intake (6 studies). Few studies were adjusted for dyslipidemia (3 cohorts) and dietary fiber intake (2 cohorts). Assessment of study quality according to NOS yielded an average score of 8.1, and all studies were of high quality (NOS >6; supplementary Table 2).
Table 1.
Characteristics of the studies on the association between whole grain consumption and the risk of all-cause, cardiovascular disease, and diabetes mortality.
3.3. All-cause mortality
Figure 2 showed the results of the pooled analysis for the relationship between whole grain consumption and the risk of all-cause mortality, which was evaluated in 10 cohorts (9 publications),[16–19,32–36] comprising 782,751 participants and 92,647 deaths. The pooled risk estimation was 0.93 (95% CI: 0.91–0.95) for every 1 serving of whole grains a day, with significant heterogeneity (I2 = 79. 0%, Pheterogeneity < 0.001). Begg test indicated no publication bias (P = 0.640; supplementary Figure 1A), but Egger test indicated possible publication bias (P = 0.002). The trim and fill analysis suggested that the imputed risk estimate was identical to our original risk estimate. No missing studies were imputed in the contour-enhanced funnel plot.
Figure 2.
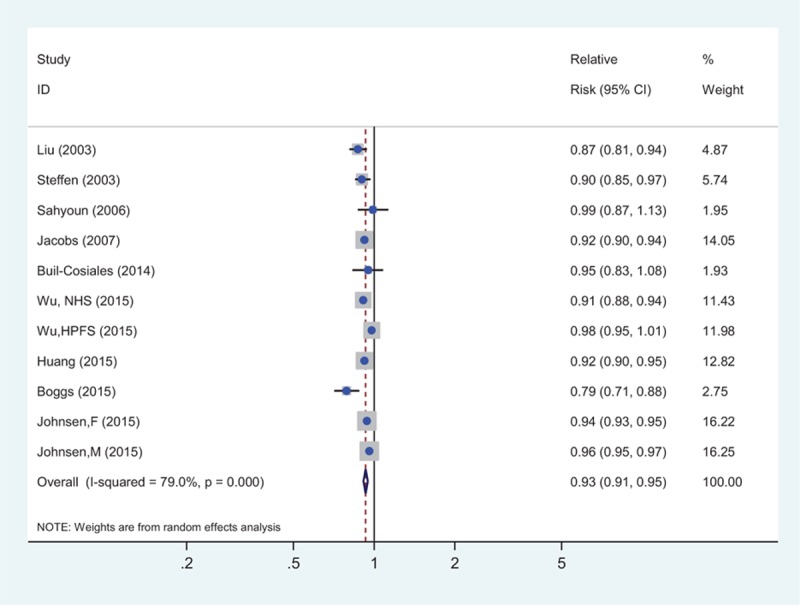
The summary risk association for 1 serving whole grains intake per day and all-cause mortality.
3.4. Cardiovascular disease-specific mortality
Figure 3A showed the pooled analysis for the relation between whole grains consumption and risk of CVD-specific mortality, which was evaluated in 8 studies (7 publications),[17–19,32,34–36] comprising 733,810 participants and 22,811 deaths from CVD. The pooled risk estimation was 0.95 (95% CI: 0.92–0.98) for an increment of 1 serving of whole grains a day, with significant heterogeneity (I2 = 68.6%, Pheterogeneity < 0.001). Both Begg test (P = 0.276; supplementary Figure 1B) and Egger test (P = 0.202) indicated no publication bias.
Figure 3.
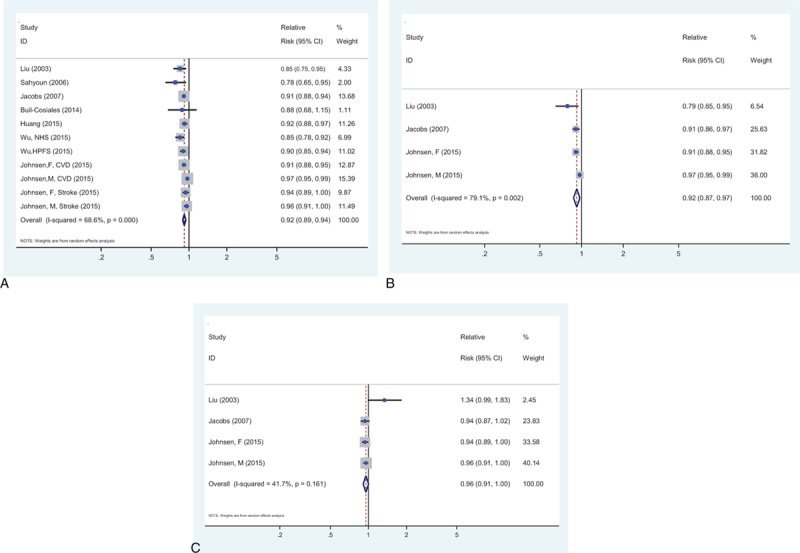
The summary risk association for 1 serving whole grains intake per day and the risk of mortality from (A) cardiovascular disease, (B) coronary heart disease, and (C) stroke.
