Supplemental Digital Content is available in the text
Keywords: HCV, health-related quality of life, interferon, ledipasvir, ribavirin, sofosbuvir
Abstract
The interferon (IFN)-free regimens for chronic hepatitis C (CHC) have high efficacy and superior health-related quality of life (HRQOL) in European/North American patients. The impact of these regimens on HRQOL of the Japanese CHC patients is not known.
The Short Form-36 was administered before, during, and after treatment to CHC patients with genotype 1 treated with ledipasvir/sofosbuvir ± ribavirin (LDV/SOF ± RBV) for 12 weeks and genotype 2 treated with SOF + RBV for 12 weeks in clinical trials. The HRQOL data were analyzed with reference to treatment regimens and clinical factors.
A total of 494 CHC patients were included (19% cirrhotic, 69% genotype 1, 52% treatment-naive; 153 received SOF + RBV, 170 received LDV/SOF + RBV, 171 received LDV/SOF). The sustained virologic response-12 rates for these regimens were 97%, 98%, and 100%, respectively. CHC patients treated with LDV/SOF, SOF + RBV, or LDV/SOF + RBV regimens had similar HRQOL scores at baseline. During treatment, more adverse events were experienced by those treated with RBV-containing regimens (46% vs 22%, P < 0.0001). The decrements in HRQOL were also significant in RBV groups: up to −3.8 points (treatment week-4), −5.2 (treatment week-12), and −3.2 (posttreatment week-12) (all P < 0.001). In contrast, RBV-free regimen (LDV/SOF) was associated with an improvement in HRQOL up to +4.1 points throughout the treatment (P < 0.01). In multivariate analysis, the use of RBV was independently associated with lower HRQOL during and after treatment (beta up to −6.4 points, P = 0.0001).
Japanese CHC patients treated with RBV-containing regimens show mild HRQOL impairment. In contrast, patients treated with LDV/SOF not only showed high efficacy but also improvement of HRQOL.
1. Introduction
Hepatitis C virus (HCV) is the most common cause of chronic liver disease in Japan.[1] The HCV infection can lead to cirrhosis and hepatocellular carcinoma. In fact, HCV is considered to be the most common (70%) etiologic cause of hepatocellular carcinoma which is, in turn, the 3rd most common cause of solid cancer in Japan.[2] Historically, treatment has been interferon (IFN)-based which for HCV genotype 1 was not only associated with relatively low efficacy but also significant side effects.[3]
In addition to the liver complications of HCV infection, it is important to recognize that HCV is a systemic disease with both hepatic and extrahepatic manifestations such as fatigue, mixed type 2 cryoglobulinemia, and type 2 diabetes.[4] Both the hepatic and extrahepatic manifestations of HCV infection lead to significant adverse clinical outcomes as well as economic burden and negative patient-reported outcomes (PROs). It is also important to note that IFN-related side effects cause significant fatigue and other negative impairment of PROs.[5,6] Although the clinical outcomes of HCV infection in Japanese patients have been widely studied, the impact of HCV and its treatment on PROs have not been studied.
In recent years, treatment of HCV has been revolutionized with the development of highly effective all-oral direct-acting antiviral agents.[7–17] These regimens are associated with high efficacy as well as improvement of PROs.[18–27] Most of patients enrolled in the initial clinical trials reporting improvement of PROs were enrolled from North America and Europe.[7–27] Recently, the efficacy of IFN-free regimen with sofosbuvir (SOF) has been demonstrated in Japanese patients with HCV infection.[28,29] The aim of this study is to assess the impact of a SOF-based regimen on PRO of HCV patients and compare changes of PROs in Japanese patients with HCV to Caucasian patients with HCV treated with the same regimens.
2. Methods
The source of data were 2 multicenter phase 3 clinical trials of SOF-based regimens for the treatment of chronic hepatitis C (CHC, GS-US-337-0113 and GS-US-337-0118) which were conducted in Japan (2013–2015). Treatment-naive or -experienced patients with or without compensated cirrhosis were enrolled to receive SOF + ribavirin (RBV) for HCV genotype 2 or ledipasvir (LDV)/SOF ± RBV for HCV genotype 1 for 12 weeks.
The PRO scores (health-related quality of life [HRQOL]) were assessed as a secondary endpoint in both trials. From medical history collected at screening for all enrolled participants, we identified patients with pretreatment history of depression or mood disorders, clinically overt fatigue, anxiety or panic disorders, insomnia or sleep disorders, as well as type 2 diabetes or hyperglycemia. Baseline hemoglobin, HCV RNA, alanine aminotransferase (ALT), HCV genotype, and presence of cirrhosis were recorded. Treatment-related adverse events (according to investigator reporting) were grouped based on the organ or body system as previously described.[8]
Patients with detectable HCV RNA at posttreatment week 4 were not followed up at subsequent visits. Patients were considered to have achieved sustained virologic response (SVR-12) if they had undetectable HCV RNA at posttreatment week 12.
2.1. Health-related quality of life
In the clinical trials, the Short Form-36 version 2 (SF-36v2) questionnaire was administered at multiple time points before, during, and after treatment to participants in their native language. The SF-36 instrument is extensively validated and has been widely used for obtaining a self-reported assessment of HRQOL from patients in various settings including clinical trials.[30] The QualityMetric Health Outcome Scoring Software v.4.5 (QualityMetric, Lincoln, RI) with the maximum data recovery and 2009 U.S. norms was used for analysis.
