Abstract
Neuropathic pain is caused by a lesion or disease of the somatosensory system, including peripheral fibres (Aβ, Aδ and C fibres) and central neurons, and affects 7–10% of the general population. Multiple causes of neuropathic pain have been described and its incidence is likely to increase owing to the ageing global population, increased incidence of diabetes mellitus and improved survival from cancer after chemotherapy. Indeed, imbalances between excitatory and inhibitory somatosensory signalling, alterations in ion channels and variability in the way that pain messages are modulated in the central nervous system all have been implicated in neuropathic pain. The burden of chronic neuropathic pain seems to be related to the complexity of neuropathic symptoms, poor outcomes and difficult treatment decisions. Importantly, quality of life is impaired in patients with neuropathic pain owing to increased drug prescriptions and visits to health care providers, as well as the morbidity from the pain itself and the inciting disease. Despite challenges, progress in the understanding of the pathophysiology of neuropathic pain is spurring the development of new diagnostic procedures and personalized interventions, which emphasize the need for a multidisciplinary approach to the management of neuropathic pain.
Although distinct definitions of neuropathic pain have been used over the years, its most recent (2011) and widely accepted definition is pain caused by a lesion or disease of the somatosensory system. The somatosensory system allows for the perception of touch, pressure, pain, temperature, position, movement and vibration. The somatosensory nerves arise in the skin, muscles, joints and fascia and include thermoreceptors, mechanoreceptors, chemoreceptors, pruriceptors and nociceptors that send signals to the spinal cord and eventually to the brain for further processing (BOX 1); most sensory processes involve a thalamic nucleus receiving a sensory signal that is then directed to the cerebral cortex. Lesions or diseases of the somatosensory nervous system can lead to altered and disordered transmission of sensory signals into the spinal cord and the brain; common conditions associated with neuropathic pain include postherpetic neuralgia, trigeminal neuralgia, painful radiculopathy, diabetic neuropathy, HIV infection, leprosy, amputation, peripheral nerve injury pain and stroke (in the form of central post-stroke pain) (FIG. 1). Not all patients with peripheral neuropathy or central nervous injury develop neuropathic pain; for example, a large cohort study of patients with diabetes mellitus indicated that the overall prevalence of neuropathic pain symptoms was 21% in patients with clinical neuropathy. However, the prevalence of neuropathic pain increased to 60% in those with severe clinical neuropathy1. Importantly, neuropathic pain is mechanistically dissimilar to other chronic pain conditions such as inflammatory pain that occurs, for example, in rheumatoid arthritis, in which the primary cause is inflammation with altered chemical events at the site of inflammation; such pain is diagnosed and treated differently2.
Box 1. Key terms.
Action potential
An electrical event in which the membrane potential of a cell in the nervous system rapidly rises and falls to transmit electrical signals from cell to cell.
Allodynia
Pain caused by a normally non-painful stimulus.
Aβ fibres
Sensory nerve fibres with a thick myelin sheath, which insulates the axon of the cell and normally promotes the conduction of touch, pressure, proprioception and vibration signals (35–90 metres per second).
Aδ fibres
Sensory nerve fibres with a myelin sheath, which insulates the axon of the cell and promotes the conduction of cold, pressure and pain signals (5–30 metres per second), that produce the acute and sharp experience of pain.
C fibres
Unmyelinated pain nerve fibres that respond to warmth and a range of painful stimuli by producing a long-lasting burning sensation due to a slow conduction speed (0.5–2 metres per second).
Chemoreceptors
Receptors that transduce chemical signals.
Complex regional pain syndromes
Also known as causalgia and reflex sympathetic dystrophy, complex regional pain syndromes are conditions that are characterized by the presence of chronic, intense pain (often in one arm, leg, hand or foot) that worsens over time and spreads in the affected area. These conditions are typically accompanied by a colour or temperature change of the skin where the pain is felt.
Conditioned pain modulation
A reduction of a painful test stimulus under the influence of a conditioning stimulus.
Dynamic mechanical allodynia
A type of mechanical allodynia that occurs when pain is elicited by lightly stroking the skin.
Expectancy-induced analgesia
A reduction of pain experience due to anticipation, desire and belief of hypoalgesia or analgesia.
Hyperalgesia
A heightened experience of pain caused by a noxious stimulus.
Hypoalgesia
A decreased perception of pain caused by a noxious stimulus.
Mechanoreceptors
A sensory receptor that transduces mechanical stimulations.
Nociceptors
A peripheral nervous system receptor that is responsible for transducing and encoding painful stimuli.
Paradoxical heat sensation
An experienced sensation of heat provoked by a cold stimulus.
Provoked pain
Pain provoked by applying a stimulus.
Pruriceptors
Sensory receptors that transduce itchy sensations.
Second-order nociceptive neurons
Nociceptive neurons in the central nervous system that are activated by the Aβ, Aδ and C afferent fibres and convey sensory information from the spinal cord to other spinal circuits and the brain.
Static pain
Another kind of mechanical hyperalgesia in those with neuropathic pain when pain is provoked after gentle pressure is applied on the symptomatic area.
Temporal summation
The phenomenon in which progressive increases in pain intensity are experienced during the repetition of identical nociceptive stimuli.
Thermoreceptors
Sensory receptors that respond to changes in temperature.
Figure 1. The peripheral and central changes induced by nerve injury or peripheral neuropathy.
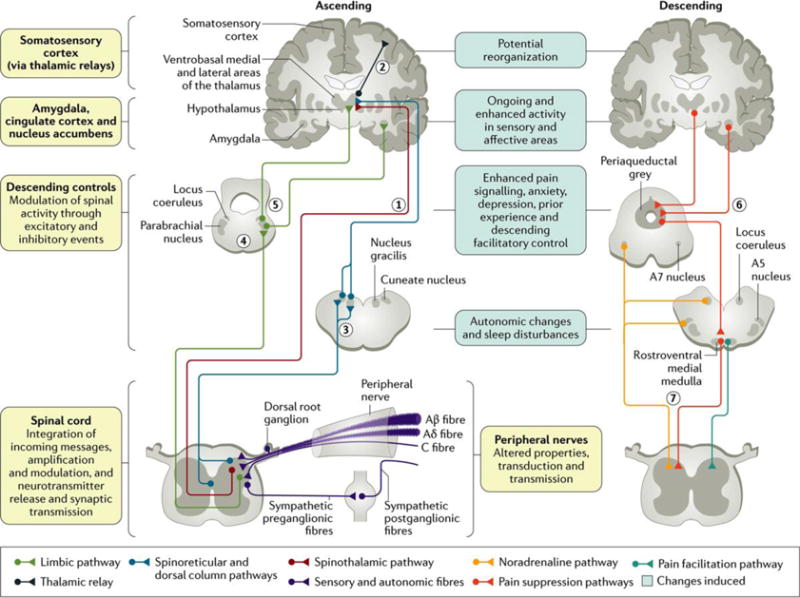
Preclinical animal studies have shown that damage to all sensory peripheral fibres (namely, Aβ, Aδ and C fibres; BOX 1) alters transduction and transmission due to altered ion channel function. These alterations affect spinal cord activity, leading to an excess of excitation coupled with a loss of inhibition. In the ascending afferent pathways, the sensory components of pain are via the spinothalamic pathway to the ventrobasal medial and lateral areas (1), which then project to the somatosensory cortex allowing for the location and intensity of pain to be perceived (2). The spinal cord also has spinoreticular projections and the dorsal column pathway to the cuneate nucleus and nucleus gracilis (3). Other limbic projections relay in the parabrachial nucleus (4) before contacting the hypothalamus and amygdala, where central autonomic function, fear and «anxiety are altered (5). Descending efferent pathways from the amygdala and hypothalamus (6) drive the periaqueductal grey, the locus coeruleus, A5 and A7 nuclei and the rostroventral medial medulla. These brainstem areas then project to the spinal cord through descending noradrenaline (inhibition via α2 adrenoceptors), and, in neuropathy, there is a loss of this control and increased serotonin descending excitation via 5-HT3 receptors (7). The changes induced by peripheral neuropathy on peripheral and central functions are shown. Adapted with permission from REF. 38, Mechanisms and management of diabetic painful distal symmetrical polyneuropathy, American Diabetes Association, 2013. Copyright and all rights reserved. Material from this publication has been used with the permission of American Diabetes Association.
Neuropathic pain is associated with increased drug prescriptions and visits to health care providers3,4. Patients typically experience a distinct set of symptoms, such as burning and electrical-like sensations, and pain resulting from non-painful stimulations (such as light touching); the symptoms persist and have a tendency to become chronic and respond less to pain medications. Sleep disturbances, anxiety and depression are frequent and severe in patients with neuropathic pain, and quality of life is more impaired in patients with chronic neuropathic pain than in those with chronic non-neuropathic pain that does not come from damaged or irritated nerves3,5.
Despite the increases of placebo responses6,7 that result in the failure of multiple new drugs in clinical trials, recent progress in our understanding of the pathophysiology of neuropathic pain provides optimism for the development of new diagnostic procedures and personalized interventions. This Primer presents the current descriptions of the presentation, causes, diagnosis and treatment of neuropathic pain, with a focus on peripheral neuropathic pain given that our knowledge is greater than that of central neuropathic pain.
Epidemiology
The estimation of the incidence and prevalence of neuropathic pain has been difficult because of the lack of simple diagnostic criteria for large epidemiological surveys in the general population. Thus, the prevalence of neuropathic pain in the chronic pain population has mainly been estimated on the basis of studies8 conducted by specialized centres with a focus on specific conditions, such as postherpetic neuralgia9, 10, painful diabetic polyneuropathy1,11–13, postsurgery neuropathic pain14, multiple sclerosis15, 16, spinal cord injury17, stroke18 and cancer19, 20.
The recent development of simple screening tools in the form of questionnaires21 has helped conduct several large epidemiological surveys in different countries (the United Kingdom, the United States, France and Brazil) and provided valuable new information on the general prevalence of neuropathic pain4. In using screening tools, such as the Douleur Neuropathique 4 questions (DN4)22 or the Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) pain scale23 (BOX 2), the prevalence of chronic pain with neuropathic characteristics has been estimated to be in the range of 7–10%8,24.
Box 2. Validated screening tools for neuropathic pain.
Symptom and clinical examination items can be assessed using distinct validated screening tools. The most common tools are listed below.
Leeds Assessment of Neuropathic Symptoms and Signs*
Four symptom items (pricking, tingling, pins and needles; electric shocks; hot or burning sensations; and pain evoked by light touching)
One item related to skin appearance (mottled or red)
Two clinical examination items (touch-evoked allodynia and altered pinprick sensation)
Douleur Neuropathique 4 questions‡
Seven symptom items (burning, painful cold, electric shocks, tingling, pins and needles, numbness and itching)
Three clinical examination items (touch hypoaesthesia (reduced sense), pinprick hypoaesthesia and brush-evoked allodynia)
Neuropathic Pain Questionnaire§
Seven sensory descriptors (burning pain, shooting pain, numbness, electrical-like sensations, tingling pain, squeezing pain and freezing pain)
Three items related to provoking factors (overly sensitive to touch, touch-evoked pain and increased pain due to weather change)
Two items describing affect (unpleasantness and overwhelming)
painDETECT||
Seven weighted symptom items (burning, tingling or prickling, touch-evoked pain, electric shocks, temperature-evoked pain, numbness and pressure-evoked pain)
Two items related to spatial (radiating pain) and temporal characteristics
ID Pain¶
Five symptom items (pins and needles, hot or burning, numbness, electrical shocks and touch-evoked pain)
One item related to location (joints)
Neuropathic Pain Symptom Inventory#
Ten descriptors (burning, pressure, squeezing, electrical shocks, stubbing, pain evoked by brushing, pain evoked by pressure, pain evoked by cold stimuli, pins and needles, and tingling)
Two temporal items (the temporal sequence of spontaneous ongoing pain and paroxysmal pain)
Five clinically relevant dimensions (evoked pain, paroxysmal pain, abnormal sensations, superficial and deep components of spontaneous ongoing pain)
*See REF 23. ‡See REF. 22. §See REF 195. ||See REF 64. ¶See REF 196. #See REF 65.
Chronic neuropathic pain is more frequent in women (8% versus 5.7% in men) and in patients >50 years of age (8.9% versus 5.6% in those <49 years of age), and most commonly affects the lower back and lower limbs, neck and upper limbs24. Lumbar and cervical painful radiculopathies are probably the most frequent cause of chronic neuropathic pain. Consistent with these data, a survey of >12,000 patients with chronic pain with both nociceptive and neuropathic pain types, referred to pain specialists in Germany, revealed that 40% of all patients experience at least some characteristics of neuropathic pain (such as burning sensations, numbness and tingling); patients with chronic back pain and radiculopathy were particularly affected25.
Mechanisms/pathophysiology
Research in the pain field has focused on understanding the plastic changes in the nervous system after nerve injury, identifying novel therapeutic targets and in facilitating the transfer of knowledge from animal models to clinical practice. We describe briefly the multiple causes of neuropathic pain and present an overview of animal and human findings that have provided insights on the pathophysiology of neuropathic pain.
Causes and distributions
Central neuropathic pain is due to a lesion or disease of the spinal cord and/or brain. Cerebrovascular disease affecting the central somatosensory pathways (poststroke pain) and neurodegenerative diseases (notably Parkinson disease) are brain disorders that often cause central neuropathic pain26. Spinal cord lesions or diseases that cause neuropathic pain include spinal cord injury, syringomyelia and demyelinating diseases, such as multiple sclerosis, transverse myelitis and neuromyelitis optica27. By contrast, the pathology of the peripheral disorders that cause neuropathic pain predominantly involves the small unmyelinated C fibres and the myelinated A fibres, namely, the Aβ and Aδ fibres5. Peripheral neuropathic pain will probably become more common because of the ageing global population, increased incidence of diabetes mellitus and the increasing rates of cancer and the consequence of chemotherapy, which affect all sensory fibres (Aβ, Aδ and C fibres). Peripheral neuropathic pain disorders can be subdivided into those that have a generalized (usually symmetrical) distribution and those that have a focal distribution (FIG. 2). The most clinically important painful generalized peripheral neuropathies include those associated with diabetes mellitus (BOX 3), pre-diabetes and other metabolic dysfunctions, infectious diseases (mainly HIV infection28 and leprosy29), chemotherapy, immune (for example, Guillain-Barre syndrome) and inflammatory disorders, inherited neuropathies and channelopathies (such as inherited erythromelalgia, a disorder in which blood vessels are episodically blocked then become hyperaemic and inflamed).
Figure 2. Neuroanatomical distribution of pain symptoms and sensory signs in neuropathic pain conditions.
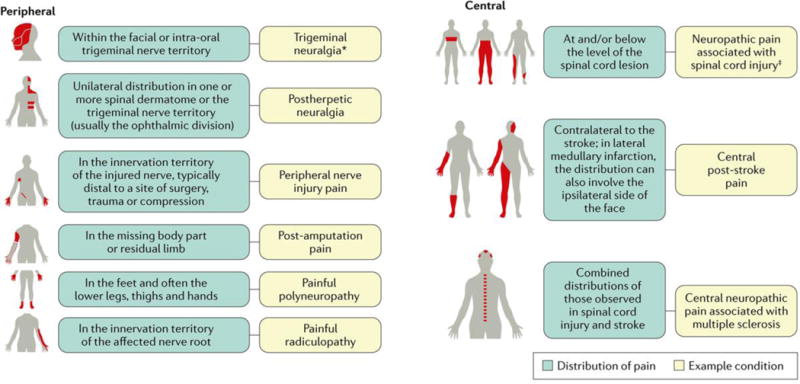
Distribution of pain and sensory signs in common peripheral and central neuropathic pain conditions. *Can sometimes be associated with central neuropathic pain. ‡Can sometimes be associated with peripheral neuropathic pain.
Box 3. Neuropathic pain and diabetes mellitus.
