Supplemental Digital Content is available in the text
Keywords: cardiovascular diseases, coronary disease, screening, type 2 diabetes mellitus, vascular complications
Abstract
Glycemic control alone does not reduce cardiovascular events in patients with type 2 diabetes (T2D), and routine screening of all T2D patients for asymptomatic coronary artery disease (CAD) is not effective for preventing acute cardiac events. We examined the effectiveness of an aggressive screening protocol for asymptomatic CAD in T2D patients with advanced vascular complications.
We designed a 3-year cohort study investigating the effectiveness of the aggressive coronary screening for T2D patients with advanced vascular complications and no known coronary events using propensity score adjusted analysis at a national center in Japan. Eligibility criteria included T2D without known coronary events and with any 1 of the following 4 complications: advanced diabetic retinopathy, advanced chronic kidney disease, peripheral artery disease, or cerebrovascular disease. In the aggressive screening group (n = 122), all patients received stress single photon emission computed tomography and those exhibiting myocardial perfusion abnormalities underwent coronary angiography. In the conventional screening group (n = 108), patients were examined for CAD at the discretion of their medical providers. Primary endpoint was composite outcome of cardiovascular death and nonfatal cardiovascular events.
Asymptomatic CAD with ≥70% stenosis was detected in 39.3% of patients completing aggressive screening. The proportions achieving revascularization and receiving intensive medical therapy within 90 days after the screening were significantly higher in the aggressive screening group than in the conventional screening group [19.7% vs 0% (P < 0.001) and 48.4% vs 9.3% (P < 0.001), respectively]. The cumulative rate of primary composite outcome was significantly lower in the aggressive screening group according to a propensity score adjusted Cox proportional hazards model (hazard ratio, 0.35; 95% confidence interval, 0.12–0.96; P = 0.04).
Aggressive coronary screening for T2D patients with advanced vascular complications reduced cardiovascular death and nonfatal cardiovascular events.
1. Introduction
The incidence of type 2 diabetes (T2D) continues to increase worldwide, and it is still difficult to halt the progression of complications such as micro- and macrovascular diseases despite adequate glycemic control.[1] Because the prognosis after acute coronary syndrome and myocardial infarction is worse in T2D patients compared with those without T2D,[2] the prevention of these coronary events is of great importance for long-term outcome. Intensive glycemic control did not prevent cardiovascular and overall mortality,[3] necessitating the development of improved screening strategies for the early detection of cardiovascular complications.
Coronary artery stenosis in T2D patients often progresses asymptomatically,[4–6] and it can result in serious acute cardiac events.[7,8] A recent report suggested that nonobstructive coronary artery disease (CAD) was associated with a significantly greater 1-year risk of myocardial infarction and all-cause mortality compared with no apparent CAD.[9] However, there is no consensus on who to screen for asymptomatic CAD among T2D patients. Traditional coronary risk factors such as hypertension and dyslipidemia are not associated with silent ischemia and asymptomatic CAD,[5,6] and recent large randomized controlled studies found that the routine screening of all T2D patients for asymptomatic CAD is not effective for preventing acute cardiac events.[10,11] Therefore, discriminative markers for aggressive coronary screening are required. Recent studies have suggested that advanced microvascular diseases such as diabetic retinopathy and nephropathy are associated with an increased risk for cardiovascular diseases.[12–14] Moreover, patients with macrovascular diseases such as peripheral arterial disease (PAD) and cerebrovascular disease often have CAD.[15,16] On the basis of these reports, we developed a novel aggressive screening strategy for asymptomatic CAD targeting T2D patients with advanced vascular complications but no known history of coronary event.[17] This screening revealed that more than half of the screened T2D patients had asymptomatic CAD with myocardial perfusion abnormalities and ≥50% coronary stenosis.[17] However, whether this aggressive coronary screening improves outcomes in T2D patients with advanced vascular complications is unclear. Therefore, we hypothesized that our aggressive coronary screening strategy targeting T2D patients with advanced vascular complications will result in decreased overall acute cardiovascular events presumably through detecting many severe coronary stenoses that majorly result in prophylactic intensive therapies. The aim of the present study is to investigate the effectiveness of the aggressive coronary screening for T2D patients with advanced vascular complications and no known coronary event.