The SF-36 instrument includes questions about patients’ health status, perception of well-being, and daily functioning, which are then used to calculate 8 domain scores. Domain scores range across 0 to 100 with higher scores indicating less disability: physical functioning (PF), role physical (RP), bodily pain (BP), general health (GH), vitality (VT), social functioning (SF), role emotional (RE), and mental health (MH). The 2 summary scores, Physical Component Summary Score (PCS) and Mental Component Summary score (MCS), summarize the physical health and MH-related components of SF-36. The summary scores are calculated as a weighted average of the domain scores linearly transformed using the population norms to the mean of 50 and a standard deviation of 10.[30]
2.2. Statistical analysis
At the 1st round of HRQOL analysis, the baseline HRQOL scores of Japanese patients were summarized and compared across the treatment regimens using Chi-square test or Kruskal–Wallis nonparametric test. The changes (decrements or improvements) in HRQOL scores from patients’ own baseline levels were calculated for each patient at each studied time point. The median changes were further compared to zero by a sign rank test for matched pairs; the null hypothesis would be the absence of a significant change from baseline. Only P-values of 0.05 or less were considered statistically significant.
Also, in Japanese patients, the independent predictors of their HRQOL summary scores at multiple time points were assessed in a series of multiple linear regressions with the treatment regimens being tested as a potential predictor. Bidirectional stepwise selection of predictors with the significance level of 0.05 for staying in the model was used. The list of potential HRQOL predictors used for the selection procedure included age, gender, body mass index (BMI), history of psychiatric disorders and type 2 diabetes, baseline hemoglobin, HCV RNA and ALT (at baseline only), cirrhosis, and history of prior anti-HCV treatment.
At the 2nd round of analysis, we selected matched controls of non-Asian ancestry from the participants of other phase 3 clinical trials treated with the same regimens who were enrolled in the US.[18–27] Matching was performed by age, gender, BMI, anti-HCV treatment history, the presence of cirrhosis, history of type 2 diabetes, and sleep disorders using a propensity score. A maximum weight bipartite matching algorithm was applied to the propensity scores to ensure that each case is matched to its unique control; then, only case–control pairs with the difference in their propensity scores of less than 0.05 were kept. Due to the extremely low prevalence of depression, anxiety, and fatigue in Japanese patients, both Japanese and American patients with history of these conditions were excluded from matching. The baseline HRQOL scores and the changes in those from baseline during and after treatment with RBV-containing and RBV-free regimens were then compared between the Japanese patients and their matched American controls using the statistical methods describe above.
All analyses were run in SAS 9.3 (SAS Institute, Cary, NC). Both studies (334-0118 and 337-0113) were separately approved by each site's Institutional Review Board.
3. Results
There were 494 Japanese HCV patients enrolled in GS-US-337-0113 and GS-US-334-0118. Of those, 153 patients with HCV genotype 2 received SOF + RBV for 12 weeks, and 341 patients with HCV genotype 1 were randomly assigned to receive either LDV/SOF + RBV (N = 170) or RBV-free LDV/SOF (N = 171) for 12 weeks.
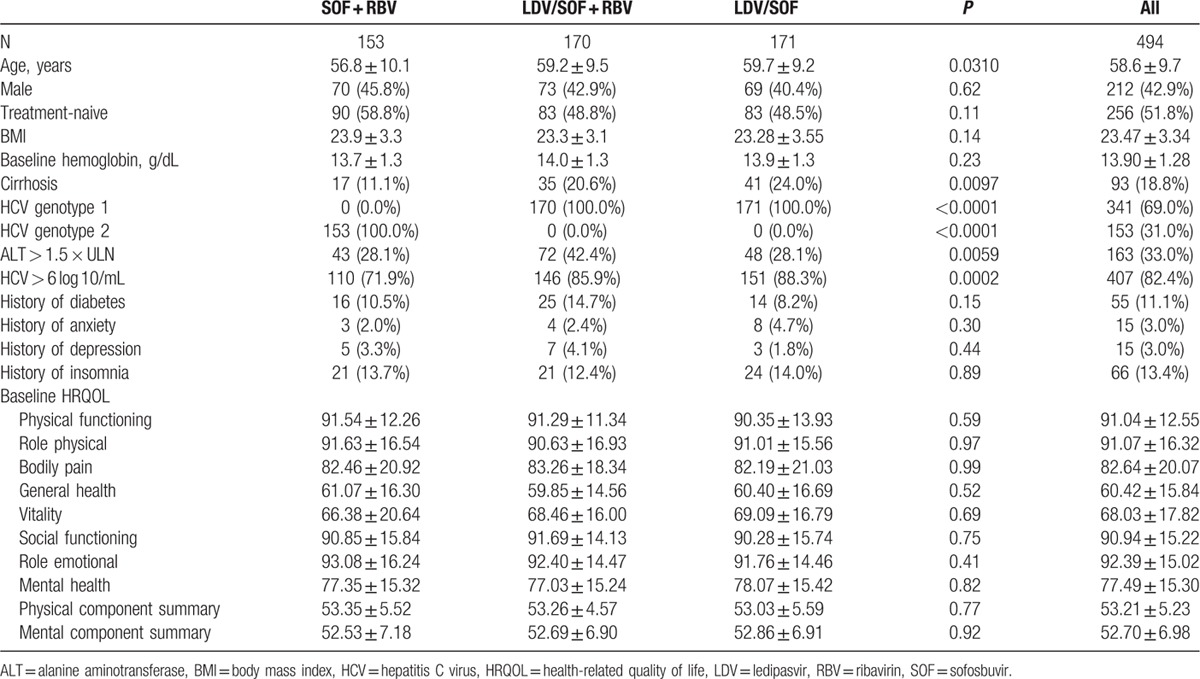
In addition to the genotype-specific treatment assignment, in patients receiving LDV/SOF with or without RBV, at baseline there was a higher proportion of patients with cirrhosis and higher levels of HCV RNA and ALT. These patients were also older than those who were assigned to receive SOF + RBV (P < 0.05). All other baseline demographic and clinical parameters were similar across the 3 treatment regimens (all P > 0.05) (Table 1). In particular, in all Japanese patients, unlike other populations used in other previously reported clinical trials of SOF-based regimens (North America, Europe, Australia, and New Zealand),[18–27] reported very low rate of “overt psychiatric disorders” (depression, anxiety) and clinically overt fatigue. This is in contrast to the baseline PRO domain scores which showed that, as compared to US patients with CHC, Japanese patients with CHC had similar score for VT domain of SF-36 and lower scores for MH and RE domains of SF-36.