Painful chronic neuropathy in patients with diabetes mellitus ranges from 10% to 26%38. Although risk factors and potential mechanisms underlying neuropathy have been studied extensively, the aetiology of the painful diabetic neuropathy is not completely known. However, findings from epidemiological studies have suggested that patients with diabetes mellitus who develop neuropathy, compared with those patients who do not, seem to have different cardiovascular function, glycaemic control, weight, rates of obesity, waist circumference, risk of peripheral arterial disease and triglyceride plasmid levels. Indeed, patients with diabetes mellitus have alterations in the peripheral and central pain pathways; other mechanistic contributors include blood glucose instability, increased peripheral nerve epineural blood flow, microcirculation of the skin of the foot, altered intraepidermal nerve fibre density, increased thalamic vascularity and autonomic dysfunction. Furthermore, methylglyoxal (a by-product of glycolysis) plasma levels are increased in patients with diabetes mellitus owing to excessive glycolysis and decreased degradation by the glyoxalase system197. This metabolite activates peripheral nerves by changing the function of Nav1.7 and Nav1.8 voltage-gated sodium channnels197 and might, therefore, have a role in painful neuropathy. Studies in animals have shown that methylglyoxal slows nerve conduction, heightens calcitonin gene-related peptide release from nerves and leads to thermal and mechanical hyperalgesia197. Notably, methylglyoxal-dependent modifications of sodium channels induce diabetes-associated hyperalgesia that is not simply due to changes in peripheral fibres197.
The topography of the pain in these disorders typically encompasses the distal extremities, often called a ‘glove and stocking’ distribution because the feet, calves, hands and forearms are most prominently affected. This distribution pattern is characteristic of dying-back, length-dependent, distal peripheral neuropathies involving a distal-proximal progressive sensory loss, pain and, less frequently, distal weakness. Less frequently, the pain has a proximal distribution in which the trunk, thighs and upper arms are particularly affected; this pattern occurs when the pathology involves the sensory ganglia. Painful focal peripheral disorders are caused by pathological processes that involve one or more peripheral nerves or nerve roots. These disorders include postherpetic neuralgia, post-traumatic neuropathy, postsurgical neuropathy, cervical and lumbar polyradiculopathies, pain associated with HIV infection, leprosy and diabetes mellitus, complex regional pain syndrome type 2 and trigeminal neuralgia30.
Rare inherited channelopathies can show characteristic pain distributions and triggering factors. For example, inherited erythromelalgia is due to mutations in SCN9A, which encodes the voltage-gated sodium channel Nav1.7 (involved in the generation and conduction of action potentials), and is characterized by pain and erythema (reddening) in the extremities, which is exacerbated by heat31. Paroxysmal extreme pain disorder is due to a distinct set of mutations in SCN9A that result in a proximal distribution of pain and erythema affecting the sacrum and mandible32; pain triggers in those with this condition can include mechanical stimuli. In approximately 30% of patients with idiopathic small-fibre neuropathy, functional mutations of the Nav1.7 sodium channel that result in hyperexcitable dorsal root ganglion neurons have been observed33.
Pain signalling changes
Peripheral neuropathy alters the electrical properties of sensory nerves, which then leads to imbalances between central excitatory and inhibitory signalling such that inhibitory interneurons and descending control systems are impaired. In turn, transmission of sensory signals and disinhibition or facilitation mechanisms are altered at the level of the spinal cord dorsal horn neurons. Indeed, preclinical studies have revealed several anatomical, molecular and electrophysiological changes from the periphery through to the central nervous system (CNS) that produce a gain of function, providing insights into neuropathic pain and its treatment (BOX 4). At the periphery, spinal cord and brain, a gain of excitation and facilitation and a loss of inhibition are apparent. These changes shift the sensory pathways to a state of hyperexcitability, and a sequence of changes over time from the periphery to the brain might contribute to the neuropathic pain state becoming chronic.
Box 4. Challenges in translating animal studies to therapeutic pharmacological targets in humans.
Translating knowledge from preclinical observations in animal models to new targeted drug therapies in the clinic has been challenging. The differences between animal behavioural tests and human neuropathic pain features, lack of long-term efficacy data in animal models and the homogeneity of animal genetic strains might contribute to these challenges. Nonetheless, a substantial part of our knowledge of neuropathic pain mechanisms is derived from animal studies. Animal models of neuropathic pain use surgical lesions of the spinal cord, cranial and peripheral sensory nerves, such as ligation, constriction or transection of parts or branches of nerves198. These animal models exhibit hypersensitivity to external stimuli, commonly to mechanical stimuli as assessed with von Frey hairs (for measuring the tactile sensitivity), but may also include hypersensitivity to thermal stimuli (especially cold). Higher-level outcome measurements that are suggestive of reward from pain relief and reflective of the spontaneous pain experienced by patients have recently been introduced in the array of animal models of neuropathic pain199. Models of diabetic neuropathy have also been affected by the ill health of the animals, but this aspect is starting to be addressed in the most recent studies38.
Notably, basic research findings have often led to the development of specific therapeutic targets. For example, the altered function of the sodium channels within the damaged peripheral nerves provides insights into the use of topical voltage-gated sodium channel blockade (such as lidocaine107 and carbamazepine186) for neuropathic pain. Moreover, the assumption of abnormal sodium channel activity has led to the use of oxcarbazepine, which has been shown to be more effective in patients with the ‘irritable nociceptor’ phenotype186. Drugs such as gabapentin and pregabalin200 (see Management) target the α2δ subunit of the voltage-dependent calcium channels that are overexpressed in patients with neuropathic pain. When given intrathecally, gabapentin inhibited hypersensitivity in animal models201 but has failed to show positive results in humans202.
Ectopic activity in primary afferent fibres might have a key role in the pathophysiology of neuropathic pain following peripheral nerve injury. Patients with painful diabetic polyneuropathy and traumatic peripheral nerve injury showed a complete loss of ipsilateral spontaneous and evoked pain when treated with a peripheral nerve block (with lidocaine, which blocks voltage-gated sodium channels)34. Similarly, a blockade of the dorsal root ganglion by intraforaminal epidural administration of lidocaine resulted in relief of painful and non-painful sensations in patients with phantom limb pain35. Microneurography studies have also identified a spontaneous activity — primarily in C fibres — that is related to pain, suggesting a potential peripheral mechanism for neuropathic pain36, 37.
Overall, the underlying hyperexcitability in neuropathic pain results from changes in ion channel function and expression, changes in second-order nociceptive neuronal function and changes in inhibitory interneuronal function.
Ion channel alterations
Neuropathy causes alterations in ion channels (sodium, calcium and potassium) within the affected nerves, which can include all types of afferent fibres that then affect spinal and brain sensory signalling. For example, increased expression and function of sodium channels at the spinal cord terminus of the sensory nerves (mirrored by an enhanced expression of the α2δ subunit of calcium channels) lead to increased excitability, signal transduction and neurotransmitter release. Indeed, the crucial role of sodium channels is shown by loss or gain of pain in humans with inherited channelopathies31. At the same time, a loss of potassium channels that normally modulate neural activity is also evident. If an afferent fibre is disconnected from the periphery due to an injury or a lesion, there will be sensory loss. However, the remnants of the fibres at the injury site can generate ectopic activity (for example, neuroma C fibre afferents), and so pain from a ‘numb’ area results38. The remaining intact fibres are hyperexcitable, so-called irritable nociceptors39. As a result, the patient can experience ongoing pain, numbness and evoked pains. The altered inputs into the spinal cord coupled with increased calcium channel function (through higher expression in the nerve terminal) result in increased neurotransmitter release and enhanced excitatory synaptic transmission in the nociceptive circuit.
Second-order nociceptive neuron alterations
Enhanced excitability of spinal neurons produces increased responses to many sensory modalities, enables low-threshold mechanosensitive Aβ and Aδ afferent fibres to activate second-order nociceptive neurons (which convey sensory information to the brain) and expands their receptive fields so a given stimulus excites more second-order nociceptive neurons, generating the so-called central sensitization40,41. In particular, ongoing discharge of peripheral afferent fibres with concomitant release of excitatory amino acids and neuropeptides leads to postsynaptic changes in second-order nociceptive neurons, such as an excess of signalling due to phosphorylation of N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors. These second-order changes plausibly explain physical allodynia and are reflected by enhanced sensory thalamic neuronal activity, as supported by data from animal42 and human studies43. Hyperexcitability can also be caused by a loss of γ-aminobutyric acid (GABA)- releasing inhibitory interneurons that can also switch to exert consequently excitatory actions at spinal levels44. In addition, there are less well-understood functional changes in non-neuronal cells within the spinal cord, such as microglia and astrocytes, which contribute to the development of hypersensitivity45.
Inhibitory modulation changes
In addition to changes in pain transmission neurons, inhibitory interneurons and descending modulatory control systems are dysfunctional in patients with neuropathic pain. Interneuron dysfunction contributes to the overall altered balance between descending inhibitions and excitations; specifically, neuropathy leads to a shift in excitation that now dominates. Consequently, the brain receives altered and abnormal sensory messages. Altered projections to the thalamus and cortex and parallel pathways to the limbic regions account for high pain ratings and anxiety, depression and sleep problems, which are relayed as painful messages that dominate limbic function.
Areas such as the cingulate cortex and amygdala have been implicated in the ongoing pain state and comorbidities associated with neuropathic pain46. Projections from these forebrain areas modulate descending controls running from the periaqueductal grey (the primary control centre for descending pain modulation) to the brainstem and then act on spinal signalling. Indeed, numerous studies have shown that the brainstem excitatory pathways are more important in the maintenance of the pain state than in its induction.
Noradrenergic inhibitions, mediated through α2-adrenergic receptors in the spinal cord, are attenuated in neuropathic pain, and enhanced serotonin signalling through the 5-HT2 and 5-HT3 serotonin receptors becomes dominant. The noradrenergic system mediates the diffuse noxious inhibitory controls (DNICs), the animal counterpart of the human conditioned pain modulation (CPM; FIG. 3), in which one pain inhibits another through descending pathways. DNICs (and CPM) are lost or at least partially impaired in those with neuropathy. Animals that recruit noradrenergic inhibitions have markedly reduced hypersensitivity after neuropathy despite identical levels of nerve damage47, explaining the advantage of using medication that manipulates the monoamine system to enhance DNICs in patients by blocking descending facilitations.
Figure 3. Schematic representation of the conditioned pain modulation.
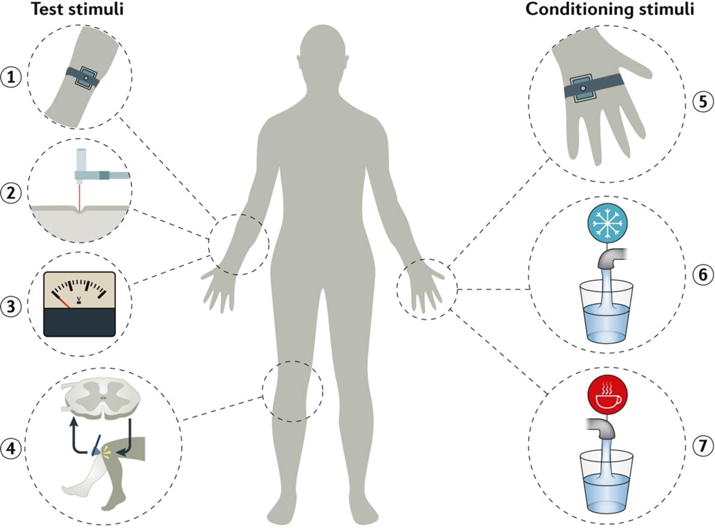
The conditioned pain modulation (CPM) paradigm is used in the research setting to assess the change of perceived pain by a test stimulus under the influence of a conditioning stimulus203. A test stimulus can be a thermal contact stimulation (1), mechanical pressure (2), an electrical stimulus (3) — for each, either pain threshold or suprathreshold magnitude estimation can be used — or nociceptive withdrawal reflex (4). A typical conditioning stimulus consists of thermal contact stimulation (5), or immersion in a cold (6) or hot (7) water bath. Other modalities can be used as well. During a CPM assessment, a test stimulus is given first, then the conditioning stimulus is given, and the test is repeated during or immediately after the conditioning.
Pain modulation mechanisms
Some patients with neuropathic pain are moderately affected, whereas others experience debilitating pain. Moreover, patients show a large variability in response to distinct pharmacological (in terms of type and dose) and non-pharmacological treatments. A key factor in this variability might be the way that the pain message is modulated in the CNS. The pain signal can be augmented or reduced as it ascends from its entry port (the dorsal horn), relayed to the CNS and arrives at the cerebral cortex (the area crucial for consciousness). The various pathways and interference can, accordingly, modify the assumed correlation between the extent of the peripheral pathology and the extent of the pain syndrome. Most patients with neuropathic pain express a pro-nociceptive pain modulation profile — that is, pain messages are augmented in the CNS48. Thus, the perception of pain can be disinhibited owing to decreased descending endogenous inhibition, which is depicted by less-efficient CPM (BOX 1), facilitated through sensitization of ascending pain pathways, which is depicted by enhanced temporal summation of painful stimulations, or both. Temporal summation is augmented in neuropathic and non-neuropathic pain, but patients with neuropathic pain present with a higher slope of increase48. CPM has been shown to be less efficient in patients with various pain syndromes than in healthy controls49.
The prospect of harnessing pain modulation seems promising for a more individualized approach to pain management. Indeed, studies have shown that the pain modulation profile can predict the development and extent of chronic postoperative pain50–52. If these findings are confirmed by larger studies, we can speculate that patients who express a facilitatory pro-nociceptive profile could be treated with a drug that reduces the facilitation (such as gabapentinoids) and patients who express an inhibitory pro-nociceptive profile could be treated with a drug that enhances the inhibitory capacity (for example, serotonin-noradrenaline reuptake inhibitors)50. Patients who express both less-efficient CPM and enhanced temporal summation might need a combination of treatments. Indeed, the level of CPM predicts the efficacy of duloxetine (a selective serotonin-noradrenaline reuptake inhibitor) in patients; CPM is restored with both duloxetine and tapentadol (a noradrenaline reuptake inhibitor). Moreover, the altered pain modulation profile of a patient can be reversed towards normality when pain is treated, as exemplified with arthroplasty surgery in patients with osteoarthritis; when the diseased joint is replaced, the majority of patients will be free of pain and the central and peripheral processes normalize34, 53, 54.
Notably, pain modulation is highly influenced by expectancy-induced analgesia, in which changes due to the beliefs and desires of patients and providers55 affect response to treatment for neuropathic pain. In laboratory settings, expectancy-induced analgesia influences clinical pain in irritable bowel syndrome56–58, idiopathic and neuropathic pain59. For example, Petersen et al.60, 61 tested expectancy-induced analgesia in patients who developed neuropathic pain after thoracotomy. Patients received lidocaine in an open (that is, patients were told: “The agent you have just been given is known to powerfully reduce pain in some patients”) or hidden (“This is a control condition for the active medication”) manner in accordance with a previously described protocol62; the results showed a large reduction of ongoing pain, maximum wind-up-like pain and an area of hyperalgesia in those in the open group, recapitulating previous reports59,60. These findings point to a clinically relevant endogenous pain inhibitory mechanism with implications for phenotyping patients with neuropathic pain in clinical trial designs and practices. Such effects should be reduced in clinical trials and intentionally enhanced in daily clinical practices as a strategy to optimize pain management.
Diagnosis, screening and prevention
A system was proposed to determine the level of certainty with which the pain in question is neuropathic as opposed, for example, to nociceptive pain5 (FIG. 4a). If the patient’s history suggests the presence of a neurological lesion or disease and the pain could be related to such (for example, using validated screening tools) and the pain distribution is neuroanatomically plausible, the pain is termed ‘possible’ neuropathic pain. ‘Probable’ neuropathic pain requires supporting evidence obtained by a clinical examination of sensory signs (for example, bedside testing and quantitative sensory testing). ‘Definite’ neuropathic pain requires that an objective diagnostic test confirms the lesion or disease of the somatosensory nervous system (for example, neurophysiological tests and skin biopsy). A minimum finding of probable neuropathic pain should lead to treatment.
