Abstract
Background:
Although gemcitabine and platinum-based agents (GP) are currently regarded as the standard chemotherapy for advanced biliary tract cancer (BTC), the prognosis remains poor. Combinations with fluoropyrimidines and targeted therapy have demonstrated modest benefits. Therefore, we conducted a meta-analysis of randomized controlled trials to evaluate the efficacy of different chemotherapy regimens.
Methods:
The PubMed, EMBASE, Cochrane Library, Scopus, and ClinicalTrials.gov registries were searched for studies published until April 2016. A meta-analysis was conducted to calculate the pooled effect size by using random effects models. Treatment efficacies were measured using progression-free survival (PFS) and overall survival. The secondary outcomes included the objective response rate (ORR), 1-year survival rate, quality of life, disease control rate, and adverse events.
Results:
Fifteen trials that involved examining 1775 patients were reviewed. Patients who received epidermal growth factor receptor (EGFR)-targeted therapy in addition to standard GP chemotherapy exhibited a significantly higher median PFS (weighted mean difference = −1.49; 95% confidence interval −2.56 to −0.43), PFS (hazard ratio = 0.79; 95% confidence interval 0.63–0.99), and ORR (odd ratio = 0.56; 95% confidence interval 0.38–0.82). Combining GP with fluoropyrimidines or vascular EGFR inhibitors (VEGFR) did not improve patient outcomes.
Conclusion:
Combining EGFR-targeted therapy with the current standard GP chemotherapy is a safe and viable option that may improve the median PFS, PFS, and ORR in patients with advanced BTC. Further research investigating the optimal dosage and drug type of EGFR inhibitors for specific BTC patient groups is warranted.
Keywords: biliary tract cancer, chemotherapy, gemcitabine, meta-analysis, targeted therapy
1. Introduction
Biliary tract cancer (BTC) includes intrahepatic cholangiocarcinoma, extrahepatic cholangiocarcinoma, gallbladder cancer, and ampullary cancer. These types of cancer are highly prevalent in East Asia and are gradually increasing in western countries.[1] Although resection is a curative treatment, only 10% of patients are diagnosed at a sufficiently early stage to undergo operation, and recurrence is common.[2] Therefore, palliative chemotherapy is essential for patients with advanced BTC.[3,4]
Combination chemotherapy with gemcitabine and platinum-based (GP) agents such as cisplatin or oxaliplatin is somewhat established as the current standard treatment according to the results from Advanced Biliary Cancer (ABC 01 and ABC 02) phase 2 and phase 3 randomized trials.[5,6] However, the prognosis remains poor because the median overall survival (OS) for patients remained less than 1 year.[5] Randomized phase 2 trials have investigated the efficacy of combination regimens with drugs, such as fluoropyrimidines and epidermal growth factor receptor (EGFR) inhibitors.[2,6–10]
Different chemotherapy regimens composed of gemcitabine, platinum-based agents, fluoropyrimidines, EGFR, and vascular EGFR inhibitors (VEGFR) have demonstrated varying progression-free survival (PFS) rate, response rate (RR), OS, and toxicity outcomes for patients with advanced BTC.[2,5–7] Several randomized controlled trials (RCTs) have investigated the effectiveness of different chemotherapy regimens. However, these studies have not been able to provide conclusive results. Recent systematic reviews have reaffirmed the efficacy of gemcitabine and cisplatin.[11,12] Nevertheless, combination therapy with other drugs, such as fluoropyrimidines and EGFR inhibitors, was not administered. Moreover, several recently published RCTs were not included.[7–10,13] Therefore, we conducted a systematic review and meta-analysis of the evidence available up to date to investigate the effectiveness of different regimens in patients with advanced BTC.
2. Methods
2.1. Inclusion and exclusion criteria
RCTs evaluating the outcome of chemotherapy in patients with advanced BTC were included in this review. Studies were also required to clearly report the inclusion and exclusion criteria for patients, the chemotherapy regimens, the stage of BTC, and the definition and evaluation of prognostic outcomes. We excluded trials that met at least 1 of the following criteria: patients who received radiotherapy <4 weeks, patients who received chemotherapy <6 months, or patient cohorts reported in duplicates.
2.2. Search strategy and study selection
The PubMed, Embase, Scopus, and Cochrane databases were electronically searched for relevant studies published until April 2016. The following Medical Subject Headings terms were used: (((biliary tract OR ampulla vater) AND (cancer OR adenocarcinoma)) OR cholangiocarcinoma), gemcitabine, cisplatin OR oxaliplatin, EGFR inhibitor OR cetuximab OR erlotinib, tyrosine kinase inhibitor OR angiogenesis inhibitor OR VEGF inhibitor OR sorafenib OR bevacizumab, fluoropyrimidine OR S-1, ((combination OR adjuvant OR targeted) AND (therapy OR chemotherapy)). The “similar articles” option in PubMed was used for broadening the search, and all retrieved abstracts, studies, and citations were reviewed. In addition, we identified other studies by using the reference sections of relevant papers and by corresponding with subject experts. Finally, unpublished studies were collected from the ClinicalTrials.gov registry (http://clinicaltrials.gov/). No language restrictions were applied. The systematic review described herein has been accepted by PROSPERO, an online international prospective register of systematic reviews, curated by the National Institute for Health Research (CRD42015029452).
