Abstract
Background:
Colonoscopic screening is recommended for first-degree relatives of patients diagnosed with colorectal cancer (CRC) or colorectal adenomatous polyps (CAP) before the age of 60 years. This has the potential to reduce CRC-related morbidity and mortality, but uptake is currently inadequate.
Methods:
The aim of the study was to compare the effectiveness of standard information versus a nurse-led tailored intervention designed to promote uptake of colonoscopy screening by siblings of CRC or CAP patients. A randomized controlled trial was conducted. Digestive surgeons and gastroenterologists recruited index patients who developed CRC or CAP before the age of 60 years. All index patients received standard screening information for their siblings, in keeping with current guidelines. Centrally computerized randomization of index patients resulted in allocating all their siblings to the same group, intervention or control. The tailored intervention targeted the index patient first, to help them convey information to their siblings. The nurse then provided the siblings with tailored information based on their answers to a self-questionnaire which explored health behaviors, derived from psychosocial models of prevention. Then the siblings were given a personalized information leaflet to hand to their regular physician. The primary endpoint was the rate of documented colonoscopy performed in siblings within 1 year after diagnosis of the index patient. The intent-to-treat analysis included siblings who refused to participate in the study. Statistical analysis was adjusted for intrafamilial correlation.
Results:
A total of 304 siblings of 125 index patients were included: 160 in the intervention group and 144 in the control group. The rate of colonoscopy uptake among siblings was 56.3% in the intervention group and 35.4% in the control group (P = 0.0027). The respective rates after exclusion of refusals were 69.2% and 37.0% (P < 0.0001). More lesions were detected in the intervention group (1 invasive cancer and 11 advanced adenomas vs 5 advanced adenomas; P = 0.022).
Conclusions:
This study demonstrates the effectiveness of a nurse-led tailored intervention designed to promote colonoscopy screening uptake by siblings of patients diagnosed with CRC or CAP before age 60 years. Such tailored interventions that also involve physicians should help to reduce CRC-related mortality.
Keywords: colonoscopy, colorectal cancer, first-degree relative, screening, tailored intervention
1. Introduction
Colorectal cancer (CRC) is the third most common cause of cancer mortality worldwide, responsible for 694,000 deaths each year.[1] In Europe, in 2012, CRC was the second most frequent cancer, with 447,000 new cases annually, and the second cause of death from cancer, with 215,000 deaths annually.[2] First-degree relatives (FDRs), and especially siblings of patients diagnosed with CRC or colorectal adenomatous polyps (CAP) are 2 to 4 times more likely than the general population to develop CRC.[3–5] Colonoscopic screening of FDRs could potentially reduce CRC-related morbidity and mortality.[6] Those countries having achieved the largest reductions in CRC mortality are characterized by better access to colonoscopic screening.[7] Screening guidelines recommend colonoscopic surveillance of high-risk individuals with a family history of either CRC or CAP in a FDR before age 60 years, or in 2 or more FDRs regardless of age of onset.[8,9] Recent data indicate that colonoscopy screening uptake by FDRs of CRC patients is inadequate (26%–54%).[10]
Previous meta-analyses have shown that tailored messages are more effective than general messages for improving adherence to cancer screening guidelines.[11,12] Two recent studies indicate that telephone-based initiatives can be successful in educating individuals with a familial or genetic risk of cancer and can encourage these at-risk individuals to undergo recommended screening procedures.[13,14] Indeed, several studies have shown that telephone-based interventions influence the CRC screening behavior of individuals at average risk, particularly those belonging to groups less likely than the general population to participate in screening.[15,16]
The impact of tailored information on organized CRC screening uptake in the medium-risk population has also been demonstrated,[17] but the effectiveness of tailored interventions targeting individuals at a high risk of CRC, including not only FDRs of CRC but also CAP, has rarely been studied, and never in Europe.[18,19] Four main factors influence screening uptake by FDRs at risk of CRC: their individual psychosocial behavioral profile, advice from a physician, the familial relationship, and the social environment.[20,21] A previous study by our research group showed the difficulties of conveying information on screening and suggested the need to reinforce the motivation of the different actors in familial screening, namely physicians, index patients, and their siblings.[22–24] These results led us to study the impact of a tailored intervention that included information and accompaniment of index patients in their transmission of messages on their siblings’ increased risk of CRC, and also the siblings’ individual perceived barriers to screening and the physicians’ awareness of screening guidelines.
