Abstract
Sex, the states of being female or male, potentially interacts with all xenobiotic exposures, both inadvertent and deliberate, and influences their toxicokinetics, toxicodynamics, and outcomes. Sex differences occur in behavior, exposure, anatomy, physiology, biochemistry, and genetics, accounting for female-male differences in responses to environmental chemicals, diet, and pharmaceuticals, including adverse drug reactions. Often viewed as an annoying confounder, researchers have studied only one sex, adjusted for sex, or ignored it. Occupational epidemiology, the basis for understanding many toxic effects in humans, usually excluded women. Likewise FDA rules excluded women of child-bearing age from drug studies for many years. Aside from sex-specific organs, sex differences and sex × age interactions occur for a wide range of disease states as well as hormone-influenced conditions and drug distribution. Women have more adverse drug reactions than men, The Classic Sex Hormone Paradigm (gonadectomy and replacement) reveals significant interaction of sex and toxicokinetics including absorption, distribution, metabolisms and elimination. Studies should be designed to detect sex differences, describe the mechanisms, and interpret these in a broad social, clinical and evolutionary context with phenomena that do not differ. Sex matters, but how much of a difference is needed to matter remains challenging.
Keywords: absorption, distribution, metabolism, excretion, sex dimorphism, sex hormones, adverse drug reactions
Introduction
Sex differences in exposure, behavior, exposure, anatomy, physiology, biochemistry and genetics, influence toxicokinetics and toxicodynamics from the molecular to whole animal level, accounting for female-male differences in responses to xenobiotics in humans and other animals. The Institute of Medicine (IOM)(Wizemann and Pardue 2001) concluded that “sex matters” and exhorted: “Being male or female is an important fundamental variable that should be considered when designing and analyzing basic and clinical research.” Aside from obvious differences related to sex-specific organs and reproductive events, xenobiotics can interact differently with the male and female sex hormones and their receptors. There remain many gaps in understanding sex differences in toxicology. Much of our understanding of toxic effects on humans stems from industrial toxicology and epidemiologic studies of workforces exposed to high levels of one or more chemicals. In addition to limitations from inexact exposure information, the traditional industrial workforces were almost exclusively male, and females were often absent or in such small numbers that they were ignored by epidemiologists (Zahm and Fraumeni, 1995; Craft, 2003; Kogevinas and Zahm 2003).
Even when a study comprises both sexes, the analysis might ignore sex differences (Wallace and Gill, 1982; Shalat et al., 2003) or adjust for them, or may lack gender-specific exposure (Greenberg and Dement, 1994). The concurrence of globalization with the widespread entry of women into factories in developing countries, offers the opportunity for new wave occupational epidemiology studies, which have the potential to fill the gaps in knowledge about exposure of both women and men to old and new industrial chemicals and particles (Gochfeld 2005).
Until the early 1990s, the U.S. Food and Drug Administration recommended against testing drugs on women (FDA 1977), particularly women of child-bearing age. In 1992, under encouragement from women's health advocates, the National Institutes of Health and the IOM reported that women were being denied evidence uniquely beneficial to their gender and that legal and ethical issues needed to be overcome (Mastroianni et al. 1994). In response to the growing disquiet and the impending IOM report, the FDA (1993) reversed its position and recommended inclusion of women in drug trials. However, researchers proved reluctant to countenance such a dramatic shift in paradigm, and women continued to be underrepresented. In many cases female participants were required to certify or even prove that they were taking contraceptives, for fear of liability in the event of pregnancy. Ironically this imposed a potent biologic influence on the very outcomes of interest (Holdcroft 2007). Contraceptives affect many clinical chemistry and physiology variables, as well as DNA methylation, endothelial function, macrophage function, and immune responses thereby compromising the drug studies supposed to represent all women (Campesi et al. 2012). I calculated that if 40% of U.S. females are of child bearing age (15 to 44 years, US Census), and 62% of women of child bearing age use contraception (Jones et al. 2012) and 68% of these use oral hormones (Guttmacher Institute 2015), then about 16% of females are represented by women in studies requiring hormonal contraception. In other cases, the warnings or releases required by Institutional Review Boards would scare away most women from volunteering, even for low risk trials.
Some studies that observe male-female differences have determined that they are all due to the average body size differences, (Schwartz, 2003) without leading to mechanistic investigation. In other cases statistical adjustment obscured differences. Laboratory research continued to focus on male animals or male cells, perhaps because of a mistaken belief that female development and physiology are intrinsically more variable than males (Itoh and Arnold 2015). The NIH has tried to engage scientists and expand the use of female animals, tissues, and cells (McCullough et al. 2014), indeed this has become a controversial requirement for grant funding (Mazure 2016).
Background
This review began with participation in two conferences in 2002 and 2003 and the literature was reviewed in 2005 (Gochfeld 2007) and again in 2015-2016 for the presentation at the Society of Toxicologic Pathology. Literature was obtained primarily through the PUBMED access to the National Library of Medicine, via the Rutgers University library portal. This was not intended as a comprehensive review, but to illustrate the areas in which sex differences had been identified.
In response to the IOM report (Wizemann and Pardue 2001), the Scientific Committee on Problems of the Environment (SCOPE) teamed with the International Union for Pure and Applied Chemistry (IUPAC) to sponsor a weeklong symposium on endocrine disruptors (Miyamoto and Burger 2003). SGOMSEC, the Scientific Group on Methodologies for the Safety Evaluation of Chemicals, an organ of SCOPE, conducted a weeklong workshop on “gender and toxicology” (Klein et al. 2007). The resulting Framework for Gender Differences in Human and Animal Toxicology (Gochfeld 2007), emphasized that sex was not adequately accounted for or analyzed in toxicology, epidemiology, and even in drug development studies. Many studies used only one sex, or if both were used, observed sex differences were unexplored (Gochfeld 2007). Toxicokinetic studies focused on metabolism, with absorption differences ignored. Physiologists and ethologists had a well-established sex hormone paradigm involving gonadectomy with and without same or opposite-sex hormone replacement, to demonstrate the primary regulatory role of the sex hormones. Toxicologists were just beginning to use this powerful approach. Other sex differences such as a direct role of genes on the X or Y chromosome were underappreciated. By 2010 there had been little improvement in attention to sex and gender in in drug research and clinical practice (Arnold 2014, Nieuwenhoven and Klinge 2010) or in pharmacology and toxicology research (Gochfeld 2010). Toxicology fared somewhat better than drug developoment, but under the keywords “gender” or “sex”, male-female differences receive scant attention in toxicology texts. For example, Klaassen (2013) has a one page entry under “gender”, none under “sex”. In an otherwise excellent chapter on solvents, Bruckner et al. (2013) cite our paper (Vahter et al. 2007), and only one 2008 paper in the section on gender.
The Framework was organized around exposure, toxicokinetics, toxicodynamics, and modulating influences (Figure 1; Gochfeld 2007, Vahter et al. 2007). The present paper focuses on toxicokinetics. Ttoxicodynamics will require a separate paper.
Figure 1.
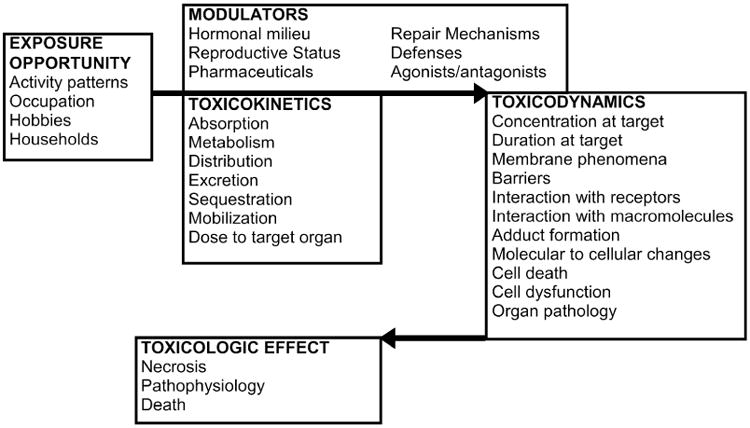
Framework for sex differences in toxicology linking exposure, toxicokinetics and toxicodynamics (after Gochfeld 2007).
Toxicokinetics encompasses absorption,distribution, metabolism and elimination.
Toxicodynamics includes the interaction of a chemical or its metabolites with targets at the molecular, subcellular, and cellular level resulting in a pathophysiologic effect or organ toxicity (Johnson et al., 1997). Both toxicokinetic and toxicodynamic actions are subject to modification, particularly by the gonadal steroids and growth hormone, by various agonists and antagonists, and by co-exposures.
Semantics
Sometime in the 1980s toxicologists began publishing on “gender differences” ratherthan “sex differences”, the terminology used in the 1960s (Dubois and Puchala 1961, Munoz et al. 1961). Perhaps it was considered impolite to talk about “sex” in public. However, “sex” referring to the genetic/gonadal/hormonal condition of an individual as male or female, is different from “gender”, a state of self-identifying as masculine or feminine---a distinction made painfully clear even to the lay public in 2016 by controversy over bathroom use by transgender individuals. This paper uses the term “sex” and the author acknowledges having capitulated to the “gender” movement in the 2007 paper. However, animals do have gender reflected in varying manifestations of aggressive or maternal traits (see below). Franconi and Campesi (2014) compromise, using the term “sex-gender differences (SGD)”. This paper uses the term “sex” to refer to male and female animals and to men and women.
Another semantic issue is the use of “fold differences” in lieu of per cent differences. Thus a 50% difference would be reported as a 1.5 fold difference. This is often clearer when talking about 10 fold or 100 fold differences in some measure. A 10 fold difference is sometimes represented as a 10 × difference or 1000% difference in the literature.
Sex Differences
Sex differences in responses to chemical, physical, biological, or psychosocial stressors are readily apparent, and dramatic differences in how males and females respond to exposures remain challenging. Of particular interest is why some chemicals produce cancer or disease in one sex but not another. Is this grounds for dismissing it or for understanding it (see Cancer section below)? Such differences in humans are not unique nor unexpected. Males and female humans have different lifestyles, experiences, and exposures (gender differences), many of which are not technically or ethically amenable to controlled trials. Animal studies, however, offer the opportunity to bypass these differences and focus on sex differences in anatomy, physiology, biochemistry, molecular biology and behavior. In addition to sex-specific organs and sexually distinctive hormonal axes, males and females may show differences in anatomy, physiology, and biochemistry of the organ systems they have in common. The classical sex hormone paradigm of agonists-antagonists-gonadectomy-replacement, is increasingly exploited, and has been supplemented in the past decade by a variety of knockout mice (Carey et al. 2007) as well as by high throughput genetic sequencing facilitating advances in various–omics.
Differences between sexes can be broken down as: 1) sociocultural, 2) exposure, 3) body size and composition, 4) genetic-molecular-biochemical, and 5) hormonal and reproductive, including pregnancy. This review focuses on the adult male and female (non-pregnant), with the recognition that differences may be altered during development, during estrous or menstrual cycles or by pregnancy. Some of the sex differences in anatomy, physiology and pharmacology, arise from the larger average size and mass of males in various species, and therefore the average larger volume and mass of male organs, compared with females. In many papers physiologic variables are ‘standardized’ for body mass or surface area that partially corrects for the body size differences (Nair and Jacob 2016). But it is too easy to “blame” observed differences on biometrics alone, while ignoring biology.
1. Adverse Drug Reactions (ADR)
Much of our knowledge of adverse drug reactions (ADRs) comes from spontaneous reports by patients or doctors to various pharmacovigilance systems in different countries (Ryu et al. 2015). These suffer from selection bias, with more severe ADRs more likely to be reported. Across nations (Ryu et al. 2015; Patel and Patel 2016) women report more adverse drug reactions than men, partly reflecting a machismo attitude among males, and partly reflecting actual susceptibilities. A Dutch study reported that women were more likely than men to volunteer for a web-based pharmacovigilance survey program for altruistic reasons (Härmark et al. 2013). Rademaker (2001) estimated a 50% increased risk of ADR for women, while in, Nakagawa and Kajiwara (2015) estimated a 2-fold increased risk across “all drug classes”, and women had more severe ADRs more likely to require hospitalization. This ADR sex difference is not apparent in childhood (Damien et al. 2016).
The heightened response of female rats to anesthesia has been known for almost a century (Nicholas and Barron 1932). Women may require higher doses of some drugs, while being more sensitive to respiratory depression, and waking sooner. ADRs can be related to overdose and toxicity of the desired pharmacologic effect or to side effects, unrelated to the primary mode of action. The former are dose-dependent, the latter more likely to be idiosyncratic and possibly dose-independent. ADRs frequently arise from drug interactions, so simply taking more medications creates both an arithmetic risk increase (more drugs leads to more risk) and a multiplicative risk (interaction). Both toxicokinetics and toxicodynamics play roles in ADRs.