Figure 4. Diagnosing neuropathic pain, a.
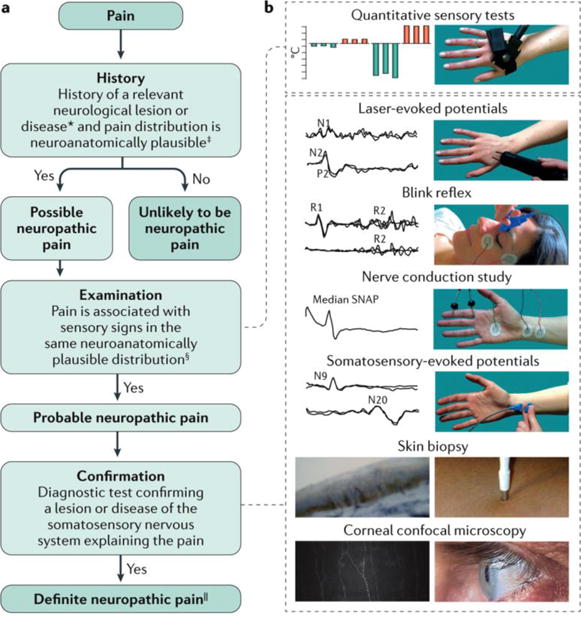
The flowchart summarizes the clinical steps in diagnosing neuropathic pain, which involves taking the patient history, examining the patient and following up with confirmatory tests. If the answer is ‘no’ after examination, the patient might still have probable neuropathic pain. In such cases, confirmation tests could be performed if sensory abnormalities are not found; for example, in some hereditary conditions, sensory abnormalities are not found at the moment of examination. *History of a neurological lesion or disease relevant to the occurrence of neuropathic pain. ‡The patient's pain distribution reflects the suspected lesion or disease. §Signs of sensory loss are generally required. However, touch-evoked or thermal allodynia might be the only finding at bedside examination. ||‘Definite’ neuropathic pain refers to a pain that is compatible with the features of neuropathic pain and confirmatory tests are consistent with the location and nature of the lesion or disease, although this may not imply any causality. b | The confirmatory tests for neuropathic pain include quantitative sensory testing (in which the patient provides a subjective report on a precise and reproducible stimulus), blink reflex testing (whereby the trigeminal afferent system is investigated by recording the R1 and R2 reflex responses recorded from the orbicularis oculi muscle) and nerve conduction study (which assesses non-nociceptive fibre function of the peripheral nerves). Somatosensory-evoked potentials (N9 is generated by the brachial plexus and N20 by the somatosensory cortex) and laser-evoked potentials (LEPs), both recorded from the scalp, are neurophysiological tools that investigate large and small afferent fibre function. The N1 LEP wave is a lateralized component and generated by the secondary somatosensory cortex, and the negative-positive complex of LEP (N2-P2) is a vertex recorded potential, which is generated by the insular cortex bilaterally and the cingulate cortex204. A skin biopsy enables the quantification of the intraepidermal nerve fibres, which provides a measure of small-fibre loss77. Finally, corneal confocal microscopy assesses corneal innervation, which consists of small nerve fibres. In most patients with neuropathic pain, standard neurophysiological testing, such as blink reflex, nerve conduction study and somatosensory-evoked potentials, is sufficient for showing the damage of the somatosensory system. However, in patients with selective damage of the nociceptive system, a nociceptive-specific tool, such as LEPs, skin biopsy or corneal confocal microscopy, is needed. Typically, tests are performed in the sequence of increasing invasiveness; that is, quantitative sensory testing, blink reflex, nerve conduction study, somatosensory-evoked potentials, LEPs, skin biopsy and corneal confocal microscopy. SNAP, sensory nerve action potential. Adapted with permission from REF 77, Macmillan Publishers Limited. The corneal innervation image in part b (left panel) is reproduced with permission from REF 86, Elsevier.
On the basis of the assumption that characteristic qualities indicative of neuropathic pain in sensory perception are present, several screening tools have been developed to identify neuropathic pain conditions or neuropathic components to chronic pain syndromes63 (BOX 2). These simple to use patient-reported questionnaires, for example, the DN4 or painDETECT22,64, assess characteristic neuropathic pain symptoms (such as burning, tingling, sensitivity to touch, pain caused by light pressure, electric shock-like pain, pain to cold or heat, and numbness) and can distinguish between neuropathic and non-neuropathic pain with high specificity and sensitivity when applied in patients with chronic pain. Other tools, such as the Neuropathic Pain Symptom Inventory (NPSI)65, have been more specifically developed for the quantification of neuropathic symptoms and dimensions and have contributed to further phenotype individual patients particularly for clinical trials.
Confirmatory tests for nerve damage
Different psychophysical and objective diagnostic tests are available to investigate somatosensory pathway function, including bedside evaluation and assessment of sensory signs as well as neurophysiological techniques, skin biopsy and corneal confocal microscopy (FIG. 4b). Of these, sensory evaluation, neurophysiological techniques and quantitative sensory testing are routinely used.
Bedside sensory assessment of sensory signs
Neuropathic pain presents as a combination of different symptoms and signs66. Touch, pinprick, pressure, cold, heat, vibration, temporal summation and after sensations can be examined at the bed side, whereby the patient describes the sensation after a precise and reproducible stimulus is applied67. To assess either a loss (negative sensory signs) or a gain (positive sensory signs) of somatosensory function, the responses are graded as normal, decreased or increased. The stimulus-evoked (positive) pain types are classified as hyperalgesic (experiencing increased pain from a stimulus that is normally perceived as less painful) or allodynic (experiencing pain from a stimulus that does not normally trigger a pain response), and according to the dynamic or static character of the stimulus.
Quantitative sensory testing
Quantitative sensory tests use standardized mechanical and thermal stimuli to test the afferent nociceptive and non-nociceptive systems in the periphery and the CNS. Quantitative sensory tests assess loss and gain of function of the entire different afferent fibre classes (Aβ, Aδ and C fibres), which is a distinct advantage over other methods68. The German Research Network on Neuropathic Pain69 proposed a battery of quantitative sensory tests that consists of 13 parameters to help identify somatosensory phenotypes of patients with neuropathic pain. These thermal and mechanical tests include the determination of detection thresholds for cold, warm, paradoxical heat sensations and touch and vibration; determination of pain thresholds for cold and heat stimulations, pinprick and blunt pressure; and determination of allodynia and pain summation. Recently, normative data from a large database of healthy individuals have helped to determine gain or loss of sensory function in age-matched and sex-matched patients with neuropathic pain70,71. Accordingly, pathological values of positive and negative signs have been determined for most variables (FIG. 5).
Figure 5. Subgrouping patients with peripheral neuropathic pain based on sensory signs.
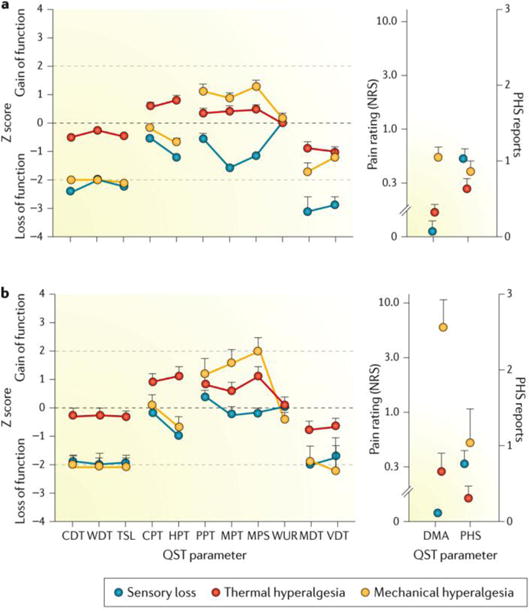
On the basis of two well-established testing (n = 902) (part a) and control (n=233) (part b) data sets69, three categories of patient phenotypes for neuropathic pain have been proposed: sensory loss, thermal hyperalgesia and mechanical hyperalgesia. Positive scores indicate positive sensory signs (hyperalgesia), and negative scores indicate negative sensory signs (hypoaesthesia or hypoalgesia). Values observed in those with neuropathic pain are significantly different from those of healthy participants when the 95% CI does not cross the zero line, which defines the average of data from normal subjects. Insets (right) show the numerical rating scale (NRS; 0–10) values for dynamic mechanical allodynia (DMA) on a logarithmic scale and the frequency of paradoxical heat sensation (PHS) on a scale of 0–3. These findings indicate that patients with neuropathic pain have different expression patterns of sensory signs. These subgroup results suggest that different mechanisms of pain generation are involved in the pain condition. Furthermore, the first clinical trial to show phenotype stratification based on these sensory profiles has predictive power for treatment response186. Error bars are the graphical representation of the variability of the data present in the database. CDT, cold detection threshold; CPT, cold pain threshold; HPT, heat pain threshold; MDT, mechanical detection threshold; MPS, mechanical pain sensitivity; MPT, mechanical pain threshold; PPT, pressure pain threshold; QST, quantitative sensory test; TSL, thermal sensory limen; VDT, vibration detection threshold; WDT, warm detection threshold; WUR, wind-up ratio. Reproduced with permission from REF 70, Baron, R. et al., Peripheral neuropathic pain: a mechanism-related organizing principle based on sensory profiles, Pain, 158, 2, 261–272, http://journals.lww.com/pain/Fulltext/2017/02000/Peripheral_neuropathic_pain___a_mechanism_related.10.aspx
Neurophysiological techniques
Laser-evoked potentials (LEPs) are widely considered the most reliable neurophysiological tool to assess nociceptive functions67,72. For example, nerve conduction studies, trigeminal reflexes and somatosensory-evoked potentials — the Aβ fibre-mediated standard neurophysiological techniques — do not provide information on nociceptive pathways. However, they are still useful to identify damage along the somatosensory pathways and are widely used for assessing peripheral and CNS diseases that cause neuropathic pain73. Laser stimulations selectively activate Aδ and C nociceptors in the superficial layers of the skin74.
LEPs related to Aδ fibre activation have been standardized for clinical application. The responses to stimulation are recorded from the scalp and consist of waveforms with different latencies. In diseases associated with damage to the nociceptive pathway, LEPs can be absent, reduced in amplitude or delayed in latency75–77. Among nociceptive-evoked potentials, contact heat- evoked potentials are also widely used in assessing neuropathic pain78. Concentric electrodes have also been introduced to measure pain-related evoked potentials and the small-fibre involvement in neuropathic pain79. Nevertheless, some studies suggest that concentric electrodes also activate non-nociceptive Aβ fibres; hence, pain-related evoked potential recording is not suitable for assessing nociceptive systems78.
Skin biopsy
Skin biopsy to assess epidermal innervation is regarded as the most sensitive tool for diagnosing small-fibre neuropathies80. The technique is useful because the skin has widespread unmyelinated C fibre terminals, with relatively few small myelinated Aδ fibres that lose their myelin sheath and reach the epidermis as unmyelinated free nerve endings81,82. However, the relationship between skin biopsy data and neuropathic pain is still unclear. One study in 139 patients with peripheral neuropathy suggested that a partial sparing of intraepidermal nerve fibres, as assessed with skin biopsy, is associated with provoked pain83.
Corneal confocal microscopy
As a non-invasive in vivo technique, corneal confocal microscopy can be used to quantify corneal nerve fibre damage (to small myelinated Aδ and unmyelinated C fibres) in patients with peripheral neuropathies84, 85. However, this technique has several limitations, such as the high cost and the reduced availability in most clinical centres. Furthermore, whether some conditions (such as dry eye syndrome and Sjögren syndrome, eye diseases or previous eye surgery) influence the corneal confocal variables is still unclear86. No study has reliably investigated the association between corneal confocal microscopy variables and neuropathic pain.
Prevention
Given that the available treatments for neuropathic pain have meaningful but modest benefits (see Management), interventions that prevent neuropathic pain can have a substantial effect on public health. Indeed, increased attention to prevention has the potential to reduce the disability experienced by many patients with chronic neuropathic pain. Leading a healthy lifestyle and education regarding pain-causing health conditions are important components of prevention, especially in those who are at greater risk of developing neuropathic pain87. Prevention programmes that combine mutually reinforcing medical and behavioural interventions might lead to greater preventive benefits.
The identification of risk factors is essential to prevent neuropathic pain developing in at-risk individuals. Primary prevention strategies (in generally healthy but at-risk individuals) include the live attenuated88,89 and subunit adjuvanted90,91 herpes zoster vaccines, which both reduce the likelihood of developing herpes zoster infections in individuals ≥50 years of age88–91, and therefore, reduce the likelihood of postherpetic neuralgia. Secondary prevention involves administering preventive interventions to individuals who are experiencing an illness, injury or treatment that can cause chronic neuropathic pain. Examples of this approach include the perioperative treatment of surgical patients to prevent chronic postsurgical pain92 and the use of antiviral or analgesic treatment in patients with herpes zoster infection93. Furthermore, proper management of health conditions, such as diabetes mellitus, may prevent neuropathic pain before it even presents94.
Management
The management of neuropathic pain generally focuses on treating symptoms because the cause of the pain can be rarely treated; furthermore, the management of aetiological conditions, such as diabetes mellitus, is typically insufficient to relieve neuropathic pain. Patients with neuropathic pain generally do not respond to analgesics such as acetaminophen, NSAIDs or weak opioids such as codeine. The traditional approach to the management of a patient with neuropathic pain is to initiate treatment with conservative pharmacological and complementary therapies before interventional strategies, such as nerve blocks and neuromodulation, are used. However, the limited efficacy of the drugs, the ageing population of patients, polypharmacy in elderly patients and opioid-related adverse effects have resulted in an increasing use of interventional therapies. Clinical studies are lacking to help guide the physician in the optimal sequence of therapy in a given patient.
Medical intervention
Numerous therapeutic recommendations, with different classes of drug, for neuropathic pain have been proposed95–99. On the basis of a systematic review and meta-analysis of all drug studies reported on since 1966, including unpublished trials100, pregabalin (a GABA analogue), gabapentin (a GABA inhibitor), duloxetine (a serotonin-noradrenaline reuptake inhibitor) and various tricyclic antidepressants have strong recommendations for use and are recommended as first-line treatments for peripheral and central neuropathic pain. High-concentration capsaicin (the active component of chili peppers) patches, lidocaine patches and tramadol (an opioid with serotonin and noradrenaline reuptake inhibition effects) have weak evidence in support of their use and are recommended as second-line treatments for peripheral neuropathic pain only. Strong opioids and botulinum toxin A (administered by specialists) have weak recommendations for use as third-line treatments. However, most of these treatments have moderate efficacy based on the number needed to treat (NNT; that is, the number of patients necessary to treat to obtain one responder more than the comparison treatment, typically placebo) for obtaining 50% of pain relief101 (TABLE 1). Furthermore, pharmacological treatments for chronic neuropathic pain are effective in <50% of patients and may be associated with adverse effects that limit their clinical utility101.
Table 1.