The aim of this randomized trial was to determine the effectiveness of a tailored intervention focusing first on patients diagnosed with CRC or CAP before age 60, then on their siblings and their siblings’ regular physicians, in terms of screening colonoscopy uptake.
2. Methods
2.1. Study population
This randomized intervention trial involved a prospective cohort of siblings of patients diagnosed with CRC or advanced CAP[8] before age 60 years in a region of western France (Poitou-Charentes: 1,792,200 inhabitants on January 1, 2013). In Poitou-Charentes, from 2010 to 2012, the age-standardized incidence rates of CRC and advanced CAP diagnosed before age 60 were, respectively, 11.9 per 100,000 (223 new cases annually) and 11.1 per 100,000 (209 new cases annually). Patients under 60 accounted for 16% of new cases of CRC and 26% of new cases of advanced CAP (medphar.univ-poitiers.fr/registre-cancers-poitou-charentes). Digestive surgeons and gastroenterologists of Poitou-Charentes were asked to prospectively propose their eligible patients to participate in the study. A monitoring of potentially eligible new cases occurring before age 60 was obtained from the general cancer registry of Poitou-Charentes, within 2 months of their notification. For patients who had not been already included, the physician was asked to check for eligibility and eventually to propose study participation.
The index patients met the following inclusion criteria: diagnosis of CRC or CAP between 2010 and 2013, age 60 years or less at diagnosis, fluency in French, existence of a sibling, and written informed consent. Index patients were not included if they had inherited CRC susceptibility confirmed by genetic testing (familial adenomatous polyposis or Lynch syndrome), a known first-degree family history of CRC or CAP, a previous history of CRC or CAP, or an inflammatory bowel disease. The investigating physician (surgeon or gastroenterologist) provided standardized information on benefits of screening, and the increased risk for siblings. The investigator then obtained information on the index patient's siblings, including their contact details; the siblings could only be contacted with the index patient's permission.
Siblings had to meet the following inclusion criteria: residence in metropolitan France, at least 1 parent in common with the index patient, fluency in French, and written informed consent. Siblings were not included in the study if their age was outside the range mentioned in French screening guidelines (under 45, or more than 5 years younger than the index patient at CRC diagnosis). Siblings who had previously undergone colonoscopy to investigate symptoms or for monitoring related to their personal history were excluded from the analysis, so as not to artificially increase the screening uptake rate.
2.2. Standard screening information
All index patients received standard screening information in keeping with current guidelines (Société Française d’Endoscopie Digestive), delivered to the index patient by the physician. This information could nevertheless be adapted to the needs of the individual sibling, at the discretion of the physician.
2.3. Randomization
Because of nonindependence between siblings belonging to the same family, clustered allocation of the intervention to whole families was randomized 1:1 between the control group and the tailored intervention group. The random allocation sequence was generated by the biostatistician before the beginning of the study using SAS-program generated random numbers, with a fixed block size of 4. Randomization was stratified according to specialty of the physicians (surgeons or gastroenterologists). Allocation was automatically assigned after sequentially entering patient's characteristics in the database and checking for eligibility. Physicians were not informed of the randomization arm.
2.4. Tailored intervention
The nurse-led tailored intervention targeting the index patients and their siblings was based on a telephone interview. The screening nurse was familiar with the constraints of colonoscopy.
The intervention first consisted of guidance and accompaniment for the index patients, to help them convey information to their siblings on their disease and its prevention. If the index patient did not wish to contact his or her siblings, the nurse asked for permission to transmit the documents and information directly to the siblings. The index patients gave their siblings a letter describing the study and its objectives, together with a consent form for study participation and a self-questionnaire.
The self-questionnaire, validated by a previous study,[22] explored health behaviors, derived from explanatory psychosocial models of prevention, namely the Health Belief Model (HBM)[25] and the Theory of Reasoned Action (TRA).[26] The questionnaire explored the perception of one's vulnerability, perceived obstacles to screening (time, cost, discomfort, anxiety), the expected benefits of colonoscopy and the utility of screening, and also motivation, fatalism, and access to colonoscopy; it also took into account whether or not the sibling had spoken to his/her other siblings and regular physician (concurring to a total of 12 factors).