Table 1.
Baseline clinico-demographic parameters and HRQOL scores of the participants of GS-US-337-0113 and GS-US-334-0118.
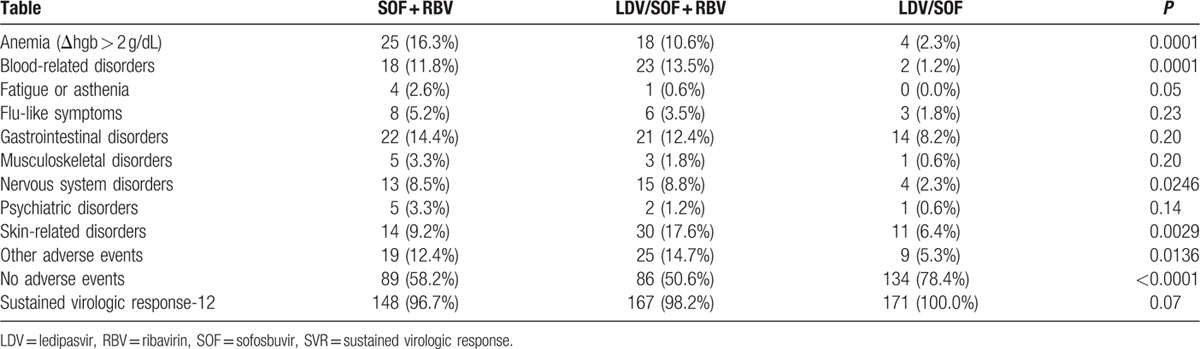
The rates of adverse events were different between RBV-free and RBV-containing regimens, including higher rates of treatment-related anemia, nervous system disorders (primarily headache), and other disorders in patients receiving SOF + RBV with or without LDV (Table 2). Additionally, in patients receiving RBV-containing regimens, there was a higher rate of skin and subcutaneous tissue-related disorders (which included predominantly pruritus and rash) in those receiving LDV/SOF + RBV than in SOF + RBV (P = 0.026). The rates of all other studied disorders were similar across the regimens, ranging from 1.0% for treatment-related fatigue to 11.5% for gastrointestinal disorders (all P > 0.05). At the same time, 78.4% of patients receiving RBV-free LDV/SOF versus 54.2% of patients receiving RBV-containing regimens experienced no treatment-related adverse events (P < 0.0001).
Table 2.
Treatment-related adverse events and SVR during treatment in GS-US-337-0113 and GS-US-334-0118.
3.1. Health-related quality of life in Japanese patients during treatment with and without RBV
Baseline HRQOL scores of Japanese patients are included in Table 1. There was no difference between the treatment regimens (all P > 0.05).
During treatment, soon after its start (week 4), there was a decrease in some SF-36 domains in patients receiving RBV-containing regimens, including PF, RP, SF, and RE by −1.4 to −3.8 points. The only HRQOL score that increased in those patients was the GH score: +2.4 points (all P < 0.002). In contrast, significant increases were noted in patients receiving RBV-free LDV/SOF, including the domains of BP, GH, VT, MH (from +2.9 to +4.1 points), and both PCS (+1.84) and MCS (+2.68) (all P < 0.05).
By the end of treatment (week 12), both HRQOL decrements in the RBV-containing group and improvements in the RBV-free group became more prominent. Indeed, the average decreases in PF, RP, SF, and RE ranged from −1.2 to −5.2 points in SOF + RBV ± LDV (all P < 0.02) accompanied by the only increase in GH (+3.1 points, P < 0.0001) in that treatment group. At the same time, the increases in LDV/SOF were from +3.1 to +4.1 points (for BP, GH, VT, MH; all P < 0.01), and no decreases were noted at this treatment group at this or any prior time point (Fig. 1A).
Figure 1.