Available pharmacotherapy for neuropathic pain
| Drug | Mechanisms of action | NNT* (range) | Adverse effects | Precautions and contraindications |
|---|---|---|---|---|
| Tricyclic antidepressants | ||||
|
| ||||
| Nortriptyline, desipramine, amitriptyline, clomipramine and imipramine | Monoamine reuptake inhibition, sodium channel blockade and anticholinergic effects | 3.6 (3–4.4) | Somnolence, anticholinergic effects and weight gain | • Cardiac disease, glaucoma, prostatic adenoma and seizure • High doses should be avoided in adults >65 years of age and in those with amyloidosis |
|
| ||||
| Serotonin-noradrenaline reuptake inhibitors | ||||
|
| ||||
| Duloxetine | Serotonin and noradrenaline reuptake inhibition | 6.4 (5.2–8.2) | Nausea, abdominal pain and constipation | • Hepatic disorder and hypertension • Use of tramadol |
|
| ||||
| Venlafaxine | Serotonin and noradrenaline reuptake inhibition | 6.4 (5.2–8.2) | Nausea and hypertension at high doses | • Cardiac disease and hypertension • Use of tramadol |
|
| ||||
| Calcium channel α2δ ligands | ||||
|
| ||||
| Gabapentin, extended-released gabapentin and enacarbil, and pregabalin | Act on the α2δ subunit of voltage-gated calcium channels, which decrease central sensitization | • 6.3 (5–8.4 for gabapentin) • 8.3 (6.2–13 for extended-released gabapentin and enacarbil) • 7.7 (6.5–9.4 for pregabalin) |
Sedation, dizziness, peripheral oedema and weight gain | Reduce dose in patients with renal insufficiency |
|
| ||||
| Topical lidocaine | ||||
|
| ||||
| Lidocaine 5% plaster | Sodium channel blockade | Not reported | Local erythema, itching and rash | None |
|
| ||||
| Capsaicin high-concentration patch (8%) | Transient receptor potential cation channel subfamily V member 1 agonist | 10.6 (7.4–19) | Pain, erythema, itching and rare cases of high blood pressure (initial increase in pain) | No overall impairment of sensory evaluation after repeated applications and caution should be taken in progressive neuropathy |
|
| ||||
| Opioids | ||||
|
| ||||
| Tramadol | μ-Receptor agonist and monoamine reuptake inhibition | 4.7 (3.6–6.7) | Nausea, vomiting, constipation, dizziness and somnolence | History of substance abuse, suicide risk and use of antidepressant in elderly patients |
|
| ||||
| Morphine and oxycodone | μ-Opioid receptor agonists; oxycodone might also cause κ-opioid receptor antagonism | 4.3 (3.4–5.8) | Nausea, vomiting, constipation, dizziness and somnolence | History of substance abuse, suicide risk and risk of misuse in the long term |
|
| ||||
| Neurotoxin | ||||
|
| ||||
| Botulinum toxin A | Acetylcholine release inhibitor and neuromuscular-blocking agent; potential effects on mechanotransduction and central effects in neuropathic pain | 1.9 (1.5–2.4) | Pain at injection site | Known hypersensitivity and infection of the painful area |
Number needed to treat (NNT) for 50% pain relief represents the number of patients necessary to treat to obtain one responder more than the comparison treatment, typically placebo101.
First-line treatments
Antidepressants and antiepileptics have been the most studied drugs in neuropathic pain. Among antidepressants, tricyclic antidepressants, such as amitriptyline, and serotonin-noradrenaline reuptake inhibitors, such as duloxetine, have confirmed efficacy in various neuropathic pain conditions. Their analgesic efficacy seems largely mediated by their action on descending modulatory inhibitory controls, but other mechanisms have been proposed (including an action on β2 adrenoceptors)102. Among antiepileptics, the efficacy of pregabalin and gabapentin, including extended-release formulations, is best established for the treatment of peripheral neuropathic pain and, to a lesser extent, spinal cord injury pain. However, the number of negative trials has increased over the past 5 years. The analgesic effects of these drugs are mainly related to a decrease in central sensitization through binding to the α2δ subunit of calcium voltage-gated channels103.
Combination of pregabalin or gabapentin with a tricyclic antidepressant or opioid at lower doses has resulted in beneficial effects as compared to monotherapy in peripheral neuropathic pain100,101,104. However, the efficacy and adverse effects of high-dose monotherapy were similar to those of moderate-dose combination therapy in patients with diabetic neuropathic pain who did not respond to monotherapy at moderate doses105. These studies provide a rationale for the use of combinations of drugs, at moderate dosages, in patients who are unable to tolerate high-dose monotherapy.
Second-line treatments
Lidocaine is thought to act on ectopic neuronal discharges through its sodium channel-blocking properties. The efficacy of lidocaine 5% patches has been assessed in focal peripheral postherpetic neuralgia, but their therapeutic gain is modest compared with placebo106,107. Capsaicin initially activates transient receptor potential cation channel subfamily V member 1 (TRPV1) ligand-gated channels on nociceptive fibres, leading to TRPV1 desensitization and defunctionalization. The sustained efficacy of a single application of a high-concentration capsaicin patch (8%) has been reported in postherpetic neuralgia108, as well as diabetic104 and non-diabetic painful neuropathies109. The long-term safety of repeated applications seems favourable based on open studies, but there are no longterm data on the effects on epidermal nerve fibres in patients with neuropathic pain101. Tramadol, an opioid agonist and serotonin-noradrenaline reuptake inhibitor, has also been shown to be effective, mainly in peripheral neuropathic pain; its efficacy is less established in central neuropathic pain101.
Third-line treatments
Botulinum toxin A is a potent neurotoxin commonly used for the treatment of focal muscle hyperactivity and has shown efficacy of repeated administrations over 6 months, with enhanced effects of the second injection110. The toxin has a beneficial role in the treatment of peripheral neuropathic pain (for example, diabetic neuropathic pain, postherpetic neuralgia and trigeminal neuralgia)110–112.
Opioid agonists, such as oxycodone and morphine, are mildly effective101, but there is concern about prescription opioid-associated overdose, death, diversion, misuse and morbidity113.
There are weak, negative or inconclusive recommendations for the use of all other drug treatments for neuropathic pain in general. Antiepileptics other than α2δ ligands (for example, topiramate, oxcarbazepine, carbamazepine, valproate, zonisamide, lacosamide and levetiracetam) fall into these categories, although some agents are probably effective in subgroups of patients. Oromucosal cannabinoids have been found to be variably effective in pain associated with multiple sclerosis and in peripheral neuropathic pain with allodynia, but several unpublished trials were negative on the primary outcome. Results for selective serotonin reuptake inhibitors, NMDA antagonists, mexiletine (a non-selective voltage-gated sodium channel blocker) and topical clonidine (an α2-adrenergic agonist and imidazoline receptor agonist) have generally been inconsistent or negative except in certain subgroups.
Emerging treatments
A few drugs targeting novel mechanisms of action are under clinical development for the treatment of peripheral neuropathic pain. These include, in particular, subtype selective sodium channel-blocking agents, particularly Nav1.7 antagonists114, and EMA401, a novel angiotensin type II antagonist that has been found to be effective in a phase II clinical trial in postherpetic neuralgia115. Although still in the preclinical phase, studies show promising results of stem cell treatment for neuropathic pain116,117.
Interventional therapies
Interventional treatments, such as nerve blocks or surgical procedures that deliver drugs to targeted areas, or modulation of specific neural structures, provide alternative treatment strategies in selected patients with refractory neuropathic pain118,119 (FIG. 6). Although generally safe (see below), spinal cord stimulation and peripheral nerve stimulation have been associated with hardware-related, biological complications, such as infections and programming-related or treatment-related adverse effects (including painful paraesthesias)120,121.
Figure 6. Example interventional treatments for neuropathic pain. a.
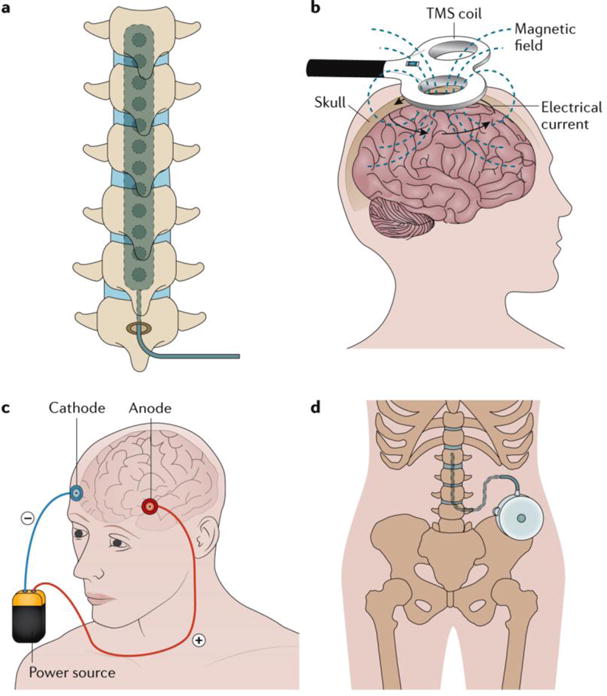
Spinal cord stimulation traditionally applies a monophasic square-wave pulse (at a frequency in the 30–100 Hz range) that results in paraesthesia in the painful region. b | Cortical stimulation involves the stimulation of the pre-central motor cortex below the motor threshold using either invasive epidural or transcranial non-invasive techniques (such as repetitive transcranial magnetic stimulation (TMS) and transcranial direct current stimulation). c | Deep brain stimulation uses high-frequency chronic intracranial stimulation of the internal capsule, various nuclei in the sensory thalamus, periaqueductal and periventricular grey, motor cortex, septum, nucleus accumbens, posterior hypothalamus and anterior cingulate cortex as potential brain targets for pain control. d | Intrathecal treatments provide a targeted drug delivery option in patients with severe and otherwise refractory chronic pain. The pumps can be refilled through an opening at the skin surface.
Neural blockade and steroid injections
A perineural injection of steroids provides transient relief (1–3 months) for trauma-related and compression-related peripheral neuropathic pain122. Systematic reviews and meta-analysis of epidural steroid injections for the treatment of cervical and lumbar radiculopathies indicate an immediate modest reduction in pain and function of <3 months duration, but had no effects on reducing the risk for subsequent surgery119,123,124. Epidural local anaesthetic and steroid nerve blocks were given a weak recommendation for the treatment of lumbar radiculopathy and acute zoster-associated neuropathic pain119. Although sympathetic ganglion blocks have been used to treat pain in some patients with complex regional pain syndromes (also known as causalgia and reflex sympathetic dystrophy), the evidence for long-term benefit is weak119.
Spinal cord stimulation
Low-intensity electrical stimulation of large myelinated Aβ fibres was introduced based on the gate control theory125 as a strategy to modulate the pain signals transmitted by the unmyelinated C fibres. The most commonly used and the best-studied neuromodulation strategy has been spinal cord stimulation, in which a monophasic square-wave pulse (frequency ranging 30–100 Hz) is applied, resulting in paraesthesia in the painful region126. Newer stimulation parameters, such as burst (40 Hz burst with five spikes at 500 Hz per burst) and high-frequency (10 kHz with sinusoidal waveforms) spinal cord stimulation, provide paraesthesia-free stimulation and equivalent or better pain relief compared with the monophasic square-wave pulse127,128.
The relative safety and reversibility of spinal cord stimulation, as well as its cost-effectiveness over the long term have made it an attractive strategy for managing patients with refractory chronic neuropathic pain129–131. Systematic reviews, randomized controlled trials and several case series provide evidence for the long-term efficacy of spinal cord stimulation when combined with medical treatment compared with medical management in various pain neuropathies132–134, and has been shown to offer sustained results at 24 months of treatment135,136. Two randomized trials in individuals with painful diabetic neuropathy reported greater reduction in pain and improvements in measures of quality of life compared with controls137,138. Current European guidelines provide a weak recommendation for spinal cord stimulation (combined with medical treatment) in, for example, diabetic neuropathic pain118,119,139. The success of spinal cord stimulation for neuropathic pain may depend on the appropriate selection of patients based on psychological traits, sensory phenotype, enhanced central sensitization and reduced CPM140,141.
Dorsal root ganglion, peripheral nerve and peripheral nerve field stimulation
Neurostimulation of afferent fibres outside the spinal cord (for example, the dorsal root ganglion, which contains the cell bodies of sensory neurons, and peripheral nerves) and subcutaneous peripheral nerve field stimulation have been reported to provide pain relief in various chronic neuropathic pain states, including occipital neuralgia and postherpetic neuralgia142,143. A multicentre prospective cohort study in patients with chronic neuropathic pain reported that dorsal root ganglion stimulation provided 56% pain reduction with a 60% responder rate (>50% reduction in pain)144. These preliminary observations are being examined with controlled trials.
Epidural and transcranial cortical neurostimulation
Epidural motor cortex stimulation (ECMS), repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) of the pre-central motor cortex at levels below the motor threshold have been proposed as treatment options for patients with refractory chronic neuropathic pain145,147. Cortical neurostimulation may reduce pain-related thalamic hyperactivity or activate descending inhibitory pathways. Meta-analysis reports suggest that 60–65% of patients respond (>40% pain reduction) to EMCS147. ECMS is a neurosurgical procedure that requires precise intra-operative placement of the stimulating electrode over the motor cortex region corresponding to the painful body part for optimal outcome.
rTMS and tDCS are non-invasive therapies that involve neurostimulation of brain areas of interest via magnetic coils or electrodes on the scalp. Repetitive sessions (5–10 sessions over 1–2 weeks) with high-frequency rTMS (5–20 Hz) have shown benefits in a mixture of central, peripheral and facial neuropathic pain states, with effects lasting >2 weeks after the stimulation. tDCS has been reported to be beneficial in reducing several peripheral neuropathic conditions148. Current European guidelines include a weak recommendation for the use of EMCS and rTMS in refractory chronic neuropathic pain and tDCS for peripheral neuropathic pain133. Contraindications of rTMS include a history of epilepsy and the presence of aneurysm clips, deep brain electrodes, cardiac pacemakers and cochlear implants.
Deep brain stimulation
The use of long-term intracranial stimulation for neuropathic pain remains controversial. Multiple sites for deep brain stimulation, including the internal capsule, various nuclei in the sensory thalamus, periaqueductal and periventricular grey, motor cortex, septum, nucleus accumbens, posterior hypothalamus and anterior cingulate cortex, have been examined as potential brain targets for pain control149. The UK National Institute for Health and Care Excellence (NICE) guidelines recognize that the procedure can be efficacious in some patients who are refractory to other forms of pain control, but current evidence on the safety of deep brain stimulation shows significant potential risks, such as intra-operative seizure, lead fractures and wound infections98. Contrary to the NICE guidelines, the current European guidelines give inconclusive recommendations139.
Intrathecal therapies
Intrathecal therapies have been developed to deliver drugs to targeted nerves through an implanted and refillable pump in patients with severe and chronic pain that is refractory to conservative treatments, including psychological, physical, pharmacological and neuromodulation therapies150,151. The report from the 2012 Polyanalgesic Consensus Conference highlighted that this therapy is associated with risks of serious morbidity and mortality and made recommendations to reduce the incidence of these serious adverse effects152. The only US FDA-approved drugs for use with such devices are morphine and ziconotide (an N-type calcium channel antagonist)153. The most frequently reported adverse reactions associated with intrathecal ziconotide are dizziness, nausea, confusion, memory impairment, nystagmus (uncontrolled movement of the eyes) and an increase in the levels of serum creatine kinase. Ziconotide is contraindicated in patients with a history of psychosis, and patients should be monitored for evidence of cognitive impairment, hallucinations or changes in mood and consciousness. No high-quality randomized trials have been conducted to assess the efficacy of ziconotide and morphine; hence, the recommendations are a consensus of experts based on clinical experience or case series.
Physical therapies
Physical therapy, exercise and movement representation techniques (that is, treatments such as mirror therapy and motor imagery that use the observation and/or imagination of normal pain-free movements) have been suggested to be beneficial in neuropathic pain management154,155. For example, mirror therapy and motor imagery are effective in the treatment of pain and disability associated with complex regional pain syndrome type I and type II156. The quality of evidence supporting these interventions for neuropathic pain is weak and needs further investigation154,157.
Psychological therapies
People with chronic pain are not passive; they actively attempt to change the causes of pain and change their own behaviour in response to pain. However, for many patients, such change without therapeutic help is unachievable, and repeated misdirected attempts to solve the problem of pain drive them further into a cycle of pain, depression and disability158. At present, there is no evidence for identifying who is at risk of untreatable, difficult to manage neuropathic pain and who might benefit from psychological intervention, although research is underway on the former159.