2. Methods
2.1. Study design, setting, and patients
We designed a 3-year cohort study investigating the effectiveness of the aggressive coronary screening for T2D patients with advanced vascular complications and no known coronary events using propensity score adjusted analysis. Eligibility criteria were the same as those in our previous study, T2D patients without suggestive symptoms and history of coronary events between the ages of 40 and 75 years who visited the clinic or were hospitalized in our department between April 2009 and August 2010. In addition, all study patients had at least 1 of the following complications: advanced diabetic retinopathy (DMR), advanced chronic kidney disease (CKD), PAD, and cerebrovascular disease. Advanced DMR was defined as proliferative diabetic retinopathy or after photocoagulation. Advanced DMR was diagnosed by at least 1 ophthalmologist. Advanced CKD was defined on the basis of estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2 or eGFR < 45 mL/min/1.73 m2 as well as albuminuria or proteinuria corresponding to urine albumin ≥30 mg/day, urine albumin ≥30 mg/g creatinine, or detection of urine protein by the paper test. The eGFR was calculated using the following formula, as recommended by the Japanese Society of Nephrology: eGFR (mL/min/1.73 m2) = 194 × Cre−1.094 × Age−0.287 (× 0.739 for women).[18] PAD was defined as an ankle-brachial index of <0.9, peripheral artery stenosis confirmed by radiological imaging, or a history of surgical treatment for PAD. Cerebrovascular disease was defined by a history of stroke or transient ischemic attack. Exclusion criteria included known or suspected coronary disease, the presence of antibodies to glutamic acid decarboxylase, acute kidney injury, and very poor prognosis and unsuitable conditions for testing. Inclusion and exclusion criteria were confirmed from clinical records, laboratory data, and questionnaires by at least 2 independent diabetologists. Disagreements between the reviewers were resolved by a third diabetologist. This study was approved by the institutional review board of the National Center for Global Health and Medicine.
2.2. Aggressive and conventional coronary screening
We assigned patients who satisfied the study participation criteria but did not undergo aggressive coronary screening between April 2009 and August 2010 to the conventional screening group. Patients in the aggressive screening group received predesigned examinations for coronary stenosis at the start of the follow-up during the same period (Supplemental Figure 1).[17]
In the aggressive screening group, we first performed myocardial perfusion imaging (MPI) and electrocardiogram-gated stress single photon emission computed tomography (SPECT) using a dual-headed gamma camera (E.cam; Siemens, Munich, Germany). Technetium-99m tetrofosmin SPECT imaging was performed using a 1-day protocol in 108 patients, and thallium-201 was used in 14 patients. Patients who exhibited myocardial perfusion abnormalities then underwent conventional coronary angiography (CAG), 64-slice multidetector-row computed tomography (MDCT) coronary angiography, or both. MPI abnormalities did not include nonperfusion abnormalities such as pulmonary uptake of isotope and electrocardiogram changes after adenosine administration. All severe coronary stenoses, which might require any revascularizations, were detected by conventional CAG. For the stress MPI SPECT examination, patients underwent exercise stress according to the Bruce protocol or pharmacologic stress using adenosine. Two experienced doctors of nuclear cardiology blinded to other clinical information independently evaluated the images. We referred to the conventional CAG findings when the patients underwent both the imaging procedures. Each coronary artery was assessed using the American Heart Association classification.[19] Conventional CAG images were interpreted by 2 experienced cardiologists blinded to other patient characteristics. The 64-slice MDCT imaging was performed with a slice thickness of 0.5 mm (Aquilion64; Toshiba Medical Systems, Otawara, Japan). If necessary and tolerated, an oral beta-blocker (metoprolol 40–100 mg) was provided before the MDCT scan to achieve a heart rate <60 beats/min. The MDCT coronary angiography was performed using 64 × 0.5 mm collimation and retrospective electrocardiographic gating. MDCT images were interpreted by an experienced cardiologist in cooperation with radiologists blinded to other clinical information. All coronary arteries and side branches with a luminal diameter of ≥2.0 mm were assessed. Significant CAD was defined as CAD with ≥70% diameter stenosis of a major epicardial coronary artery. The findings of the aggressive coronary screening were open for the patients’ medical providers. After aggressive coronary screening, patients received appropriate treatments, as determined by their medical doctors.
In the conventional screening group, patients received conventional examinations for suspected coronary events according to the symptoms at the discretion of their medical providers. Management decisions for patients in both the screening groups were made according to the best judgment of the patients’ personal medical providers. Patients in the aggressive screening group were followed up for 3 years from the day of consent (April 2009 and August 2010), whereas the conventionally screened patients were followed up for 3 years from the day (within the same enrolment period) on which eligibility criteria were met.
2.3. Outcome measurements
The primary outcome was the composite endpoint of cardiovascular death and nonfatal cardiovascular events. Acute myocardial infarction (ICD-10: I21), stroke (I60–64), and PAD (I73) during the study period were defined as cardiovascular events. Unexpected sudden death was regarded as cardiovascular death. Angina pectoris (I20), heart failure (I50), and asymptomatic coronary stenosis (I25.0 and I25.1) were not included among cardiovascular outcomes in this study. The outcomes were independently checked using clinical records and radiological images by 2 diabetologists blinded to patient screening group. Secondary outcomes included death from any cause, cardiovascular death, and cardiovascular events. In addition, we also assessed the prevalence of significant CAD in patients receiving aggressive coronary screening and of treatment intervention within 90 days after the aggressive coronary screening. Revascularization procedures included percutaneous coronary intervention and coronary artery bypass grafting. Intensive medical therapy after the screening was defined as the addition of low-dose aspirin and the addition or increased dose of a statin drug, antihypertensive agents, or antidiabetic medicines.