Calabrese (1985) published a list of pharmaceuticals already known to affect males and females differently. Compared with men, women respond well at lower doses of psychoactive drugs (Seeman 2004, Maron et al. 2004). At the same dosage regimen of fluoxetine (Prozac) women showed a greater cortisol reduction than men (Bano et al. 2004). This could be due to a higher concentration of the drug at the target (toxicokinetic) or altered metabolic profile. The difference in rates of ADR probably has multiple causes, both pharmacokinetic and pharmacodynamics (Miller 2001; Anderson 2008). Most of the FDA drug bans are based on toxic effects in women (Heinrich 2001). These effects might have been recognized earlier if women had been represented in the drug development trials.
Is the ADR risk because women take relatively higher doses? Are the relatively higher doses a function of inadequately studied pharmacokinetics or pharmacodynamics. Do women take more medications in general, thus increasing their risk arithmetically, or is it ‘simply’ that women are more likely to report, perhaps more mild events. All of these probably contribute (Anderson 2008). Paradoxically one study found that the ADR reports by women are more serious and more likely to result in hospitalization (Miller 2001), for example sodium phosphate induced nephropathy (Ehrenpreis et al. 2011). But a U.S. study of 2341 fatal ADRs found males over-represented (Shepherd et al. 2012). Females also experience more allergic and hepatotoxic effects from a variety of drugs (Anderson 2008).
Using the Dutch National Medical Registry, Rodenburg et al. (2012) found about 1/3 of adverse drug reactions (ADR) requiring hospital admissions were attributable to cardiovascular drugs, with women only slightly over-represented (55%). Women were significantly more likely to suffer from diuretics and cardiac glycosides and less prone to ADR from coronary vasodilators. In Korea, women were over-represented in ADRs due to drugs but not due to contrast-media ADRs (Ryu et al. 2015).
Among the most serious adverse drug reactions are cardiac arrhythmias which can be caused by cardiovascular drugs or non-cardiovascular drugs (Frommeyer and Eckardt 2016). Non-cardiac medications may cause Brugada syndrome (males at greater risk) or long-QT interval (females at greater risk), both of which may lead to fatal arrhythmias (Konigstein et al. 2016). The commonly used opiate pain medication, tramadol, has primarily nervous system toxicity, but also affects the electrophysiology of the heart. Among 1400 tramadol poisoning cases, EKG abnormalities were identified in a third, with males significantly over-represented among the tachycardia and S-wave abnormalities (P<0.0001) and females significantly over-represented among long QT interval and right bundle branch block (Ghamsari et al. 2016).
2. Anatomy, Body and Organ Size, Composition and Physiology
Across human societies, males average taller and heavier than females with relatively more muscle mass and lower body fat. These anatomical differences have received more attention than physiologic differences. The body weight difference is used to correct organ weights and physiologic metrics or to scale therapeutic doses for females (Nair and Jacob 2016). The International Commission on Radiological Protection (ICRP) developed its “reference man” to assist in calculating radiation doses to organs. ICRP 89 (Valentin, 2001) provided mean values for percent body composition for adult males and females. (Table 1). The data were not intended for physiologically-based pharmacokinetic modeling (PBPK) and were therefore limited by the lack of distribution parameters (Gochfeld 2007). Some of the key variables such as renal clearance are not provided.
Table 1.
Absolute and relative body composition for adult Caucasian men and women extracted from “Reference Man Table” 2.8 in the International Commission on Radiological Protection (ICRP Publication 89, Valentin 2001). Relative organ mass standardized by dividing by body mass. (used with permission of Environmental and Occupational Health Sciences Institute).
| Organ as % of body mass | Organ mass for adult male | Organ mass for adult female | Female:male ratio of relative organ mass |
|---|---|---|---|
| Total body mass | 73 kg | 60 kg | |
| Adipose tissue | 25% | 37% | 150% |
| Skeletal muscle | 40% | 29% | 73% |
| Cortical bone | 6.0% | 5.3% | 88% |
| Trabecular bone | 1.51% | 1.33% | 88% |
| Cartilage | 1.51% | 1.50% | 99% |
| Blood | 7.7% | 6.8% | 89% |
| Skin | 4.5% | 3.8% | 85% |
| GI Tract | 1.66% | 1.9% | 114% |
| Lungs | 0.69% | 0.7% | 102% |
| Liver | 2.45% | 2.33% | 95% |
| Kidney | 0.43% | 0.46% | 108% |
| Brain | 2.0% | 2.2% | 109% |
To standardize the morphological differences, the ICRP data were converted to percentages of organ weight divided by body weight (given as 73kg for adult males and 60 kg for adult females), and I examined the ratio of these relative organ weights for females versus males (Table 1). For most organs there is less than a 10% difference in the weight-corrected values, however the ratio for adipose tissue was 50% higher in females. Females also had relatively heavier pituitaries (21%), adrenals (13%) and GI tract (14%), while males had higher total lung capacity and vital capacity (13%), blood (11%), bone (12%), skin (15%), and particularly skeletal muscle (27%) (Valentin, 2001). There was only a slight difference (6%) in the body mass to surface area, 38.4 kg/m2 for males vs 36.1 kg/m2 for females. Thus substantial differences persist even after scaling for body weight.
Male and female adult mice are generally very similar in body weight. B6C3F1 average 46.2 g (males) and 46.4 g (females), with males weighing more in youth and females more at 18 months in some strains (Rao 1999). Males tend to grow fast and are then slightly surpassed in weight by adult females. Rats, on the other hand show much greater sex dimorphism. Pooling control rats from 26 studies, Bailey et al (2004) found quite different organ/body weight regressions between females and males for liver and kidney with greater similarity with brain weight as the denominator.
Related to anatomic size differences are average physiologic differences between men and women. The differences in forced vital capacity are documented in reference standards for spirometry, but there are inconsistencies: with heavy exercise women have higher maximum ventilation rate (from table 2.32 in Valentin 2001). Relative cardiac output is higher in women (1.1 fold) while men have higher blood volume (1.2 fold: Valentin 2001). The Basal Metabolic Rate (BMR) of women is given as 52 kcal/hr compared with 68 for men, thus standardized for body mass the female BMR is 93% compared with men, while weight standardized total energy expenditure is 78% of male (i.e. 22% or 1.3 fold lower energy intake per day), reflected in average calories consumed. Risk assessors use a default value of 2 L/day for adult drinking water intake, which is a good approximation of the 1960 ml/day for females, but underestimates the 2600 ml/day for males (from table 2.30 in Valentin, 2001).
3. Sex Hormones and the Sex Hormone Paradigm
Mammals have heterogametic sex determined in the embryo by whether it is XX (female) or XY (male). The Y chromosome carries the sry gene that directs the sex differentiation, formation of the fetal testis, and whether the fetus will be bathed in testosterone and develop into a male. Genes on the sex chromosomes directly influence brain development as well as determining the hormones which indirectly exert many effects. In both sexes, androgens influence brain function (NRC 2005). Steroids can modify gene expression, even stimulating neurons to generate new or discard old synapses (Kawata et al. 1994).
The estrogenic and androgenic hormones and their receptors vary throughout the life cycle. The estrous/menstrual cycle in females superimposes a dramatic short-term cycle as well. Since the mid-1900s physiologists and ethologists (Fraps 1955; Murton et al. 1973) and more recently toxicologists have explored the influence of estrogenic and androgenic hormone systems on a wide variety of behavioral and physiologic phenomena. A typical experimental sequence would include 1) observing sex differences, 2) gonadectomy, 3) replacement with same sex hormone, 4) replacement with opposite sex hormone. Later modifications included: 5) experiments with agonists and antagonists, 6) studies with genetically modified animals (knockouts), and finally 7) separation of genetic sex from gonadal sex by manipulating the sry testis-determining gene (Arnold and Burgoyne 2004). See Arnold et al. (2014) for recent summary.
Some of the genes that have very great fold differences are located on the Y and X chromosomes (Yang et al. 2012). To what extent do these impact development separately from determining the sex hormone environment. This required the separation of genetic and hormonal sex. Arnold and Burgoyne (2004) relocated the testis-determining sry gene from the Y chromosome to an autosome by transgenic insertion. Upon successful breeding this produced XX as well as XY males and XY as well as XX females. They found fewer than 10 genes differentially expressed in XX vs XY males and XX vs XY females indicating few genes are regulated directly in the absence of sex hormones (van Nas et al. 2009, Arnold et al. 2014).
Organisms, such as almost all vertebrates, that have separate sexes, manifest dimorphic behavior, particularly with regard to reproduction, controlled by sex specific brain centers that may add new neurons or contract under hormonal control. These are linked to various cycles including annual photoperiod. Significant sex differences occur in centers corresponding to touch and olfaction in nocturnal mammals and to vision and hearing in birds (Nottebohm and Arnold 1976).
An attractive feature of the sex hormone paradigm is its elegant logic. However, it sometimes raises more questions than it answers----not just is some variable influenced by hormones, but how, when, and even why? In some cases there are disconnects, when gonadectomy reduces a behavior, but replacement does not restore it. For example, in male animals estrogen does not always have the same effect as castration. Two examples, of the “classic sex hormone paradigm” are shown in Table 2.
Table 2.
Application of the Classic Sex Hormone Paradigm to examine sex differences in elimination of perfluoroctanoic acid and in the expression of CYP2J5 in mouse kidney.
| Procedure | Perfluoroctanoic acid clearance | CYP2J5 expression in mouse kidney |
|---|---|---|
| Citation | Kudo et al. (2002) | Ma et al. (2004) |
| Intact male | Much slower than female | Males have more protein |
| Treat intact male with estradiol | Inceased clearance | Reduced expression |
| Gonadectomized male | Clearance increased 14× | Reduced expression |
| Treat gonadectomized male with testosterone | Reverse the increase | Increased expression |
| Intact female | Rapid clearance | Low expression |
| Gonadectomize female | Greatly reduced clearance | Increased expression |
| Treat intact female with testosterone | Greatly reduced clearance | DHT raises female expression to male level |
| Treat gonadectomized female with estradiol | Reverse the reduction | Decreased expression |
Some examples of renal clearance fit the classic sex hormone paradigm. Perfluorooctanoic acid (PFOA) has attracted increasing attention because detectable residues of this persistent organic pollutant (POP) are almost universal in human and animal tissue. PFOA is eliminated via an organic anion transporter (OAT) in the renal tubule, but the half life is 70 times longer in male than female rats (Worley and Fisher 2015). Estradiol treatment or castration speed elimination in males, while subsequent testosterone treatment lengthens it. Ovariectomy slowed clearance in females and estradiol restored it. (Worley and Fisher 2015).
4. Intrauterine Position: Rodents have Gender
Nature conducts its own sex hormone intervention in animals that have litters and perhaps in humans. In boy-girl (MF) twin gestations there is inconsistent evidence regarding possible masculinization of the female sib, exposed to her brother's androgens through Twin Testosterone Transfer (TTT) For example, cognitive effects have been reported, such asgreater mathematics aptitude, reviewed skeptically by Ahrenfeldt et al. 2015). Also females from MF twins had larger tooth dimensions than those from FF twins (Ribeiro et al. 2013). The evidence is sufficient to warrant further investigation in humans (Tapp et al. 2011), however, Ahrenfeld et al's (2015) study of 9th graders found no difference in school performance on either visuospatial or language skills between girls from FF or MF twins. Since twin studies are popular, the paucity of evidence indicative of TTT in human twins suggests that it is minor and/or rare.
In animals, however, testosterone transfer is well established in mice and gerbils that have large litters (vom Saal and Bronson 1980). Females embryos lying between two males (2M females), later exhibit more male-typical behavior than 0M or 1M females, including mounting receptive females. Similarly 2F males have different behavior and lower reproductive success than 2M males. The intrauterine hormonal environment affects anatomy (anogenital distance), maturation (vaginal opening), sexual maturity, aggressive behavior, hormone production, mating and fertilization, and fecundity (Clark and Galef 1998). These “gender differences” are a significant potential source of individual variability with impacts particularly on behavioral and reproductive toxicology. Having a contiguous male fetus increased masculine behavior of male rats, decreased female behavior in female, and increased weights at gestation day 21 of both sexes, without affecting anogenital distance (Hernández-Tristan et al. 1999).
5. Growth Hormone
Growth hormone plays multiple roles in regulation of sex-dependent gene expression (Waxman and O'Connor 2006). Many of the cyp450 genes are up regulated by growth hormone. In rats and mice the male-female differences in CYP expression may exceed 500 fold, such that some isoforms appear sex-specific. Similar but smaller differences are seen in humans. The transcription factor and signal transduction cascade was worked out by Waxman and O'Connor (2006). The schedule of growth hormone release differs. In human females growth hormone is released at a relatively continuous level which increases CYP3A4 expression in the liver in response to dexamethasone, while pulsatile releases characteristic of males suppress it (Dhir et al. 2006). This effect is less marked in humans than in rats. Similarly CYP2C11 expression is regulated by growth hormone in the spleen and thymus (Thangavel et al. 2007).