2.3. Data extraction
Baseline and outcome data were independently abstracted by 2 reviewers (LC and CC), and the study designs, study population characteristics, inclusion and exclusion criteria, chemotherapy regimens, complications, and post-treatment parameters were extracted. Decisions individually recorded by the reviewers were compared, and disagreements were resolved by a third reviewer (KWT). The authors of the studies were contacted for additional information.
2.4. Methodological quality appraisal
Two reviewers (LC and CC) independently assessed the methodological quality of each study by using the risk of bias method recommended by the Cochrane Collaboration. Several domains were assessed, including the adequacy of the randomization, allocation concealment, blinding of the patients and outcome assessors, length of follow-up, information provided to the patients regarding study withdrawals, whether intention-to-treat analysis was performed, and freedom from other biases.
2.5. Outcomes
The primary outcomes were PFS and OS. Secondary outcomes included ORR, 1-year survival, quality of life (QoL), disease control rate (DCR), and adverse events (AEs). ORRs were determined according to the Response Evaluation Criteria in Solid Tumors 1.0 and 1.1.[14] QoL was measured with the European Organization for Research and Treatment of Cancer QLQ-C30 version 3.0.[15]
2.6. Statistical analyses
Data were entered and analyzed using Review Manager, version 5.3 (The Cochrane Collaboration, Oxford, UK). The meta-analysis was performed in accordance with the PRISMA guidelines.[16] Standard deviations were estimated from the provided confidence interval (CI) limits or standard error. Dichotomous outcomes were analyzed using odds ratios (ORs) as the summary statistic. Furthermore, continuous outcomes were analyzed using the weighted mean difference (WMD), and the time-related endpoints (PFS and OS) were reported as hazard ratios (HRs). The precision levels of the effect sizes were reported as 95% CI. A pooled estimate of the WMD was computed using the DerSimonian and Laird random effect model.[17]
To evaluate the statistical heterogeneity and inconsistency of treatment effects across studies, Cochrane Q tests and I2 statistics, respectively, were used. Statistical significance was set at P < 0.10 for Cochrane Q tests. Moreover, statistical heterogeneity across studies was assessed using the I2 statistics, which quantified the proportion of the total outcome variability across the studies.
This study used published data and thus ethical approval was not necessary.
3. Results
3.1. Trial characteristics
Figure 1 presents a flowchart describing the trial screening and selection procedure. The initial search strategy yielded 2131 studies, and 1517 among these were ineligible on the basis of the criteria used for screening titles and abstracts. Therefore, the full texts of 614 studies were retrieved. However, most were excluded from our final review because of the following reasons: 346 were non-RCTs; 65 were cellular or animal experiments; 126 included other treatments; and 62 included other cancer types. Hence, 15 studies were included in the current study.[2,5–10,13,18–24]
Figure 1.
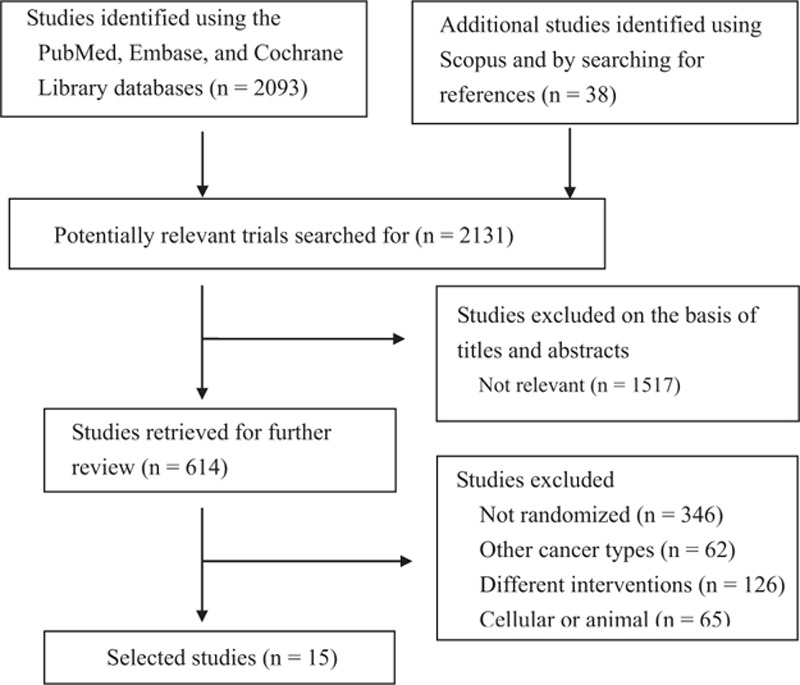
Flowchart describing the inclusion of studies.
These 15 studies were published between 2010 and 2015, and had sample sizes ranging from 62 to 410 patients. Among the included trials, Lee et al,[6] Choi et al,[18] and Kim et al[19] have published 3 trials investigating different outcome measurements from the same patients.[17] Valle et al[21,22] also published a trial that included data from their previous study.[5] All trials have enrolled patients with advanced, metastatic, or unresectable BTC. Patients received varying gemcitabine treatment regimens in different studies. The median PFS was investigated in 11 studies, and 7 studies evaluated its HS. OS was also investigated in 9 studies, and 7 studies evaluated its HR. Chemotherapy sessions lasted approximately 3 hours and treatment durations were adjusted according to various protocols. Baseline characteristics in the treatment groups of the included RCTs were balanced (Table 1).