Figure 3B showed the pooled analysis for the relation between whole grains consumption and risk of CHD-specific mortality, which was evaluated in 3 studies,[17,32,36] comprising 240,532 participants and 2678 deaths from CHD. The pooled risk estimation was 0.92 (95% CI: 0.88–0.97) for every 1 serving of whole grains a day, with significant heterogeneity (I2 = 79.1%, Pheterogeneity = 0.002).
Figure 3C showed the pooled analysis for the relation between whole grains consumption and risk of stroke-specific mortality, which was evaluated in 3 studies,[17,32,36] comprising 240,532 participants and 840 deaths from stroke. The pooled risk estimation was 0.96 (95% CI: 0.91–1.01) for every 1 serving of whole grains a day, with no evidence of significant heterogeneity (I2 = 41.7%, Pheterogeneity = 0.161).
3.5. Diabetes-specific mortality
Figure 4 showed the pooled analysis for the relation between whole grains consumption and risk of diabetes-specific mortality, which was evaluated in 2 studies,[31,35] comprising 487,451 participants and 465 deaths. The definition of diabetes-specific mortality was based on International Classification of Diseases (ICD), 9th and 10th Revision (ICD-9, 250; ICD-10, E10–E14) in 1 article,[35] but not available in the other article.[31] The pooled risk estimation was 0.91 (95% CI: 0.76–1.11) for every 1 serving of whole grains a day, with significant heterogeneity (I2 = 84.0%, Pheterogeneity = 0.002).
Figure 4.
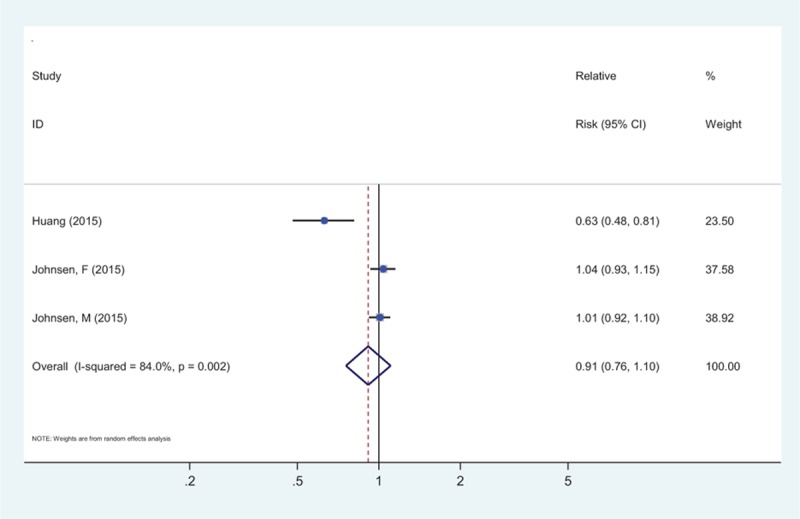
The summary risk association for 1 serving whole grains intake per day and the risk of diabetes-specific mortality.
3.6. Subgroup, meta-regression, and sensitivity analyses
Subgroup analyses were shown in Table 2. For the risk of all-cause mortality, the sex-specific SRR were 0.95 (95% CI: 0.93–0.99; 3 studies) for men and 0.92 (95% CI: 0.89–0.94; 4 studies) for women. The difference in SRRs across gender strata was not significant (P = 0.169). There was no statistically significant difference in the association between the USA (SRR = 0.92, 95% CI: 0.89–0.94) and Europe (SRR = 0.95, 95% CI: 0.93–0.97; Pdifference = 0.268). No significant differences were shown among strata of duration of follow-up (≤14 years: SRR = 0.92, 95% CI: 0.90–0.93; >14 years: SRR = 0.94, 95% CI: 0.91–0.96). Restricting analysis to studies adjusted for diabetes, dyslipidemia, hypertension, dietary fiber intake, and multivitamins intake, respectively, resulted in nearly identical pooled estimates.