6. Exposure
The 2007 Framework addressed sex differences in exposure opportunity and contact related to behavior and occupation in the home, community and workplace environment (Gochfeld 2007). The present paper assumes many actual or potential sex and gender differences in exposure. Behavioral differences subsumed under “lifestyle” directly affect contact and intake. For examples, smoking is damaging to both sexes, but males and females smoked differently, with males inhaling more per cigarette, while women held cigarettes close to their face and experienced greater sidestream smoke (Burger and Gochfeld 1990). Beyond humans, male and female mammals and birds exhibit different individual and social behavior which influences consumption of nutrients and toxicants (Burger 2005). Sexual dimorphism, dominance and social groupings are important in the wild and intrude on laboratory studies, as well. Males and females differ in locomotor and exploratory activities and energy intake related to dominance, mating and reproduction. Assuring equal dietary intake usually requires paired gavage feeding, bypassing important parts of the upper GI tract.
7. Endocrine Disruption
There are major sex differences in targets of endocrine disruption. Peakall et al (1975) presented mechanisms by which chlorinated hydrocarbon pesticides induced hormones that degraded estrogen, interfering with egg production in birds such as pelicans, eagles and falcons, resulting in extinction of some bird populations within a 20 year period. Publication of “Our Stolen Future” (Colburn et al., 1996) revived and focused interest in endocrine active substances in the environment affecting humans, resulting in substantial research (see papers in Miyamoto and Burger, 2003) on sex differences including, for example:
Synthesis and release of proteins and hormones
Up or down-regulation of receptors
Non-steroidal agonists (activation binding)
Non-activating receptor binding (competitive inhibition)
Non-hormonal mimics or antagonists (receptor or non-receptor mediated)
Altered metabolism (increased or decreased breakdown)
Altered transport quantity or free:bound ratios
This rapidly expanding field of inquiry intersects toxicokinetics and toxicodynamics and is beyond the scope of the present paper.
Toxicokinetics
Toxicokinetics (TK) is divided into four processes: absorption, distribution, metabolism and elimination (ADME). These can be viewed as taking over where exposure ends. Toxicokinetics determines the amount of an active agent circulating in the blood and delivered to a target organ, where many other factors combine to determine whether there is a toxic effect such as an ADR (Leming and Baitang 2012). Xenobiotics can be inhaled from air, ingested from food or water, absorbed through skin, or injected. Absorption through the alveolar-capillary membrane (lung), the GI mucosa, or the skin depends on a variety of chemical, medium, and intrinsic biologic factors. Substances may differ in their bioaccesibility (ease of release from the environmental media, diet, or tablet) or in the absorptive capacity of the organ-blood interface (Burger et al. 2003). Absorption may be passive (diffusion) or active, facilitated by transporters. Together these phenomena influence the circulating concentration of the xenobiotic, its peak concentration (Cmax) available to reach target organs (their biologically effective dose) and the duration of effect measured by the area-under-the curve (AUC), which can be terminated at any time or carried out to infinity. The total volume in which a substance might be distributed at any time, the volume of distribution, is designated Vd. It comprises the blood, extracellular fluid and intracellular fluid and body fat. The time from dosing to reach Cmax is designated Tmax. The phenomenologic approach to toxicokinetics can be seen in reports of blood or plasma concentrations, area-under the curve (AUC) or half-lives (T1/2) of chemicals, particularly pharmaceuticals. Sex differences in these metrics may arise due to differences in any of the stages of toxicokinetics.
Xenobiotic concentrations in blood depend on the volume of distribution and on rates of clearance (Cl) from the blood. Even corrected for body weight, males have higher blood volume and females higher body fat content (Anderson 2008). Females have somewhat lower average renal clearance (lower GFR). Xenobiotics are eliminated from the body (or in some cases deposited in repository organs), and the elimination or clearance curves have been quantified for many substances, each with its own half-life (T1/2) in the body as a whole and in different compartments.
Many of the toxicokinetic studies of xenobiotics, focus on the blood level of the drug (or active metabolite) without being able to or even trying to define how such differences arise or persist the black box approach. Greater body mass and blood and fluid volume and lower body fat, explain only part of the difference (Schwartz 2003).
Fletcher et al (1994) identified 30 studies from 1980-1993, reporting sex differences in toxicokinetics for nutrients, ethanol, and pharmaceuticals. Clearance was one of the common measures with males exceeding females in 17 of 29 cases, a few by a large margin. Clewell et al. (2002) reviewed toxicokinetic data to identify age-specific or sex-specific vulnerabilities for risk assessment and found many age differences but not many sex differences (designated M=F in Table 3 adapted from Clewell et al. 2002). For most toxicokinetic functions the data were deemed “insufficient. Most differences identified, were subtle.
Table 3.
Clewell et al (2002) reviewed the literature on toxicokinetics with regard to male/female similarities or differences. This summarizes their conclusion.
| Route of Exposure | Difference |
|---|---|
| Absorption | |
| Oral for Lipophilic agents | M=F |
| Oral for Hydrophilic agents | M=F |
| Inhalation for lipophilic | M=F (only methylene chloride) |
| Inhalation for hydrophilic | Insufficienta |
| Inhalation for particulates | M=F |
| Dermal for lipophilic | Insufficienta |
| Dermal for hydrophilic | Insufficienta |
| Distribution | |
| Lipophilic | F>M |
| Hydrophilic | F<M |
| Protein bound | F<M |
| Metabolism | |
| Glutathione S transferase | Insufficienta |
| Sulfotransferase | Insufficienta |
| Glucuronyl transferase | F<M |
| P450 | F<M |
| Carboxyesterase | Insufficienta |
| Alcoholdehydrogenase | Insufficienta |
| Elimination | |
| Glomerular filtration | M=F |
| Tubular secretion | Insufficienta |
| Tubular reabsorption | Insufficienta |
Insufficient indicates too few data to analyze.
1. Absorption
Absorption or permeability refers to the ease with which a substance that is bioavailable can be transferred into the blood either through passive diffusion or active transport. The intravenous route for a dissolved substance results in 100% transfer to the blood stream. Comparison of the blood level achieved by other routes is considered a measure of bioavailability. Absorption can occur through the lung, GI tract or skin.
Although bioavialability has been studied extensively, absorption has proven less tractable. Studies of sex differences in GI absorption have been sparse (Walsh 1997, Clewell et al. 2002). Schwartz (2003) believed that absorption was generally less efficient in females for a variety of substances. Sex differences in the metabolic enzyme expression or activity in the gut wall could modify absorption (Beierle et al. 1999).
Once a substance contacts the alveolar-capillary membrane, the GI mucosa, or the skin, there is an opportunity for passage into the blood stream and thence throughout the body. The substance may undergo metabolism by the microbiota or by enzymes in the respiratory or intestinal tracts. Toxicologists often choose a parenteral dosing route specifically to avoid the vagaries of intake and absorption. Hydrophilic and lipophilic substances have different absorption properties. This spectrum of properties is often described by the octanol:water partition coefficient between two phases or media. Transport across the mucosal surface may be by passive diffusion (lipophilic xenobiotics) or by carrier-mediated facilitated transport. Energy-dependent active transport results in absorption against a concentration gradient. Non-polar solvents are generally efficiently absorbed (the usual assumption is 100%), so there is little room for male-female differences in absorption for this class of compounds. Many risk assessments simply assumed 100% absorption, essentially ignoring that important phenomenon (Burger et al. 2003).
Strongly hydrophilic substances are poorly absorbed. Vesicle packets involved in endocytosis and exocytosis are important for certain large molecules, such as the absorption of vitamin B-12. Beierle et al. (1999) did not find support for consistent sex differences in GI absorption prompting Clewell et al. (2002) to list M=F for both lipophilic and hydrophilic substances.
A. Gastrointestinal Tract
In the GI tract, absorption is influenced by pH, gastric emptying time, and intestinal transit (Fletcher et al. 1994), and by surface area of the organ, as well as the presence of transporters, concurrent intake and the microbiome (Dietert and Silbergeld 2015). Women secrete less gastric acid and have slower gastric emptying (Clewell et al. 2002), and this difference is heightened in pregnancy (Fletcher et al. 1994).
Absorption can be passive or active (Rozman and Klaassen 2001), and great advances have been made in characterizing transporters and identifying their ligands as well as the underlying control of expression. It has long been known that children absorb a much higher percentage of ingested lead (ca 50%) than do adults (ca 10%) (ATSDR, 1999). It is likely that this reflects up regulation of divalent cation transport in order to maximize the absorption of calcium needed for the growing skeleton---lead just comes along for the ride (Vahter et al. 2007). Women are much more likely than men to have depleted iron stores, and they have significantly greater absorption of cadmium from the diet than men or than women with normal iron stores (Vahter et al. 2002; Berglund et al. 1994). Acetaminophen was administered to rats, and intestinal absorption was quicker and greater in females (Raheja et al. 1983.)
Several sugars characterized by absorption in different parts of the intestinal tract are used clinically to determine permeability of the GI tract. A study of age × sex interaction found that permeability for lactulose and sucralose declined with age in females, but not in males (McOmber et al. 2010). The authors could only speculate that this is “hormonal”, perhaps related to glucocorticoids which alter permeability in rats as well as human.
B. Respiratory Tract
In humans, air flow through the pulmonary tree may differ due to geometry, resulting in different flow rates, impaction and turbulence (Valentin 2001). Male mice are slightly larger than females and have higher tidal volume, flow rates, and ventilation rates (L/min). The higher tidal volume in male mice is not solely anatomical, since ER-alpha knockout mice show a reversed pattern (higher tidal volume in females; Card et al. 2006).
There are few sex differences in absorption of airborne substances, either volatile or particulate from lung, based on a small sample of 8 men and 9 women (Ernstgård et al. 2003), Clewell et al. (2002) found data only for methylene chloride and for particulates also with no male-female difference. In rats, males absorb more manganese from a manganese sulfate aerosol than females (Dorman et al., 2004). Conversely, exposure to inhaled lead acetate resulted in higher lead concentration in the lung of females, but greater cytotoxicity in males (Fortoul et al. 2005).
C. Dermal Absorption
Area of contact, skin thickness, intactness, keratinization, and blood flow influence uptake through the skin. Sex was not addressed in their review of dermal absorption (Wester and Maibach, 1997; Bronaugh and Maibach, 1999). The ICRP reference reports no difference in epidermal thickness by sex (Valentin, 2001). Bronaugh et al. (1983) documented some differences in rat skin permeability. A human study showed that men had slightly but significantly higher transepidermal water loss than women (Chilcott and Farrar, 2000).
Dermal absorption of bis(2-chloroethoxy)methane (CEM) was compared in male and female rats and mice. Absorption was more rapid and higher in rats than mice, and females of both species had higher absorption reflected in higher Cmax and AUC (Waidyanatha et al. 2011). Bathwater exposure to chloroform resulted in equal uptake (measured by exhaled breath) in men and women at 40C, while men absorbed about 50% more at 30C (Corley et al 2000).
Trichloroethylene (TCE) is absorbed through the skin more by females than males. In rats dermal penetration was 2-fold greater in females than males. Gonadectomy of males increased TCE uptake in a rat skin preparation, reflected in the AUC. Testosterone decreased dermal penetration in skin from females (McCormick and Abdel-Rahman 1991).
2. Distribution
A. Transport
Once absorbed, a xenobiotic distributes itself in the blood, and lipophilic substances quickly move into fat, such that fat becomes part of the volume of distribution. For a lipophilic drug, women had a 28% greater Vd, while for water-soluble drugs the Vd was 36% lower in females (Wilson 1984). The transport and distribution of lipophilic and hydrophilic substances was one of the few areas of sex difference reported by Clewell et al (2002), and for some chemicals normalizing for lean body mass, erased much of the sex difference (Beierle et al. 1999). The xenobiotics and their metabolites are transported in the blood either “free”: dissolved or in ionic form, or “bound to protein. Albumin is the major transport protein for the many chemicals and their metabolites. Nutritional factors influence the availability of protein for binding, but this is probably not a limiting factor. Metallothioneins (MT) are low molecular weight proteins rich in sulfhydryl groups that bind cations. The physiologic role is to transport zinc, but other metals have a high affinity for MT, which can transfer the metal to the kidney for elimination. After a cadmium injection, male mice (9 wks old) had greater MT-mRNA than females, but this was not apparent at 4 or 46 weeks. Ovariectomy resulted in a 1.25 fold increase in MT-mRNA that was reversed by estradiol, and conversely MT-mRNA was reduced in males receiving estradiol (Sogawa et al. 2001). Females average more body fat and greater storage of lipophilic contaminants (Valentin 2001).
The free:bound ratio influences the bioavailability of the substance, changes the chemical properties, influencing how transport occurs between the capillary and interstitial space (O'Flaherty 1997).
B. Transporters into Cells
The endothelial wall and adjacent cell membrane poses a potential barrier to substances in the blood stream. Lipophilic substances readily pass from blood to extracellular fluid and into cells. Males and females may differ in the expression or function of transporters. Female mice retain more ascorbate than males, attributed to a difference in the ascorbate transporter (Kuo et al. 2004). The world of transporters has grown dramatically in the past decade. Rapid progress means simultaneous discoveries, and variable nomenclature. The ABCG2 efflux transporter, also known as the breast cancer resistant protein (BCRP) from its role in resistance to chemotherapeutic drugs, shows different expression patterns in rats and mice and humans. Male rats and mice had higher expression than females. Sex hormone manipulation revealed that the expression in male rat kidney was due to suppressive effect of estradiol, while in mouse liver it was induced by testosterone (Tanaka et al. 2004).