The nurse then provided the siblings with tailored information based on their answers to the self-questionnaire and on their behavioral stages.[27] Tailoring has been defined as: “Any combination of information or change strategies intended to reach one specific person, based on characteristics that are unique to that person, related to the outcome of interest, and have been derived from an individual assessment,”[28] which involves the creation of a message adapted to each particular individual.[29] A computer algorithm was developed to summarize the 12 factors, with a score of 100 corresponding to a positive perception (low fatalism, no perceived obstacle). The scores were then ordered to customize the telephone intervention on 4 to 5 low-scored factors. Phone call was followed by the dispatch of a tailored information leaflet bearing the sibling's name, focusing messages on the lowest scored factors. Tailored follow-up phone calls were used, if needed, to encourage the siblings to accept colonoscopy. The principles of the tailored intervention were based on a meta-analysis which showed that those tailored interventions with the biggest impact tend to focus on screening and preventive behaviors, involve repeated contacts, use a control group rather than a comparison group, have relatively short follow-up, and base their tailoring on 4 or 5 of the most salient factors.[12]
In addition, the siblings were provided with a letter of information and a personalized leaflet to give to their regular physicians, informing them of screening guidelines for individuals at a high risk of CRC, to encourage the physician to advise the patient to consult a gastroenterologist for colonoscopy.
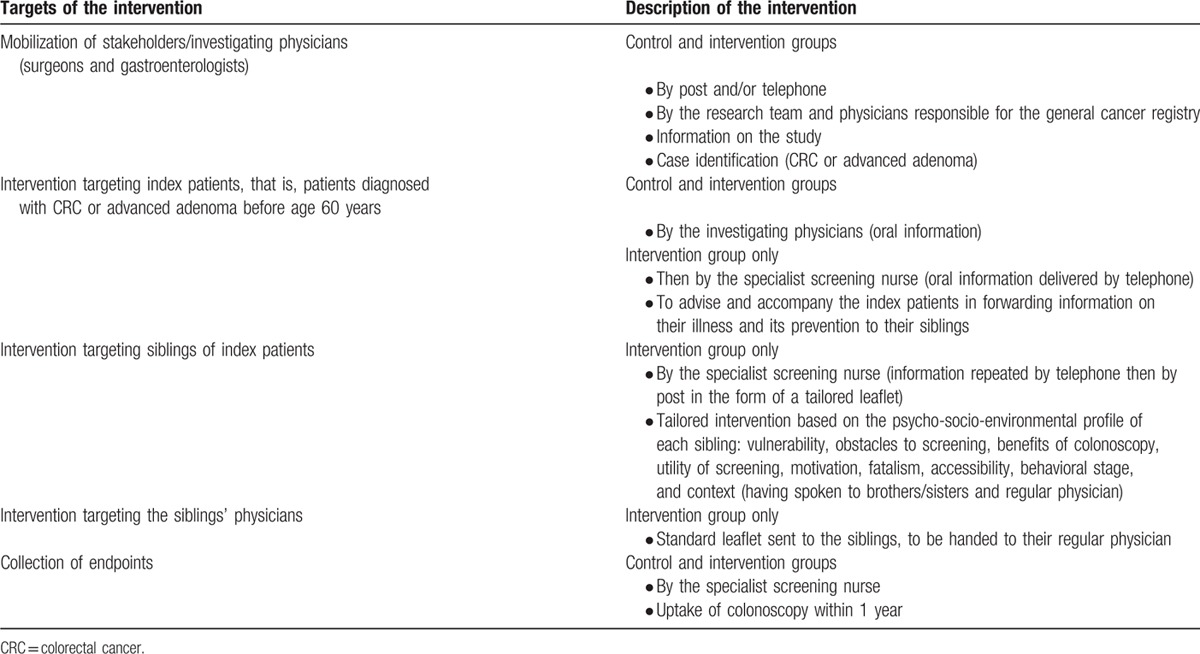
In summary, this intervention included: a telephone interview designed to inform the index patients and to help them pass on the message to their brothers and sisters concerning their diagnosis and their siblings’ increased risk of CRC; repeated tailored interventions designed to increase awareness among the siblings regarding the benefits of colonoscopy, taking into account their individual psychosocial environmental profile, first by telephone and then by mail; and education on screening guidelines, targeting the siblings’ regular physicians, in the form of a personalized leaflet handed to them by the siblings themselves, to encourage them to inform and motivate the patient to consult a gastroenterologist for colonoscopy (Table 1).
Table 1.
Implementation of the intervention designed to promote colonoscopy among patients at an increased risk of CRC.
2.5. Outcome
One year after inclusion, the nurse asked the siblings in both groups whether they had undergone colonoscopy, and, if so, collected the documented results. In the control group, this was the only contact between the siblings and the nurse.