Psychological interventions are designed to promote the management of pain and to reduce its adverse consequences. Treatments are often provided after pharmacological or physical interventions have failed, although they could be introduced earlier and in concert with non-psychological interventions. Cognitive-behavioural therapy (CBT) has received the most research attention; however, CBT is not a single treatment and can be usefully thought of as a family of techniques that are woven together by a clinical narrative of ‘individual change’ delivered by therapists who actively manage treatment. Such treatments address mood (typically anxiety and depression), function (including disability) and social engagement, as well as indirectly targeting analgesia. Secondary outcomes are sometimes reported because they are deemed important to treatment delivery (for example, therapeutic alliance and self-efficacy) or because they are valued by one or more stakeholder (for example, return to work and analgesic use).
A Cochrane systematic review of psychological interventions for chronic pain analysed data from 35 trials, which showed small-to-moderate effects of CBT over comparisons such as education, relaxation and treatment as usual160. In a companion review of 15 trials delivering treatment via the Internet, a similar broadly positive conclusion emerged, although the confidence in the estimates of effects was low161. Psychological treatments other than behavioural therapy and CBT were considered in this review, but none was of sufficient quality to include. Another Cochrane review of trials specifically undertaken in patients with neuropathic pain found no evidence for or against the efficacy and safety of psychological interventions for chronic neuropathic pain162, which is not surprising given the similar findings for non-psychological interventions163. An urgent need for studies of treatments that are designed specifically for patients with neuropathic pain exists, in particular, those with painful diabetic neuropathy, which is a growing problem164. Specifically, studies of CBT are needed with content that is specifically designed to meet the psychosocial needs of patients with neuropathy, in particular, with regard to the multiple sensory challenge, comorbidity and polypharmacy165. A recognition that neuropathic pain increases with age will also mean that an understanding of later-life accommodation to illness will be important166. In addition, a methodological focus on individual experience and trajectories of change is needed, either through single case experiments or through ecological momentary assessment167. Furthermore, communication technology, in particular, the use of mobile health innovation, is likely to play an important part in future solutions. However, how to manage effective therapeutic relationships at a distance, and how technology can augment and improve face-to-face CBT remain to be clarified168. Technical psychological variables — such as catastrophic thinking, acceptance or readiness to change — should be relegated to process variables. Conversely, a pragmatic focus on patient-reported outcomes will be essential to reduce pain, improve mood and reduce disability, which will ultimately improve quality of life.
Quality of life
Neuropathic pain can substantially impair quality of life as it often associates with other problems, such as loss of function, anxiety, depression, disturbed sleep and impaired cognition. Measures of health-related quality of life (HRQOL) that capture broad dimensions of health including physical, mental, emotional and social functioning are increasingly used when assessing the efficacy of different interventions to manage chronic neuropathic and non-neuropathic pain. It is mainly useful when calculating quality-adjusted life years, which are necessary for cost-utility analyses.
The most commonly used HRQOL instruments are general, whereas others have been designed specifically for those with neuropathic pain. Meyer-Rosberg and colleagues validated both the 36-Item Short Form Health Survey (SF-36) and the Nottingham Health Profile (NHP) in the assessment of HRQOL in neuropathic pain related to peripheral nerve or nerve root lesions in patients attending multidisciplinary pain clinics169. The scores of all eight dimensions (vitality, physical functioning, bodily pain, general health perceptions, physical role functioning, emotional role functioning, social role functioning and mental health) in the SF-36 were significantly lower in those with neuropathic pain than in the general population, which is in line with another study170.
The onset of neuropathy in patients with diabetes mellitus has been shown to significantly decrease all aspects of quality of life171. If diabetic polyneuropathy is accompanied by pain, both physical and mental components of quality of life are further affected172. A recent study also showed that both EuroQol five dimensions (EQ-5D) and Short Form-6 dimension (SF-6D) questionnaires can discriminate between chronic pain with or without neuropathic pain173. Furthermore, the role of psychological factors in impairing quality of life in neuropathic pain has been analysed174, showing, for example, that pain catastrophizing was associated with decreased HRQOL174. The SF-36 and the EQ-5D have been the most commonly used instruments in clinical trials to assess the efficacy of treatments, such as gabapentin in postherpetic neuralgia175, diabetic polyneuropathy176 and neuropathic pain due to peripheral nerve injury170; the efficacy of duloxetine in diabetic peripheral neuropathy177; and the efficacy of spinal cord stimulation in diabetic polyneuropathy178.
Outlook
Although nervous system mechanisms underlying chronic neuropathic pain have been uncovered through animal and human research, the development of novel interventions with improved efficacy and tolerability has been slow. New therapeutic approaches as well as improved clinical trial designs, specifically addressing genotypic and phenotypic profiles, have great promise to build on recent advances in basic and translational research.
Clinical trial design
The explanations for the slow progress in identifying treatments with improved efficacy that are receiving the greatest attention are inadequate clinical trial assay sensitivity and the need to target treatment to patients who are most likely to respond179,180. Assay sensitivity refers to the ability of a clinical trial to distinguish an efficacious treatment from placebo (or another comparator). The possibility that recent neuropathic pain clinical trials suffer from limited assay sensitivity is consistent with the observation that a considerable number of recent trials in patients with neuropathic pain investigating medications with well-established efficacy have returned negative results7,181. For example, a recent analysis of neuropathic pain trials showed that assay sensitivity was compromised by including patients with highly variable baseline pain ratings182, which suggests that trials might have greater assay sensitivity if highly variable baseline pain ratings were an exclusion criterion115.
The outcomes of clinical trials in neuropathic pain have generally shown modest efficacy, with the NNTs for 50% pain relief ranging from six to eight for positive studies in the latest meta-analysis101. Several reasons could account for these results179,181, including high placebo responses, variability in the diagnostic criteria used for neuropathic pain in clinical trials and limited assay sensitivity. Thus, it has been proposed that an alternative therapeutic approach to neuropathic pain should incorporate stratification of patients according to clinical phenotypes (signs and symptoms)66,77,183,184, whereas most trials have simply classified patients according to aetiology.
Phenotyping
Several clinical trials provide support for the relevance of phenotypic subgrouping of patients, which has the potential to lead to a more personalized pain therapy in the future107,110,185,186. In particular, two phenotypes — the presence of mechanical allodynia and preserved nociceptive function — are often combined and seem to predict the response to systemic and topical sodium channel blockers, botulinum toxin A and clonidine gel in recent clinical trials107,110,185. Indeed, any personalized pain treatments will rely on the ability to select patients who are likely to respond187.
The strongest evidence showing that profiles of signs and symptoms can identify treatment responders stems from a trial in which patients who were defined as having an irritable nociceptor phenotype experienced a greater decrease in pain with oxcarbazepine versus placebo than those without this phenotype186. This is the only trial in which a pre-specified primary analysis demonstrated a difference in treatment versus placebo response in patient subgroups identified by phenotyping. These results are very promising, but require replication as well as use of phenotyping measures that would be suitable for larger confirmatory trials and use in clinical practice188. Phenotyping could also be used to test whether certain patients have a more robust response to non-pharmacological treatments, for example, invasive, psychological and complementary interventions188, as well as to identify which patients are most likely to respond to combinations of treatments. Indeed, given the importance of expectations and psychological and social factors — including adaptive coping and catastrophizing — in the development and maintenance of chronic neuropathic pain, it would not be surprising if phenotyping has a great part to play in demonstrating the efficacy of psychological interventions as it does for medications.
To advance the design, execution, analysis and interpretation of clinical trials of pain treatments, several public-private partnerships have undertaken systematic efforts to increase assay sensitivity and provide validated approaches for phenotyping patients and identifying those who are most likely to respond to treatment. These efforts — which include ACTTION (www.acttion.org), EuroPain (www.imieuropain.org) and the German Research Network on Neuropathic Pain (www.neuro.med.tu-muenchen.de/dfns/) — are providing an evidence base for the design of future neuropathic pain clinical trials and for the development of mechanism-based approaches to personalized neuropathic pain treatment.
Personalized pain medicine
Personalized medical care refers to the principle that patients can be stratified such that each patient receives the most effective and tolerable treatment for their individual needs. Patients can be stratified on several levels: clinical phenotype, detailed sensory profiling, genetics and potentially (in the future) using cellular models to facilitate treatment choice. Close consultation with the patient is required and this involves complex discussions around the uncertainties of genetic risk and the balance between efficacy and tolerability of potential treatments. Human genetics studies have demonstrated that Nav1.7 is a crucial pain target189, and therapeutics aimed at targeting Nav1.7 provide an example of a situation in which testing for specific genetic mutations can inform patient care. Loss-of-function mutations lead to congenital insensitivity to pain and gain-of-function mutations cause rare inherited pain disorders, including inherited erythromelalgia31, paroxysmal extreme pain disorder32 and idiopathic small-fibre neuropathy (which involves pain and small-fibre degeneration in the extremities)33.
Genetic information can, therefore, inform diagnostics; however, the interpretation of genetic results is complex and should be accompanied by functional analysis of mutant ion channels wherever possible190. For instance, in the context of small-fibre neuropathy, mutations might not be fully penetrant. Finding a mutation in SCN9A may have immediate implications for treatment in choosing a drug with activity against voltage-gated sodium channels (not normally first-line agents in the treatment of neuropathic pain), such as mexiletine, which is not recommended in the treatment of neuropathic pain but is used in inherited erythromelalgia, in which mexiletine has proven efficacy in normalizing abnormal channel properties in vitro191 and clinical efficacy in individual cases. A further step has been taken in using structural modelling of Nav1.7 to predict what treatment a specific mutation will respond to192; the modelling results were used to predict the efficacy of carbamazepine (a voltage-gated sodium channel blocker) in inherited erythromelalgia associated with the SCN9A S241T mutation193. Furthermore, the generation of nociceptors in vitro using patient-derived induced pluripotent stem cells is now possible. In rare Mendelian pain disorders (such as inherited erythromelalgia), these nociceptors have been shown to be hyperexcitable194. Treatments targeting Nav1.7 can be screened in such cellular models and related to clinical efficacy as proof of concept before their use in patients (these nociceptors have been shown to be hyperexcitable in inherited erythromelalgia194).
Genetic stratification is more challenging in common acquired neuropathic pain states, such as painful diabetic neuropathy, because such conditions are polygenic and subject to considerable environmental interaction. Thus, the relevance of an individual target such as Nav1.7 in these conditions is less clear. Despite these limitations, the prospect of personalized medicine is a step forward towards promising pain management strategies.
Supplementary Material
Acknowledgments
L.C. acknowledges support from the UMB and the National Institute of Dental and Craniofacial Research (NIDCR) at the US NIH (R01DE025946). A.H.D. and D.L.B. acknowledge support from the Wellcome Trust Pain Consortium. R.B. acknowledges support from the European Union Project No. 633491: DOLORisk, IMI Europain, the German Federal Ministry of Education and Research (ERA_NET NEU-RON/IM-PAIN Project) and the German Research Network on Neuropathic Pain, NoPain system biology and the German Research Foundation. R.B. also acknowledges support from the German Federal Ministry of Education and Research (BMBF), the ERA_NET NEURON/IM-PAIN Project (01EW1503), the German Research Network on Neuropathic Pain (01EM0903), NoPain system biology (0316177C) and the German Research Foundation (DFG). D.Y. acknowledges support from the Israel Science Foundation, European Horizons 2020 and the US Department of Defense. S.N.R. acknowledges support from the NIH (NS26363).
Footnotes
Author contributions
Introduction (L.C. and T.L.); Epidemiology (D.B.); Mechanisms/ pathophysiology (A.H.D., L.C., D.Y. and R.F.); Diagnosis, screening and prevention (R.B., A.T. and R.H.D.); Management (N.A., N.B.F, S.N.R. and C.E.); Quality of life (E.K.); Outlook (R.H.D. and D.L.B.); Overview of the Primer (L.C.).
Competing interests
L.C. has received lecture honoraria (Georgetown University and Stanford University) and has acted as speaker or consultant for Grünenthal and Emmi Solution. R.B. is an industry member of AstraZeneca, Pfizer, Esteve, UCB Pharma, Sanofi Aventis, Grünenthal, Eli Lilly and Boehringer Ingelheim; has received lecture honoraria from Pfizer, Genzyme, Grünenthal, Mundipharma, Sanofi Pasteur, Medtronic Inc. Neuromodulation, Eisai, Lilly, Boehringer Ingelheim, Astellas, Desitin, Teva Pharma, Bayer-Schering, MSD and Seqirus; and has served as a consultant for Pfizer, Genzyme, Grünenthal, Mundipharma, Sanofi Pasteur, Medtronic Inc. Neuromodulation, Eisai, Lilly, Boehringer Ingelheim Pharma, Astellas, Desitin, Teva Pharma, BayerSchering, MSD, Novartis, Bristol-Myers Squibb, Biogen idec, AstraZeneca, Merck, AbbVie, Daiichi Sankyo, Glenmark Pharmaceuticals, Seqirus, Genentech, Galapagos NV and Kyowa Hakko Kirin. A.H.D. has acted as speaker or consultant forSeqirus, Grünenthal, Allergan and Mundipharma. D.B. has acted as a consultant for Grünenthal, Pfizer and Indivior. D.L.B. has acted as a consultant for Abide, Eli Lilly, Mundipharma, Pfizer and Teva. D.Y. received a lecture honorarium from Pfizer and holds equity in BrainsGate and Theranica. R.F. has acted as an advisory board member for Abide, Astellas, Biogen, Glenmark, Hydra, Novartis and Pfizer. A.T. has received research funding, lecture honoraria and acted as speaker or consultant for Mundipharma, Pfizer, Grünenthal and Angelini Pharma. N.A. has received honoraria for participation in advisory boards or speaker bureau by Astellas, Teva, Mundipharma, Johnson and Johnson, Novartis and Sanofi Pasteur MSD. N.B.F. has received honoraria for participation in advisory boards from Teva Pharmaceuticals, Novartis and Grünenthal, and research support from EUROPAIN Investigational Medicines Initiative (IMI). E.K. has served on the advisory boards of Orion Pharma and Grünenthal, and received lecture honoraria from Orion Pharma and AstraZeneca. R.H.D. has received research grants and contracts from the US FDA and the US NIH, and compensation for activities involving clinical trial research methods from Abide, Aptinyx, Astellas, Boston Scientific, Centrexion, Dong-A, Eli Lilly, Glenmark, Hope, Hydra, Immune, Novartis, NsGene, Olatec, Phosphagenics, Quark, Reckitt Benckiser, Relmada, Semnur, Syntrix, Teva, Trevena and Vertex. S.N.R. has received a research grant from Medtronic Inc. and honoraria for participation in advisory boards of Allergan, Daiichi Sankyo, Grünenthal USA Inc. and Lexicon Pharmaceuticals. C.E. and T.L. declare no competing interests.