One group of transporters attracting attention are the Multidrug and Toxin Extrusion (MATE) transporters, moving organic molecules (including anticancer drugs) into and out of cells which are expressed differently in males and females (Lickteig et al. 2008).
3. Metabolism
Metabolism offers a rich literature on sex differences, particularly for organic, xenobiotics and Phase I metabolism. There is great variability among species, strains, developmental stages and sexes, as well as among organs and chemical substrates. Gene expression differences can be reflected in measurements of specific mRNA, protein, or in enzyme activity. There may be sex differences in constitutive levels as well as in inducibility by different substrates (Iba et al. 1999).). The well-established sensitivity of female rats to barbiturates attributed to hepatic metabolism is an example of different amounts or activities of phase I enzymes (Czerniak, 2001
In our laboratory at Rutgers University, we developed an ecotoxicology model based on the ubiquitous, native, long-lived, White-footed Mouse (Peromyscus leucopus). We analyzed a wide variety of phase I and phase II enzymes in both sexes at different ages from 6 to 48 months (Guo et al. 1993), and showed that males had significantly higher microsomal P450 content as well as NADPH-cytochrome P450 oxidoreductase activity at all ages, and many of the P-450 enzyme activities were higher in males. Females showed age-dependent decreases for several enzymes between 6 and 24 months of age, which was less apparent in males.
A. Phase I and Cytochrome P450s
Phase I metabolism involves a variety of reactions including oxidation, hydroxylation, and epoxide formation which occur in many organs, predominantly in the liver. Metabolism changes the chemistry, distribution properties, and toxicity of the original xenobiotic, detoxifying some chemicals and activating others. Metrics include concentration of the xenobiotic or metabolite in blood or their ratio, measures of enzyme amount or activity, and measures of gene-specific mRNA.
Attention has focused particularly on the cytochrome P450 families (Parkinson 2001), with measurement of the specific mRNA expression, or the amount of enzyme protein, and/or the enzyme activity (ratio of parent to metabolite). In the 1980s, when researchers worked with microsomal fractions and P450s were lumped together as “mixed function oxidases” (MFOs), before the classification of families and isoforms, sex differences were already apparent. Raheja et al. (1983) recognized that females were less susceptible to substances “metabolized by the P-450 MFO system.” They dosed rats with acetaminophen and documented that liver damage appeared earlier and progressed to greater severity in male rats. By six hours females had eliminated 24% of the dose compared with 17% for males (about 1.4 fold difference). Females also showed greater depletion of glutathione. Sex differences for several of the CYP isoforms for several studies are summarized in Table 4.
Table 4. Male-female expression and/or activity comparisons for selected CYP ENZYMES (H)=Human, (M)=Mouse, (R)=Rat.
| CYP isoform | Anderson 2008 (H) | Schwartz 2003 (H) | Yang 2012 (H) | Huang 2011 (R) |
|---|---|---|---|---|
| 1A1 | ||||
| 1A2 | M>F | M>F | M>F | |
| 2A1 | F>M | |||
| 2A6 | F>M | |||
| 2B3 | M>F | |||
| 2B6 | F>M | |||
| 2C7 | F>M | |||
| 2C9 | M=F | |||
| 2C11 2C12 2C13 | M>F | |||
| 2C16 | M>F | |||
| 2D1 | M>F | |||
| 2D6 | M=F | M>F | M>F | |
| 2D26 | F>M | |||
| 2E1 | M>F | M>F | M>F | |
| 3A2 | M>F | |||
| 3A4 | F>M | F>M | ||
| 7D1 |
In their review, Clewell et al. (2002) found evidence of higher activity of CYP1A2, CYP3A4, CYP2D6 and CP2E1 in males. In humans, Mahgoub et al (1977) found a bimodal distribution in the debrisoquine/4-OH debrisoquine ratio, attributable to a single autosomal gene mutation and Kahn et al. (1985) found slower hydroxylation in female mice of several strains. “Poor” versus “extensive” metabolism of this antihypertensive drug, is now known to be related partly to polymorphisms in CYP2D6, one of the main drug-metabolizing enzymes.
In some cases the sex difference is greater than 10 fold. For example, male rats treated with monochloroacetate had a 45 × increase in liver injury enzymes after exposure to chloroform, reflecting a sex difference susceptibility (Davis and Berndt, 1992 Sex differences in CYP1A1 and CYP1A2 are well-documented. In untreated rats CYP1A1 protein and mRNA were detected in kidney and lung of female rats, but not males (Iba et al. 1998). CYP1A2 was detected in liver of both sexes. In untreated rats, CYP1A1 activity was significantly higher in females than males in liver (1.8×) and kidney (16×) but not in lung (Iba et al. 1998). Pyridine induction increases CYP1A1 mRNA and protein in liver, kidney and lung in females. Pyridine upregulated CYP1A1 mRNA without a concomitant increase in protein, in liver and lung of male rats. Nicotine induced CYP1A1 in rat lung tissue, males > females (Iba et al. 1998). The response of CYP1A2 to pyridine was different. Both mRNA and protein increased in liver of female rats, while in males the protein (but not the mRNA were increased).
Rodents have some sex-related P450s, such as CYP2C11 and 3A2 in male rats and CYP2C12 in females. Schwartz (2003) reported that CYP2C9 and CYP2C19 levels are similar between males and females, but treatment with estradiol and progesterone reduced CYP2C19 activity (Palovaara et al. 2003). Sexual and developmental differences expression in the CYP3A enzyme family has been demonstrated in rodent (Jan et al. 2006). CYP3A1 is virtually absent in adult male rats, but is highly inducible while 3A2 protein is present constitutively. CYP3A9 is a female predominant enzyme while CYP3A18 occurs in males (Mahnke et al. 1997). CYP3A18 and CYP3A23 proteins were present before and after puberty, about 1.2 fold higher in male rats than females. CYP3A9 was detected only in adult rats, about 2× higher in females, while CYP3A2 was detected only in male rats (Mahnke et al. 1997).
Jan et al. 2006 detected CYP3A2, CYP3A18, CYP3A9 and CYP3A1 constitutively in males rats, while females had very low levels of all four proteins, although all (particularly CYP3A2) proved inducible by pregnenolone 16α-carbonitrile in both sexes.
Differences in P450 expression or activity can be due to genetics, sex hormones, body weight and fat, or to enzyme inducers in the diet. CYP1A2 is involved in metabolism of PAHs and various amines. It is expressed mainly in the liver with higher activity in males (Beierle et al. 1999) and has been implicated in the activation of xenobiotics. A wide range of substances can induce CYP1A2, and variation in this enzyme level contributes to variable risk of amine related cancers (Clewell et al. 2002). More than half of drugs studied are metabolized by CYP3A4 (Tanaka 1999) which occurs in the GI tract and liver. In humans, men have somewhat higher CYP1A2 andCYP2E1 while women “appear” to have greater CYP3A activity (Scandlyn et al. 2008). One study showed a 1.4 fold higher level in young women reflecting induction by progesterone. Other studies have found higher CYP3A4 levels in males, which would result in faster metabolism of drugs. CYP2C19 showed higher activity in males in one study and females in another (Clewell et al. 2002). Males tend to have higher alcohol hydrogenase activity, while females achieve higher blood alcohol levels for the same intake, again influenced by body composition.
Penaloza et al. (2014) were able to demonstrate that methylation altered production of CYP450 and other proteins were influenced by stress or estradiol, and that different gene methylation patterns “partially explain the sex-based differences in expression.”(Penaloza et al. 2014).
et alCells isolated from male and female mice younger than 10 days, pre-sex hormone production, showed sex differences in enzyme profiles with females having 2 to >300 × higher methylation of X-ist (a gene involved in inactivating one × chromosome in females), as well as CYP1A1 and other P450s (Penaloza et al. 2014). The authors concluded that different methylation rates explained some of the sex differences in CYP expression, resulting in different metabolic profiles even at birth.
Enzyme inducibility can differ between males and females. St. Johnswort increased CYP3A4 levels by 50% in 8 men versus 90% in 8 women while CYP1A2 increased 20% in women but not at all in men (Wenk et al. 2004). Pre-treatment with rifampin induced CYP3A in the intestine (male > female), with faster oral clearance for the drug midazolam. However, after IV midazolam, there was greater CYP3A induction in females (Gorski et al., 2003). Male mice treated with acrylonitrile developed higher blood cyanide levels, attributable to differential action of CYP2E1. Expression of cyp2e1 was higher in male kidney than female, with no sex difference in the liver (Chanas et al., 2003). Iba et al. (1998) showed that whereas pyridine induction of CYP1A2 was greater in females than males, the reverse was true for induction by beta-naphthoflavone.
An example of a classically direct sex hormone effect on P450 is CYP2J5 in the mouse kidney which is up-regulated by testosterone and down-regulated by estrogen (see Table 2).
This is one of the P450s which converts arachidonic acid (AA) to epoxyeicosatrienoic acid (EET). Males have higher CYP2J5 protein than females, and castration decreases expression. Dihydrotestosterone (DHT) administered to females or castrated males, increased the protein to almost normal male levels (Ma et al. 2004). Treatment of ovariectomized females or castrated males with estradiol, reduces CYP2J5. Mice with reduced androgen receptor have lower levels of CYP2J5 and do not respond to DHT. The androgen receptor antagonist, flutamide, likewise lowers CYP2J5 levels (Ma et al. 2004).
CYP1A1 (formerly known the aryl hydrocarbon hydroxylase) is inducible by a variety of polycyclic aromatic carcinogens that bind to the aromatic hydrocarbon receptor (AhR; Harper et al. 1991). It is involved in metabolic activation of carcinogens in tobacco smoke. Levels of CYP1A1 differ between males and females. Benzo [a]pyrene induced greater CYP1A1 in male rats than in females, while fluroanthene induction did not produce a sex difference (Ramesh et al. 2000). A dose of TCDD that was lethal to wild-type male mice was not lethal to wild-type females or to CYP1A1 null males (Uno et al., 2004).
Researchers are now examining networks of co-expressed genes. For example the relationship between CYP3A4, GST genes, and other drug metabolizing enzymes and transporter genes (DMETs). Yang et al. (2012) identified 80 genes (out of 19,541) differentially expressed in male and female liver samples; including 7 genes on the X and 12 on the Y chromosomes. Ten of the DMET genes had more than a 1.5 fold difference including SLC3A1, CYP7A1, ACSL4, CYP3A7, GSTA1, CYP3A4, GSTA2, UGT2B17, SLC13A1 and ADH1A, and they have a wide variety of actions. Huang et al (2011) characterized many proteins using what they characterize as “Quantitative shot-gun proteomics”, identifying many sex-biased CYP enzymes. CYP2D1 metabolism in females was 70% that of males, corresponding to the amount of protein, rather than a difference in activity.
B. Phase II--conjugation
Many drugs and their metabolites are not water soluble, and they are eliminated via the bile and feces. Phase II metabolism, typically involves conjugation to an organic moiety which renders the substance water soluble for excretion in the urine. Phase II reactions include glucuronidation, acetylation, methylation, sulfation, or conjugation with glutathione. Clewell et al. (2002) found “no evident differences” between sexes, and some reactions involving acetyltransferase, show no sex difference (Schwartz 2003), particularly when corrected for body weight. Phase II is essential for renal clearance of many xenobiotics, and it is generally faster in men (Schwartz 2003). Rats administered acetaminophen excreted it quickly in the urine. The glucuronide conjugate was significantly greater in females, while the sulfate conjugate was higher in males (Raheja et al. 1983). In our studies of the White-footed Mouse, we found that at all ages up to 48 months, males had about 3× higher phase II metabolizing UDP-glucuronosyl-transferase than females (Figure 2)(Guo et al 1993).
Figure 2.
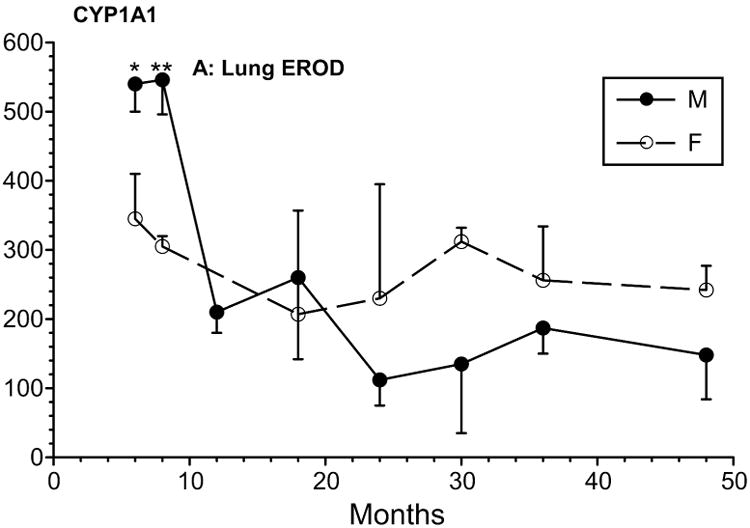
Sex differences in CYP1A1 across the life cycle in a laboratory population of the White-footed Mouse (Peromyscus leucopus)(Guo et al. 1993). Non-overlapping error bars indicate significant difference (P<0.05).