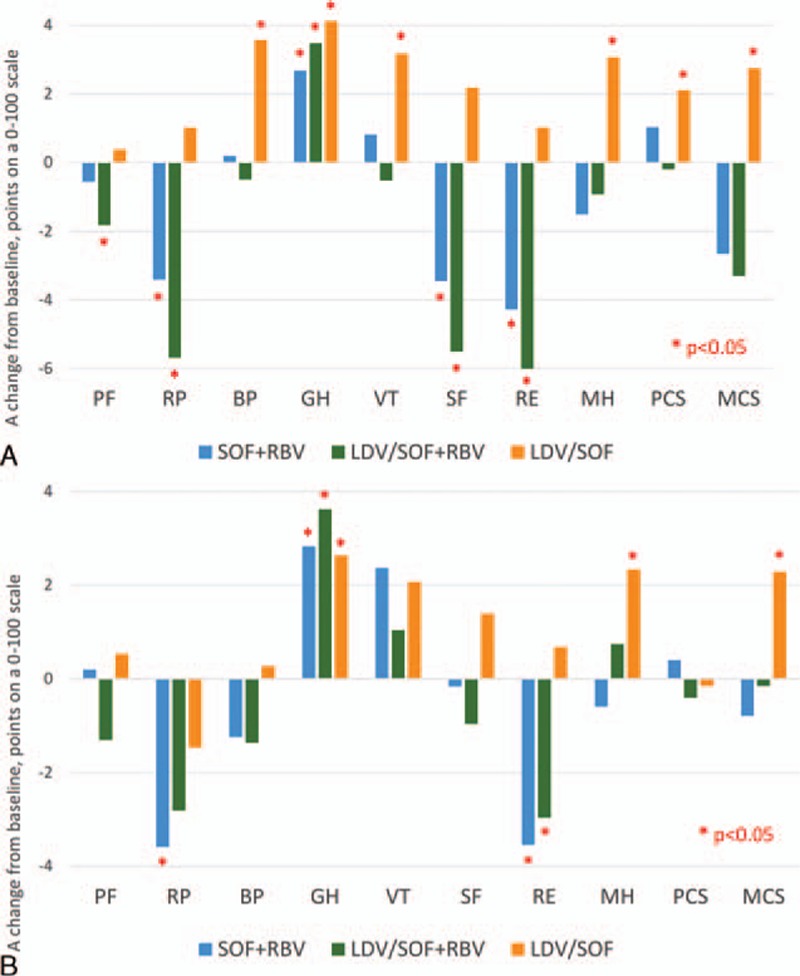
Treatment-emergent (A) and posttreatment week 12 (B) changes in HRQOL scores in Japanese patients treated with different anti-HCV regimens. A positive change indicates improvement of HRQOL. HCV = hepatitis C virus, HRQOL = health-related quality of life.
Soon after treatment cessation (follow-up week 4), HRQOL decrements in the RBV-containing group became smaller in magnitude but still significant for RP, SF, and RE (−2.6 to −3.5 points, all P < 0.025). Later in follow-up (week 12), in patients with SVR, the decrements in PF, RP, and RE were still significant in patients who completed an RBV-containing regimen while the decrement in SF was no longer significant, and there was also a moderate improvement in VT (+1.7 points, P = 0.04). Posttreatment HRQOL improvements in patients who completed the RBV-free LDV/SOF regimen remained at the level observed at the end of treatment (Fig. 1B).
Independent predictors of lower HRQOL summary scores in Japanese patients at most of the studied time points included older age (−0.05 to −0.09 points per each additional year of age to PCS), female gender (−1.3 to points to −2.1 points to PCS), the presence of cirrhosis (−1.4 to −1.7 points to PCS only), and history of insomnia or sleep disorders (−1.5 to −6.4 points to PCS and MCS) (all P < 0.04). After adjustment, the use of an RBV-containing treatment regimen was independently associated with −1.7 to −3.1 points to MCS at multiple time points during treatment (all P < 0.04) (Supplementary Table 1). There was no association of the treatment regimen used with the HRQOL scores after treatment cessation (all P > 0.05).
3.2. Comparison of HRQOL between American and Japanese patients before, during, and after treatment with RBV-containing regimens
Of 494 Japanese patients enrolled in GS-US-337-0113 and GS-US-334-0118, only 346 had matched controls that were enrolled in the U.S. to receive similar treatment (SOF + RBV, LDV/SOF + RBV, or RBV-free LDV/SOF). These patients were matched by age, gender, BMI, the presence of cirrhosis, history of diabetes, and history of prior HCV treatment (Table 3).
Table 3.
Comparison of HRQOL in Japanese patients and American matched controls.
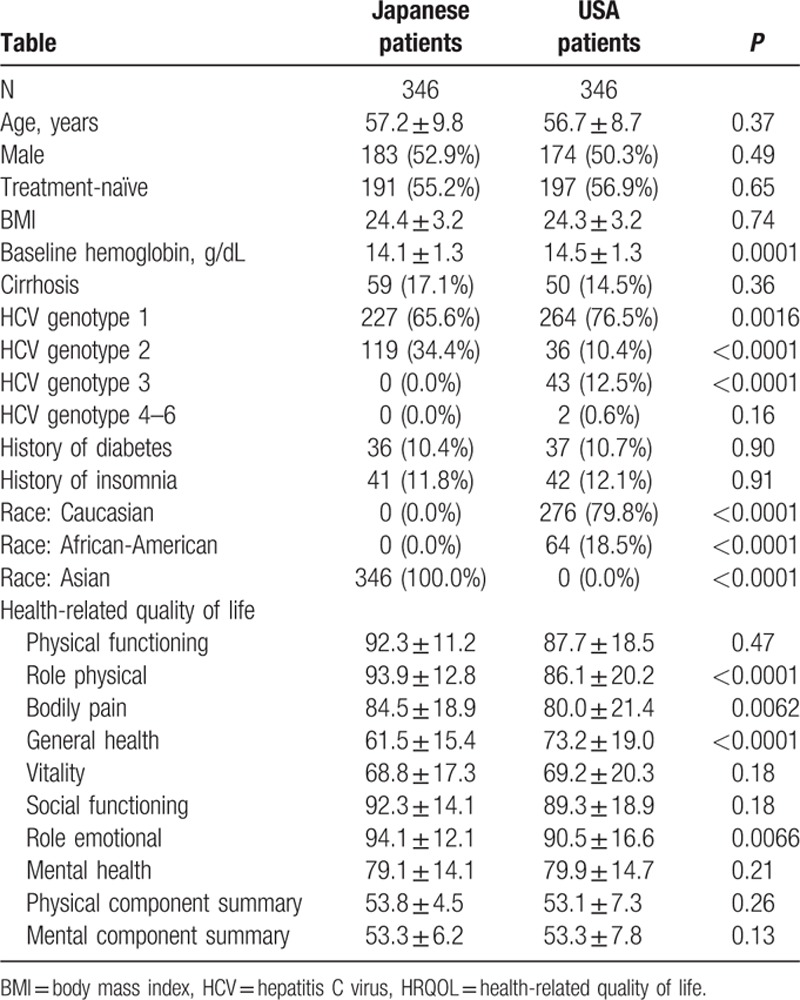
A number of the baseline physical health-related HRQOL scores of Japanese patients were significantly higher in comparison to the American HCV controls (RP, BP, and RE; Table 3). The greatest difference was observed for the RP score which was, on average, 7.8 points higher in Japanese patients (P < 0.0001). Despite this, the GH score was significantly lower in HCV patients who were enrolled in Japan (by −11.8 points, P < 0.0001) (Table 3).