Table 2.
Subgroup and meta-regression analyses for the association between whole grains consumption and risk of all-cause and cardiovascular disease mortality.
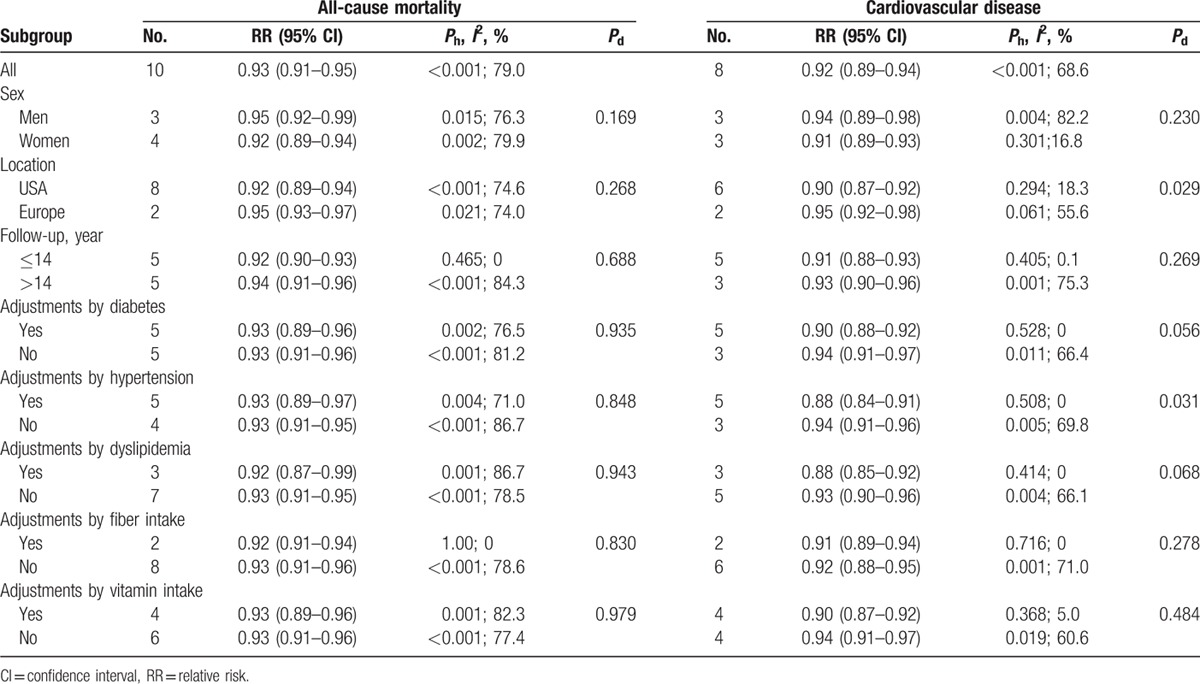
For the risk of CVD-specific mortality, every 1 serving increase in whole grain consumption was associated with a decreased risk of deaths from CVD in both males (SRR = 0.94, 95% CI: 0.89–0.98; n = 3) and females (SRR = 0.91, 95% CI: 0.89–0.93; n = 3). There was statistically significant difference in the association between the USA (SRR = 0.90, 95% CI: 0.87–0.92) and Europe (SRR = 0.95, 95% CI: 0.92–0.98; Pdifference = 0.029). No significant differences were shown among strata of duration of follow-up (≤14 years: SRR = 0.91, 95% CI: 0.88–0.93; >14 years: SRR = 0.93, 95% CI, 0.90–0.96, Pdifference = 0.269). Restricting analysis to studies adjusted for history of diabetes, dyslipidemia, hypertension, dietary fiber intake, and multivitamins intake, respectively, yielded a statistical inverse association with low/no between-study heterogeneity (Table 2). When the overall homogeneity and effect size were calculated by excluding 1 study at a time, the results were not affected by the removal of 1 study (Supplementary Figure 2A and B).
3.7. Nonlinear association
Using a restricted cubic spline model, we observed some evidence of a significant nonlinear association between whole grain intake and the risk of mortality from all-cause (P = 0. 001 for nonlinearity, Supplemental Figure 3A) and CVD (P = 0. 003 for nonlinearity, Supplemental Figure 3B).