Change during the estrous cycle can be dramatic. Estradiol level affected UDP-glucuronyl transferase and NADPH-cytochrome c reductase (Watanabe et al. 1997). Glucuronidation rate was higher in liver of male rats compared with females, while the reverse was true in kidney. Castration increased urinary excretion of a glucuronide by males almost to the female level, which was suppressed by testosterone treatment. Conversely glucuronide excretion by females was reduced by ovariectomy or testosterone (Palylyk-Colwell and Jamali 2004). Males rats had lower UDP-glucuronosyltransferase activity than females. Gonadectomy increased activity in males and decreased it in females. Females had higher biliary excretory rates for bilirubin (Muraca etal. 1983). et al
4. Elimination
Xenobiotics or their metabolites are excreted primarily through the urine and through the bile into feces, but also through the lungs, skin, sweat, saliva, and milk. For urinary excretion, the compound must be delivered to the kidney or converted there to a water soluble form usually by phase II metabolism. Renal excretion can be viewed as three phases: glomerular filtration sensitive to blood flow and hydrostatic pressure, tubular reabsorption, and tubular secretion. At the glomerulus most of the fluid, electrolytes, small molecules and even small proteins are filtered. Many of these are too precious to waste and must be retrieved quickly from the tubule into the capillaries wrapped around them. Reabsorption removes 90% plus of the water, all of the sugar and aminoacids, most of the electrolytes ---basically reabsorbing anything the body doesn't want to waste from the tubular contents. As urine formation continues, tubular secretion eliminates substances that aren't completely filtered including creatinine and some drugs, as well as H+ and NH4+ to maintain blood pH. Renal clearance, particularly glomerular filtration rate (GFR) is a critical variable which is typically higher in men than women (Kadiri and Ajayi, 2000), allowing women to retain more contaminant, even after conjugation. Female rats also have lower GFR associated with lower renal blood flow (Cerrutti et al. 2001).
Clewell et al (2002) reported generally higher (but inconsistent) GFR in males, and found no information on sex differences in tubular secretion. Tubular secretion typically involves transporters moving substances into the urine, against the reabsorption of water. Kidney transporters of the ABC family (efflux transporters) and of the Solute Carrier (SLC family of uptake transporters) play a role in drug disposition and elimination. Substantial differences between men and women influence the movement of compounds into and out of renal tubular cells (Yang et al. 2013).
OATs are active in the renal tubule, and their expression is modified in complex ways by sex hormones (Kudo et al. 2002). As an example, PFOA, a ubiquitous environmental contaminant, is persistent in the environment and is not metabolized in animals, but is eliminated mainly in urine. PFOA shows sex-differences in renal elimination, related to sex-specific expression of OATs in proximal renal tubule cells, accounting for much higher and faster excretion in female vs male rats (Worley & Fisher 2015). Similar sex difference occurs in Fathead Minnow (Pimephales promelas), and androgen treatment slows the elimination in females, but estrogen did not accelerate elimination by males (Lee and Schultz 2010). Thus this appears to be a widespread phenomenon, but may have different adaptive significance in terrestrial and aquatic organisms.
Joseph et al (2015) analyzed expression of 30 ABC efflux transporters and SCL uptake transporters in kidneys of 61 men and 34 women and reported no differences across 24 genes. Theirs is a good example of the misleading use of Bonferonni corrections for multiple comparisons, which is prone to type II errors, especially when there are many independent variables. This led Joseph et al (2015) to report no significant differences by sex. However, from their data-rich graph, I calculated individual t scores, finding that at least SCL31A1 (p<0.001) was significantly lower in females (Figure 3). ATP2B2 and ABC5 appear statistically lower in females (P=0.05), while ATP7B is statistically higher in females (P<0.02). Overcorrecting obscures differences that deserve investigation. Of the 22 transporters that showed an apparent sex difference, 21 had higher expression in males (binomial test P<0.0001). Even if all eight transporters with no apparent difference were female-biased, the male predominance (21 out of 30) would still be significant (binomial test P=0.02).
Figure 3.
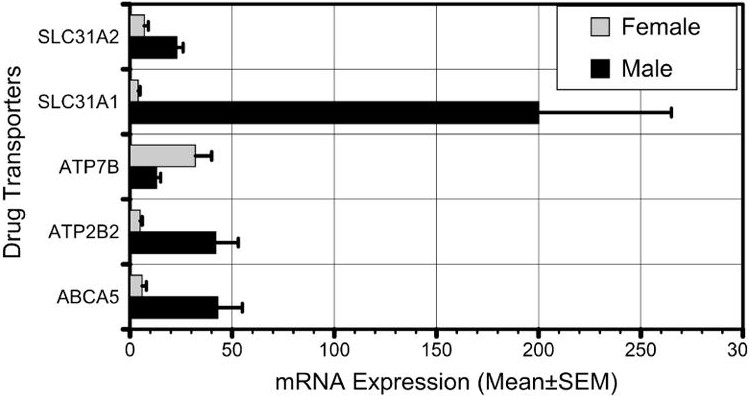
Five of the 24 genes examined by Joseph et al. (2015) appear to show significant sex differences. Most of the genes showed higher expression in males.
SCL31A1, a member of the solute carrier family 31, is a high-affinity copper transporter in cell membrane. ATP7B (Wilson Disease Protein), the significantly female biased transporter, is active in liver and brain, eliminating excess copper into the bile and plasma. ATP2B2 is a calcium ion efflux transporter maintaining intracellular homeostasis. It is not obvious why these primary functions should be sex-biased, and it is likely that additional functions will be identified for these transporters. It is also possible that some of these differences will prove unstable if they are replicated or tested in other systems.
The uptake, distribution and elimination of metals can also vary by sex. A single dose of methylmercury was followed by higher urinary elimination of mercury in males vs. females with a concomitant reduction in brain and blood, but not kidney mercury concentrations (Hirayama and Yasutake 1986).
Dose to Organs
The toxicokinetic processes influence the blood level at any point in time. Absorption increases the blood level, while distribution to a target organ and elimination, result in clearing the substance from the blood. At any time point the blood level determines the amount available for transfer into organs: target organs, storage depots, or elimination.
1. Liver
Of the studies reviewed for this paper, there were differences in gene expression, protein product, and enzyme activity, with varying substrates and varying induction, without producing a clear and consistent pattern across species and studies.
Many of the toxicokinetic differences occur in the liver and have been studied both in vivo and in vitro. The liver is the first pass recipient of nutrients and toxicants directly from the intestine through the portal circulation. The branches of the portal vein and hepatic artery run parallel through the liver to the lobules. As blood moves through liver lobule from the portal vein to the hepatic vein, its contents are subjected to the body's most active metabolic tissue as well as to a decrease in oxygen tension as it moves away from proximity to the hepatic artery. Here is a key intersection between toxicokinetics (delivery) and toxicodynamics (response).
Women are less tolerant to both acute CNS and hepatotoxic effects of ethanol than males. They experience higher blood alcohol levels for the same intake, and women develop liver damage sooner (Ashley et al. 1977) attributed to toxicokinetics and toxicodynamics (Ronis et al. 2004). In rats, transporters of the multidrug resistance-associated protein (Mrp2 and Mrp3) were higher in females than males, while member of the OAT family were either equal or higher in males. Androgen treatment increased Mrp3 in females only (Rost et al. 2005).
2. Brain
Imaging reveals a complex pattern of sexual dimorphism in the adult human brain (Goldstein et al. 2001). This sexual dimorphism in size and organization begins early in life. The X and Y chromosomes have a relatively large number of genes related to brain structure, influenced directly before gonadal hormones are available (Arnold 2004). In early embryonic life, genes on the Y chromosome begin to exert an effect on brain development. As the embryo develops its own hormonal capability, the sex steroids can modify brain development (Kawata et al 1994). The hormones may exert their effect at critical periods in development and throughout life (NRC 2005).
The BBB keeps substances circulating in the blood stream from entering the brain circulation. The barrier is comprised of astrocytes and endothelial cells with tight junctions that allow passage only of small, lipophilic substances. Pakulski et al (2000) made measurements during neurosurgery and reported slightly lower, but not significant albumin permeability in females compared with males. Saija et al (1990) measured the blood-to-brain transfer in rats of aminoisobutyric acid, finding higher permeability at estrus compared with proestrus, and there was a “marked increase” in permeability after ovariectomy.
After pre-treatment with lipopolysaccharide, mice injected with sodium selenite showed greater brain accumulation in females than in males (Minami et al., 2002). Conversely dietary selenite protected the BBB, resulting in lower permeability during chemically-induced convulsions in female rats, while male rats experienced higher permeability related to convulsions (Seker et al. 2008). However, the combination of selenium and vitamin E reduced permeability during hyperthermic convulsions in both sexes (Oztas et al 2007).
Carcinogens and Cancer
Apart from cancers of the respective male and female sex organs, there are sex differences in cancer risks and rates that involve both toxicokinetics and toxicodynamics (Crump et al. 1999). Cancer depends in part on how efficiently a xenobiotic is metabolized and whether the metabolite is itself active (Zang and Wynder, 1996). For the same lifetime smoking, women have up to a 70% higher lung cancer risk than men (Zang and Wynder 1996). Observational studies of smoking behavior indicate that women have higher sidestream smoke than men (Burger and Gochfeld, 1990), but it may also reflect respiratory physiology, metabolism or other susceptibility factors.
It is perplexing when a particular cancer is increased in one sex and not the other, given similar or even controlled ‘identical’ exposures. The dioxin release in Seveso, Italy in 1976, was followed by a 5× increase in lymphoreticulosarcoma in men, and a similar increase in myeloid leukemia and multiple myeloma in women (Bertazzi et al. 1993). Gallo et al. (1986) had identified TCDD binding to the Ah receptor downregulating the estrogen receptor, and had predicted a possible protective effect against breast and endometrial cancer which was borne out by the Bertazzi et al. (1993) cancer data on the dioxin-exposed population around Seveso.
Chemicals such as TCE and PERC increase kidney tumor rates in male rats, but not in female rats or male or female mice. Green et al. (1990) suggested that this related to a unique alpha-2 globulin present in the male rat kidney tubule where it undergoes hydrolysis, which contributes to cell necrosis, proliferation and malignancy (Bruckner et al. 2013). Thus the cancer in male rats is often considered inapplicable to human risk assessment. However, reports that both TCE and PERC have also been implicated in increased rates of kidney cancer in humans, leding to an NAS (2009) conclusion of “limited/suggestive evidence of an association” for PERC as a human renal carcinogen.
The National Toxicology Program's cancer bioassays are a rich resource for comparing cancer incidence rates among male and female control rats and mice. Moore et al (2013) reviewed 180 studies of B6C3F1 with both sexes, for the incidence of primary pulmonary neoplasms. The median incidence for alveolar bronchiolar adenomas and carcinomas was 16% and 8% in males compared with 5% and 2% in females.
This paper has identified examples of differences in toxicokinetics, some at the gene expression or transporter level. If metabolism or elimination is slowed, prolonged exposure may occur. For drugs with narrow therapeutic windows, even modest differences may result in adverse drug reactions based on toxicity or on therapeutic failure. The NAT-2 gene (arylamine N-acetyl transferase) has common single nucleotide polymorphisms that determine slow, intermediate, or rapid acetylation in Phase II metabolism. These differences have been implicated as a cancer risk, although a recent meta-analysis did not find breast cancer differences related to this common polymorphism (Wang and Marei 2016). Modest differences in drug metabolizing enzymes or transporters (DMET expression), for example in CYP3A4 and or GSTA1 might increase cancer risk, without causing any clinically apparent “disease” (Yang et al. 2012).
Of the 278 carcinogens reviewed, 201 (72%) had significant sex differences (p ≤ .05) in at least one nonreproductive organ; 130 showed male bias and 59 female bias (Kadekar et al. 2012). Using only substances with strong evidence in the NTP bioassay data base 68 carcinogens increased cancer in males versus only 19 in females (Kadekar et al. 2012). One hundred thirty of these 201 chemicals induced gender-specific tumors in male rats and 59 in female rats. Male-specific tumors included pancreatic and skin tumors, and female-specific tumors included lung tumors. For some tumor sites, these differences in gender susceptibility can be associated with literature data on sex hormone receptor expression. In conclusion, gender-specific tumors were common. The male dominance is in line with recent human data, and the male susceptibility to carcinogens should be further studied.