References
- 1.Abbott CA, Malik RA, van Ross ER, Kulkarni J, Boulton AJ. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care. 2011;34:2220–2224. doi: 10.2337/dc11-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. This review presents differences and commonalities among distinct chronic pain states. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attal N, Lanteri-Minet M, Laurent B, Fermanian J, Bouhassira D. The specific disease burden of neuropathic pain: results of a French nationwide survey. Pain. 2011;152:2836–2843. doi: 10.1016/j.pain.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 4.Torrance N, Smith BH, Bennett MI, Lee AJ. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. J Pain. 2006;7:281–289. doi: 10.1016/j.jpain.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Finnerup NB, et al. Neuropathic pain: an updated grading system for research and clinical practice. Pain. 2016;157:1599–1606. doi: 10.1097/j.pain.0000000000000492. This is an updated grading system to guide clinical diagnosis of neuropathic pain by illustrating the significance of confirmatory tests, the role of screening tools and potential uncertainties about anatomical pain distributions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dolgin E. Fluctuating baseline pain implicated in failure of clinical trials. Nat Med. 2010;16:1053. doi: 10.1038/nm1010-1053a. [DOI] [PubMed] [Google Scholar]
- 7.Tuttle AH, et al. Increasing placebo responses over time in U.S. clinical trials of neuropathic pain. Pain. 2015;156:2616–2626. doi: 10.1097/j.pain.0000000000000333. This study explores factors explaining why novel analgesics that were designed to treat neuropathic pain failed. [DOI] [PubMed] [Google Scholar]
- 8.van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain. 2014;155:654–662. doi: 10.1016/j.pain.2013.11.013. This is one of the first attempts to review epidemiological studies of neuropathic pain in the general population; however, the heterogeneity of the studies precluded meta-analysis, indicating a need for standardized tools and diagnostic approaches. [DOI] [PubMed] [Google Scholar]
- 9.Bouhassira D, et al. Patient perspective on herpes zoster and its complications: an observational prospective study in patients aged over 50 years in general practice. Pain. 2012;153:342–349. doi: 10.1016/j.pain.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Helgason S, Petursson G, Gudmundsson S, Sigurdsson JA. Prevalence of postherpetic neuralgia after a first episode of herpes zoster: prospective study with long term follow up. BMJ. 2000;321:794–796. doi: 10.1136/bmj.321.7264.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daousi C, et al. Chronic painful peripheral neuropathy in an urban community: a controlled comparison of people with and without diabetes. Diabet Med. 2004;21:976–982. doi: 10.1111/j.1464-5491.2004.01271.x. [DOI] [PubMed] [Google Scholar]
- 12.Davies M, Brophy S, Williams R, Taylor A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care. 2006;29:1518–1522. doi: 10.2337/dc05-2228. [DOI] [PubMed] [Google Scholar]
- 13.Bouhassira D, Letanoux M, Hartemann A. Chronic pain with neuropathic characteristics in diabetic patients: a French cross-sectional study. PLoS ONE. 2013;8:e74195. doi: 10.1371/journal.pone.0074195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen MP, Chodroff MJ, Dworkin RH. The impact of neuropathic pain on health-related quality of life: review and implications. Neurology. 2007;68:1178–1182. doi: 10.1212/01.wnl.0000259085.61898.9e. [DOI] [PubMed] [Google Scholar]
- 15.Solaro C, et al. The prevalence of pain in multiple sclerosis: a multicenter cross-sectional study. Neurology. 2004;63:919–921. doi: 10.1212/01.wnl.0000137047.85868.d6. [DOI] [PubMed] [Google Scholar]
- 16.Osterberg A, Boivie J, Thuomas KA. Central pain in multiple sclerosis — prevalence and clinical characteristics. Eur J Pain. 2005;9:531–542. doi: 10.1016/j.ejpain.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Siddall PJ, McClelland JM, Rutkowski SB, Cousins MJA. longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain. 2003;103:249–257. doi: 10.1016/S0304-3959(02)00452-9. [DOI] [PubMed] [Google Scholar]
- 18.Klit H, Finnerup NB, Andersen G, Jensen TS. Central poststroke pain: a population-based study. Pain. 2011;152:818–824. doi: 10.1016/j.pain.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 19.Rayment C, et al. Neuropathic cancer pain: prevalence, severity, analgesics and impact from the European Palliative Care Research Collaborative-Computerised Symptom Assessment study. Palliat Med. 2013;27:714–721. doi: 10.1177/0269216312464408. [DOI] [PubMed] [Google Scholar]
- 20.Bennett MI, et al. Prevalence and aetiology of neuropathic pain in cancer patients: a systematic review. Pain. 2012;153:359–365. doi: 10.1016/j.pain.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 21.Bouhassira D, Attal N. Diagnosis and assessment of neuropathic pain: the saga of clinical tools. Pain. 2011;152:S74–S83. doi: 10.1016/j.pain.2010.11.027. This review describes the main clinical tools that are used for the screening and measurement of neuropathic pain, focusing on the potential value and limitation of each tool. [DOI] [PubMed] [Google Scholar]
- 22.Bouhassira D, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4) Pain. 2005;114:29–36. doi: 10.1016/j.pain.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Bennett M. The LANSS Pain Scale: the Leeds assessment of neuropathic symptoms and signs. Pain. 2001;92:147–157. doi: 10.1016/s0304-3959(00)00482-6. [DOI] [PubMed] [Google Scholar]
- 24.Bouhassira D, Lanteri-Minet M, Attal N, Laurent B, Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain. 2008;136:380–387. doi: 10.1016/j.pain.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 25.Freynhagen R, et al. Screening of neuropathic pain components in patients with chronic back pain associated with nerve root compression: a prospective observational pilot study (MIPORT) Curr Med Res Opin. 2006;22:529–537. doi: 10.1185/030079906X89874. [DOI] [PubMed] [Google Scholar]
- 26.Borsook D. Neurological diseases and pain. Brain. 2012;135:320–344. doi: 10.1093/brain/awr271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watson JC, Sandroni P. Central neuropathic pain syndromes. Mayo Clin Proc. 2016;91:372–385. doi: 10.1016/j.mayocp.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 28.Stavros K, Simpson DM. Understanding the etiology and management of HIV-associated peripheral neuropathy. Curr HIV/AIDS Rep. 2014;11:195–201. doi: 10.1007/s11904-014-0211-2. [DOI] [PubMed] [Google Scholar]
- 29.Thakur S, Dworkin RH, Haroun OM, Lockwood DN, Rice AS. Acute and chronic pain associated with leprosy. Pain. 2015;156:998–1002. doi: 10.1097/j.pain.0000000000000178. [DOI] [PubMed] [Google Scholar]
- 30.Freeman R. Not all neuropathy in diabetes is of diabetic etiology: differential diagnosis of diabetic neuropathy. Curr Diab Rep. 2009;9:423–431. doi: 10.1007/s11892-009-0069-7. This article discusses common disorders in the differential diagnosis of peripheral neuropathy. [DOI] [PubMed] [Google Scholar]
- 31.Yang Y, et al. Mutations in SCN9A, encoding a sodium channel alpha subunit, in patients with primary erythermalgia. J Med Genet. 2004;41:171–174. doi: 10.1136/jmg.2003.012153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fertleman CR, et al. SCN9A mutations in paroxysmal extreme pain disorder: allelic variants underlie distinct channel defects and phenotypes. Neuron. 2006;52:767–774. doi: 10.1016/j.neuron.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 33.Faber CG, et al. Gain of function Nav1.7 mutations in idiopathic small fiber neuropathy. Ann Neurol. 2012;71:26–39. doi: 10.1002/ana.22485. [DOI] [PubMed] [Google Scholar]
- 34.Haroutounian S, et al. Primary afferent input critical for maintaining spontaneous pain in peripheral neuropathy. Pain. 2014;155:1272–1279. doi: 10.1016/j.pain.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 35.Vaso A, et al. Peripheral nervous system origin of phantom limb pain. Pain. 2014;155:1384–1391. doi: 10.1016/j.pain.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 36.Serra J, et al. Microneurographic identification of spontaneous activity in C-nociceptors in neuropathic pain states in humans and rats. Pain. 2012;153:42–55. doi: 10.1016/j.pain.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 37.Kleggetveit IP, et al. High spontaneous activity of C-nociceptors in painful polyneuropathy. Pain. 2012;153:2040–2047. doi: 10.1016/j.pain.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 38.Tesfaye S, Boulton AJ, Dickenson AH. Mechanisms and management of diabetic painful distal symmetrical polyneuropathy. Diabetes Care. 2013;36:2456–2465. doi: 10.2337/dc12-1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fields HL, Rowbotham M, Baron R. Postherpetic neuralgia: irritable nociceptors and deafferentation. Neurobiol Dis. 1998;5:209–227. doi: 10.1006/nbdi.1998.0204. [DOI] [PubMed] [Google Scholar]
- 40.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–S15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baron R, Hans G, Dickenson AH. Peripheral input and its importance for central sensitization. Ann Neurol. 2013;74:630–636. doi: 10.1002/ana.24017. This article presents the main mechanisms of pain states that begin with damage to the nerves in the periphery and lead to enhanced transmitter release within the spinal cord and central sensitization — emphasizing the need for multimodal approaches that target central sensitization and/or its peripheral drivers. [DOI] [PubMed] [Google Scholar]
- 42.Patel R, Dickenson AH. Neuronal hyperexcitability in the ventral posterior thalamus of neuropathic rats: modality selective effects of pregabalin. J Neurophysiol. 2016;116:159–170. doi: 10.1152/jn.00237.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peyron R. Functional brain imaging: what has it brought to our understanding of neuropathic pain? A special focus on allodynic pain mechanisms. Pain. 2016;157:S67–S71. doi: 10.1097/j.pain.0000000000000387. [DOI] [PubMed] [Google Scholar]
- 44.Gagnon M, et al. Chloride extrusion enhancers as novel therapeutics for neurological diseases. Nat Med. 2013;19:1524–1528. doi: 10.1038/nm.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsuda M, Beggs S, Salter MW, Inoue K. Microglia and intractable chronic pain. Glia. 2013;61:55–61. doi: 10.1002/glia.22379. [DOI] [PubMed] [Google Scholar]
- 46.Navratilova E, Atcherley CW, Porreca F. Brain circuits encoding reward from pain relief. Trends Neurosci. 2015;38:741–750. doi: 10.1016/j.tins.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bannister K, Dickenson AH. What the brain tells the spinal cord. Pain. 2016;157:2148–2151. doi: 10.1097/j.pain.0000000000000568. [DOI] [PubMed] [Google Scholar]
- 48.Yarnitsky D. Role of endogenous pain modulation in chronic pain mechanisms and treatment. Pain. 2015;156:S24–S31. doi: 10.1097/01.j.pain.0000460343.46847.58. [DOI] [PubMed] [Google Scholar]
- 49.Lewis GN, Rice DA, McNair PJ. Conditioned pain modulation in populations with chronic pain: a systematic review and meta-analysis. J Pain. 2012;13:936–944. doi: 10.1016/j.jpain.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 50.Yarnitsky D, Granot M, Nahman-Averbuch H, Khamaisi M, Granovsky Y. Conditioned pain modulation predicts duloxetine efficacy in painful diabetic neuropathy. Pain. 2012;153:1193–1198. doi: 10.1016/j.pain.2012.02.021. This article presents a new perspective of predicting drug responses in patients with painful diabetic neuropathy based on CPM mechanisms. [DOI] [PubMed] [Google Scholar]
- 51.Wilder-Smith OH, Schreyer T, Scheffer GJ, Arendt-Nielsen L. Patients with chronic pain after abdominal surgery show less preoperative endogenous pain inhibition and more postoperative hyperalgesia: a pilot study. J Pain Palliat Care Pharmacother. 2010;24:119–128. doi: 10.3109/15360281003706069. [DOI] [PubMed] [Google Scholar]
- 52.Petersen KK, Graven-Nielsen T, Simonsen O, Laursen MB, Arendt-Nielsen L. Preoperative pain mechanisms assessed by cuff algometry are associated with chronic postoperative pain relief after total knee replacement. Pain. 2016;157:1400–1406. doi: 10.1097/j.pain.0000000000000531. [DOI] [PubMed] [Google Scholar]
- 53.Kosek E, Ordeberg G. Lack of pressure pain modulation by heterotopic noxious conditioning stimulation in patients with painful osteoarthritis before, but not following, surgical pain relief. Pain. 2000;88:69–78. doi: 10.1016/S0304-3959(00)00310-9. [DOI] [PubMed] [Google Scholar]
- 54.Graven-Nielsen T, Wodehouse T, Langford RM, Arendt-Nielsen L, Kidd BL. Normalization of widespread hyperesthesia and facilitated spatial summation of deep-tissue pain in knee osteoarthritis patients after knee replacement. Arthritis Rheum. 2012;64:2907–2916. doi: 10.1002/art.34466. [DOI] [PubMed] [Google Scholar]
- 55.Colloca L, Miller FG. Role of expectations in health. Curr Opin Psychiatry. 2011;24:149–155. doi: 10.1097/YCO.0b013e328343803b. [DOI] [PubMed] [Google Scholar]
- 56.Hall KT, et al. Conscientiousness is modified by genetic variation in catechol-O-methyltransferase to reduce symptom complaints in IBS patients. Brain Behav. 2015;5:39–44. doi: 10.1002/brb3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaptchuk TJ, et al. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ. 2008;336:999–1003. doi: 10.1136/bmj.39524.439618.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vase L, Robinson ME, Verne GN, Price DD. The contributions of suggestion, desire, and expectation to placebo effects in irritable bowel syndrome patients. An empirical investigation. Pain. 2003;105:17–25. doi: 10.1016/s0304-3959(03)00073-3. [DOI] [PubMed] [Google Scholar]
- 59.Vase L, Skyt I, Hall KT. Placebo, nocebo, and neuropathic pain. Pain. 2016;157:S98–S105. doi: 10.1097/j.pain.0000000000000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petersen GL, et al. Placebo manipulations reduce hyperalgesia in neuropathic pain. Pain. 2012;153:1292–1300. doi: 10.1016/j.pain.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 61.Petersen GL, et al. Expectations and positive emotional feelings accompany reductions in ongoing and evoked neuropathic pain following placebo interventions. Pain. 2014;155:2687–2698. doi: 10.1016/j.pain.2014.09.036. [DOI] [PubMed] [Google Scholar]
- 62.Colloca L, Lopiano L, Lanotte M, Benedetti F. Overt versus covert treatment for pain, anxiety, and Parkinson’s disease. Lancet Neurol. 2004;3:679–684. doi: 10.1016/S1474-4422(04)00908-1. [DOI] [PubMed] [Google Scholar]
- 63.Bennett MI, et al. Using screening tools to identify neuropathic pain. Pain. 2007;127:199–203. doi: 10.1016/j.pain.2006.10.034. [DOI] [PubMed] [Google Scholar]
- 64.Freynhagen R, Baron R, Gockel U, Tolle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22:1911–1920. doi: 10.1185/030079906X132488. [DOI] [PubMed] [Google Scholar]
- 65.Bouhassira D, et al. Development and validation of the Neuropathic Pain Symptom Inventory. Pain. 2004;108:248–257. doi: 10.1016/j.pain.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 66.Baron R, Forster M, Binder A. Subgrouping of patients with neuropathic pain according to pain-related sensory abnormalities: a first step to a stratified treatment approach. Lancet Neurol. 2012;11:999–1005. doi: 10.1016/S1474-4422(12)70189-8. [DOI] [PubMed] [Google Scholar]
- 67.Cruccu G, et al. EFNS guidelines on neuropathic pain assessment: revised 2009. Eur J Neurol. 2010;17:1010–1018. doi: 10.1111/j.1468-1331.2010.02969.x. [DOI] [PubMed] [Google Scholar]
- 68.Backonja MM, et al. Value of quantitative sensory testing in neurological and pain disorders: NeuPSIG consensus. Pain. 2013;154:1807–1819. doi: 10.1016/j.pain.2013.05.047. [DOI] [PubMed] [Google Scholar]
- 69.Rolke R, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. 2006;123:231–243. doi: 10.1016/j.pain.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 70.Baron R, et al. Peripheral neuropathic pain: a mechanism-related organizing principle based on sensory profiles. Pain. 2017;158:261–272. doi: 10.1097/j.pain.0000000000000753. This study shows how patients with neuropathic pain present with different expression patterns of sensory signs, which suggests various mechanisms of neuropathic pain generation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Magerl W, et al. Reference data for quantitative sensory testing (QST): refined stratification for age and a novel method for statistical comparison of group data. Pain. 2010;151:598–605. doi: 10.1016/j.pain.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 72.Haanpaa M, et al. NeuPSIG guidelines on neuropathic pain assessment. Pain. 2011;152:14–27. doi: 10.1016/j.pain.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 73.Garcia-Larrea L. Objective pain diagnostics: clinical neurophysiology. Neurophysiol Clin. 2012;42:187–197. doi: 10.1016/j.neucli.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 74.Truini A, et al. Pathophysiology of pain in postherpetic neuralgia: a clinical and neurophysiological study. Pain. 2008;140:405–410. doi: 10.1016/j.pain.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 75.Truini A, et al. Differential involvement of A-delta and A-beta fibres in neuropathic pain related to carpal tunnel syndrome. Pain. 2009;145:105–109. doi: 10.1016/j.pain.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 76.Truini A, et al. Mechanisms of pain in distal symmetric polyneuropathy: a combined clinical and neurophysiological study. Pain. 2010;150:516–521. doi: 10.1016/j.pain.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 77.Truini A, Garcia-Larrea L, Cruccu G. Reappraising neuropathic pain in humans — how symptoms help disclose mechanisms. Nat Rev Neurol. 2013;9:572–582. doi: 10.1038/nrneurol.2013.180. [DOI] [PubMed] [Google Scholar]
- 78.Truini A, et al. Trigeminal small-fibre function assessed with contact heat evoked potentials in humans. Pain. 2007;132:102–107. doi: 10.1016/j.pain.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 79.Hansen N, et al. Amplitudes of pain-related evoked potentials are useful to detect small fiber involvement in painful mixed fiber neuropathies in addition to quantitative sensory testing — an electrophysiological study. Front Neurol. 2015;6:244. doi: 10.3389/fneur.2015.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tesfaye S, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285–2293. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lauria G, et al. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol. 2010;17:903–912. doi: 10.1111/j.1468-1331.2010.03023.x. [DOI] [PubMed] [Google Scholar]