Table 1.
Characteristics of the included randomized controlled trials.
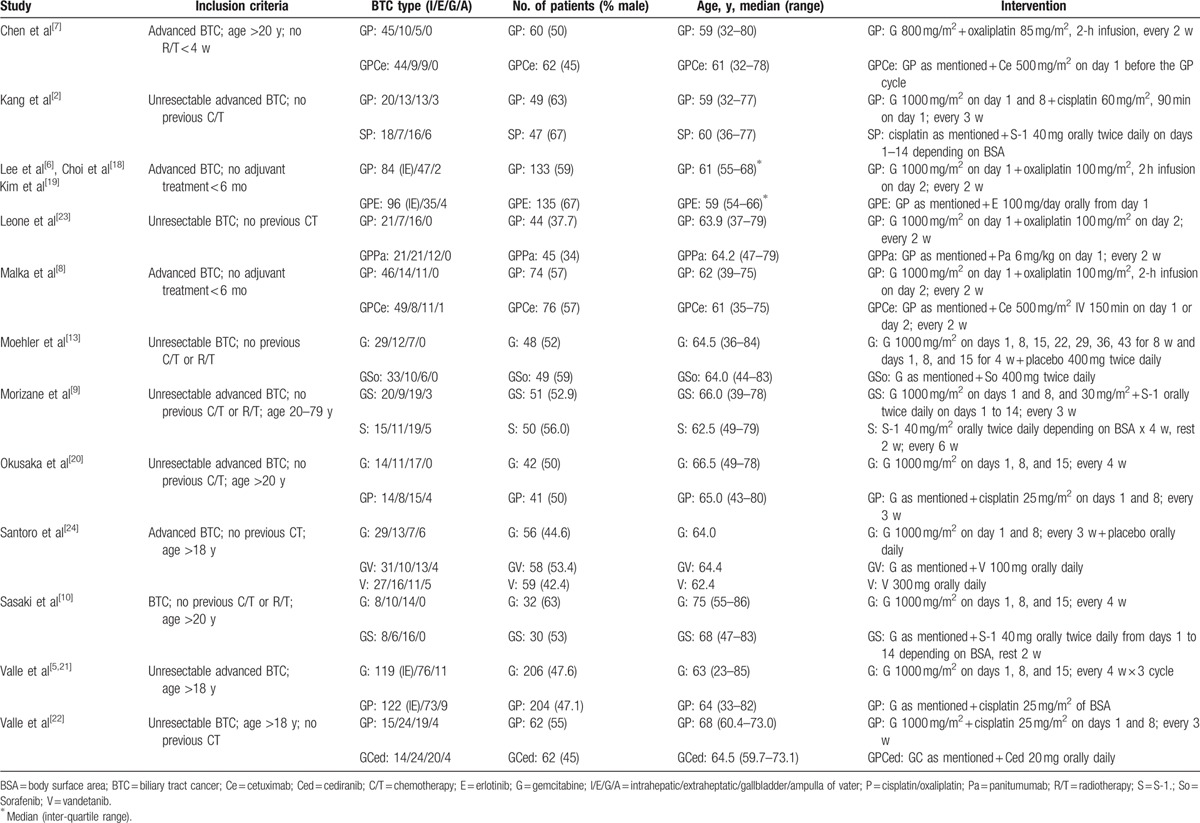
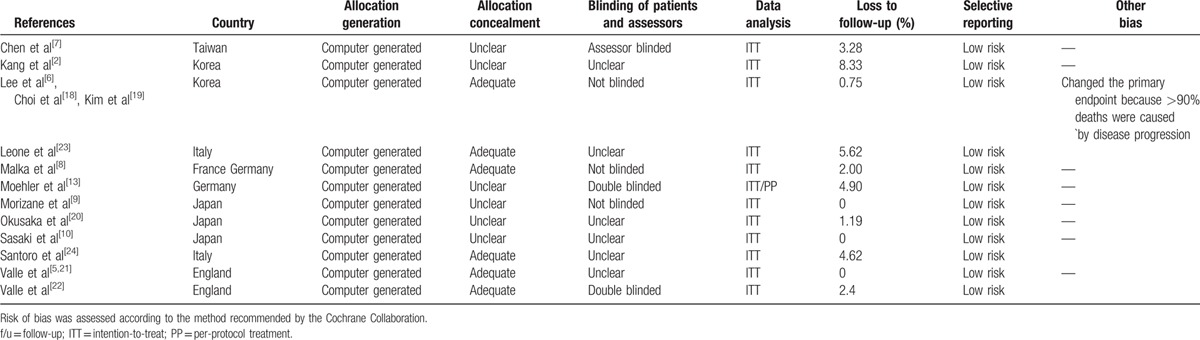
Table 2 summarizes a summary of the methodological quality of the included trials. All 9 studies reported acceptable methods of randomization. Six studies have not described allocation concealment details. Furthermore, 6 studies did not describe the blinding of patients and outcome assessors. All analyses were performed on an intention-to-treat basis and 1 study included data on per-protocol treatment.[13] The number of patients lost to follow-up was acceptable (<20%) in all studies. One study changed its primary endpoint because more than 90% of the deaths were caused by disease progression.[6] Other biases observed in the studies included stratification imbalances and crude primary endpoint selection.[6,8]
Table 2.