4. Discussion
Our meta-analyses of prospective cohort studies confirm an inverse association between whole grain consumption and the risk of all-cause and CVD-specific mortality in both men and women: individuals eating each additional 1 serving of whole grains daily would reduce all-cause mortality by 7% and CVD-specific mortality by 8%. These findings were consistent across subgroup and sensitivity analyses. Furthermore, there was significant association for the risk of mortality from CHD, but no associations for the risk of mortality from stroke and diabetes.
A causal link between whole grains consumption and mortality risk cannot be proven using observational studies. To date, there are many controlled trials that directly investigated the effects of whole-grain interventions on putative intermediate biomarkers.[9,37] For example, results from a clinical trial in North Europe suggested that a greater proportion of whole grain rye in a Nordic diet is associated with favorable outcomes in LDL-cholesterol and log triglyceride concentrations.[9] A randomized cross-over trial suggested that consumption of unfermented and fermented whole grain rye crisp bread led to lower postprandial glucose and insulin response compared with refined wheat crisp bread.[37] All such clinical trials recruited small numbers of participants and were of short-term duration (<1 year). In addition, participants always consumed controlled portions of the foods, but not mimic ordinary daily consumption. Owing to the likely long pathogenesis of CVD and the difficulty in controlling consumption of food over long-enough duration, it is unfeasible to perform long-term randomized trials.
The negative association observed between whole grains intake and mortality risk agrees with previous reports. Greater adherence to a Mediterranean diet, with a relatively large amount of whole grains, has been shown to be related with the lower risk of total mortality and mortality from CVDs.[38,39] Recently, results from a meta-analysis showed that compared with persons who ate the lowest level of whole grains, individuals consuming 48 to 80 g whole grain/day had a 26% lower risk of type 2 diabetes (95% CI: 0.69, 0.80) and 21% lower risk of CVD (95% CI: 0.74, 0.85).[10] These findings were confirmed by Tang et al's meta-analysis,[4] which identified a lower CVD risk of around 21%(95% CI: 0.74–0.83) for high whole grains consumers compared with the lowest consumers.
Because fiber and whole grain consumption are likely to correlate highly, it remains a challenge to examine whether dietary fiber intake is a surrogate marker for whole grain intake and the potential beneficial compounds within whole grains, or whether it is the fiber component of whole grains that confers the protective associations observed with greater intake. In the current meta-analysis, we found that the inverse associations between intake of whole grains and risks of deaths from all-cause and CVD did not change after adjustment for cereal fiber, suggesting that other ingredients may partly account for the whole-grain effects. Furthermore, our subgroup analyses observed that the pooled risk estimations from American studies were significantly lower than those from European studies (P = 0.029). There were differences in classifications and types of whole grains consumption according to geographical locations, which would account for the heterogeneity between study locations. However, these results should be interpreted with caution, as a small number of studies could be included in each subgroup analysis.
Several plausible mechanisms exist for these associations between consumption of whole grains and mortality. Whole grains are good sources of minerals (magnesium, potassium, and calcium), fiber, B-group vitamins, vitamin E, and phytochemicals. Many observational studies have suggested that intake of fiber was inversely associated with risks of several chronic diseases, including CHD,[40] stroke,[41] and certain neoplasms.[42,43] Cereal fiber was able to prevent the oxidation of cholesterol and to increase the formation of endothelial prostacyclin, which may lead to the reduction of vascular tone and inhibition of platelet aggregation.[44] Furthermore, antioxidant phytochemicals, such as phenolic acids and alkylresorcinols, may modulate cellular oxidative status and prevent biologically important molecules such as DNA, proteins, and membrane lipids from oxidative damage.[45] In addition, intakes of magnesium and calcium have been reported to be inversely associated with the risk of mortality[46,47] and several diseases.[48,49]
Our meta-analysis has several strengths. First, this meta-analysis included only prospective cohort studies, which may help minimize potential recall or selection bias. Second, our meta-analysis included a total of 782 thousand subjects and 92 thousand cases of all-cause mortality; we therefore had adequate statistical power to detect moderate associations. Third, the estimates from the fully adjusted models (such as age, sex, smoking, alcohol use, physical activity, BMI, and its intermediate biomarkers) for each study were used in our analyses to reduce the potential of confounding. Moreover, the nonlinear dose–response analysis was conducted, which can help to quantify the associations and test the shape of these possible associations.