- 82.Nolano M, et al. Cutaneous innervation of the human face as assessed by skin biopsy. J Anat. 2013;222:161–169. doi: 10.1111/joa.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Truini A, et al. Does the epidermal nerve fibre density measured by skin biopsy in patients with peripheral neuropathies correlate with neuropathic pain? Pain. 2014;155:828–832. doi: 10.1016/j.pain.2014.01.022. This study presents the advantages and limitations of using skin biopsy and epidermal nerve fibre density measurement for the diagnosis of neuropathic pain. [DOI] [PubMed] [Google Scholar]
- 84.Papanas N, Ziegler D. Corneal confocal microscopy: recent progress in the evaluation of diabetic neuropathy. J Diabetes Investig. 2015;6:381–389. doi: 10.1111/jdi.12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ziegler D, et al. Early detection of nerve fiber loss by corneal confocal microscopy and skin biopsy in recently diagnosed type 2 diabetes. Diabetes. 2014;63:2454–2463. doi: 10.2337/db13-1819. [DOI] [PubMed] [Google Scholar]
- 86.Tavakoli M, et al. Corneal confocal microscopy: a novel noninvasive test to diagnose and stratify the severity of human diabetic neuropathy. Exp Neurol. 2010;223:245–250. doi: 10.2337/dc10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Smith BH, Torrance N. Epidemiology of neuropathic pain and its impact on quality of life. Curr Pain Headache Rep. 2012;16:191–198. doi: 10.1007/s11916-012-0256-0. [DOI] [PubMed] [Google Scholar]
- 88.Oxman MN, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 89.Schmader KE, et al. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50–59 years. Clin Infect Dis. 2012;54:922–928. doi: 10.1093/cid/cir970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lal H, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372:2087–2096. doi: 10.1056/NEJMoa1501184. This randomized, placebo-controlled, phase III study evaluates the efficacy and safety of a herpes zoster virus subunit vaccine; the vaccine reduced the risk of herpes zoster infection similarly in adults across several age brackets. [DOI] [PubMed] [Google Scholar]
- 91.Cunningham AL, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375:1019–1032. doi: 10.1056/NEJMoa1603800. [DOI] [PubMed] [Google Scholar]
- 92.Gewandter JS, et al. Research design considerations for chronic pain prevention clinical trials: IMMPACT recommendations. Pain. 2015;156:1184–1197. doi: 10.1097/j.pain.0000000000000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dworkin RH, et al. Recommendations for the management of herpes zoster. Clin Infect Dis. 2007;44:S1–S26. doi: 10.1086/510206. [DOI] [PubMed] [Google Scholar]
- 94.Smith AG, et al. Lifestyle intervention for prediabetic neuropathy. Diabetes Care. 2006;29:1294–1299. doi: 10.2337/dc06-0224. [DOI] [PubMed] [Google Scholar]
- 95.Attal N, et al. EFNS guidelines on pharmacological treatment of neuropathic pain. Eur J Neurol. 2006;13:1153–1169. doi: 10.1111/j.1468-1331.2006.01511.x. [DOI] [PubMed] [Google Scholar]
- 96.Dworkin RH, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132:237–251. doi: 10.1016/j.pain.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 97.Moulin D, et al. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Res Manag. 2014;19:328–335. doi: 10.1155/2014/754693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tan T, Barry P, Reken S, Baker M, Guideline Development Group Pharmacological management of neuropathic pain in non-specialist settings: summary of NICE guidance. BMJ. 2010;340:c1079. doi: 10.1136/bmj.c1079. [DOI] [PubMed] [Google Scholar]
- 99.Attal N, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17:1113–e88. doi: 10.1111/j.1468-1331.2010.02999.x. [DOI] [PubMed] [Google Scholar]
- 100.Finnerup NB, Attal N. Pharmacotherapy of neuropathic pain: time to rewrite the rulebook? Pain Manag. 2016;6:1–3. doi: 10.2217/pmt.15.53. [DOI] [PubMed] [Google Scholar]
- 101.Finnerup NB, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14:162–173. doi: 10.1016/S1474-4422(14)70251-0. This systematic review updates the recommendations for the pharmacological management of neuropathic pain, emphasizing that modest efficacy, considerable placebo responses, heterogeneous diagnostic criteria and poor phenotypic profiling account for moderate trial outcomes and unmet needs of the patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yalcin I, et al. β2-Adrenoceptors are critical for antidepressant treatment of neuropathic pain. Ann Neurol. 2009;65:218–225. doi: 10.1002/ana.21542. [DOI] [PubMed] [Google Scholar]
- 103.Luo ZD, et al. Upregulation of dorsal root ganglion α2δ calcium channel subunit and its correlation with allodynia in spinal nerve-injured rats. J Neurosci. 2001;21:1868–1875. doi: 10.1523/JNEUROSCI.21-06-01868.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vinik AI, et al. Capsaicin 8% patch repeat treatment plus standard of care (SOC) versus SOC alone in painful diabetic peripheral neuropathy: a randomised, 52-week, open-label, safety study. BMC Neurol. 2016;16:251. doi: 10.1186/s12883-016-0752-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tesfaye S, et al. Duloxetine and pregabalin: high-dose monotherapy or their combination? The “COMBO-DN study” — a multinational, randomized, double-blind, parallel-group study in patients with diabetic peripheral neuropathic pain. Pain. 2013;154:2616–2625. doi: 10.1016/j.pain.2013.05.043. [DOI] [PubMed] [Google Scholar]
- 106.Binder A, et al. Topical 5% lidocaine (lignocaine) medicated plaster treatment for post-herpetic neuralgia: results of a double-blind, placebo-controlled, multinational efficacy and safety trial. Clin Drug Investig. 2009;29:393–408. doi: 10.2165/00044011-200929060-00003. [DOI] [PubMed] [Google Scholar]
- 107.Demant DT, et al. Pain relief with lidocaine 5% patch in localized peripheral neuropathic pain in relation to pain phenotype: a randomised, double-blind, and placebo-controlled, phenotype panel study. Pain. 2015;156:2234–2244. doi: 10.1097/j.pain.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 108.Backonja M, et al. NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia: a randomised, double-blind study. Lancet Neurol. 2008;7:1106–1112. doi: 10.1016/S1474-4422(08)70228-X. [DOI] [PubMed] [Google Scholar]
- 109.Burness CB, McCormack PL. Capsaicin 8% patch: a review in peripheral neuropathic pain. Drugs. 2016;76:123–134. doi: 10.1007/s40265-015-0520-9. [DOI] [PubMed] [Google Scholar]
- 110.Attal N, et al. Safety and efficacy of repeated injections of botulinum toxin A in peripheral neuropathic pain (BOTNEP): a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2016;15:555–565. doi: 10.1016/S1474-4422(16)00017-X. This randomized, double-blind, placebo-controlled trial in peripheral neuropathic pain indicates that botulinum toxin A reduces pain intensity over 24 weeks compared with a placebo treatment. [DOI] [PubMed] [Google Scholar]
- 111.Shackleton T, et al. The efficacy of botulinum toxin for the treatment of trigeminal and postherpetic neuralgia: a systematic review with meta-analyses. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122:61–71. doi: 10.1016/j.oooo.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 112.Lakhan SE, Velasco DN, Tepper D. Botulinum toxin-A for painful diabetic neuropathy: a meta-analysis. Pain Med. 2015;16:1773–1780. doi: 10.1111/pme.12728. [DOI] [PubMed] [Google Scholar]
- 113.Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Prescription of long-acting opioids and mortality in patients with chronic noncancer pain. JAMA. 2016;315:2415–2423. doi: 10.1001/jama.2016.7789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Emery EC, Luiz AP, Wood JN. Nav1.7 and other voltage-gated sodium channels as drug targets for pain relief. Expert Opin Ther Targets. 2016;20:975–983. doi: 10.1517/14728222.2016.1162295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rice AS, et al. EMA401, an orally administered highly selective angiotensin II type 2 receptor antagonist, as a novel treatment for postherpetic neuralgia: a randomised, double-blind, placebo-controlled phase 2 clinical trial. Lancet. 2014;383:1637–1647. doi: 10.1016/S0140-6736(13)62337-5. [DOI] [PubMed] [Google Scholar]
- 116.Chen G, Park CK, Xie RG, Ji RR. Intrathecal bone marrow stromal cells inhibit neuropathic pain via TGF-beta secretion. J Clin Invest. 2015;125:3226–3240. doi: 10.1172/JCI80883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Braz JM, et al. Forebrain GABAergic neuron precursors integrate into adult spinal cord and reduce injury-induced neuropathic pain. Neuron. 2012;74:663–675. doi: 10.1016/j.neuron.2012.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cruccu G, et al. EFNS guidelines on neurostimulation therapy for neuropathic pain. Eur J Neurol. 2007;14:952–970. doi: 10.1111/j.1468-1331.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- 119.Dworkin RH, et al. Interventional management of neuropathic pain: NeuPSIG recommendations. Pain. 2013;154:2249–2261. doi: 10.1016/j.pain.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mekhail NA, et al. Retrospective review of 707 cases of spinal cord stimulation: indications and complications. Pain Pract. 2011;11:148–153. doi: 10.1111/j.1533-2500.2010.00407.x. [DOI] [PubMed] [Google Scholar]
- 121.Eldabe S, Buchser E, Duarte R. V Complications of spinal cord stimulation and peripheral nerve stimulation techniques: a review of the literature. Pain Med. 2016;17:325–336. doi: 10.1093/pm/pnv025. [DOI] [PubMed] [Google Scholar]
- 122.Bhatia A, Flamer D, Shah PS. Perineural steroids for trauma and compression-related peripheral neuropathic pain: a systematic review and meta-analysis. Can J Anaesth. 2015;62:650–662. doi: 10.1007/s12630-015-0356-5. [DOI] [PubMed] [Google Scholar]
- 123.Cohen SP, Bicket MC, Jamison D, Wilkinson I, Rathmell JP. Epidural steroids: a comprehensive, evidence-based review. Reg Anesth Pain Med. 2013;38:175–200. doi: 10.1097/AAP.0b013e31828ea086. [DOI] [PubMed] [Google Scholar]
- 124.Chou R, et al. Epidural corticosteroid injections for radiculopathy and spinal stenosis: a systematic review and meta-analysis. Ann Intern Med. 2015;163:373–381. doi: 10.7326/M15-0934. [DOI] [PubMed] [Google Scholar]
- 125.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 126.Yearwood TL, Hershey B, Bradley K, Lee D. Pulse width programming in spinal cord stimulation: a clinical study. Pain Physician. 2010;13:321–335. [PubMed] [Google Scholar]
- 127.De Ridder D, Plazier M, Kamerling N, Menovsky T, Vanneste S. Burst spinal cord stimulation for limb and back pain. World Neurosurg. 2013;80:642–649.e1. doi: 10.1016/j.wneu.2013.01.040. [DOI] [PubMed] [Google Scholar]
- 128.Russo M, Van Buyten JP. 10-kHz high-frequency SCS therapy: a clinical summary. Pain Med. 2015;16:934–942. doi: 10.1111/pme.12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Manca A, et al. Quality of life, resource consumption and costs of spinal cord stimulation versus conventional medical management in neuropathic pain patients with failed back surgery syndrome (PROCESS trial) Eur J Pain. 2008;12:1047–1058. doi: 10.1016/j.ejpain.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 130.Kumar K, Rizvi S. Cost-effectiveness of spinal cord stimulation therapy in management of chronic pain. Pain Med. 2013;14:1631–1649. doi: 10.1111/pme.12146. [DOI] [PubMed] [Google Scholar]
- 131.Deer TR, et al. The appropriate use of neurostimulation: new and evolving neurostimulation therapies and applicable treatment for chronic pain and selected disease states. Neuromodulation Appropriateness Consensus Committee. Neuromodulation. 2014;17:599–615. doi: 10.1111/ner.12204. [DOI] [PubMed] [Google Scholar]
- 132.North RB, et al. Spinal cord stimulation versus re-operation in patients with failed back surgery syndrome: an international multicenter randomized controlled trial (EVIDENCE study) Neuromodulation. 2011;14:330–335. doi: 10.1111/j.1525-1403.2011.00371.x. [DOI] [PubMed] [Google Scholar]
- 133.Kumar K, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain. 2007;132:179–188. doi: 10.1016/j.pain.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 134.Simpson EL, Duenas A, Holmes MW, Papaioannou D, Chilcott J. Spinal cord stimulation for chronic pain of neuropathic or ischaemic origin: systematic review and economic evaluation. Health Technol Assess. 2009;13:1–154. doi: 10.3310/hta13170. [DOI] [PubMed] [Google Scholar]
- 135.Kumar K, et al. The effects of spinal cord stimulation in neuropathic pain are sustained: a 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery. 2008;63:762–770. doi: 10.1227/01.NEU.0000325731.46702.D9. [DOI] [PubMed] [Google Scholar]
- 136.Kemler MA, De Vet HC, Barendse GA, Van Den Wildenberg FA, Van Kleef M. The effect of spinal cord stimulation in patients with chronic reflex sympathetic dystrophy: two years’ follow-up of the randomized controlled trial. Ann Neurol. 2004;55:13–18. doi: 10.1002/ana.10996. [DOI] [PubMed] [Google Scholar]
- 137.de Vos CC, et al. Spinal cord stimulation in patients with painful diabetic neuropathy: a multicentre randomized clinical trial. Pain. 2014;155:2426–2431. doi: 10.1016/j.pain.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 138.Slangen R, et al. Spinal cord stimulation and pain relief in painful diabetic peripheral neuropathy: a prospective two-center randomized controlled trial. Diabetes Care. 2014;37:3016–3024. doi: 10.2337/dc14-0684. [DOI] [PubMed] [Google Scholar]
- 139.Cruccu G, et al. EAN guidelines on central neurostimulation therapy in chronic pain conditions. Eur J Neurol. 2016;23:1489–1499. doi: 10.1111/ene.13103. These up-to-date guidelines on neurostimulation for neuropathic pain by the European Academy of Neurology indicate poor-to-moderate quality of evidence for non-invasive and invasive neurostimulation. [DOI] [PubMed] [Google Scholar]
- 140.Campbell CM, Jamison RN, Edwards RR. Psychological screening/phenotyping as predictors for spinal cord stimulation. Curr Pain Headache Rep. 2013;17:307. doi: 10.1007/s11916-012-0307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Campbell CM, et al. Dynamic pain phenotypes are associated with spinal cord stimulation-induced reduction in pain: a repeated measures observational pilot study. Pain Med. 2015;16:1349–1360. doi: 10.1111/pme.12732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Krames ES. The role of the dorsal root ganglion in the development of neuropathic pain. Pain Med. 2014;15:1669–1685. doi: 10.1111/pme.12413. [DOI] [PubMed] [Google Scholar]
- 143.Petersen EA, Slavin KV. Peripheral nerve/field stimulation for chronic pain. Neurosurg Clin N Am. 2014;25:789–797. doi: 10.1016/j.nec.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 144.Liem L, et al. One-year outcomes of spinal cord stimulation of the dorsal root ganglion in the treatment of chronic neuropathic pain. Neuromodulation. 2015;18:41–48. doi: 10.1111/ner.12228. [DOI] [PubMed] [Google Scholar]
- 145.Sukul VV, Slavin KV. Deep brain and motor cortex stimulation. Curr Pain Headache Rep. 2014;18:427. doi: 10.1007/s11916-014-0427-2. [DOI] [PubMed] [Google Scholar]
- 146.Moore NZ, Lempka SF, Machado A. Central neuromodulation for refractory pain. Neurosurg Clin N Am. 2014;25:77–83. doi: 10.1016/j.nec.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lefaucheur JP. Cortical neurostimulation for neuropathic pain: state of the art and perspectives. Pain. 2016;157:S81–S89. doi: 10.1097/j.pain.0000000000000401. In this paper, various types of stimulation, including EMCS, rTMS and anodal tDCS, are discussed as therapeutic strategies when neuropathic pain is lateralized and stimulation is applied to the motor cortex contralateral to the pain side. [DOI] [PubMed] [Google Scholar]
- 148.Lefaucheur JP, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS) Clin Neurophysiol. 2016;128:56–92. doi: 10.1016/j.clinph.2016.10.087. [DOI] [PubMed] [Google Scholar]
- 149.Keifer OP, Jr, Riley JP, Boulis NM. Deep brain stimulation for chronic pain: intracranial targets, clinical outcomes, and trial design considerations. Neurosurg Clin N Am. 2014;25:671–692. doi: 10.1016/j.nec.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Prager J, et al. Best practices for intrathecal drug delivery for pain. Neuromodulation. 2014;17:354–372. doi: 10.1111/ner.12146. [DOI] [PubMed] [Google Scholar]
- 151.Bolash R, Mekhail N. Intrathecal pain pumps: indications, patient selection, techniques, and outcomes. Neurosurg Clin N Am. 2014;25:735–742. doi: 10.1016/j.nec.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 152.Deer TR, et al. Polyanalgesic Consensus Conference — 2012: recommendations to reduce morbidity and mortality in intrathecal drug delivery in the treatment of chronic pain. Neuromodulation. 2012;15:467–482. doi: 10.1111/j.1525-1403.2012.00486.x. [DOI] [PubMed] [Google Scholar]
- 153.Pope JE, Deer TR, Bruel BM, Falowski S. Clinical uses of intrathecal therapy and its placement in the pain care algorithm. Pain Pract. 2016;16:1092–1106. doi: 10.1111/papr.12438. [DOI] [PubMed] [Google Scholar]
- 154.Dobson JL, McMillan J, Li L. Benefits of exercise intervention in reducing neuropathic pain. Front Cell Neurosci. 2014;8:102. doi: 10.3389/fncel.2014.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kluding PM, et al. The effect of exercise on neuropathic symptoms, nerve function, and cutaneous innervation in people with diabetic peripheral neuropathy. J Diabetes Compl. 2012;26:424–429. doi: 10.1016/j.jdiacomp.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Smart KM, Wand BM, O’Connell NE. Physiotherapy for pain and disability in adults with complex regional pain syndrome (CRPS) types I and II. Cochrane Database Syst Rev. 2016;2:CD010853. doi: 10.1002/14651858.CD010853.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Thieme H, Morkisch N, Rietz C, Dohle C, Borgetto B. The efficacy of movement representation techniques for treatment of limb pain — a systematic review and meta-analysis. J Pain. 2016;17:167–180. doi: 10.1016/j.jpain.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 158.Eccleston C, Crombez G. Worry and chronic pain: a misdirected problem solving model. Pain. 2007;132:233–236. doi: 10.1016/j.pain.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 159.DOLORisk. Objectives. DOLORisk. 2015 http://dolorisk.eu/project/objectives.