During treatment with RBV-containing regimens, the rates of nearly all treatment-related adverse events were significantly higher in American patients with the only exceptions of treatment-related anemia and flu-like symptoms, which were reported at similar rates in both groups (Table 4). The greatest difference was observed in the rates of recorded treatment-related fatigue and psychiatric disorders. In fact, only 28.0% of American patients versus 56.8% of Japanese had no treatment-related side effects recorded during treatment with RBV-containing regimens (P < 0.0001).
Table 4.
Treatment-related adverse events during treatment with and without RBV in Japanese and American patients.
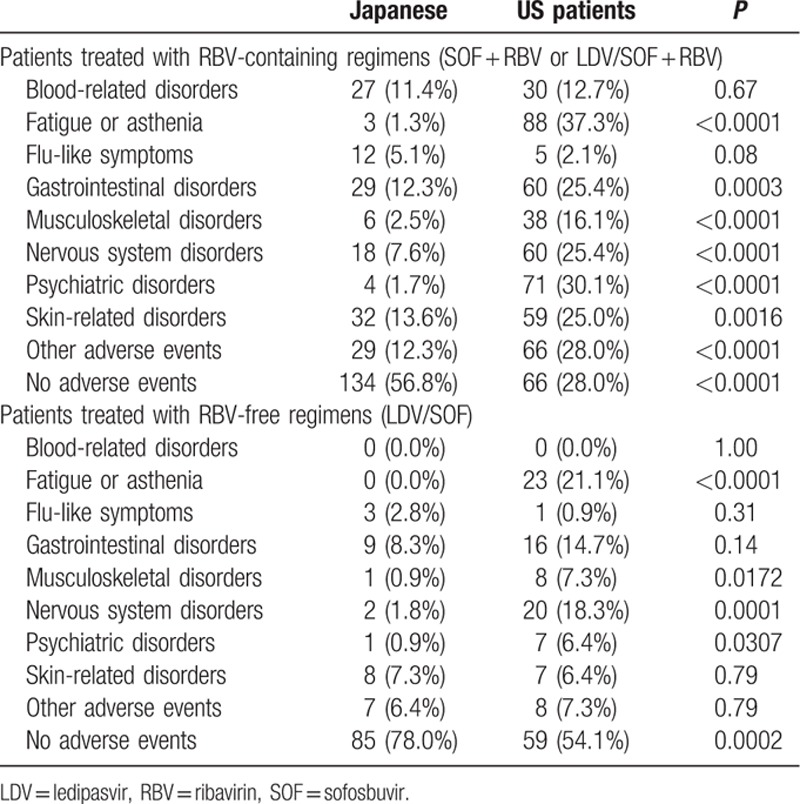
Consistent with substantially greater rates of recorded adverse events in American patients, the decrements in HRQOL during treatment with RBV-containing regimens also differed between Japanese and American patients (Fig. 2A). Indeed, for PF and VT scores and their derived PCS score, the decrements were significant in American (those ranged from −5.1 to −2.0 points on average, respectively, all P < 0.05) but not in Japanese patients (on average, −1.1 to +1.0 points, all P > 0.05).
Figure 2.
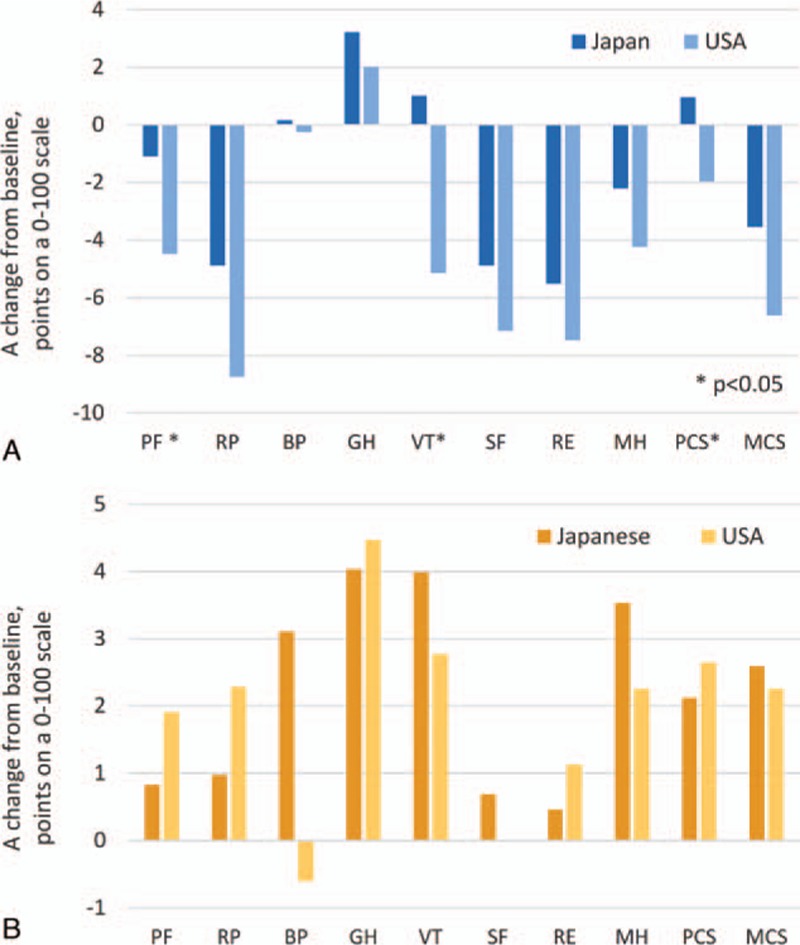
Treatment-emergent HRQOL changes in Japanese and American patients treated with interferon-free RBV-containing regimens (A) and with RBV-free regimens (B). A positive change indicates improvement of HRQOL. HRQOL = health-related quality of life, RBV = ribavirin.