Assessment of methodological quality of included studies.
3.1.1. Gemcitabine and cisplatin
Two studies[5,20] investigated the efficacy of GP combination chemotherapy with gemcitabine monotherapy and reported a significantly higher median PFS (WMD = −2.60; 95% CI −3.81 to −1.40) and PFS (HR = 0.63; 95% CI 0.52–0.76) for the gemcitabine–cisplatin group (Fig. 2). GP combination chemotherapy group also exhibited a significantly higher median OS (WMD = −3.25; 95% CI −5.14 to −1.35; Fig. 2) and OS (HR = 0.65; 95% CI 0.53–0.79; Fig. 3), and objective RR (ORR) (OR = 0.53; 95% CI 0.31–0.88; Fig. 4).
Figure 2.
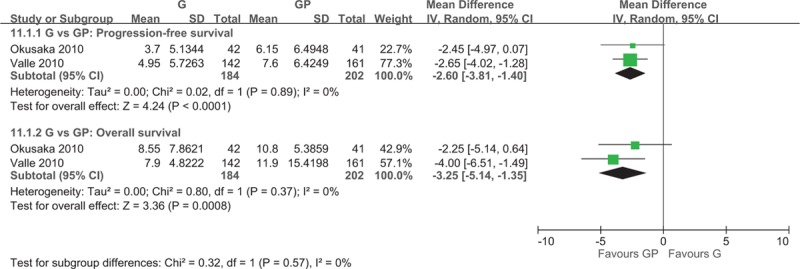
Forest plot of comparison between gemcitabine and gemcitabine and cisplatin. The outcome was the duration of 11.1.1 Median progression-free survival, 11.1.2 Median overall survival.
Figure 3.
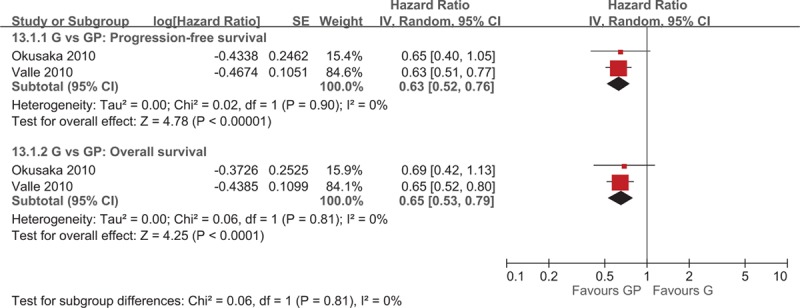
Forest plot of comparison between gemcitabine and gemcitabine and cisplatin. The outcome was hazard ratio of 13.1.1 Progression-free survival, 13.1.2 Overall survival.
Figure 4.
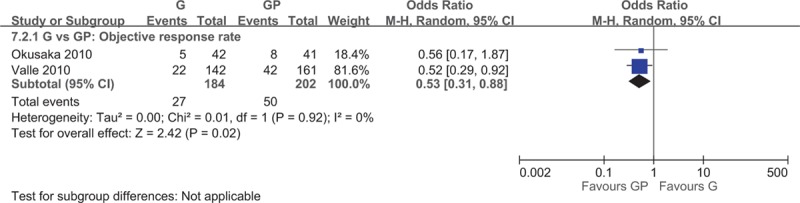
Forest plot of comparison between gemcitabine and gemcitabine and cisplatin, objective response rate.
3.1.2. Fluoropyrimidine
Sasaki et al[10] observed no significant difference in the median OS (WMD = 1.05; 95% CI −4.33 to 6.43) and ORR (OR = 0.41; 95% CI 0.09–1.83) for the gemcitabine and S-1 group compared with the gemcitabine monotherapy group.
Morizane et al[9] examined the effectiveness of gemcitabine and S-1 with that of S-1 monotherapy and reported a significantly lower median PFS (WMD = 3.40; 95% CI 1.54–5.26) for the S-1 monotherapy treatment group. The ORR (OR = 2.71; 95% CI 1.02–7.23) for the combination group was significantly higher than the S-1 monotherapy group. Kang et al[2] reported no difference in the median PFS (WMD = 0.25; 95% CI −2.06 to 2.56) between the gemcitabine and cisplatin and S-1 plus cisplatin groups. Moreover, no significant difference was observed in the median OS (WMD = 3.40; 95% CI 1.54–5.26) and OS (HR = 0.73; 95% CI 0.45–1.17), and ORR (OR = 0.83; 95% CI 0.30–2.28) between the 2 groups.