2.6. Statistical analysis
For an average rate of colonoscopy uptake of 50% in the nonintervention group, an expected difference between the groups of 20%, with a 1-sided 5% alpha risk and a power of 95%, required a total of 300 eligible siblings after taking intrafamilial correlation into account (r = 0.15).[24]
On an intent-to-treat (ITT) basis, the primary analysis population comprised all eligible siblings of the eligible index patients, including refusals to participate in the study (considered to represent the absence of colonoscopy), to avoid a differential selection bias. Indeed, refusal of the intervention or the investigation was not, a priori, independent of the achievement of screening colonoscopy. The secondary analysis population consisted of the assessable population at 1 year (excluding refusals), with the aim of documenting the impact of the intervention in the participating population. The primary endpoint was the rate of colonoscopy uptake within the first year. The secondary endpoint was the result of colonoscopy: not done, normal or nonmalignant (hyperplastic polyps), simple adenoma, advanced adenoma (high-grade dysplasia, size >10 mm, villous histology),[30] or in situ or invasive carcinoma.
Statistical analysis used SAS v9.3 software (Cary, NC). Univariate and multivariate analyses of the primary endpoint (colonoscopy performed at 1 year) were adjusted for intrafamilial correlation using a logistic model based on generalized estimating equations. Effect size estimation was based on the odds ratio (OR) and 95% confidence interval (CI) derived from the logistic model. The analysis of the secondary endpoint (result of colonoscopy) used the exact trend test.
2.7. Ethical review
Written informed consent was obtained from all the study participants, and the study protocol was approved by the French regulatory authorities, Comité de Protection des Personnes CPP Ouest III the 09/06/2010 (No. 10.07.13) and Commission Nationale Informatique et Liberté the 11/18/2010 (DR-2010-269). The study was registered by the Agence Nationale de Sécurité du Médicament et des produits de santé (ANSM-RCB 2010-A00706-33).
3. Results
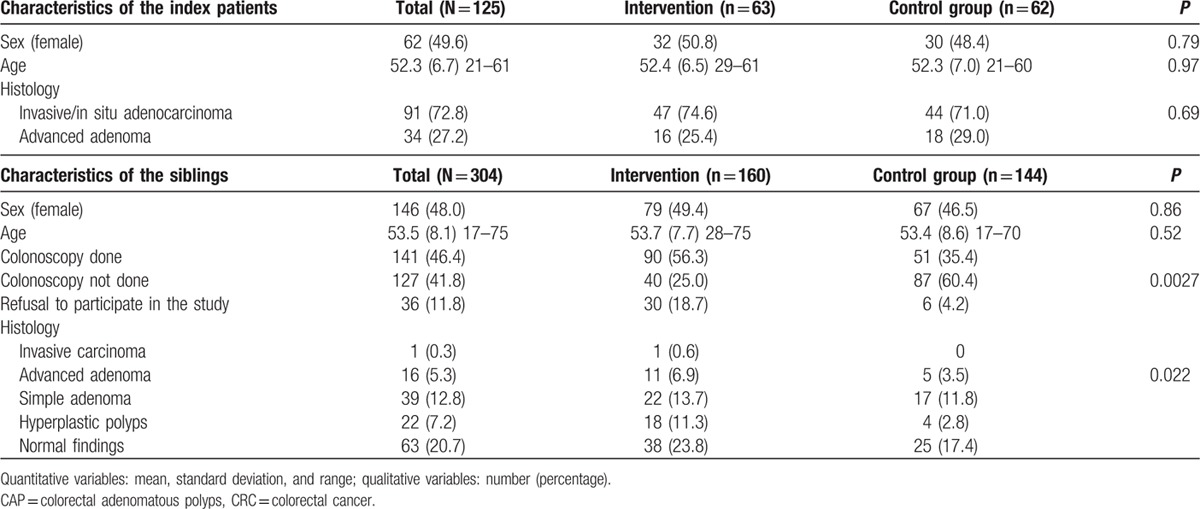
Of the 138 index patients identified by 35 physicians (9 surgeons and 26 gastroenterologist), 125 patients were included (Fig. 1). Six index patients withdrew their consent when asked to provide contact details for their siblings. Two index patients gave contact details for only some of their siblings, as they were no longer in contact with the others. Contact details were collected for 366 siblings, of whom 304 were included in the primary analysis: 160 in the intervention group (including 30 refusals to participate), and 144 in the control group (including 6 refusals to participate). The sociodemographic characteristics and tumor histologies of the index patients did not differ between the groups, neither did the age or sex distribution of the siblings (Table 2).