Despite this, post-SVR improvements in HRQOL scores after treatment with RBV-containing regimens were more prominent in American patients (Fig. 3A). In fact, there were no residual decrements in HRQOL observed in American patients with SVR by follow-up week 12, and significant improvements were noted in RP (+3.6), GH (+4.1), VT (+6.4), MH (+1.3), and both PCS (+3.8) and MCS (+2.1) (all P < 0.05). In contrast, in Japanese patients, significant decrements in RP and RE (−3.8 and −3.2 points, respectively) were noted, while improvements were observed in GH and VT only (+3.4 to +2.2 points, respectively) (all P < 0.05).
Figure 3.
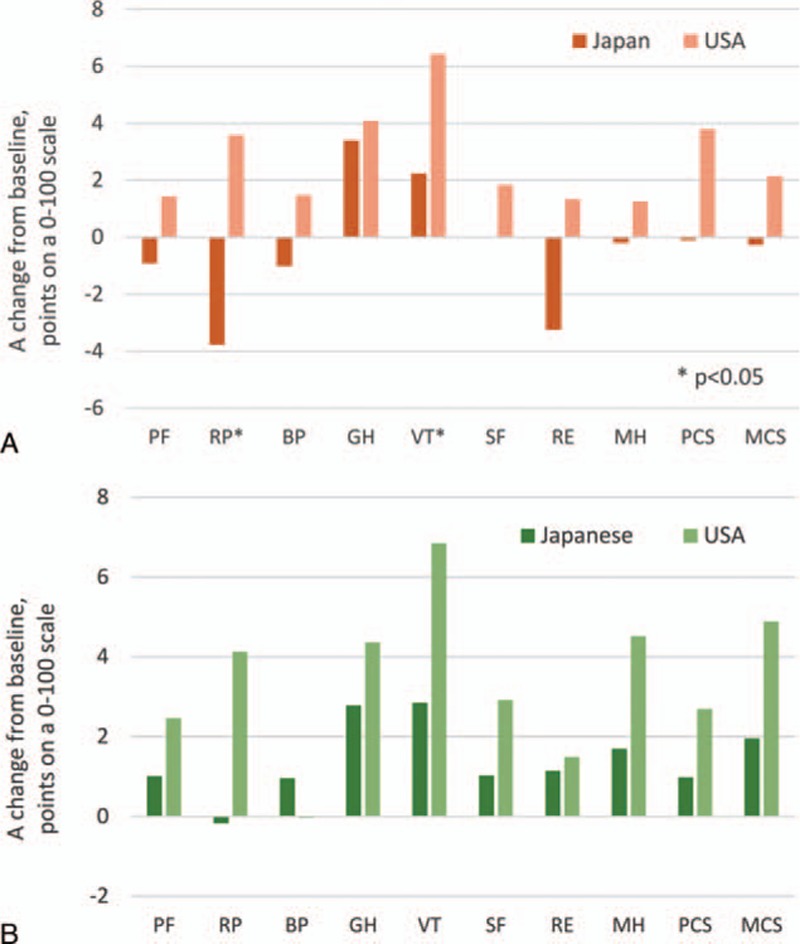
Post-SVR HRQOL changes in Japanese and American patients treated with interferon-free RBV-containing regimens (A) and with RBV-free regimens (B). A positive change indicates improvement of HRQOL. HRQOL = health-related quality of life, RBV = ribavirin, SVR = sustained virologic response.
Similar to RBV-containing regimens, in patients treated with RBV-free regimens, the rates of treatment-related adverse events were significantly higher in American patients, primarily in the domains of nervous system disorders and fatigue (Table 3). Indeed, only 54.1% of American controls did not experience any treatment-related adverse events during treatment with LDV/SOF versus 78.0% of Japanese patients (P = 0.0002).
Similarly to those treated with RBV-containing regimens, in the RBV-free LDV/SOF group, the treatment-emergent changes in HRQOL followed the trend of treatment-related adverse events. Indeed, improvements in HRQOL scores in Japanese patients during treatment tended to be more prominent in comparison to their American controls (Fig. 2B). Those included significant improvements in GH and VT by treatment week 4 (by +3.1 and +3.3 points, respectively), and the same domain scores plus MH and both summary scores PCS and MCS by treatment week 12 (+2.1 to +4.0 points) (all P < 0.05). Improvements observed in American controls by the end of treatment with RBV-free LDV/SOF were substantially less pronounced (all P > 0.025 except for GH where an average improvement was +4.5, P = 0.0002).
However, similarly to the RBV-containing arm, after cessation of treatment with LDV/SOF and after achieving SVR-12, the changes from baseline in HRQOL scores became more prominent in American patients (Fig. 3B). In fact, there were no substantial improvement of HRQOL scores in Japanese patients in comparison to the end-of-treatment time point (all P > 0.05) while improvements in American patients were substantial (+2.5 to +6.9 points from baseline in all but 2 HRQOL domains and both summary scores; all P < 0.05).