3.1.3. Targeted therapy
A total of 7 studies investigated the efficacy of targeted therapies including EGFR and VEGFR inhibitors.[6–8,13,22–24] Four trials examined the efficacy of incorporating targeted therapies in addition to GP chemotherapy[6–8,23] and reported a significantly higher median PFS (WMD = −1.49; 95% CI −2.56 to −0.43) for patients who used EGFR inhibitors in addition to combination chemotherapy (Fig. 5). Chen et al[7] and Malka et al[8] have not reported the HR of PFS, whereas Lee et al[6] and Leone et al[23] reported the PFS (HR = 0.79; 95% CI 0.63 to 0.99) significantly favoring the target therapy group (Fig. 6). No significant difference was observed in the median OS for patients receiving the additional EGFR-targeted therapy (WMD = −0.07; 95% CI −1.91 to 1.77; Fig. 5). Lee et al[6] and Leone et al[23] reported no difference in OS (HR = 0.90; 95% CI 0.70–1.15) between groups. The ORR (OR = 0.56; 95% CI 0.38–0.82) was significantly higher for patients who received EGFR-targeted therapy (Fig. 7).
Figure 5.
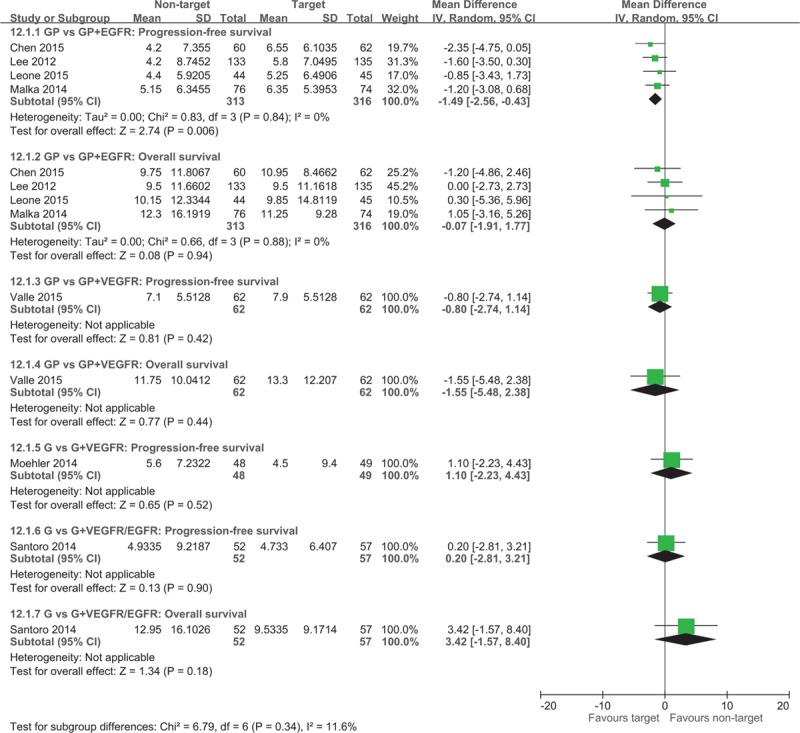
Forest plot of comparison between non-target therapy and target therapy. The outcome was the duration of 12.1.1 Median progression-free survival, 12.1.2 Median overall survival.
Figure 6.
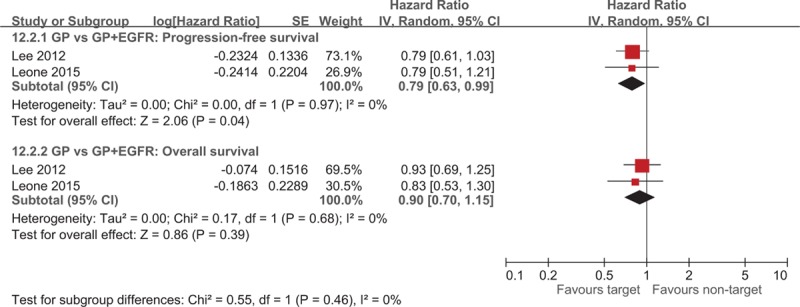
Forest plot of comparison between nontarget therapy and target therapy. The outcome was hazard ratio of 12.2.1 Progression-free survival, 12.2.2 Overall survival.
Figure 7.
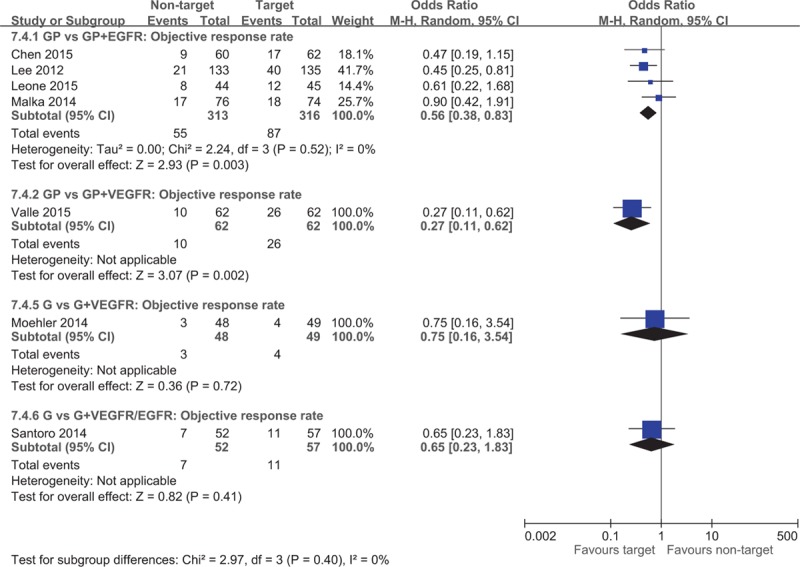
Forest plot of comparison between nontarget therapy and target therapy, objective response rate.