Our study has some limitations. First, all but one studies assessed dietary intake by using FFQs, errors in measurement of whole grain intake were inevitable.[50,51] However, the self-administered FFQs used in these studies were validated against multiple diet records, and reasonable correlation coefficients were observed between these assessments of whole grains consumption. In addition, the definition of whole grains differed in some of the studies. For example, 2 studies[16,19] considered whole grain foods using the proportion of whole grain or bran ≥25% as the criterion, and other studies did not show the definition of whole grains. Owing to the prospective study design in the included studies, misclassification of whole grains consumption was independent of study outcome ascertainment, which is more likely to attenuate the associations toward the null.
Second, unmeasured or residual confounding cannot be completely ruled out. All the included studies adjusted for the conventional risk factors, such as smoking, alcohol use, obesity, and physical inactivity, all of which have been shown to increase the risk of all-cause mortality[52] and CVD. However, few studies adjusted for other dietary factors (such as dietary fiber and multivitamins intake, etc.) and for baseline vascular drug use (such as antihypertensives, statins, aspirin, etc.).
Third, significant heterogeneity was observed among studies, which may be explained in part by the inconsistency in study locations, number of cases, baseline exclusion criteria, population demographic and lifestyle characteristics, methods of dietary assessments, and adjustment for confounders. Indeed, in the meta-regression analysis of CVD mortality, studies from the USA showed little heterogeneity, while studies from Europe showed significant heterogeneity.
Fourth, the participants included in our meta-analysis were predominantly from the United States and Europe. Therefore, the results are applicable to the United States and Europe but cannot be extended to other demographic or ethnic groups.
Finally, we could not completely deny the possibility of publication bias. Actually, Egger test did show a significant publication bias for the association between whole grain consumption and all-cause mortality. The application of the trim and fill method did not alter the summary effect size, further suggesting that results were not affected by publication bias.
In conclusion, our meta-analysis provides further evidence that higher intake of whole grains is inversely related to the risk of deaths from all causes, particularly from CVDs. Consumption of whole grains was not associated with risk of stroke and diabetes-specific deaths. The results support current recommendations to increase whole grain consumption to promote health and overall longevity.
Acknowledgments
The authors thank National Natural Scientific Foundation of China (No. 81201780) and Changhai Scientific Foundation of China (No. CH125520706) for the support.
Supplementary Material
Footnotes
Abbreviations: CHD = coronary heart disease, CI = confidence interval, CVD = cardiovascular disease, FFQ = Food Frequency Questionnaire, ICD = International Classification of Diseases, NOS = Newcastle–Ottawa quality assessment scale, RR = relative risk.
BL and GZ contributed equally to this work.
Funding/support: This work was supported by the National Natural Scientific Foundation of China (No. 81201780) and Changhai Scientific Foundation of China (No. CH125520706).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Okarter N, Liu RH. Health benefits of whole grain phytochemicals. Crit Rev Food Sci Nutr 2010; 50:193–208. [DOI] [PubMed] [Google Scholar]
- 2.Rautiainen S, Levitan EB, Orsini N, et al. Total antioxidant capacity from diet and risk of myocardial infarction: a prospective cohort of women. Am J Med 2012; 125:974–980. [DOI] [PubMed] [Google Scholar]
- 3.Fang L, Li W, Zhang W, et al. Association between whole grain intake and stroke risk: evidence from a meta-analysis. Int J Clin Exp Med 2015; 8:16978–16983. [PMC free article] [PubMed] [Google Scholar]
- 4.Tang G, Wang D, Long J, et al. Meta-analysis of the association between whole grain intake and coronary heart disease risk. Am J Cardiol 2015; 115:625–629. [DOI] [PubMed] [Google Scholar]