4. Discussion
This is the first study providing in-depth assessment of HRQOL in Japanese patients with CHC treated with IFN-free regimens. The data analysis provides evidence for very high efficacy of IFN-free regimens in Japanese patients with CHC. Furthermore, after 12 weeks of treatment, SVR-12 for HCV genotype 2 who were treated with SOF + RBV was 96.7% while SVR-12 for HCV genotype 1 who were treated with LDV/SOF + RBV was 98% and those who were treated with LDV/SOF was 100%. In addition to clinical efficacy (SVR), we also show the impact of these regimens on patients’ HRQOL. As previously indicated, RBV-containing regimens (SOF + RBV or LDV/SOF + RBV) cause mild and reversible decrement in HRQOL during treatment. Nevertheless, our multivariate analysis demonstrated that in addition to previously described predictors of HRQOL, RBV containing regimens were independently associated with some aspects of HRQOL impairment.[18–27]
In contrast, IFN- and RBV-free regimens (LDV/SOF) lead to the improvement of HRQOL during treatment. In fact, this is the 1st study showing improvement of HRQOL during the treatment of Japanese patients with HCV infection.
Another interesting finding of our study was the difference of HRQOL in HCV patients enrolled from the U.S. and those who were enrolled from Japan. Our data show some differences in HRQOL domains. Although Japanese patients with HCV had less impairment in the areas of physical health and functioning, they still reported more impairment in their GH. This is in contrast to clinically overt fatigue and psychiatric disorders before treatment and during treatment which were significantly lower in Japanese patients with CHC. It is important to note the clinical history was based on what was reported by clinicians and patients while HRQOL data were based on a validated questionnaire. These inconsistencies between HRQOL scores and clinical reports in the 2 cohorts of patients with CHC may be based on cultural differences including how patients may express themselves to their caregivers and how caregivers may interpret the symptoms. Future studies are needed to explore the validity of these differences and whether this could lead to different strategies for the treatment of CHC in Japanese patients.
Finally, our data show that regardless of the regimen and country of enrollment, SVR-12 results in the improvement of HRQOL domains. Nevertheless, these improvements of HRQOL are most prominent in those patients who received IFN- and RBV-free regimens with LDV/SOF.
The limitations of this study include a relatively short follow-up, a very small number of patients without SVR which did not allow to us evaluate its effect on posttreatment PROs, and unclear generalizability of the results of these clinical trials to the rest of HCV population. It is possible that some aspects of PROs may be influenced by cost and access to treatment regimens. This issue requires additional research. In this analysis, we also were not able to study the effect of this treatment in important HCV subpopulations such as those with decompensated cirrhosis, hepatitis B virus or human immunodeficiency virus coinfection, postliver transplant patients, and patients with clinically significant nonhepatic comorbidities; in all these patients, PROs may be different from those in patients enrolled in these clinical trials who were required to be otherwise healthy.
In summary, our data demonstrate that IFN-free regimens for the treatment of Japanese patients with HCV infection lead to very high efficacy and improvement of HRQOL. Although clearance of HCV infection with all regimens led to improvement of HRQOL, these improvements were more obvious during treatment with LDV/SOF. The study points to the important concept that treatment for HCV must show comprehensive benefit not only to include clinical outcomes (such as SVR) but also PROs such as HRQOL.
Supplementary Material
Footnotes
Abbreviations: ALT = alanine aminotransferase, BMI = body mass index, BP = bodily pain, CHC = chronic hepatitis C, GH = general health, HCV = hepatitis C virus, HRQOL = health-related quality of life, IFN = interferon, LDV = ledipasvir, MCS = Mental Component Summary Score, MH = mental health, PCS = Physical Component Summary Score, PF = physical functioning, PRO = patient-reported outcome, RBV = ribavirin, RE = role emotional, RP = role physical, SF = social functioning, SF-36 = Short Form-36, SOF = sofosbuvir, SVR = sustained virologic response, VT = vitality.
Funding/support: This study was funded by Gilead Sciences, ZMY, MO, and MM have received research funding from Gilead Sciences.