Moehler et al[13] found no difference in the median PFS (WMD = 1.10; 95% CI −2.23 to 4.43) and PFS (HR = 1.28; 95% CI 0.81–2.02) for the gemcitabine monotherapy group compared with the VEGFR-targeted therapy group. The median OS reported by Moehler et al[13] was not pooled because CIs were not reported, and no difference was observed in OS (HR = 1.20; 95% CI 0.75–1.93). Furthermore, Moehler et al[13] observed no difference in ORR (OR = 0.75; 95% CI 0.16–3.54) between the groups (Fig. 7). Valle et al[22] also found no difference in the median PFS, PFS HR, OS, and OS HR whether or not VEGFR-targeted therapy was administered in addition to standard GP chemotherapy. However, the ORR was significantly higher for the GP and VEGFR-targeted therapy group. Santoro et al[24] reported no difference in median PFS, OS, and ORR for gemcitabine monotherapy with or without VEGFR and EGFR-targeted therapy.
3.2. Other measurements
Other measurements include the 1-year survival, QoL, and DCR. Okusaka et al[20] reported a higher 1-year survival rate for the gemcitabine and cisplatin group (39.0%) than the gemcitabine monotherapy group (31.0%). Morizane et al[9] found a significantly higher 1-year survival rate for the gemcitabine plus S-1 group (52.9%; 95% CI 38.5–65.5) than the S-1 monotherapy group (40%; 95% CI 26.5–53.1). Furthermore, Moehler et al[13] reported that the QoL measurements did not generally favor any treatment group. Lee et al[6] also included the QoL as a secondary endpoint but did not include the data because of low compliance rates. Chen et al[7] observed a significantly higher DCR (P = 0.02) for the GP and EGFR-targeted therapy group (58%) than for the GP-only group (37%). Valle et al[22] found no difference in DCR between the GP-only group and the GP and VEGFR-targeted therapy group. Santoro et al[24] reported no significant difference in DCR whether or not VEGFR-targeted therapy was applied in combination with gemcitabine monotherapy.
3.3. Complications
Two studies reported significantly more grade 3 to 4 hematologic AEs, including anemia (OR = 2.84; 95% CI 1.38–5.83) and leukopenia (OR = 1.76; 95% CI 1.04–2.97), in the GP combination chemotherapy group than in the gemcitabine monotherapy group.[5,20] Other hematologic AEs, such as thrombocytopenia and raised alanine aminotransferase (ALT), levels were also higher in the monotherapy group, although statistically insignificant. No differences were observed in nonhematologic AEs such as anorexia, vomiting, and diarrhea.
Three studies investigated in the efficacy of fluoropyrimidines.[2,9,10] Two studies have compared gemcitabine and S-1 with gemcitabine or S-1 monotherapy.[9,10] Grade 3 to 4 leukopenia events (OR = 1.82, 95% CI 0.57–5.83) were significantly less frequent in the S-1 monotherapy group compared with the gemcitabine and S-1. Patients who received gemcitabine monotherapy also reported less leukopenia events, but the difference was statistically insignificant. No differences were observed in hematologic AEs, including anemia, thrombocytopenia, raised ALT levels, and nonhematologic AEs such as anorexia, vomiting, and diarrhea. Moreover, Kang et al[2] reported significantly more frequent 3 to 4 anemia, leukopenia, thrombocytopenia in the GP group than in the S-1 and cisplatin group. The study reported no differences in raised ALT levels, anorexia, vomiting, and diarrhea.[2]
All 4 studies investigating EGFR inhibitors reported no significant differences in hematologic AEs and nonhematologic AEs.[6–8,23] The 2 studies that compared gemcitabine and GP with or without VEGFR-targeted therapy both found no differences for both hematologic and nonhematologic AEs whether or not VEGFR-targeted therapy was used added.[13,22] Santoro et al[24] compared gemcitabine with or without EGFR and VEGFR-targeted therapy and reported no differences in hematologic and nonhematologic AEs.
4. Discussion
The results of the present meta-analysis reveal that GP doublet chemotherapy is the most effective regimens among other combinations of gemcitabine, platinum-based agents, and fluoropyrimidines. One trial that compared cisplatin and S-1 chemotherapy with the standard GP regimen reported no statistically significant difference in the median PFS, median OS, OS HR, and ORR.[2] However, other combinations with fluoropyrimidines, including S-1 monotherapy and gemcitabine and S-1 chemotherapy, were significantly less effective, with S-1 monotherapy demonstrating the least favorable outcomes.[9,10] Regarding targeted therapies, the addition of VEGFR-targeted therapy to gemcitabine or GP chemotherapy did not improve patient outcomes.[13,24] Combined VEGFR and EGFR tyrosine kinase inhibitor in addition to gemcitabine also did not improve PFS or OS.[24] In contrast, incorporating EGFR-targeted therapy with the recently established standard GP chemotherapy demonstrated certain advantages over GP chemotherapy alone. As compared with GP chemotherapy, EGFR-targeted therapy and GP chemotherapy was associated with a significantly higher median PFS, PFS HR, and ORR.