2.4. Statistical methods
Patient characteristics are described separately for the 2 groups. Data are presented as the number (%) or the mean with standard deviation. Continuous variables were compared using t-tests and categorical variables using Chi-square tests or Fisher exact tests as appropriate. Average treatment effect on the treated was estimated by a Cox proportional hazard model using a propensity score. The propensity score estimating the proportion of those assigned to the aggressive screening group was derived using a logistic regression model that included the following predictors: age range (<60, 60−70, or ≥70 years), sex, body mass index, smoking status, hypertension, use of angiotensin II receptor blockers or angiotensin-converting enzyme inhibitors, dyslipidemia, use of statin, duration of diabetes, hemoglobin A1c level, use of insulin, advanced DMR, CKD stage (categorized as requiring hemodialysis, requiring peritoneal dialysis, or eGFR of <30, 30−60, or ≥60 mL/min/1.73 m2), history of stroke and PAD, use of aspirin, and welfare benefit status. We chose these variables on the assumption that they would likely be used by physicians to decide suitability for aggressive coronary screening because they are predictive of cardiovascular outcomes. We then generated an appropriate weight from the propensity score on the basis of inverse probability of treatment weighting method.[20] Kaplan–Meier analyses were used to assess primary and secondary endpoints, and the groups were compared using the log-rank test. P values of <0.05 were considered statistically significant for all tests. All analyses were performed using Stata software, version 12.1 (Stata Corp., College Station, TX).
3. Results
A total of 230 patients with T2D between the ages of 40 and 75 years met the eligibility criteria for this study of whom 122 underwent aggressive coronary screening and 108 conventional screening (Fig. 1). A total of 91.7% of the study patients completed 3 years of follow-up. Table 1 summarizes that the baseline clinical and demographic characteristics were well-matched between the screening groups. In the aggressive screening group, 10 patients with myocardial perfusion abnormalities as detected by SPECT underwent neither conventional CAG nor 64-slice MDCT. Of the remaining 112 patients who completed the aggressive screening protocol, 44 (39.3%) had significant asymptomatic CAD (Fig. 2A). Among 44 patients with significant CAD, 14 (31.8%) had 1-vessel disease and 30 (68.2%) had multi-vessel disease with stenosis ≥50% of the vessel diameter. Asymptomatically significant CAD was observed in 38.6%, 61.1%, 52.4%, and 37.2% patients with advanced DMR, advanced CKD, PAD, and cerebrovascular disease, respectively. Additional treatments administered within 90 days after screening are presented in Fig. 2B. In total, 19.7% of the aggressive screening group achieved revascularization, and 48.4% received intensive medical therapy within 90 days after the screening; these proportions were significantly higher than those within 90 days of enrolment for the conventional group (P < 0.001 for both treatments). The proportion of the additional or higher dose of antidiabetic medication was insignificantly higher in the aggressive screening group than in the conventional screening group (11.5% vs 4.6%; P = 0.09). Within the aggressive screening group, the proportions of the revascularization and the intensive medical therapies were significantly higher in the patients with significant CAD than for those without significant CAD [54.6% vs 0% (P < 0.001) and 84.1% vs 32.4% (P < 0.001), respectively]. These proportions did not differ significantly between the aggressively screened patients without any significant SPECT abnormalities and the conventionally screened patients.
Figure 1.
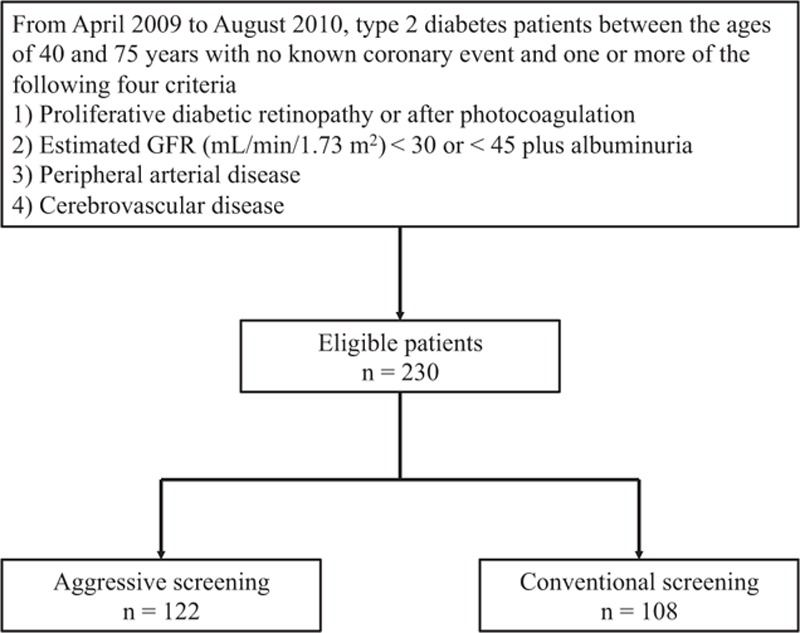
Flowchart of study participants. GFR = glomerular filtration rate.