- 160.Williams AC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev. 2012;11:CD007407. doi: 10.1002/14651858.CD007407.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Eccleston C, et al. Psychological therapies (internet-delivered) for the management of chronic pain in adults. Cochrane Database Syst Rev. 2014;2:CD010152. doi: 10.1002/14651858.CD010152.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Eccleston C, Hearn L, Williams AC. Psychological therapies for the management of chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2015;10:CD011259. doi: 10.1002/14651858.CD011259.pub2. This systematic review indicates a lack of evidence on the efficacy and safety of psychological interventions for chronic neuropathic pain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Moore A, Derry S, Eccleston C, Kalso E. Expect analgesic failure; pursue analgesic success. BMJ. 2013;346:f2690. doi: 10.1136/bmj.f2690. [DOI] [PubMed] [Google Scholar]
- 164.Otis JD, et al. A randomized controlled pilot study of a cognitive-behavioral therapy approach for painful diabetic peripheral neuropathy. J Pain. 2013;14:475–482. doi: 10.1016/j.jpain.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 165.Eccleston C. Embodied: The Psychology of Physical Sensation. Oxford Univ. Press; 2016. [Google Scholar]
- 166.Eccleston C, Tabor A, Edwards RT, Keogh E. Psychological approaches to coping with pain in later life. Clin Geriatr Med. 2016;32:763–771. doi: 10.1016/j.cger.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 167.Morley S, Williams A, Eccleston C. Examining the evidence about psychological treatments for chronic pain: time for a paradigm shift? Pain. 2013;154:1929–1931. doi: 10.1016/j.pain.2013.05.049. [DOI] [PubMed] [Google Scholar]
- 168.Duggan GB, et al. Qualitative evaluation of the SMART2 self-management system for people in chronic pain. Disabil Rehabil Assist Technol. 2015;10:53–60. doi: 10.3109/17483107.2013.845696. [DOI] [PubMed] [Google Scholar]
- 169.Meyer-Rosberg K, et al. Peripheral neuropathic pain — a multidimensional burden for patients. Eur J Pain. 2001;5:379–389. doi: 10.1053/eujp.2001.0259. [DOI] [PubMed] [Google Scholar]
- 170.Gordh TE, et al. Gabapentin in traumatic nerve injury pain: a randomized, double-blind, placebo-controlled, cross-over, multi-center study. Pain. 2008;138:255–266. doi: 10.1016/j.pain.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 171.Ahroni JH, Boyko EJ. Responsiveness of the SF-36 among veterans with diabetes mellitus. J Diabetes Compl. 2000;14:31–39. doi: 10.1016/s1056-8727(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 172.Van Acker K, et al. Prevalence and impact on quality of life of peripheral neuropathy with or without neuropathic pain in type 1 and type 2 diabetic patients attending hospital outpatients clinics. Diabetes Metab. 2009;35:206–213. doi: 10.1016/j.diabet.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 173.Torrance N, et al. Estimating the burden of disease in chronic pain with and without neuropathic characteristics: does the choice between the EQ-5D and SF-6D matter? Pain. 2014;155:1996–2004. doi: 10.1016/j.pain.2014.07.001. This study presents the advantage of using EQ-5D over SF-6D for estimating the burden of chronic pain in those with or without neuropathic clinical characteristics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Geelen CC, Kindermans HP, van den Bergh JP, Verbunt JA. Perceived physical activity decline as a mediator in the relationship between pain catastrophizing, disability, and quality of life in patients with painful diabetic neuropathy. Pain Pract. 2016 doi: 10.1111/papr.12449. http://dx.doi.org/10.1111/papr.12449. [DOI] [PubMed]
- 175.Backonja M, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA. 1998;280:1831–1836. doi: 10.1001/jama.280.21.1831. [DOI] [PubMed] [Google Scholar]
- 176.Rowbotham M, Harden N, Stacey B, Bernstein P, Magnus-Miller L. Gabapentin for the treatment of postherpetic neuralgia: a randomized controlled trial. JAMA. 1998;280:1837–1842. doi: 10.1001/jama.280.21.1837. [DOI] [PubMed] [Google Scholar]
- 177.Ogawa K, Fujikoshi S, Montgomery W, Alev L. Correlation between pain response and improvements in patient-reported outcomes and health-related quality of life in duloxetine-treated patients with diabetic peripheral neuropathic pain. Neuropsychiatr Dis Treat. 2015;11:2101–2107. doi: 10.2147/NDT.S87665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Duarte RV, Andronis L, Lenders MW, de Vos CC. Quality of life increases in patients with painful diabetic neuropathy following treatment with spinal cord stimulation. Qual Life Res. 2016;25:1771–1777. doi: 10.1007/s11136-015-1211-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dworkin RH, et al. Considerations for improving assay sensitivity in chronic pain clinical trials: IMMPACT recommendations. Pain. 2012;153:1148–1158. doi: 10.1016/j.pain.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 180.Gewandter JS, et al. Research designs for proof-of-concept chronic pain clinical trials: IMMPACT recommendations. Pain. 2014;155:1683–1695. doi: 10.1016/j.pain.2014.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dworkin RH, et al. Assay sensitivity and study features in neuropathic pain trials: an ACTTION meta-analysis. Neurology. 2013;81:67–75. doi: 10.1212/WNL.0b013e318297ee69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Farrar JT, et al. Effect of variability in the 7-day baseline pain diary on the assay sensitivity of neuropathic pain randomized clinical trials: an ACTTION study. Pain. 2014;155:1622–1631. doi: 10.1016/j.pain.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 183.Attal N, et al. Assessing symptom profiles in neuropathic pain clinical trials: can it improve outcome? Eur J Pain. 2011;15:441–443. doi: 10.1016/j.ejpain.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 184.Finnerup NB, Jensen TS. Mechanisms of disease: mechanism-based classification of neuropathic pain — a critical analysis. Nat Clin Pract Neurol. 2006;2:107–115. doi: 10.1038/ncpneuro0118. [DOI] [PubMed] [Google Scholar]
- 185.Campbell CM, et al. Randomized control trial of topical clonidine for treatment of painful diabetic neuropathy. Pain. 2012;153:1815–1823. doi: 10.1016/j.pain.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Demant DT, et al. The effect of oxcarbazepine in peripheral neuropathic pain depends on pain phenotype: a randomised, double-blind, placebo-controlled phenotype-stratified study. Pain. 2014;155:2263–2273. doi: 10.1016/j.pain.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 187.Dworkin RH, McDermott MP, Farrar JT, O’Connor AB, Senn S. Interpreting patient treatment response in analgesic clinical trials: implications for genotyping, phenotyping, and personalized pain treatment. Pain. 2014;155:457–460. doi: 10.1016/j.pain.2013.09.019. This article indicates within-patient variation and treatment-by-patient interaction as two sources of variance that contribute to differences between patients in responding to efficacious analgesic treatments. [DOI] [PubMed] [Google Scholar]
- 188.Edwards RR, et al. Patient phenotyping in clinical trials of chronic pain treatments: IMMPACT recommendations. Pain. 2016;157:1851–1871. doi: 10.1097/j.pain.0000000000000602. This article proposes core phenotyping domains to estimate the likelihood to experience pain relief. Recommended measures for each domain include psychosocial factors, symptom characteristics, sleep patterns, responses to noxious stimulation, endogenous pain-modulatory processes and response to pharmacological challenge. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bennett DL, Woods CG. Painful and painless channelopathies. Lancet Neurol. 2014;13:587–599. doi: 10.1016/S1474-4422(14)70024-9. This paper provides an introduction to the discovery of genetic variants that can alter the individual perception of pain, including the inactivating mutations in SCN9A, resulting in congenital insensitivity to pain. Furthermore, other genetic variations that contribute to risk or severity of more complex pain phenotypes are presented. [DOI] [PubMed] [Google Scholar]
- 190.Waxman SG, et al. Sodium channel genes in pain-related disorders: phenotype-genotype associations and recommendations for clinical use. Lancet Neurol. 2014;13:1152–1160. doi: 10.1016/S1474-4422(14)70150-4. [DOI] [PubMed] [Google Scholar]
- 191.Cregg R, Cox JJ, Bennett DL, Wood JN, Werdehausen R. Mexiletine as a treatment for primary erythromelalgia: normalization of biophysical properties of mutant L858F NaV1.7 sodium channels. Br J Pharmacol. 2014;171:4455–4463. doi: 10.1111/bph.12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yang Y, et al. Structural modelling and mutant cycle analysis predict pharmacoresponsiveness of a Nav 1.7 mutant channel. Nat Commun. 2012;3:1186. doi: 10.1038/ncomms2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Geha P, et al. Pharmacotherapy for pain in a family with inherited erythromelalgia guided by genomic analysis and functional profiling. JAMA Neurol. 2016;73:659–667. doi: 10.1001/jamaneurol.2016.0389. [DOI] [PubMed] [Google Scholar]
- 194.Cao L, et al. Pharmacological reversal of a pain phenotype in iPSC-derived sensory neurons and patients with inherited erythromelalgia. Sci Transl Med. 2016;8:335ra56. doi: 10.1126/scitranslmed.aad7653. [DOI] [PubMed] [Google Scholar]
- 195.Krause SJ, Backonja MM. Development of a neuropathic pain questionnaire. Clin J Pain. 2003;19:306–314. doi: 10.1097/00002508-200309000-00004. [DOI] [PubMed] [Google Scholar]
- 196.Portenoy R. Development and testing of a neuropathic pain screening questionnaire: ID Pain. Curr Med Res Opin. 2006;22:1555–1565. doi: 10.1185/030079906X115702. [DOI] [PubMed] [Google Scholar]
- 197.Bierhaus A, et al. Methylglyoxal modification of Nav1.8 facilitates nociceptive neuron firing and causes hyperalgesia in diabetic neuropathy. Nat Med. 2012;18:926–933. doi: 10.1038/nm.2750. [DOI] [PubMed] [Google Scholar]
- 198.Burma NE, Leduc-Pessah H, Fan CY, Trang T. Animal models of chronic pain: advances and challenges for clinical translation. J Neurosci Res. 2016 doi: 10.1002/jnr.23768. http://dx.doi.org/10.1002/jnr.23768. [DOI] [PubMed]
- 199.Okun A, et al. Hedonic and motivational responses to food reward are unchanged in rats with neuropathic pain. Pain. 2016;157:2731–2738. doi: 10.1097/j.pain.0000000000000695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Patel R, Dickenson AH. Mechanisms of the gabapentinoids and alpha 2 delta-1 calcium channel subunit in neuropathic pain. Pharmacol Res Perspect. 2016;4:e00205. doi: 10.1002/prp2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Dias QM, et al. The effect of intrathecal gabapentin on neuropathic pain is independent of the integrity of the dorsolateral funiculus in rats. Life Sci. 2012;91:837–842. doi: 10.1016/j.lfs.2012.08.032. [DOI] [PubMed] [Google Scholar]
- 202.Rauck R, et al. Intrathecal gabapentin to treat chronic intractable noncancer pain. Anesthesiology. 2013;119:675–686. doi: 10.1097/ALN.0b013e3182a10fbf. [DOI] [PubMed] [Google Scholar]
- 203.Nir RR, Yarnitsky D. Conditioned pain modulation. Curr Opin Support Palliat Care. 2015;9:131–137. doi: 10.1097/SPC.0000000000000126. [DOI] [PubMed] [Google Scholar]
- 204.Valeriani M, Pazzaglia C, Cruccu G, Truini A. Clinical usefulness of laser evoked potentials. Neurophysiol Clin. 2012;42:345–353. doi: 10.1016/j.neucli.2012.05.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