All included trials have reported the analysis patients with cholangiocarcinoma and gallbladder cancer, while 8 of the trials included Ampulla of Vater cancer cases.[2,5,6,8,9,22,24] Overall, 67.3% of the studied population had intra or extrahepatic cholangiocarcinoma, 28.5% had gallbladder cancer, and 4.0% had Ampulla of Vater cancer. The treatment outcomes in different cancer types were not reported separately; therefore, we cannot analyze the respective efficacy of chemotherapies in each cancer type. Patients with cholangiocarcinoma typically have the least favorable outcomes. Because the aforementioned results were mostly obtained from patients with cholangiocarcinoma, we believe that the GP doublet chemotherapy can be equally or more effective in patients with gallbladder and Ampulla of Vater cancer. In the future, more research investigating the dosage and frequency of chemotherapies for different BTC types may reduce AEs while retaining its treatment effect.
The dosage and frequency of drug administration may influence treatment outcomes. In the 3 trials that investigated the efficacy of S-1, Kang et al[2] reported that S-1 and cisplatin chemotherapy is as effective as the standard GP chemotherapy. Interestingly, in this study, S-1 was administered with only 1 week of rest every 2 weeks, a notably shorter period than the other studies.[2] Therefore, although chemotherapies with S-1 have not yielded desirable results, well-designed RCTs may be warranted for evaluating the efficacy and safety of higher dosage and higher frequency of S-1 in treating BTC.
Targeted therapy has shown certain benefits in other cancer types,[25] and its effects for BTC are being investigated. In our meta-analysis, VEGFR-targeted therapy could not improve BTC patient outcomes. Sorafenib, the multi-tyrosine kinase inhibitor used in this study, functions on the basis of the expression levels of their targeted proteins in the tumor tissue.[26] The absence of targeted proteins, such as c-kit and VEGFR-2, may have contributed to its low efficacy.[13] Fortunately, EGFR-targeted therapy and GP demonstrated a significantly higher median PFS, PFS HR, and ORR than the standard GP chemotherapy. However, our included trials[6,8] have applied diverse drug types and dosages. Additional investigations are required for clarifying the optimal drug dosage and type of EGFR inhibitors for specific BTC patient groups.
In the included trials, Chen et al[7] stratified randomized the patients according to their KRAS mutation status, and Malka et al[8] evaluated 4-mouth PFS according to KRAS, BRAF, and EGFR tumor status; both trials revealed that the prognostic outcomes did not correlate with the tumor mutation status. Lee et al[6] obtained 60 tissue specimens in 133 patients treated with chemotherapy and erlotinib for DNA analysis; EGFR overexpression was recorded in 12 of 28 (3%) patients, and only 6 of 60 (10%) patients had a KRAS mutation (codon 12), because the predictive value of tumor mutation status for response to erlotinib is limited by the small number of tissues; further investigation is needed to identify those patients who are likely to respond to erlotinib.
The studies included in our analysis demonstrated considerable heterogeneity because of various clinical factors. First, the drug dose and frequency were inconsistent among the studies. Second, BTC involved numerous carcinomas types. Finally, ethnic diversities among the patient population may also contribute to different outcomes. Such diversities among studies resulted in heterogeneity.
This study has several limitations. First, certain trials have enrolled a relatively small sample size of patients recruited per treatment group. Second, several of the primary and secondary outcomes were variably reported. Moreover, 1 study design included stratification factors of center and the presence of measurable disease, thus resulting in imbalanced population considering primary tumor location.[6] Another study design[8] precluded formal statistical comparisons, thus potentially limiting the inferences based on our analysis.
5. Conclusions
Our meta-analysis revealed that GP combination chemotherapy is the most effective regimen among other combinations of gemcitabine, platinum-based agents, and fluoropyrimidines. Individual RCTs did not report significant treatment effects; however, our pooled data revealed promising results of EGFR-targeted therapy in increasing the survival rate of advanced BTC patients. This finding may facilitate the effective treatment of patients with cancer.
Footnotes
Abbreviations: AEs = adverse events, BTC = biliary tract cancer, CI = confidence interval, DCR = disease control rate, EGRF = epidermal growth factor receptor, GP = gemcitabine and platinum-based, HR = hazard ratios, OR = odd ratios, ORR = objective response rate, OS = overall survival, PFS = progression-free survival, QoL = quality of life, RCT = randomized controlled trials, RR = response rate, VEGF = vascular epidermal growth factor receptor inhibitors, WMD = weighted mean difference.
LC and CC contributed equally to this work.
Funding: This research was supported by Cochrane Taiwan, Taipei Medical University, Taipei, Taiwan.