Table 1.
Baseline characteristics of study patients∗.
Figure 2.
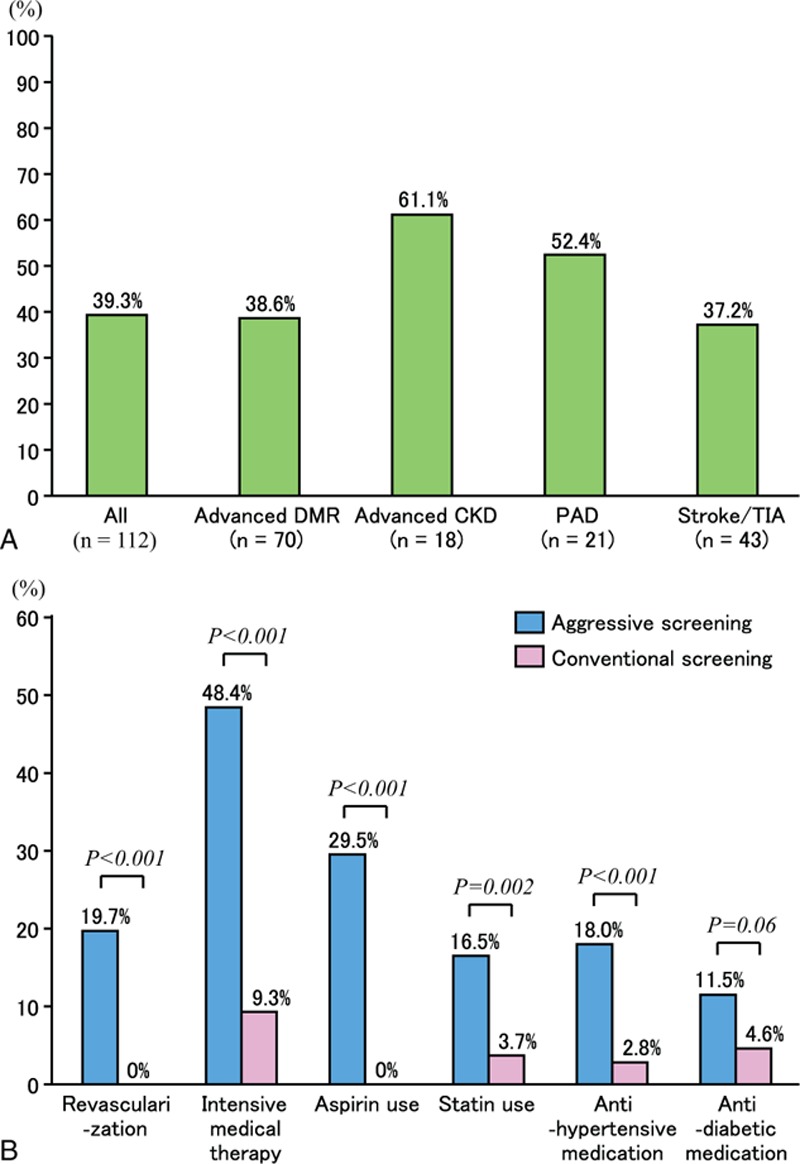
Prevalence of significant asymptomatic CAD and additional treatments after screening. Prevalence of severe coronary stenosis (A) and additional treatment within 90 days after screening (B). CAD = coronary artery disease.
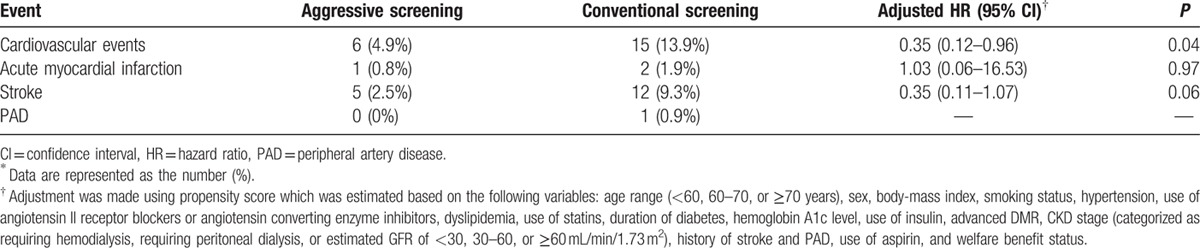
The unadjusted cumulative rate of the composite outcome, including cardiovascular death and nonfatal cardiovascular events, was significantly lower in patients in the aggressive screening group (hazard ratio, 0.34; 95% confidence interval, 0.13–0.89; P = 0.02), than those in the conventional screening group (Fig. 3). The propensity score adjusted Kaplan–Meier survival curves for these composite events are shown in Fig. 4. The cumulative events rate was also significantly lower in the aggressive screening group using a propensity score adjusted Cox proportional hazards model (hazard ratio, 0.35; 95% confidence interval, 0.12–0.96; P = 0.04). A total of 8 patients died during follow-up: 2 patients in the aggressive screening group and 6 patients in the conventional screening group. Cumulative rate of death from any cause is shown in Fig. 5A and that of cardiovascular events as well as death from any other causes is shown in Fig. 5B; both the rates were lower in the aggressive screening group than in the conventional screening group. Cardiovascular events in both groups during the follow-up period are summarized in Table 2. Incidences of acute myocardial infarction, stroke, and PAD were all lower in the aggressive screening group than in the conventional screening group, although these differences did not reach statistical significance. Acute myocardial infarction occurred in 1 patient (0.8%) in the aggressive screening group, and the event occurred during the percutaneous coronary intervention.