Figure 1.
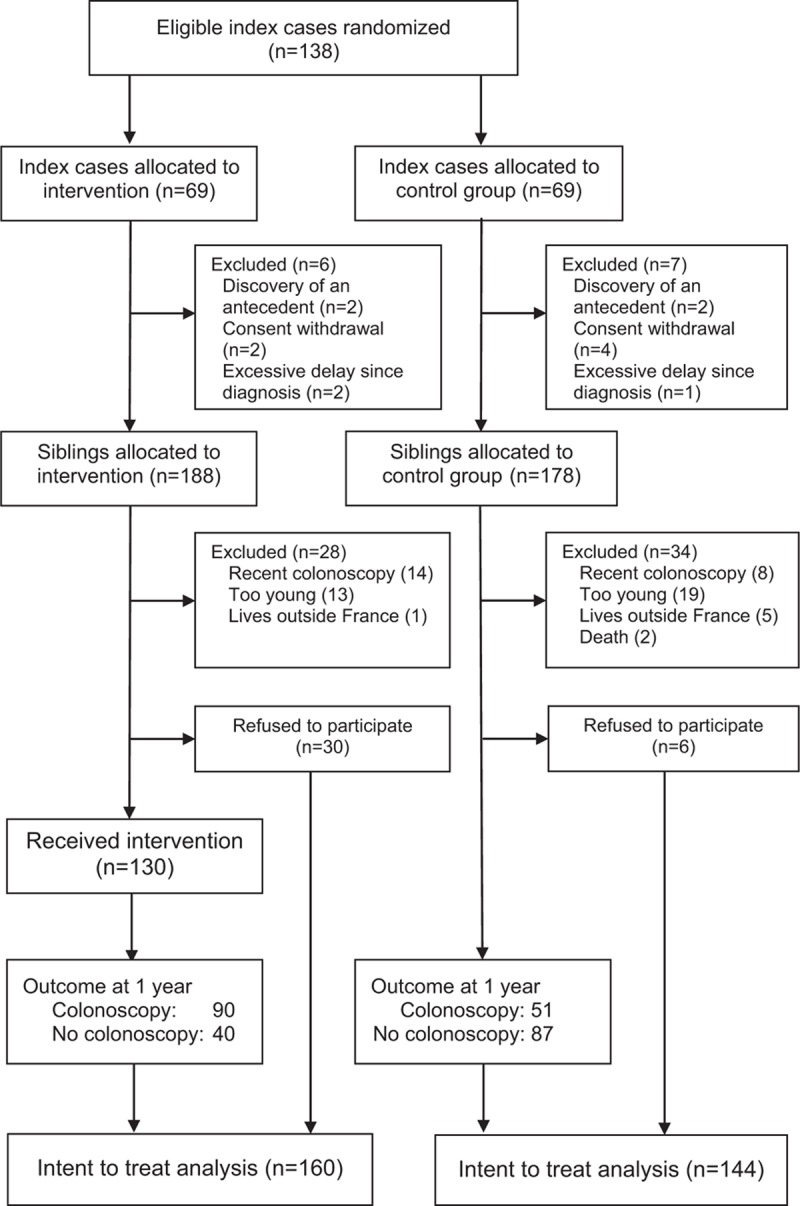
Study flowchart: selection of index patients and siblings.
Table 2.
Uptake of screening colonoscopy by siblings of index CRC/CAP patients (univariate analysis).
3.1. Uptake of colonoscopy
The colonoscopy uptake rates among the siblings within 1 year after diagnosis of the index patient were 56.3% in the intervention group and 35.4% in the control group (P = 0.0027), with estimated effect size OR = 2.37 (1.35; 4.15) in favor of the intervention. The respective rates in the population excluding refusals were 69.2% and 37.0% (P < 0.0001), which correspond to an effect size OR = 3.94 (2.13; 7.26). The effect of the intervention remained significant (P = 0.0036) in the multivariate analysis including age, sex, number of siblings, and histology of the index lesion (carcinoma vs adenoma). More lesions were detected by colonoscopy in the intervention group (1 invasive cancer and 11 cases of advanced CAP) than in the control group (5 cases of advanced CAP) (P = 0.022 in the primary ITT population; P = 0.0015 in the secondary population excluding refusals to participate) (Table 2).