The authors report no conflicts of interest.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin 2007; 57:43–66. [DOI] [PubMed] [Google Scholar]
- 2.Kang MJ, Lee JL, Kim TW, et al. Randomized phase II trial of S-1 and cisplatin versus gemcitabine and cisplatin in patients with advanced biliary tract adenocarcinoma. Acta Oncol 2012; 51:860–866. [DOI] [PubMed] [Google Scholar]
- 3.Glimelius B, Hoff man K, Sjoden PO, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol 1996; 7:593–600. [DOI] [PubMed] [Google Scholar]
- 4.Thongprasert S. The role of chemotherapy in cholangiocarcinoma. Ann Oncol 2005; 16 Suppl 2:ii93–ii96. [DOI] [PubMed] [Google Scholar]
- 5.Valle J, Wasan H, Palmer DH, et al. ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010; 362:1273–1281. [DOI] [PubMed] [Google Scholar]
- 6.Lee J, Park SH, Chang HM, et al. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2012; 13:181–188. [DOI] [PubMed] [Google Scholar]
- 7.Chen JS, Hsu C, Chiang NJ, et al. Taiwan Cooperative Oncology Group. A KRAS mutation status-stratified randomized phase II trial of gemcitabine and oxaliplatin alone or in combination with cetuximab in advanced biliary tract cancer. Ann Oncol 2015; 26:943–949. [DOI] [PubMed] [Google Scholar]
- 8.Malka D, Cervera P, Foulon S, et al. BINGO investigators. Gemcitabine and Oxaliplatin with or without cetuximab in advanced biliary-tract cancer (BINGO): a randomised, open-label, non-comparative phase 2 trial. Lancet Oncol 2014; 15:819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morizane C, Okusaka T, Mizusawa J, et al. Randomized phase II study of gemcitabine plus S-1 versus S-1 in advanced biliary tract cancer: a Japan Clinical Oncology Group trial (JCOG 0805). Cancer Sci 2013; 104:1211–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sasaki T, Isayama H, Nakai Y, et al. A randomized phase II study of gemcitabine and S-1 combination therapy versus gemcitabine monotherapy for advanced biliary tract cancer. Cancer Chemother Pharmacol 2013; 71:973–979. [DOI] [PubMed] [Google Scholar]
- 11.Fiteni F, Nguyen T, Vernerey D, et al. Cisplatin/gemcitabine or oxaliplatin/gemcitabine in the treatment of advanced biliary tract cancer: a systematic review. Cancer Med 2014; 3:1502–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park JO, Oh DY, Hsu C, et al. Gemcitabine plus cisplatin for advanced biliary tract cancer: a systematic review. Cancer Res Treat 2015; 47:343–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moehler M, Maderer A, Schimanski C, et al. Working Group of Internal Oncology. Gemcitabine plus sorafenib versus gemcitabine alone in advanced biliary tract cancer: a double-blind placebo-controlled multicentre phase II AIO study with biomarker and serum programme. Eur J Cancer 2014; 50:3125–3135. [DOI] [PubMed] [Google Scholar]
- 14.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. E J Natl Cancer Inst 2000; 92:205–216. [DOI] [PubMed] [Google Scholar]
- 15.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993; 85:365–376. [DOI] [PubMed] [Google Scholar]
- 16.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009; 62:e1–e34. [DOI] [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188. [DOI] [PubMed] [Google Scholar]
- 18.Choi MK, Choi JY, Lee J, et al. Prognostic and predictive value of metabolic tumor volume on (18)F-FDG PET/CT in advanced biliary tract cancer treated with gemcitabine/oxaliplatin with or without erlotinib. Med Oncol 2014; 31:23. [DOI] [PubMed] [Google Scholar]
- 19.Kim ST, Jang KT, Lee J, et al. Molecular subgroup analysis of clinical outcomes in a phase 3 study of gemcitabine and oxaliplatin with or without erlotinib in advanced biliary tract cancer. Transl Oncol 2015; 8:40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okusaka T, Nakachi K, Fukutomi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer 2010; 103:469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valle JW, Wasan H, Johnson P, et al. Gemcitabine alone or in combination with cisplatin in patients with advanced or metastatic cholangiocarcinomas or other biliary tract tumours: a multicentre randomised phase II study—The UK ABC-01 Study. Br J Cancer 2009; 101:621–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valle JW, Wasan H, Lopes A, et al. Cediranib or placebo in combination with cisplatin and gemcitabine chemotherapy for patients with advanced biliary tract cancer (ABC-03): a randomised phase 2 trial. Lancet Oncol 2015; 16:967–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leone F, Marino D, Cereda S, et al. Panitumumab in combination with gemcitabine and oxaliplatin does not prolong survival in wild-type KRAS advanced biliary tract cancer: a randomized phase 2 trial (Vecti-BIL study). Cancer 2016; 122:574–581. [DOI] [PubMed] [Google Scholar]
- 24.Santoro A, Gebbia V, Pressiani T, et al. A randomized, multicenter, phase II study of vandetanib monotherapy versus vandetanib in combination with gemcitabine versus gemcitabine plus placebo in subjects with advanced biliary tract cancer: the VanGogh study. Ann Oncol 2015; 26:542–547. [DOI] [PubMed] [Google Scholar]
- 25.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007; 25:1960–1966. [DOI] [PubMed] [Google Scholar]
- 26.Wehler TC, Hamdi S, Maderer A, et al. Single-agent therapy with sorafenib or 5-FU is equally effective in human colorectal cancer xenograft: no benefit of combination therapy. Int J Colorectal Dis 2013; 28:385–398. [DOI] [PMC free article] [PubMed] [Google Scholar]