Figure 3.
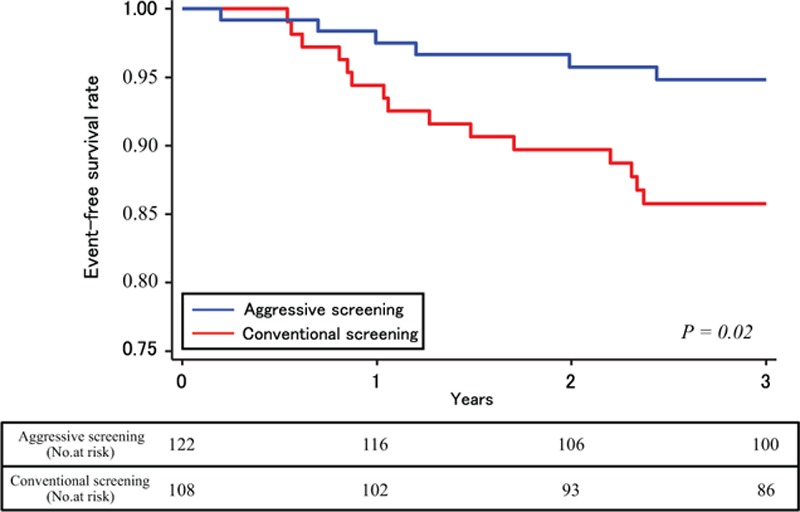
Rates of freedom from cardiovascular death and nonfatal cardiovascular events in unadjusted Cox proportional hazards model.
Figure 4.
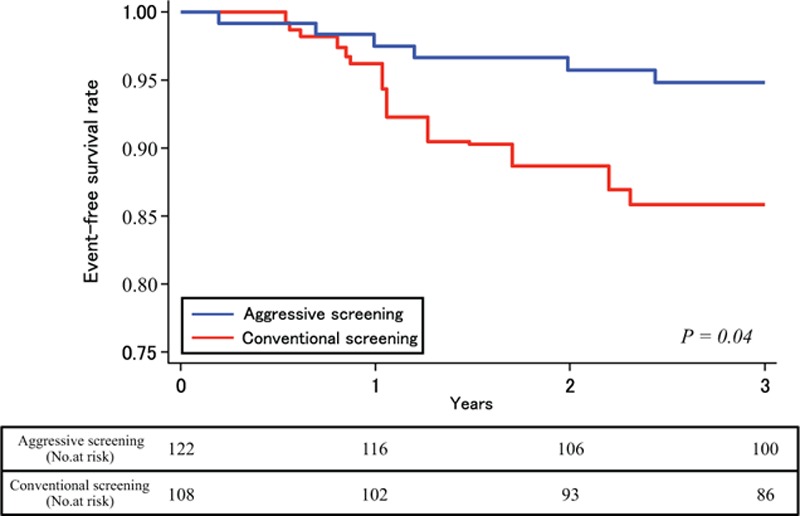
Rates of freedom from cardiovascular death and nonfatal cardiovascular events in adjusted Cox proportional hazards model.
Figure 5.