3.2. Intervention
In the intervention group, 4 index patients requested that the information be given to their siblings (n = 15) by the specialized screening nurse. Then individual tailoring of the intervention was based on 4 or 5 of the most salient factors identified by the algorithm. The targeted intervention by the nurse enabled a majority of siblings to improve their awareness of the importance of screening. Indeed, 25 siblings declared they had not been aware of their increased risk of CRC or polyps, and had not received advice on screening. Thirty-one siblings had not discussed the index patient's illness or the possibility of screening with their physician. The nurse began the intervention by encouraging those siblings to discuss screening with their brothers and sisters and/or to discuss the index patient's illness and screening with their physician.
Vulnerability, that is to say the siblings’ impression that they were not more likely to get cancer than people of the same age, was the psychosocial factor that most often obtained the lowest score in the questionnaire; the screening nurse thus prioritized the excess risk for the individual in question in 44.7% of cases (51/114 responding siblings). Fatalism and/or obstacles to screening were the main focus of the intervention in, respectively, 34.2% and 28.9% of cases. Regarding obstacles to screening, the nurse reassured the siblings as to the time taken by screening, the cost of colonoscopy and its inconvenience, and also related worries or concerns about follow-up. These arguments were reinforced in a tailored document sent to each sibling, focusing on those factors with low scores.
4. Discussion
This randomized trial demonstrates the effectiveness of a tailored intervention designed to improve the uptake of screening colonoscopy by siblings of patients diagnosed with CRC or CAP, who are at high risk of CRC according to current guidelines. In the intervention group, colonoscopy uptake was increased by over 20% in the primary (ITT) analysis and by over 30% in the secondary analysis excluding refusals to participate in the study.
Few prospective studies have focused on interventions designed to modify factors that influence the uptake of colonoscopy by individuals at a high risk of CRC, even though several studies have clearly identified these factors.[21] In FDRs of CRC survivors, tailored written information (based on the HBM model) did not improve adhesion to screening (fecal occult blood, sigmoidoscopy, or colonoscopy) when compared with generic written information (14% and 21%, respectively, within 3 months).[31] A tailored face-to-face colon cancer risk counseling among relatives of CRC patients, dispensed by a nurse educator and based on a theoretical model, led to a 13% increase within 4 months in adherence to an examination appropriate to their level of risk, by comparison with general health counseling (20% and 7% uptake, respectively).[32] These 2 studies did not select the index patients based on age at diagnosis, meaning that the level of risk and screening guidelines were not identical for all the included relatives. In siblings of patients diagnosed with CRC before age 60, tailored interventions (tailored print or tailored print plus phone) significantly increased colonoscopy uptake by 11% and 12% compared with a standard intervention (25% and 26% vs 14%, respectively).[33] Recently, the Family Health Promotion Project conducted a randomized study which showed that a tailored telephone intervention based on psychosocial models among individuals at a high risk of CRC, identified from a CRC family registry, increased the colonoscopy uptake rate at 24 months by 11% compared with mailed general information on screening (54% vs 43%, respectively).[19]
In our study, the ITT estimated rate of colonoscopy uptake by the siblings within 1 year after diagnosis of CRC or CAP in the index patient was 56.3% in the intervention group and 35.4% in the control group (P = 0.0027), which is an increase of 20.9%. Unlike previous studies, the intervention by a specialized screening nurse involved the index patients, resulting in better transmission of information to their siblings. The fact that the study involved gastroenterologists, oncologists, and surgeons, who have a key role in transmitting information to index patients, and the fact that the intervention was conducted by a healthcare professional (nurse), enhanced its credibility among the patients and physicians.[21] Information was also provided to the siblings’ regular physicians regarding their role in providing information on prevention guidelines, as few of the siblings had raised the index patient's illness with their physician.
This study was based on a randomized design with adequate statistical power, together providing strong evidence. Collection of the index patients’ ages and histological diagnoses allowed us to accurately identify subjects at a high risk of CRC. The endoscopic and pathological reports of colonoscopies performed in the siblings were also collected to obtain reliable endpoints.
Study limits were inherent to a randomized intervention trial design. Although this design is best-suited to provide the highest level of evidence of the efficacy of the intervention, its experimental nature prevents immediate reproducibility or generalizability. Moreover, as expected, sibling refusals to participate were more frequent in the intervention group (19%), mostly related to the specific administrative constraints of a research study (more procedures, poor understanding of the need for written consent). Inclusion of refusals in the ITT analysis thus likely underestimated the effectiveness of the intervention (21%, compared with a 32% increase in the secondary analysis excluding refusals). Effective colonoscopy uptake in the intervention group thus ranged between 56% and 69%.