- 5.Chanson-Rolle A, Meynier A, Aubin F, et al. Systematic review and meta-analysis of human studies to support a quantitative recommendation for whole grain intake in relation to type 2 diabetes. PLoS One 2015; 10:e0131377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seal CJ, Brownlee IA. Whole-grain foods and chronic disease: evidence from epidemiological and intervention studies. Proc Nutr Soc 2015; 74:313–319. [DOI] [PubMed] [Google Scholar]
- 7.Aune D, Chan DS, Lau R, et al. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ 2011; 343:d6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agurs-Collins T, Rosenberg L, Makambi K, et al. Dietary patterns and breast cancer risk in women participating in the Black Women's Health Study. Am J Clin Nutr 2009; 90:621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magnusdottir OK, Landberg R, Gunnarsdottir I, et al. Whole grain rye intake, reflected by a biomarker, is associated with favorable blood lipid outcomes in subjects with the metabolic syndrome – a randomized study. PLoS One 2014; 9:e110827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye EQ, Chacko SA, Chou EL, et al. Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain. J Nutr 2012; 142:1304–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pereira MA, Jacobs DR, Jr, Pins JJ, et al. Effect of whole grains on insulin sensitivity in overweight hyperinsulinemic adults. Am J Clin Nutr 2002; 75:848–855. [DOI] [PubMed] [Google Scholar]
- 12.Katz DL, Nawaz H, Boukhalil J, et al. Effects of oat and wheat cereals on endothelial responses. Prev Med 2001; 33:476–484. [DOI] [PubMed] [Google Scholar]
- 13.Buyken AE, Goletzke J, Joslowski G, et al. Association between carbohydrate quality and inflammatory markers: systematic review of observational and interventional studies. Am J Clin Nutr 2014; 99:813–833. [DOI] [PubMed] [Google Scholar]
- 14.Fardet A. New hypotheses for the health-protective mechanisms of whole-grain cereals: what is beyond fibre? Nutr Res Rev 2010; 23:65–134. [DOI] [PubMed] [Google Scholar]
- 15.Jonnalagadda SS, Harnack L, Liu RH, et al. Putting the whole grain puzzle together: health benefits associated with whole grains – summary of American Society for Nutrition 2010 Satellite Symposium. J Nutr 2011; 141:1011S–1022S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steffen LM, Jacobs DR, Jr, Stevens J, et al. Associations of whole-grain, refined-grain, and fruit and vegetable consumption with risks of all-cause mortality and incident coronary artery disease and ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr 2003; 78:383–390. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs DR, Jr, Andersen LF, Blomhoff R. Whole-grain consumption is associated with a reduced risk of noncardiovascular, noncancer death attributed to inflammatory diseases in the Iowa Women's Health Study. Am J Clin Nutr 2007; 85:1606–1614. [DOI] [PubMed] [Google Scholar]
- 18.Wu H, Flint AJ, Qi Q, et al. Association between dietary whole grain intake and risk of mortality: two large prospective studies in US men and women. JAMA Intern Med 2015; 175:373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sahyoun NR, Jacques PF, Zhang XL, et al. Whole-grain intake is inversely associated with the metabolic syndrome and mortality in older adults. Am J Clin Nutr 2006; 83:124–131. [DOI] [PubMed] [Google Scholar]
- 20.He M, van Dam RM, Rimm E, et al. Whole-grain, cereal fiber, bran, and germ intake and the risks of all-cause and cardiovascular disease-specific mortality among women with type 2 diabetes mellitus. Circulation 2010; 121:2162–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Accessed June 15, 2012]. [Google Scholar]
- 23.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188. [DOI] [PubMed] [Google Scholar]
- 24.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 1992; 135:1301–1309. [DOI] [PubMed] [Google Scholar]
- 25.Orsini NBR, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata J 2006; 6:40–57. [Google Scholar]
- 26.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Royston P. A strategy for modelling the effect of a continuous covariate in medicine and epidemiology. Stat Med 2000; 19:1831–1847. [DOI] [PubMed] [Google Scholar]
- 28.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50:1088–1101. [PubMed] [Google Scholar]
- 29.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000; 56:455–463. [DOI] [PubMed] [Google Scholar]
- 31.Johnsen NF, Frederiksen K, Christensen J, et al. Whole-grain products and whole-grain types are associated with lower all-cause and cause-specific mortality in the Scandinavian HELGA cohort. Br J Nutr 2015; 114:608–623. [DOI] [PubMed] [Google Scholar]