The remaining authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Sievert W, Altraif I, Razavi HA, et al. A systematic review of hepatitis C virus epidemiology in Asia, Australia and Egypt. Liver Int 2011; 31 Suppl 2:61–80. [DOI] [PubMed] [Google Scholar]
- 2.Umemura T, Ichijo T, Yoshizawa K, et al. Epidemiology of hepatocellular carcinoma in Japan. J Gastroenterol 2009; 44 Suppl 19:102–107. [DOI] [PubMed] [Google Scholar]
- 3.Chayama K, Hayes CN, Ohishi W, et al. Treatment of chronic hepatitis C virus infection in Japan: update on therapy and guidelines. J Gastroenterol 2013; 48:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Younossi ZM, Kanwal F, Saab S, et al. The impact of hepatitis C burden: an evidence-based approach. Aliment Pharmacol Ther 2014; 39:518–531. [DOI] [PubMed] [Google Scholar]
- 5.Younossi Z, Henry L. Systematic review: patient-reported outcomes in chronic hepatitis C – the impact of liver disease and new treatment regimens. Aliment Pharmacol Ther 2015; 41:497–520. [DOI] [PubMed] [Google Scholar]
- 6.Younossi Z, Henry L. The impact of the new antiviral regimens on patient reported outcomes and health economics of patients with chronic hepatitis C. Dig Liver Dis 2014; 46 Suppl 5:S186–S196. [DOI] [PubMed] [Google Scholar]
- 7.Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014; 370:1889–1898. [DOI] [PubMed] [Google Scholar]
- 8.Zeuzem S, Dusheiko GM, Salupere R, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med 2014; 370:1993–2001. [DOI] [PubMed] [Google Scholar]
- 9.Kowdley KV, Gordon SC, Reddy KR, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med 2014; 370:1879–1888. [DOI] [PubMed] [Google Scholar]
- 10.Andreone P, Colombo MG, Enejosa JV, et al. ABT-450, ritonavir, ombitasvir, and dasabuvir achieves 97% and 100% sustained virologic response with or without ribavirin in treatment-experienced patients with HCV genotype 1b infection. Gastroenterology 2014; 147:359–365. [DOI] [PubMed] [Google Scholar]
- 11.Zeuzem S, Jacobson IM, Baykal T, et al. Retreatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med 2014; 370:1604–1614. [DOI] [PubMed] [Google Scholar]
- 12.Nelson DR, Cooper JN, Lalezari JP, et al. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology 2015; 61:1127–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawitz E, Sulkowski MS, Ghalib R, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet 2014; 384:1756–1765. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson IM, Gordon SC, Kowdley KV, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med 2013; 368:1867–1877. [DOI] [PubMed] [Google Scholar]
- 15.Poordad F, Hezode C, Trinh R, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med 2014; 370:1973–1982. [DOI] [PubMed] [Google Scholar]
- 16.Charlton M, Everson GT, Flamm SL, et al. Ledipasvir and sofosbuvir plus ribavirin for treatment of HCV infection in patients with advanced liver disease. Gastroenterology 2015; 149:649–659. [DOI] [PubMed] [Google Scholar]
- 17.Afdhal N, Reddy KR, Nelson DR, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med 2014; 370:1483–1493. [DOI] [PubMed] [Google Scholar]
- 18.Younossi ZM, Stepanova M, Afdhal N, et al. Improvement of health-related quality of life and work productivity in chronic hepatitis C patients with early and advanced fibrosis treated with ledipasvir and sofosbuvir. J Hepatol 2015; 63:337–345. [DOI] [PubMed] [Google Scholar]
- 19.Younossi ZM, Stepanova M, Sulkowski M, et al. Sofosbuvir and ribavirin for treatment of chronic hepatitis c in patients co-infected with hepatitis C virus and HIV: the impact on patient-reported outcomes. J Infect Dis 2015; 212:367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Younossi ZM, Stepanova M, Nader F, et al. Patient-reported outcomes in chronic hepatitis C patients with cirrhosis treated with sofosbuvir-containing regimens. Hepatology 2014; 59:2161–2169. [DOI] [PubMed] [Google Scholar]
- 21.Younossi ZM, Stepanova M, Henry L, et al. Minimal impact of sofosbuvir and ribavirin on health related quality of life in chronic hepatitis C (CH-C). J Hepatol 2014; 60:741–747. [DOI] [PubMed] [Google Scholar]
- 22.Younossi ZM, Stepanova M, Henry L, et al. Effects of sofosbuvir-based treatment, with and without interferon, on outcome and productivity of patients with chronic hepatitis C. Clin Gastroenterol Hepatol 2014; 12:1349.e13–1359.e13. [DOI] [PubMed] [Google Scholar]
- 23.Gerber L, Estep M, Stepanova M, et al. Effects of viral eradication with ledipasvir and sofosbuvir, with or without ribavirin, on measures of fatigue in patients with chronic hepatitis C virus infection. Clin Gastroenterol Hepatol 2016; 14:156.e3–164.e3. [DOI] [PubMed] [Google Scholar]
- 24.Younossi ZM, Stepanova M, Pol S, et al. The impact of ledipasvir/sofosbuvir on patient-reported outcomes in cirrhotic patients with chronic hepatitis C: the SIRIUS study. Liver Int 2016; 36:42–48. [DOI] [PubMed] [Google Scholar]
- 25.Younossi ZM, Stepanova M, Marcellin P, et al. Treatment with ledipasvir and sofosbuvir improves patient-reported outcomes: results from the ION-1, -2, and -3 clinical trials. Hepatology 2015; 61:1798–1808. [DOI] [PubMed] [Google Scholar]
- 26.Younossi ZM, Stepanova M, Zeuzem S, et al. Patient-reported outcomes assessment in chronic hepatitis C treated with sofosbuvir and ribavirin: the VALENCE study. J Hepatol 2014; 61:228–234. [DOI] [PubMed] [Google Scholar]
- 27.Younossi ZM, Stepanova M, Nader F, et al. The patient's journey with chronic hepatitis C from interferon plus ribavirin to interferon- and ribavirin-free regimens: a study of health-related quality of life. Aliment Pharmacol Ther 2015; 42:286–295. [DOI] [PubMed] [Google Scholar]
- 28.Omata M, Nishiguchi S, Ueno Y, et al. Sofosbuvir plus ribavirin in Japanese patients with chronic genotype 2 HCV infection: an open-label, phase 3 trial. J Viral Hepat 2014; 21:762–768. [DOI] [PubMed] [Google Scholar]
- 29.Mizokami M, Yokosuka O, Takehara T, et al. Ledipasvir and sofosbuvir fixed-dose combination with and without ribavirin for 12 weeks in treatment-naive and previously treated Japanese patients with genotype 1 hepatitis C: an open-label, randomised, phase 3 trial. Lancet Infect Dis 2015; 15:645–653. [DOI] [PubMed] [Google Scholar]
- 30.Ware JE, Kosinski M. Interpreting SF-36 summary health measures: a response. Qual Life Res 2001; 10:405–413. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