5. Conclusions
This study demonstrates the effectiveness of a tailored intervention administered by a specialized screening nurse in promoting screening uptake by siblings of patients diagnosed with CRC or CAP before age 60 years. The next step will be to integrate this type of intervention in a public health program, and to measure its impact, notably on CRC-related morbidity and mortality, and to evaluate its medico-economic impact. This type of intervention may also be applicable to other familial cancers (breast cancer, melanoma, etc.) or noncancer diseases (inherited high cholesterol, etc.),[34] for which screening uptake is still inadequate.
Acknowledgments
The authors thank the surgeons (Drs Barthes, Beydoun, Cervi, De Wailly, Doucet, Faure, Smirnoff and Zaranis) and gastroenterologists (Drs Ballorain, Bonneau, Bouffard, Chanteloup, Croquet, De Bayser, Decottignies, Gros, Haineaux, Hallak, Kitmacher, Letard, Levy, Magois-Pilon, Michaudel, Moulin, Moullot, Ouali, Popot, Saade, Toillon, Vasseur, Verneau, Vuillemin et Wangermez) for patient enrolment, Vianney Jouhet for developing the IT algorithms used to generate score-based tailored information documents, Myriam Taouqi for her involvement in the implementation of the study, Marie-Annie Guibert for the tailored interventions, and David Young for the English translation of the manuscript.
The authors thank l’Agence Régionale de Santé for support.
Footnotes
Abbreviations: CAP = colorectal adenomatous polyps, CRC = colorectal cancer, FDR = first-degree relative, HBM = Health-belief Model, ITT = intent-to-treat, OR = odds ratio, TRA = Theory of Reasoned Action.
Funding: The study received financial support from the Institut de Recherche en Santé Publique (IReSP) and Institut National du Cancer (INCa).
The authors have no conflicts of interest to disclose..
References
- 1.Globocan 2012. Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012 [Internet]. Lyon: International Agency for Research on Cancer [cited 2015 Nov 7] Available at: http://globocan.iarc.fr (accessed August 4, 2016). [Google Scholar]
- 2.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013; 49:1374–1403. [DOI] [PubMed] [Google Scholar]
- 3.Butterworth AS, Higgins JPT, Pharoah P. Relative and absolute risk of colorectal cancer for individuals with a family history: a meta-analysis. Eur J Cancer 2006; 42:216–227. [DOI] [PubMed] [Google Scholar]
- 4.Tuohy TM, Rowe KG, Mineau GP, et al. Risk of colorectal cancer and adenomas in the families of patients with adenomas: a population-based study in Utah. Cancer 2014; 120:35–42. [DOI] [PubMed] [Google Scholar]
- 5.Ng SC, Lau JY, Chan FK, et al. Risk of advanced adenomas in siblings of individuals with advanced adenomas: a cross-sectional study. Gastroenterology 2016; 150:608–616. [DOI] [PubMed] [Google Scholar]
- 6.Lieberman DA. Cost-effectiveness model for colon cancer screening. Gastroenterology 1995; 109:1781–1790. [DOI] [PubMed] [Google Scholar]
- 7.Ait Ouakrim D, Pizot C, Boniol M, et al. Trends in colorectal cancer mortality in Europe: retrospective analysis of the WHO mortality database. BMJ 2015; 351:h4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology 2008; 134:1570–1595. [DOI] [PubMed] [Google Scholar]
- 9.ANAES–Service des recommandations professionnelles. Endoscopie digestive basse. Indications en dehors du dépistage en population. Recommandations pour la pratique clinique: Avril 2004. Oncologie 2005; 7:70–75.[French]. [Google Scholar]
- 10.Ait Ouakrim D, Lockett T, Boussioutas A, et al. Screening participation for people at increased risk of colorectal cancer due to family history: a systematic review and meta-analysis. Fam Cancer 2013; 12:459–472. [DOI] [PubMed] [Google Scholar]
- 11.Krebs P, Prochaska JO, Rossi JS. A meta-analysis of computer-tailored interventions for health behavior change. Prev Med 2010; 51:214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noar SM, Benac CN, Harris MS. Does tailoring matter? Meta-analytic review of tailored print health behavior change interventions. Psychol Bull 2007; 133:673–693. [DOI] [PubMed] [Google Scholar]