Microbiome
Animals and humans have microbiome ecosystems on skin, in axillae, inguinal region, in the respiratory tract, and at various points in the GI tract. These organisms and their interactions play active roles in metabolism, influencing the metabolites produced and their bioaccessibility. Recently the microbiome has been recognized for its important role in toxicokinetics (metabolism) and as a target “organ” as well (Dietert and Silbergeld 2015). The microbiome is examined in detail by Silbergeld in this issue. et alet alet alet al
Discussion
The measurable concentration of any agent in the blood at any time is a dynamic result of the four toxicokinetic processes: absorption, distribution (including clearance), metabolism and elimination.
This review has provided examples of many areas where sex differences exist, without accounting for the areas where they don't exist. There are two caveats before proceeding with discussion: 1) many studies have been one of a kind and have not been replicated. 2) there is no consistent criterion for how much of a fold-difference is “important” either clinically or evolutionarily. What fold difference in what enzyme system would encourage or require the EPA to take action on an environmental contaminant, FDA to take action on a drug, or a clinician or patient to adjust a dose.
Clinical evidence is mostly phenomenologic and not linked with toxicokinetic estimates. Meibohm et al. (2002) concluded that sex differences in drug responses, including the roles of physiology and metabolism, were “generally only subtle”, but they singled out drugs effecting the QT interval and some CNS responses. Women were more responsive than men to opioids (Craft 2003), while the converse is true in studies of female rodents, and Craft (2003) was cautious about attributing differences to toxicokinetics or toxicodynamics. Many of the reported sex differences in response to drugs are probably primarily toxicokinetic influencing the Cmax and AUC. For examples, women had a 20-30% greater response to muscle relaxants, while males were up to 40% more responsive to the sedative effects of propofol (Pleym et al 2003).
Data Limitations
This paper has focused on studies that report some differences between males and females. Many of the reports are one of a kind, and may not be replicated, so it becomes important to search for patterns that explain the observations. Small fold differences may be due to error and should not be over-interpreted. For a comprehensive picture, however, the reported sex difference need to be juxtaposed to studies or phenomena that looked for sex differences with adequate power, and found them absent. These may be buried among studies that didn't even look for sex differences and studies that lacked power. Unfortunately it is more difficult to search for “non-sex difference” than for “sex difference.”
Although granting agencies usually require power calculations, the majority of papers reviewed make no mention of their power. Moreover, there is always a suspicion that studies that are “negative” because of lack of power never get published (“negative publication bias”). Epidemiology research is intrinsically conservative, biased towards avoiding type I errors, even at the risk of type II errors (Gochfeld 2003). Many human studies of toxicokinetics have fewer than 10 males and 10 females, and many animal studies include six or fewer animals in each dosage group. Such small samples compromise the ability to detect and publish small differences, and conversely, when differences are detected, one wonders how much credence to place in the result.
A reasonable guidance is to scrutinize positive reports of sex difference to rule out possible confounders such as a difference in age, while negative reports of no sex difference should be examined to ascertain that there was adequate power to detect a difference, let's say a 1.5 fold or 50% difference (Gochfeld 2007). In this review my access to literature benefitted from the essentially free access accorded by the Rutgers University library system. Although my primary references were through PUBMED, I encountered many citations to new, on-line open access journals which are not themselves indexed on PUBMED. Whereas I previously cautioned against “Abstract based science” (Gochfeld 2007), I now worry about the proliferation of hundreds of for-profit journals offering rapid on-line publication, with claimed but unproven peer review practices. Caveat emptor.
Does Sex Matter?
Our attention to sex differences was focused by the Institute of Medicine's conclusion (Wizemann and Pardue 2001) that “sex matters”. The current review as well as the previous review (Gochfeld 2007), estimated that many toxicokinetic differences are on the order of about 1.2 fold (20%) uncorrect for body size, although some enzyme expression ratios are many fold, resulting in higher susceptibility of females to some xenobiotics and a higher risk of ADR. There is generally little quantification of the impact of such differences, and although drug development studies now include females, it is not apparent that differences discovered can or should influence prescribing practices (Schwartz, 2003). Numeracy, the ability to interpret numbers such as percentages is a challenge for both patients (Schapira et al. 2008) and clinicians (Taylor and Byrne-Davis 2016) and are context dependent. A 20% increase in cancer risk might influence behavior. How can a patient or physician interpret or respond to a 20% increase in the Cmax of a drug or its duration of effect?
Managing prescriptions in an era of polypharmacy is challenging enough, particularly if patients are exhorted to “ask your doctor” or “be sure to tell your doctor”. Doctors can’t always welcome and process such information in a brief clinical encounter. Using sex-difference information in toxicokinetics to fine-tune doses should, in principle, reduce the number of adverse drug reactions. In the foreseeable future, however, patients are likely to bear the burden of recognizing when a side-effect becomes enough of an ADR to warrant discontinuance and reporting. It is quite clear that sex matters both with regard to differential responses to xenobiotics and willingness to report. It is not clear whether the medical profession and society are prepared to make use of the information.
Conclusion
Between exposure and toxicodynamics, toxicokinetics determines how much of a pharmacologic or toxicologic dose reaches target organs. Studies of sex differences in absorption, distribution, metabolism and elimination can identify magnitudes of difference (fold differences), for those substances being measured. What fold difference corresponds to increase susceptibility or risk, varies greatly and risk itself, whether environmental cancer or adverse drug reaction, is continuous rather than categorical/ The academic's mantra that “more research is needed” remains true. Descriptions of sex differences and the mechanisms underlying or modifying them apply broadly to environmental chemicals and pharmaceuticals. Extrapolating from inbred rodents to outbred humans has always been challenging.
But there is a final consideration. No human studies select participants at random from the demographic they are hopefully representing. It is understood that any metric will show variability, a composite of individual variation, analytic variation and what statisticians call “error”. Statisticians accommodate this variation with a standard deviation or confidence limits, without having to acknowledge that the sample actually comprises heterogeneous individuals some of which, particularly the outliers, may not be at all representative, with regard to some toxicokinetic process, for example proximal tubular reabsorption or renin secretion (Schnermann 2015). Toxicology has the luxury of replicates, seldom available in human studies, but it is sobering that even inbred mice may manifest consistent inter-individual variability, for example in arterial blood pressure (Schnermann 2015), variability that we pay good money to erase when we buy inbred strains.
Post Script: I came away from this review with innumerable questions about whether particular findings were species-specific, substrate-specific, and generalizable versus idiosyncratic. I wonder “why” such differences exist, what adaptive value they may have for different sexes. How many differences are artefacts of particular methodologies, or of inbred laboratory animals, and why some differences seem tightly controlled and others not. I think that any generalizations about toxicokinetic differences, must be viewed through an evolutionary framework which interprets the sex differences as adaptations to the somewhat different lives experienced by males and females (Hrdy 1981).
Acknowledgments
I want to thank Justin Vidal and Dinesh Stanislaus, for the invitation to participate in the symposium on the Influence of Age, Hormones, and the Microbiome on Variation in Toxicologic Responses at the 35th annual meeting of the Society of Toxicologic Pathology (San Diego, June 2016). I want to thank all of my co-participants in the 2003 SGOMSEC workshop on gender, particularly our host Erminio Marafante, as well as Werner Klein, Bernard Goldstein, Deborah Cory-Slechta, and co-chairs Joanna Burger and Marie Vahter. Thirty years of interactions with colleagues at the Environmental and Occupational Health Sciences Institute and Rutgers University, particularly Joanns Burger, Michael Gallo, Howard Kipen, Jeffrey Laskin, Robert Laumbach, Michael Pratt, Ken Reuhl, and Iris Udasin have been particularly valuable. My research in environmental toxicology has been supported by many agencies including the National Institute for Environmental Health Sciences (ESO 5022) and the Department of Energy through the Consortium for Risk Evaluation with Stakeholder Participation.
References
- Ahrenfeldt L, Petersen I, Johnson W, Christensen K. Academic performance of opposite-sex and same-sex twins in adolescence: a Danish national cohort study. Horm Behav. 2015;69:123–131. doi: 10.1016/j.yhbeh.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Burgoyne PS. Are XX and XY brain cells intrinsically different? Trends Endocrinol Metabol. 2004;15:6–11. doi: 10.1016/j.tem.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Chen X, Itoh Y. What a difference an X or Y makes: sex chromosomes, gene dose, and epigenetics in sexual differentiation. Handb Exp Pharmacol. 2014;214:67–88. doi: 10.1007/978-3-642-30726-3_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GD. Gender differences in pharmacological response. Int Rev Neurobiol. 2008;83:1–10. doi: 10.1016/S0074-7742(08)00001-9. [DOI] [PubMed] [Google Scholar]
- Ashley MJ, Olin JS, le Riche WH, Kornaczewski A, Schmidt W, Rankin JG. Morbidity in alcoholics. Evidence for accelerated development of physical disease in women. Arch Intern Med. 1977;137:883–887. doi: 10.1001/archinte.137.7.883. [DOI] [PubMed] [Google Scholar]
- ATSDR. Agency for Toxic Substances and Disease Registry. Centers for Disease Control; Atlanta, GA: 1999. Toxicological Profile for Lead. [Google Scholar]
- Bailey SA, Zidell RH, Perry RW. Relationship between organ weight and body/brain weight in the rat: what is the best analytical endpoint? Toxicol Pathol. 2004;32:448–466. doi: 10.1080/01926230490465874. [DOI] [PubMed] [Google Scholar]
- Bano S, Akhter S, Afridi MI. Gender based response to fluoxetine hydrochloride medication in endogenous depression. J Coll Physicians Surg Pak. 2004;14:161–165. [PubMed] [Google Scholar]
- Beierle I, Meibohm B, Derendorf H. Gender differences in pharmacokinetics and pharmacodynamics. Int J Clin Pharmacol Ther. 1999;37:529–47. [PubMed] [Google Scholar]
- Berglund M, Akesson A, Nermell B, Vahter M. Intestinal absorption of dietary cadmium in women depends on body iron stores and fiber intake. Environ Health Perspect. 1994;1102:1058–1066. doi: 10.1289/ehp.941021058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertazzi A, Pesatori AC, Consonni D, Tironi A, Landi MT, Zocchetti C. Cancer incidence in a population accidentally exposed to 2,3,7,8-tetrachlorodibenzo-para-dioxin. Epidemiol. 1993;4:398–406. doi: 10.1097/00001648-199309000-00004. [DOI] [PubMed] [Google Scholar]
- Bronaugh RL, Maibach HI. Percutaneous Absorption: Drugs, Cosmetics, Mechanisms, Methodology. Marcel Dekker; New York: 1999. [Google Scholar]
- Bronaugh RL, Stewart RF, Congdon ER. Differences in permeability of rat skin related to sex and body site. J Soc Cosmet Chem. 1983;34:127–135. [Google Scholar]