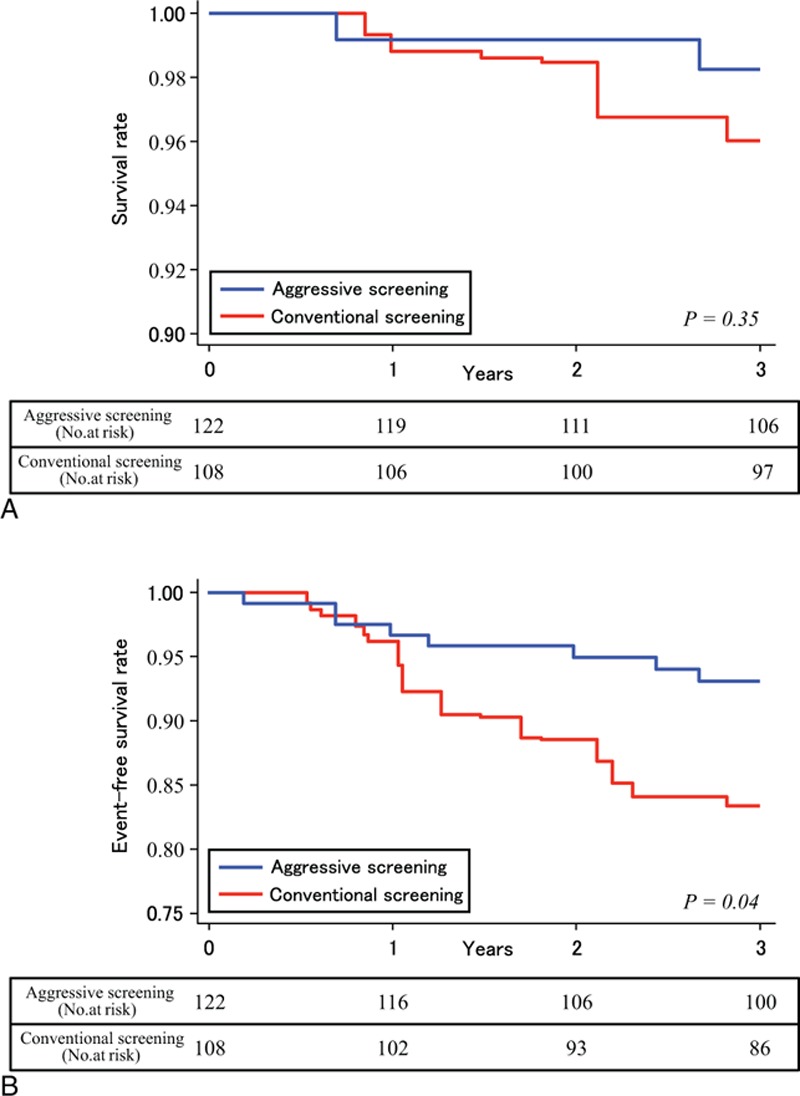
Rates of survival and freedom from cardiovascular events. Rates of survival (A) and rates of freedom from death and cardiovascular events (B).
Table 2.
Cardiovascular events in study patients∗.
4. Discussion
This study suggests that aggressive coronary screening for T2D patients with advanced vascular complications can reveal several cases of having asymptomatically significant CAD that put patients at an elevated risk of severe coronary events. Detection resulted in timely intervention to reverse coronary stenosis as well as earlier initiation of other treatments, including low-dose aspirin and addition or increased doses of statins, antihypertensives, and antidiabetic medications. These treatments may have resulted in the observed reduction in composite outcome of cardiovascular death and nonfatal cardiovascular events. Aggressive coronary screening targeting this patient group may be an effective method for improving prognosis.
Recent large randomized controlled trials found no benefit of routine screenings for patients with diabetes.[10,11] In a previous study, however, early detection of high-risk SPECT findings in asymptomatic diabetes patients prolonged survival.[21] Screening benefits may depend on the pretest probability of asymptomatic CAD. Therefore, aggressive screening may improve outcome, particularly in T2D patients at an extremely high risk for asymptomatic CAD. There are several important differences between the previous studies and our study, most notably in baseline prevalence of micro- and macrovascular diseases, possibly due to different inclusion criteria. For example, only approximately 15% of the patients in the DIAD study had diabetic retinopathies, including simple diabetic retinopathy, whereas approximately 60% of the patients in our study had advanced retinopathies such as proliferative diabetic retinopathy. Because vascular complications may reflect systemic vascular damage, including damage to coronary arteries,[12–16,22–25] the pretest probability of asymptomatic CAD may be significantly higher using our criteria. In fact, 10% to 20% of the patients in the DIAD study and in the FACTOR-64 study had silent ischemia or CAD, whereas approximately 80% of our patients had myocardial perfusion abnormalities[17] and 40% had asymptomatically significant CAD. As these vascular complications are irreversible, they may be more useful markers of systemic vascular damage than conventional risk factors such as hypertension and dyslipidemia. In addition, patients with progressive diabetic complications may have autonomic denervation of the heart, which could prevent sensation of symptoms even when coronary stenosis is severe and requires early treatment.[26,27] The present study suggests that aggressive screening in T2D with advanced vascular complications may identify those patients at a high risk of the composite endpoint of cardiovascular events and death. The potential benefits of this are that essential treatment, such as revascularization and intensive medical therapy, may appropriately be administered and also that the attitude of the patient and their medical providers may favorably be changed to prevent cardiovascular events. However, because aggressive coronary screening is expensive and involves exposure to radiation, patients chosen for screening for asymptomatic CAD should be carefully selected.
Previous studies have suggested that silent ischemia may result in serious cardiac events and death.[7,8] On the basis of our result that approximately 40% of patients with advanced micro- or macrovascular complications had asymptomatic but significant CAD with myocardial perfusion abnormalities, intensive medical therapy should be considered when advanced vascular complications are found in T2D patients.[28,29] Intensive medical therapies such as the addition of low-dose aspirin and the addition or increased dose of a statin drug may have contributed to the reduction in cardiovascular events. Although low-dose aspirin as primary prevention does not reduce the risk of cardiovascular events in all T2D patients,[30,31] it may have significant benefits in T2D patients with significant pre-existing vascular damage. Intensive medical therapies may be beneficial for T2D patients with advanced vascular complications regardless of whether they have the traditional coronary risk factors. However, statin use at the baseline between April 2009 and August 2010 was approximately 39%, which is low, and therefore, the results of this study may not easily be generalized. Further studies are required to identify reliable treatment indications for these intensive therapies.