- 13.Kinney AY, Boonyasiriwat W, Walters ST, et al. Telehealth personalized cancer risk communication to motivate colonoscopy in relatives of patients with colorectal cancer: the family CARE randomized controlled trial. J Clin Oncol 2014; 32:654–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwartz MD, Valdimarsdottir HB, Peshkin BN, et al. Randomized noninferiority trial of telephone versus in-person genetic counseling for hereditary breast and ovarian cancer. J Clin Oncol 2014; 32:618–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basch CE, Wolf RL, Brouse CH, et al. Telephone outreach to increase colorectal cancer screening in an urban minority population. Am J Public Health 2006; 96:2246–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myers RE, Sifri R, Daskalakis C, et al. Increasing colon cancer screening in primary care among African Americans. J Natl Cancer Inst 2014; 106:dju344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skinner CS, Halm EA, Bishop WP, et al. Impact of risk assessment and tailored versus nontailored risk information on colorectal cancer testing in primary care: a randomized controlled trial. Cancer Epidemiol Biomarkers Prev 2015; 24:1523–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edwards AG, Naik G, Ahmed H, et al. Personalised risk communication for informed decision making about taking screening tests. Cochrane Database Syst Rev 2013; 2:CD001865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lowery JT, Horick N, Kinney AY, et al. A randomized trial to increase colonoscopy screening in members of high-risk families in the colorectal cancer family registry and cancer genetics network. Cancer Epidemiol Biomarkers Prev 2014; 23:601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madlensky L, Esplen MJ, Gallinger S, et al. Relatives of colorectal cancer patients: factors associated with screening behavior. Am J Prev Med 2003; 25:187–194. [DOI] [PubMed] [Google Scholar]
- 21.Ait Ouakrim D, Lockett T, Boussioutas A, et al. Screening participation predictors for people at familial risk of colorectal cancer: a systematic review. Am J Prev Med 2013; 44:496–506. [DOI] [PubMed] [Google Scholar]
- 22.Ingrand I, Dujoncquoy S, Migeot V, et al. Interactions among physicians, patients and first-degree relatives in the familial screening of colorectal cancer in France. Patient Prefer Adherence 2008; 2:47–55. [PMC free article] [PubMed] [Google Scholar]
- 23.Ingrand I, Dujoncquoy S, Beauchant M, et al. General practitioner and specialist views on colonoscopic screening of first-degree relatives of colorectal cancer patients. Cancer Epidemiol 2009; 33:223–230. [DOI] [PubMed] [Google Scholar]
- 24.Taouqi M, Ingrand I, Beauchant M, et al. Determinants of participation in colonoscopic screening by siblings of colorectal cancer patients in France. BMC Cancer 2010; 10:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janz NK, Becker MH. The health belief model: a decade later. Health Educ Q 1984; 11:1–47. [DOI] [PubMed] [Google Scholar]
- 26.Ajzen I. The theory of planned behavior. Organ Behav Hum Decis Process 1991; 50:179–211. [Google Scholar]
- 27.Prochaska JO, Redding CA, Evers KE. Glanz K, Rimer BK, Viswanath K. The transtheoretical model and stages of change. Health Behavior and Health Education: Theory, Research and Practice 4th edSan Francisco, CA: Jossey-Bass; 2009. 97–121. [Google Scholar]
- 28.Kreuter MW, Skinner CS. Tailoring: what's in a name? Health Educ Res 2000; 15:1–4. [DOI] [PubMed] [Google Scholar]
- 29.Rimer BK, Kreuter MW. Advancing tailored health communication: a persuasion and message effects perspective. J Commun 2006; 56 (suppl):S184–S201. [Google Scholar]
- 30.Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012; 143:844–857. [DOI] [PubMed] [Google Scholar]
- 31.Rawl SM, Champion VL, Scott LL, et al. A randomized trial of two print interventions to increase colon cancer screening among first-degree relatives. Patient Educ Couns 2008; 71:215–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glanz K, Steffen AD, Taglialatela LA. Effects of colon cancer risk counseling for first-degree relatives. Cancer Epidemiol Biomarkers Prev 2007; 16:1485–1491. [DOI] [PubMed] [Google Scholar]
- 33.Manne SL, Coups EJ, Markowitz A, et al. A randomized trial of generic versus tailored interventions to increase colorectal cancer screening among intermediate risk siblings. Ann Behav Med 2009; 37:207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manne S, Jacobsen PB, Ming ME, et al. Tailored versus generic interventions for skin cancer risk reduction for family members of melanoma patients. Health Psychol 2010; 29:583–593. [DOI] [PMC free article] [PubMed] [Google Scholar]