- Bruckner JV, Anand SS, Warren DA. Toxic effects of solvents and vapors. In: Klaassen CD, editor. Casarett and Doull's Toxicology. McGraw Hill; New York: 2013. pp. 1031–1112. [Google Scholar]
- Burger J. Framework and methodology for incorporating gender-related issues in wildlife risk assessment: metals as a case study. Ecotoxicol Environ Safety. 2005;104:153–162. doi: 10.1016/j.envres.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Burger J, Diaz-Barriga F, Marafante E, Pounds J, Robson R. Methodologies to examine the importance of host factors in bioavailability of metals. Ecotoxicol Environ Safety. 2003;56:20–31. doi: 10.1016/s0147-6513(03)00047-2. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M. Natural observations of smoking behavior: are there sex, age, context- or activity- related differences. Addictive Behav. 1990;15:309–317. doi: 10.1016/0306-4603(90)90040-5. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Toxic Susceptibility: Male/Female Differences. Wiley-Interscience; New York: 1985. [Google Scholar]
- Campesi I, Sanna M, Zinellu A, Carru C, Rubattu L, Bulzomi P, Seghieri G, Tonolo G, Palermo M, Rosano G, Marino M, Franconi F. Oral contraceptives modify DNA methylation and monocyte-derived macrophage function. Biol Sex Diff. 2012;3:4–15. doi: 10.1186/2042-6410-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card JW, Carey MA, Bradbury JA, DeGraff LM, Morgan DL, Moorman MP, Zeldin DC. Gender differences in murine airway responsiveness and lipopolysaccharide-induced inflammation. J Immunol. 2006;177:621–630. doi: 10.4049/jimmunol.177.1.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey MA, Card JW, Bradbury JA, Moorman MP, Haykal-Coates N, Gavett SH, Zhu D. Spontaneous airway hyperresponsiveness in estrogen receptor-α–deficient mice. Am J Respir Crit Care Med. 2007;175:126–135. doi: 10.1164/rccm.200509-1493OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerrutti JA, Quaglia NB, Torres AM. Characterization of the mechanisms involved in the gender differences in p-aminohippurate renal elimination in rats. Canadian J Physiol Pharmacol. 2001;79:805–813. [PubMed] [Google Scholar]
- Chanas B, Wang H, Ghanayem BI. Differential metabolism of acrylonitrile to cyanide is responsible for the greater sensitivity of male vs female mice: role of CYP2E1 and epoxide hydrolases. Toxicol Appl Pharmacol. 2003;193:293–301. doi: 10.1016/j.taap.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Chilcott RP, Farrar R. Biophysical measurements of human forearm skin in vivo: effects of site, gender, chirality and time. Skin Res Technol. 2000;6:64–69. doi: 10.1034/j.1600-0846.2000.006002064.x. [DOI] [PubMed] [Google Scholar]
- Clark MM, Galef BG., Jr Prenatal influences on reproductive life-history strategies. Trends in Ecology and Evolution. 1995;10:151–153. doi: 10.1016/s0169-5347(00)89025-4. [DOI] [PubMed] [Google Scholar]
- Clewell HJ, Teeguarden J, McDonald T, Sarangapani R, Lawrence G, Covington T, Gentry R, Shipp A. Review and evaluation of the potential impact of age- and gender-specific pharmacokinetic differences on tissue dosimetry. Crit Rev Toxicol. 2002;32:329–389. doi: 10.1080/20024091064264. [DOI] [PubMed] [Google Scholar]
- Colburn T, Dumanski D, Myers JP. Our Stolen Future. Dutton; New York: 1996. [Google Scholar]
- Corley RA, Gordon SM, Wallace LA. Physiologically based pharmacokinetic modeling of the temperature-dependent dermal absorption of chloroform by humans following bath water exposures. Toxicol Sci. 2000;53:13–23. doi: 10.1093/toxsci/53.1.13. [DOI] [PubMed] [Google Scholar]
- Craft RM. Sex differences in drug- and non-drug induced analgesia. Life Sci. 2003;72:2675–2688. doi: 10.1016/s0024-3205(03)00178-4. [DOI] [PubMed] [Google Scholar]
- Crump KS, Krewski D, Van Landingham C. Estimates of the proportion of chemicals that were carcinogenic or anticarcinogenic in bioassays conducted by the National Toxicology Program. Environ Health Perspect. 1999;107:83–88. doi: 10.1289/ehp.9910783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerniak R. Gender-based differences in pharmacokinetics in laboratory animal models. Internatl J Toxicol. 2001;20:161–163. doi: 10.1080/109158101317097746. [DOI] [PubMed] [Google Scholar]
- Damien S, Patural H, Trombert-Paviot B, Beyens MN. Adverse drug reactions in children: 10 years of pharmacovigilance. Arch Pediatr. 2016;23:468–476. doi: 10.1016/j.arcped.2016.01.015. [DOI] [PubMed] [Google Scholar]
- Davis ME, Berndt WO. Sex differences in monochloroacetate pretreatment effects on chloroform toxicity in rats. Fund Applied Toxicol. 1992;18:66–71. doi: 10.1016/0272-0590(92)90196-o. [DOI] [PubMed] [Google Scholar]
- Dhir RN, Dworakowski W, Thangavel C, Shapiro BH. Sexually dimorphic regulation of hepatic isoforms of human cytochrome p450 by growth hormone. J Pharmacol Exp Ther. 2006;316:87–94. doi: 10.1124/jpet.105.093773. [DOI] [PubMed] [Google Scholar]
- Dietert RR, Silbergeld EK. Biomarkers for the 21st century: listening to the microbiome. Toxicol Sci. 2015;144:208–216. doi: 10.1093/toxsci/kfv013. [DOI] [PubMed] [Google Scholar]
- Dorman DC, McManus BE, Marshall MW, James RA, Struve MF. Old age and gender influence the pharmacokinetics of inhaled manganese sulfate and manganese phosphate in rats. Toxicol Appl Pharmacol. 2004;197:113–24. doi: 10.1016/j.taap.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Dubois KP, Puchala E. Studies on the sex difference in toxicity of a cholinergic phosphorothioate. Proc Soc Exp Biol Med. 1961;107:908–911. doi: 10.3181/00379727-107-26791. [DOI] [PubMed] [Google Scholar]
- Ehrenpreis ED, Parakkal D, Semer R, Du H. Renal risks of sodium phosphate tablets for colonoscopy preparation: a review of adverse drug reactions reported to the US Food and Drug Administration. Colorectal Dis. 2011;13:270–275. doi: 10.1111/j.1463-1318.2011.02679.x. [DOI] [PubMed] [Google Scholar]
- Ernstgård L, Sjogren B, Warholm M, Johanson G. Sex differences in the toxicokinetics of inhaled solvent vapors in humans 2. 2-propanol. Toxicol Appl Pharmacol. 2003;193:158–167. doi: 10.1016/j.taap.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Fletcher CV, Acosta EP, Strykowski JM. Gender differences in human pharmacokinetics and pharmacodynamics. J Adolesc Health. 1994;15:619–629. doi: 10.1016/s1054-139x(94)90628-9. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration (FDA) Guideline: General Considerations for the Clinical Evaluation of Drugs. Rockville MD: U.S. Food and Drug Administration; 1977. [Google Scholar]
- Food and Drug Administration (FDA) Guideline for the study and evaluation of gender differences in the clinical evaluation of drugs; notice. Fed Register. 1993;58:39406–39416. [PubMed] [Google Scholar]
- Fortoul TI, Moncada-Hernandez S, Saldivar-Osorio L, Espejel-Maya G, Mussali-Galante P, del Carmen Avila-Casado M, Colin-Barenque L, Hernandez-Serrato MI, Avila-Costa MR. Sex differences in bronchiolar epithelium response after the inhalation of lead acetate (Pb) Toxicol. 2005;207:323–330. doi: 10.1016/j.tox.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Franconi F, Campesi I. Pharmacogenomics, pharmacokinetics and pharmacodynamics: interaction with biological differences between men and women. Brit J Pharmacol. 2014;171:580–594. doi: 10.1111/bph.12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraps RM. The varying effects of sex hormones in birds. Memoirs Soc Endocrinol. 1955;4:205–218. [Google Scholar]
- Frommeyer G, Eckardt L. Drug-induced proarrhythmia: risk factors and electrophysiological mechanisms. Nat Rev Cardiol. 2016;13:36–47. doi: 10.1038/nrcardio.2015.110. [DOI] [PubMed] [Google Scholar]
- Gallo MA, Hesse EJ, Macdonald GJ, Umbreit TH. Interactive effects of estradiol and 2,3,7,8-tetrachlorodibenzo-p-dioxin on hepatic cytochrome P-450 and mouse uterus. Toxicol Lett. 1986;32:123–132. doi: 10.1016/0378-4274(86)90058-5. [DOI] [PubMed] [Google Scholar]
- Ghamsari AA, Dadpour B, Najari F. Frequency of Electrocardiographic Abnormalities in Tramadol Poisoned Patients; a Brief Report. Emerg. 2016;4:151–154. [PMC free article] [PubMed] [Google Scholar]
- Gochfeld M. Why epidemiology of endocrine disruptors warrants the precautionary principle. Pure Appl Chem. 2003;75:2521–2529. [Google Scholar]
- Gochfeld M. Occupational Medicine Practice in the United States since the Industrial Revolution. J Occup Environ Med. 2005;47:115–131. doi: 10.1097/01.jom.0000152918.62784.5a. [DOI] [PubMed] [Google Scholar]
- Gochfeld M. Framework for gender differences in human and animal toxicology. Environ Res. 2007;104:4–21. doi: 10.1016/j.envres.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Gochfeld M. Sex-gender research sensitivity and healthcare disparities. J Women Health. 2010;19:189–194. doi: 10.1089/jwh.2009.1632. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, jr, Faraone SV, Tsuang MT. Normal Sexual Dimorphism of the Adult Human Brain Assessed by Magnetic Resonance Imaging. Cerebral Cortex. 2001;11:490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- Gorski JC, Vannaprasaht S, Hamman MA, Ambrosius WT, Bruce MA, Haehner-Daniels B, Hall SD. The effect of age, sex, and rifampin administration on intestinal and hepatic cytochrome P450 3A activity. Clin Pharmacol Therap. 2003;74:275–287. doi: 10.1016/S0009-9236(03)00187-5. [DOI] [PubMed] [Google Scholar]
- Green T, Odum J, Nash JA. Perchlorethylene-induced rat kidney tumors: an investigation of the mechanisms involved and their relevance to humans. Toxicol Applied Pharmacol. 1990;103:77–89. doi: 10.1016/0041-008x(90)90264-u. [DOI] [PubMed] [Google Scholar]
- Greenberg GN, Dement JM. Exposure assessment and gender differences. J Occup Med. 1994;36:907–912. [PubMed] [Google Scholar]
- Guo Z, Wang M, Tian G, Burger J, Gochfeld M, Yang CS. Age-and gender-related variations in the activities of drug-metabolizing and antioxidant enzymes in the white-footed mouse (Peromyscus leucopus) Growth Dev Aging. 1993;57:85–100. [PubMed] [Google Scholar]
- Guttmacher Institute. Contraceptive Use in the United States. Guttmacher Institute Fact-Sheet. [accessed Sept 7, 2016];2015 Web site: https://https://www.guttmacher.org/factsheet/contraceptiveuseunitedstates.
- Härmark L, Lie-Kwie M, Berm L, Gier H, Grootheest K. Patients' motives for participating in active post-marketing surveillance. Pharmacoepidemiol Drug Saf. 2013;22:70–6. doi: 10.1002/pds.3327. [DOI] [PubMed] [Google Scholar]
- Harper PA, Prokipcak RD, Bush LE, Golas CL, Okey AB. Detection and characterization of the Ah receptor for 2,3,7,8-tetrachlorodibenzo-p-dioxin in the human colon adenocarcinoma cell line LS180. Arch Biochem Biophys. 1991;290:27–36. doi: 10.1016/0003-9861(91)90587-9. [DOI] [PubMed] [Google Scholar]
- Hernández-Tristán R, Arevalo C, Canals S. Effect of prenatal uterine position on male and female rats sexual behavior. Physiol Behav. 1999;67:401–408. doi: 10.1016/s0031-9384(99)00077-3. [DOI] [PubMed] [Google Scholar]
- Heinrich J. Drug safety: Most drugs withdrawn in recent years had greater health risks for women. Washington: US General Accounting Office; 2001. GAO-01-286R. [Google Scholar]
- Hirayama K, Yasutake A. Sex and age differences in mercury distribution and excretion in methylmercury-administered mice. J Toxicol Environ Health. 1986;18:49–60. doi: 10.1080/15287398609530847. [DOI] [PubMed] [Google Scholar]
- Holdcroft A. Gender bias in research: how does it affect evidence based medicine? J Royal Soc Med. 2007;100:2–3. doi: 10.1258/jrsm.100.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrdy SB. The Woman that Never Evolved. Harvard University Press; New York: 1981. [Google Scholar]
- Huang HJ, Tsai ML, Chen YW, Chen SH. Quantitative shot-gun proteomics and MS-based activity assay for revealing gender differences in enzyme contents for rat liver microsome. J Proteomics. 2011;74:2734–2744. doi: 10.1016/j.jprot.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Iba MM, Fung J, Thomas PE, Park Y. Constitutive and induced expression by pyridine and beta-naphthoflavone of rat CYP1A is sexually dimorphic. Arch Toxicol. 1999;73:208–216. doi: 10.1007/s002040050608. [DOI] [PubMed] [Google Scholar]
- Iba MM, Scholl H, Fung J, Thomas PE, Alam J. Induction of pulmonary CYP1A1 by nicotine. Xenobiotica. 1998;28:827–43. doi: 10.1080/004982598239083. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Arnold AP. Are females more variable than males in gene expression? Meta-analysis of microarray datasets. Biol Sex Diff. 2015;6:18–27. doi: 10.1186/s13293-015-0036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan YH, Mishin Busch CM, Thomas PE. Generation of specific antibodies and their use to characterize sex differences in four rat P450 3A enzymes following vehicle and pregnenolone 16α-carbonitrile treatment. Arch Biochem Biophys. 2006;446:101–110. doi: 10.1016/j.abb.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Johnson DE, Wolfgtang GHI, Gledin MA, Braeckman RA. Toxicokinetics and toxicodynamics. In: Williams PD, Hottendorf GH, editors. Comprehensive Toxicology Vol 2: Toxicological Testing and Evaluation. Pergamon; New York: 1997. pp. 169–182. [Google Scholar]