Our study had several limitations. First, this study was performed at a single national medical center and was limited to a specific geographical area. Therefore, a large-scale randomized controlled study at multiple centers is required to confirm these results. Second, missing data, limited samples, and nonrandomization may have influenced the results and statistical analyses. Because of missing data on blood pressure levels, we could not fully calculate the risk of greater than 10% of myocardial infarction. However, we conducted this study using propensity score adjusted analysis to minimize potential biases between the groups. Thus, we believe that our study demonstrates the benefits of aggressive screening for T2D patients with advanced vascular complications. Third, the difference of the follow-up examination frequency between the 2 groups might influence the results. The main results of our study, however, would not change because the aggressive screening could result in frequent follow-up that would find cardiovascular events earlier in the aggressive screened patients than in the conventional screened patients and the difference of primary and secondary outcomes between the 2 groups might be even larger. Fourth, the incidence of acute myocardial infarction did not differ significantly between the groups. In this study as well, cardiac events did not include angina pectoris and heart failure. A longer and larger-scale follow-up will be required. Fifth, treatment strategies and interventions before and after screening were not designed; each patient received treatment that was optimal for them, as determined by their own physician with reference to the guideline-directed medical therapy. Sixth, this was an exploratory pilot study, and therefore, we did not calculate sample size and study power. Thus, our findings may not go much beyond the hypothesis and need to be validated by a randomized controlled trial with an appropriate sample size.
In conclusion, aggressive coronary screening for T2D patients with advanced vascular complications may identify those at a high risk of the composite endpoint of cardiovascular events and death. Further studies are needed to evaluate the effects of aggressive coronary screening in T2D patients.
Supplementary Material
Footnotes
Abbreviations: CAD = coronary artery disease, CAG = conventional coronary angiography, CKD = chronic kidney disease, DMR = diabetic retinopathy, eGFR = estimated glomerular filtration rate, MDCT = multidetector-row computed tomography, MPI = myocardial perfusion imaging, PAD = peripheral arterial disease, SPECT = single photon emission computed tomography, T2D = type 2 diabetes.
Authorship: TT conceived the study. TT and TS designed the protocol. MM and KK evaluated the SPECT images, and MK and HH evaluated the 64-slice MDCT and conventional CAG images. TT, TS, RY-H, HK, and MKi contributed to data collection. TT, TS, RY-H, HN, MKa, and MN analyzed all the data. TT, TS, RY-H, and MN wrote the report. All the authors contributed to the interpretation of the results and approved the final version. TT had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding/support: This research was funded by a grant from the Japan Diabetes Foundation.
Mitsuhiko Noda has received speaker honoraria from Sanofi, Mitsubishi Tanabe Pharma, Daiichi Sankyo, Eli Lilly Japan, MSD, Sanwa Kagaku Kenkyusho, Ono Pharmaceutical Co. Ltd, Takeda Pharmaceutical Co. Ltd, Astellas, Kowa Pharmaceutical Co. Ltd, Taisho Toyama Pharmaceutical Co. Ltd, Kissei Pharmaceutical Co. Ltd, Meiji Seika Pharma Co. Ltd, Kyowa Hakko Kirin Co. Ltd, ABBVie Inc., and Johnson & Johnson K.K., and research grants from Takeda Pharmaceutical Co. Ltd, Daiichi Sankyo, Mitsubishi Tanabe Pharma, and Kyowa Hakko Kirin Co. Ltd. The other authors have no conflicts of interest to disclose.
This study was approved by the institutional review board of the National Center for Global Health and Medicine.
Supplemental Digital Content is available for this article.