- 32.Liu S, Sesso HD, Manson JE, et al. Is intake of breakfast cereals related to total and cause-specific mortality in men? Am J Clin Nutr 2003; 77:594–599. [DOI] [PubMed] [Google Scholar]
- 33.Boggs DA, Ban Y, Palmer JR, et al. Higher diet quality is inversely associated with mortality in African-American women. J Nutr 2015; 145:547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buil-Cosiales P, Zazpe I, Toledo E, et al. Fiber intake and all-cause mortality in the Prevencion con Dieta Mediterranea (PREDIMED) study. Am J Clin Nutr 2014; 100:1498–1507. [DOI] [PubMed] [Google Scholar]
- 35.Huang T, Xu M, Lee A, et al. Consumption of whole grains and cereal fiber and total and cause-specific mortality: prospective analysis of 367,442 individuals. BMC Med 2015; 13:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnsen NF, Frederiksen K, Christensen J, et al. Whole-grain products and whole-grain types are associated with lower all-cause and cause-specific mortality in the Scandinavian HELGA cohort. Br J Nutr 2015; 1–16. [DOI] [PubMed] [Google Scholar]
- 37.Johansson DP, Lee I, Riserus U, et al. Effects of unfermented and fermented whole grain rye crisp breads served as part of a standardized breakfast, on appetite and postprandial glucose and insulin responses: a randomized cross-over trial. PLoS One 2015; 10:e0122241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fung TT, Rexrode KM, Mantzoros CS, et al. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation 2009; 119:1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tektonidis TG, Akesson A, Gigante B, et al. A Mediterranean diet and risk of myocardial infarction, heart failure and stroke: a population-based cohort study. Atherosclerosis 2015; 243:93–98. [DOI] [PubMed] [Google Scholar]
- 40.Wu Y, Qian Y, Pan Y, et al. Association between dietary fiber intake and risk of coronary heart disease: a meta-analysis. Clin Nutr 2015; 34:603–611. [DOI] [PubMed] [Google Scholar]
- 41.Threapleton DE, Greenwood DC, Evans CE, et al. Dietary fiber intake and risk of first stroke: a systematic review and meta-analysis. Stroke 2013; 44:1360–1368. [DOI] [PubMed] [Google Scholar]
- 42.Ben Q, Sun Y, Chai R, et al. Dietary fiber intake reduces risk for colorectal adenoma: a meta-analysis. Gastroenterology 2014; 146:689–699.e686. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Z, Xu G, Ma M, et al. Dietary fiber intake reduces risk for gastric cancer: a meta-analysis. Gastroenterology 2013; 145:113–120.e113. [DOI] [PubMed] [Google Scholar]
- 44.Sanchez-Muniz FJ. Dietary fibre and cardiovascular health. Nutr Hosp 2012; 27:31–45. [DOI] [PubMed] [Google Scholar]
- 45.Kim YJ, Ahn YH, Lim Y, et al. Daily nutritional dose supplementation with antioxidant nutrients and phytochemicals improves DNA and LDL stability: a double-blind, randomized, and placebo-controlled trial. Nutrients 2013; 5:5218–5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guasch-Ferre M, Bullo M, Estruch R, et al. Dietary magnesium intake is inversely associated with mortality in adults at high cardiovascular disease risk. J Nutr 2014; 144:55–60. [DOI] [PubMed] [Google Scholar]
- 47.Levitan EB, Shikany JM, Ahmed A, et al. Calcium, magnesium and potassium intake and mortality in women with heart failure: the Women's Health Initiative. Br J Nutr 2013; 110:179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu T, Chen GC, Zhai L, et al. Nonlinear reduction in risk for type 2 diabetes by magnesium intake: an updated meta-analysis of prospective cohort studies. Biomed Environ Sci 2015; 28:527–534. [DOI] [PubMed] [Google Scholar]
- 49.Asemi Z, Saneei P, Sabihi SS, et al. Total, dietary, and supplemental calcium intake and mortality from all-causes, cardiovascular disease, and cancer: a meta-analysis of observational studies. Nutr Metab Cardiovasc Dis 2015; 25:623–634. [DOI] [PubMed] [Google Scholar]
- 50.Keogh RH, White IR, Rodwell SA. Using surrogate biomarkers to improve measurement error models in nutritional epidemiology. Stat Med 2013; 32:3838–3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boeing H. Nutritional epidemiology: new perspectives for understanding the diet-disease relationship? Eur J Clin Nutr 2013; 67:424–429. [DOI] [PubMed] [Google Scholar]
- 52.Ekelund U, Ward HA, Norat T, et al. Physical activity and all-cause mortality across levels of overall and abdominal adiposity in European men and women: the European Prospective Investigation into Cancer and Nutrition Study (EPIC). Am J Clin Nutr 2015; 101:613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