References
- 1.Gregg EW, Li Y, Wang J, et al. Changes in diabetes-related complications in the United States, 1990-2010. N Engl J Med 2014; 370:1514–1523. [DOI] [PubMed] [Google Scholar]
- 2.Franklin K, Goldberg RJ, Spencer F, et al. Implications of diabetes in patients with acute coronary syndromes. The Global Registry of Acute Coronary Events. Arch Intern Med 2004; 164:1457–1463. [DOI] [PubMed] [Google Scholar]
- 3.Kelly TN, Bazzano LA, Fonseca VA, et al. Systematic review: glucose control and cardiovascular disease in type 2 diabetes. Ann Intern Med 2009; 151:394–403. [DOI] [PubMed] [Google Scholar]
- 4.Hammoud T, Tanguay JF, Bourassa MG. Management of coronary artery disease: therapeutic options in patients with diabetes. J Am Coll Cardiol 2000; 36:355–365. [DOI] [PubMed] [Google Scholar]
- 5.Scognamiglio R, Negut C, Ramondo A, et al. Detection of coronary artery disease in asymptomatic patients with type 2 diabetes mellitus. J Am Coll Cardiol 2006; 47:65–71. [DOI] [PubMed] [Google Scholar]
- 6.Wackers FJ, Young LH, Inzucchi SE, et al. Detection of silent myocardial ischemia in asymptomatic diabetic subjects: the DIAD study. Diabetes Care 2004; 27:1954–1961. [DOI] [PubMed] [Google Scholar]
- 7.Deedwania PC, Carbajal EV. Silent ischemia during daily life is an independent predictor of mortality in stable angina. Circulation 1990; 81:748–756. [DOI] [PubMed] [Google Scholar]
- 8.Yeung AC, Barry J, Orav J, et al. Effects of asymptomatic ischemia on long-term prognosis in chronic stable coronary disease. Circulation 1991; 83:1598–1604. [DOI] [PubMed] [Google Scholar]
- 9.Maddox TM, Stanislawski MA, Grunwald GK, et al. Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA 2014; 312:1754–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young LH, Wackers FJ, Chyun DA, et al. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA 2009; 301:1547–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muhlestein JB, Lappe DL, Lima JA, et al. Effect of screening for coronary artery disease using CT angiography on mortality and cardiac events in high-risk patients with diabetes: the FACTOR-64 randomized clinical trial. JAMA 2014; 312:2234–2243. [DOI] [PubMed] [Google Scholar]
- 12.Cheung N, Wang JJ, Klein R, et al. Diabetic retinopathy and the risk of coronary heart disease: the Atherosclerosis Risk in Communities Study. Diabetes Care 2007; 30:1742–1746. [DOI] [PubMed] [Google Scholar]
- 13.Tonelli M, Jose P, Curhan G, et al. Proteinuria, impaired kidney function, and adverse outcomes in people with coronary disease: analysis of a previously conducted randomised trial. BMJ 2006; 332:1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351:1296–1305. [DOI] [PubMed] [Google Scholar]
- 15.Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease: morbidity and mortality implications. Circulation 2006; 114:688–699. [DOI] [PubMed] [Google Scholar]
- 16.Bhatt DL, Steg PG, Ohman EM, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA 2006; 295:180–189. [DOI] [PubMed] [Google Scholar]
- 17.Tsujimoto T, Kajio H, Takahashi Y, et al. Asymptomatic coronary heart disease in patients with type 2 diabetes with vascular complications: a cross-sectional study. BMJ Open 2011; 1:e000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53:982–992. [DOI] [PubMed] [Google Scholar]
- 19.Austen WG, Edwards JE, Frye RL, et al. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation 1975; 51:5–40. [DOI] [PubMed] [Google Scholar]
- 20.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011; 46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorajja P, Chareonthaitawee P, Rajagopalan N, et al. Improved survival in asymptomatic diabetic patients with high-risk SPECT imaging treated with coronary artery bypass grafting. Circulation 2005; 112:I311–316. [DOI] [PubMed] [Google Scholar]
- 22.Targher G, Bertolini L, Zenari L, et al. Diabetic retinopathy is associated with an increased incidence of cardiovascular events in Type 2 diabetic patients. Diabet Med 2008; 25:45–50. [DOI] [PubMed] [Google Scholar]
- 23.Rajagopalan N, Miller TD, Hodge DO, et al. Identifying high-risk asymptomatic diabetic patients who are candidates for screening stress single-photon emission computed tomography imaging. J Am Coll Cardiol 2005; 45:43–49. [DOI] [PubMed] [Google Scholar]
- 24.Doobay AV, Anand SS. Sensitivity and specificity of the ankle-brachial index to predict future cardiovascular outcomes: a systematic review. Arterioscler Thromb Vasc Biol 2005; 25:1463–1469. [DOI] [PubMed] [Google Scholar]
- 25.van Hecke MV, Dekker JM, Stehouwer CD, et al. Diabetic retinopathy is associated with mortality and cardiovascular disease incidence: the EURODIAB prospective complications study. Diabetes Care 2005; 28:1383–1389. [DOI] [PubMed] [Google Scholar]
- 26.Pop-Busui R, Evans GW, Gerstein HC, et al. Effects of cardiac autonomic dysfunction on mortality risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care 2010; 33:1578–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spallone V, Ziegler D, Freeman R, et al. Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev 2011; 27:639–653. [DOI] [PubMed] [Google Scholar]
- 28.Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007; 356:1503–1516. [DOI] [PubMed] [Google Scholar]
- 29.Group BDS, Frye RL, August P, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009; 360:2503–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogawa H, Nakayama M, Morimoto T, et al. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA 2008; 300:2134–2141. [DOI] [PubMed] [Google Scholar]
- 31.Pignone M, Alberts MJ, Colwell JA, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: a position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Diabetes Care 2010; 33:1395–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