- Jones J, Mosher WD, Daniels K. Current contraceptive use in the United States, 2006–2010, and changes in patterns of use since 1995. [accessed Sept 7, 2016];National Health Statistics Reports, 2012, No 60. 2012 Website: http://www.cdc.gov/nchs/data/nhsr/nhsr060.pdf. [PubMed]
- Joseph S, Nicolson TJ, Hammons G, Word B, Green-Knox B, Lyn-Cook B. Expression of drug transporters in human kidney: impact of sex, age, and ethnicity. Biol Sex Differ. 2015;6:4–21. doi: 10.1186/s13293-015-0020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadekar S, Peddada S, Silins I, French JE, Högberg J, Stenius U. Gender differences in chemical carcinogenesis in National Toxicology Program 2-year bioassays. Toxicol Pathol. 2012;40:1160–1168. doi: 10.1177/0192623312446527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadiri S, Ajayi SO. Variability in the relationship between serum creatinine and creatinine clearance in hypertensives and normotensives with normal renal function. African J Med Sci. 2000;29:93–96. [PubMed] [Google Scholar]
- Kahn GC, Rubenfield MA, Davies RC, Murray DS, Boobis AR. Sex and strain differences in hepatic debrisoquine 4-hydroxylase activity of the rat. Drug Metabol Dispos. 1985;13:510–516. [PubMed] [Google Scholar]
- Kawata M, Yuri K, Morimoto M. Steroid hormone effects on gene expression, neuronal structure, and differentiation. Horm Behav. 1994;28:477–482. doi: 10.1006/hbeh.1994.1045. [DOI] [PubMed] [Google Scholar]
- Klaassen CD. Cassarett and Doull's Toxicology: The Basic Science of Poisons. 8th. McGraw-Hill; New York: 2013. [Google Scholar]
- Klein W, Gochfeld M, Davis B. Background on the Scientific Group on Methodologies for the Safety Evaluation of Chemicals and Workshop 16: Gender differences. Environ Res. 2007;104:2–3. [Google Scholar]
- Kogevinas M, Zahm SH. Introduction: epidemiologic research on occupational health in women. Amer J Industr Med. 2003;44:565–575. doi: 10.1002/ajim.10317. [DOI] [PubMed] [Google Scholar]
- Konigstein M, Rosso R, Topaz G, Postema PG, Friedensohn L, Heller K, et al. Drug-induced Brugada syndrome: Clinical characteristics and risk factors. Heart Rhythm. 2016;13:1083–1087. doi: 10.1016/j.hrthm.2016.03.016. [DOI] [PubMed] [Google Scholar]
- Kudo N, Katakura M, Sato Y, Kawashima Y. Sex hormone-regulated renal transport of perfluorooctanoic acid. Chem Biol Interact. 2002;139:301–316. doi: 10.1016/s0009-2797(02)00006-6. [DOI] [PubMed] [Google Scholar]
- Kuo SM, MacLean ME, McCormick K, Wilson JX. Gender and sodium-ascorbate transporter isoforms determine scorbate concentrations in mice. J Nutr. 2004;134:2216–2221. doi: 10.1093/jn/134.9.2216. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Schultz IR. Sex differences in the uptake and disposition of perfluorooctanoic acid in fathead minnows after oral dosing. Environ Sci Technol. 2010;44:491–496. doi: 10.1021/es901838y. [DOI] [PubMed] [Google Scholar]
- Leming S, Baitang N. Sex Differences in the Expression of Drug-Metabolizing and Transporter Genes in Human Liver. J Drug Metabol Toxicol. 2012;3:3–12. doi: 10.4172/2157-7609.1000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lickteig AJ, Cheng X, Augustine LM, Klaassen CD, Cherrington NJ. Tissue distribution, ontogeny and induction of the transporters Multidrug and toxin extrusion (MATE) 1 and MATE2 mRNA expression levels in mice. Life Sci. 2008;83:59–64. doi: 10.1016/j.lfs.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Graves J, Bradbury JA, Zhao Y, Swope DL, King L, et al. Regulation of mouse renal CYP2J5 expression by sex hormones. Mol Pharmacol. 2004;65:730–743. doi: 10.1124/mol.65.3.730. [DOI] [PubMed] [Google Scholar]
- Mahgoub A, Idle JR, Dring LG, Lancaster R, Smith RL. Polymorphic hydroxylation of Debrisoquine in man. Lancet. 1977;2:584–586. doi: 10.1016/s0140-6736(77)91430-1. [DOI] [PubMed] [Google Scholar]
- Mahnke A, Strotkamp D, Roos PH, Hanstein WG, Chabot GG, Nef P. Expression and inducibility of cytochrome P450 3A9 (CYP3A9) and other members of the CYP3A subfamily in rat liver. Arch Biochem Biophys. 1997;337:62–68. doi: 10.1006/abbi.1996.9752. [DOI] [PubMed] [Google Scholar]
- Maron E, Toru I, Vasar V, Shlik J. The effect of 5-hydroxytryptophan on cholecystokinin-4-induced panic attacks in healthy volunteers. J Psychopharmacol. 2004;18:194–199. doi: 10.1177/0269881104042619. [DOI] [PubMed] [Google Scholar]
- Mastroianni AC, Faden R, Federman D. Women and Health Research: Ethical and Legal Issues of Including Women in Clinical Studies. Institute of Medicine, National Academy Press; Washington D.C: 1994. [PubMed] [Google Scholar]
- Mazure C. Our evolving science: studying the influence of sex in preclinical research. Biol Sex Diff. 2016;7:15–18. doi: 10.1186/s13293-016-0068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick K, Abdel-Rahman MS. The role of testosterone in trichloroethylene penetration in vitro. Environ Res. 1991;54:82–92. doi: 10.1016/s0013-9351(05)80196-3. [DOI] [PubMed] [Google Scholar]
- McCullough LD, de Vries GJ, Miller VN, Becker JB, Sandberg K, McCarthy MM. NIH initiative to balance sex of animals in preclinical studies: generative questions to guide policy, implementation, and metrics. Biol Sex Diff. 2014;5:15–22. doi: 10.1186/s13293-014-0015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McOmber ME, Ching-Nan O, Shulman RJ. Gastrointestinal Permeability Testing in Children and Adults. J Pediatr Gastroenterol Nutr. 2010;50:269–275. doi: 10.1097/MPG.0b013e3181aa3aa9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meibohm B, Beierle I, Derendorf H. How important are gender differences in pharmacokinetics. Clin Pharmacokinet. 2002;41:329–342. doi: 10.2165/00003088-200241050-00002. [DOI] [PubMed] [Google Scholar]
- Miller MA. Gender-based differences in the toxicity of pharmaceuticals--the Food and Drug Administration's perspective. Int J Toxicol. 2001;20:149–152. doi: 10.1080/109158101317097728. [DOI] [PubMed] [Google Scholar]
- Minami T, Sakita Y, Ichida S, Dohi Y. Gender difference regarding selenium penetration into the mouse brain. Biol Trace Elem Res. 2002;89:85–93. doi: 10.1385/BTER:89:1:85. [DOI] [PubMed] [Google Scholar]
- Miyamoto J, Burger J. Implications of Endocrine Active Substances for Humans and Wildlife. International Union for Pure and Applied Chemistry and Scientific Committee on Problems of the Environment. Pure Appl Chem. 2003;75:1–2615. [Google Scholar]
- Moore NP, McFadden LG, Landenberger BD, Thomas J. Gender differences in the incidence of background and chemically induced primary pulmonary neoplasms in B6C3F1 mice: a retrospective analysis of the National Toxicology Program (NTP) carcinogenicity bioassays. Exp Toxicol Pathol. 2013;65:1109–1115. doi: 10.1016/j.etp.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Munoz C, Guerrero S, Paeile C, Campos I. Sexual differences in the toxicity of procaine in rats. Toxicol Appl Pharmacol. 1961;3:445–454. doi: 10.1016/0041-008x(61)90059-x. [DOI] [PubMed] [Google Scholar]
- Muraca M, De Groote J, Fevery J. Sex Differences of Hepatic Conjugation of Bilirubin Determine its Maximal Biliary Excretion in Non-Anaesthetized Male and Female Rats. Clin Sci. 1983;64:85–90. doi: 10.1042/cs0640085. [DOI] [PubMed] [Google Scholar]
- Murton RK, Thearle RJP, Lofts B. The endocrine basis of breeding behavior in the feral pigeon (Columba livia): I. Effects of exogenous hormones on the pre-incubation behaviour of intact males. Animal Behaviour. 1969;17:286–306. doi: 10.1016/0003-3472(69)90014-1. [DOI] [PubMed] [Google Scholar]
- Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7:27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa K, Kajiwara A. Female sex as a risk factor for adverse drug reactions. Nihon Rinsho. 2015;73:581–585. [PubMed] [Google Scholar]
- National Academy of Science (NAS) Contaminated water Supplies at Camp Lejeune: Assessing Potential Health Effects. National Academy of Sciences; Washington D.C: 2009. [PubMed] [Google Scholar]
- National Research Council (NRC) Application of Toxicogenomics to Cross-species Extrapolation. National Academy Press; Washington D.C: 2005. [PubMed] [Google Scholar]
- Nicholas JS, Barron DH. The use of sodium amytal in the production of anesthesia in the rat. J Pharmacol Exper Therap. 1932;46:125–129. [Google Scholar]
- Nieuwenhoven L, Klinge I. Lessons in applying sex- and gender-sensitive methods. J Women's Health. 2010;19:313–321. doi: 10.1089/jwh.2008.1156. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Arnold AP. Sexual dimorphism in vocal control areas of the songbird brain. Sci. 1976;194:211–213. doi: 10.1126/science.959852. [DOI] [PubMed] [Google Scholar]
- O'Flaherty EJ. Toxicokinetics: distribution of toxicants. In: Bond J, editor. In Comprehensive Toxicology Vol 1: General Principles. Pergamon; New York: 1997. pp. 115–134. [Google Scholar]
- Oztas B, Akgul S, Seker FB. Gender difference in the influence of antioxidants on the blood-brain barrier permeability during pentylenetetrazol-induced seizures in hyperthermic rat pups. Biol Trace Elem Res. 2007;118:77–83. doi: 10.1007/s12011-007-0020-1. [DOI] [PubMed] [Google Scholar]
- Pakulski C, Drobnik L, Millo B. Age and sex as factors modifying the function of the blood-cerebrospinal fluid barrier. Medical Sci Monit. 2000;6:314–318. [PubMed] [Google Scholar]
- Palovaara S, Typbring G, Laine K. The effect of ethinylestradiol and levonorgestrel on the CYP2C19-mediated metabolism of omperazole in healthy female subjects. Brit J Clin Pharmacol. 2003;56:232–237. doi: 10.1046/j.1365-2125.2003.01868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palylyk-Colwell EL, Jamali F. Effect of gonadectomy and hormones on sex differences in ketoprofen enantiomer glucuronidation and renal excretion of formedglucuronides in the rat. Pharm Res. 2004;21:989–95. doi: 10.1023/b:pham.0000029288.48673.c2. [DOI] [PubMed] [Google Scholar]
- Parkinson A. Biotransformation of xenobiotics. In: Klaassen CD, editor. Casarett & Doull's Toxicology: The Basic Science of Poisons. McGraw-Hill; New York: 2001. pp. 133–223. [Google Scholar]
- Patel TK, Patel PB. Incidence of Adverse Drug Reactions in Indian Hospitals: A Systematic Review of Prospective Studies. Curr Drug Saf. 2016;11:128–136. doi: 10.2174/1574886310666150921104523. [DOI] [PubMed] [Google Scholar]
- Peakall DB, Miller DS, Kinter WB. Prolonged eggshell thinning caused by DDE in the duck. Nature. 1975;254:421. doi: 10.1038/254421a0. [DOI] [PubMed] [Google Scholar]
- Penaloza CG, Estevez B, Han DM, Norouzi M, Lockshin RA, Zakeri Z. Sex-dependent regulation of cytochrome P450 family members Cyp1a1, Cyp2e1, and Cyp7b1 by methylation of DNA. FASEB J. 2014;28:966–977. doi: 10.1096/fj.13-233320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleym H, Spigset O, Kharasch ED, Dale O. Gender differences in drug effects: implications for anesthesiologists. Acta Anaesthesiol Scand. 2003;47:241–259. doi: 10.1034/j.1399-6576.2003.00036.x. [DOI] [PubMed] [Google Scholar]
- Rademaker M. Do women have more adverse drug reactions? Am J Clin Dermatol. 2001;2:349–351. doi: 10.2165/00128071-200102060-00001. [DOI] [PubMed] [Google Scholar]
- Raheja KL, Linscheer WG, Cho C. Hepatotoxicity and metabolism of acetaminophen in male and female rats. J Toxicol Environ Health. 1983;12:143–158. doi: 10.1080/15287398309530413. [DOI] [PubMed] [Google Scholar]
- Ramesh A, Inyang F, Hood DB, Knuckles ME. Aryl hydrocarbon hydroxylase activity in F-344 rats subchronically exposed to benzo(a)pyrene and fluoranthene through diet. J Biochem Molec Toxicol. 2000;14:155–161. doi: 10.1002/(sici)1099-0461(2000)14:3<155::aid-jbt5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Rao GN. Growth, body weight patterns and life span of the B6C3F1 mouse. In: Maronpot RR, editor. Pathology of the Mouse: Reference and Atlas. Cache River Press; Saint Louis, MO: 1999. pp. 7–12. [Google Scholar]
- Ribeiro DC, Brook AH, Hughes TE, Sampson WJ, Townsend GC. Intrauterine hormone effects on tooth dimensions. J Dent Res. 2013;92:425–431. doi: 10.1177/0022034513484934. [DOI] [PubMed] [Google Scholar]
- Rodenburg EM, Stricker BH, Visser LE. Sex differences in cardiovascular drug-induced adverse reactions causing hospital admissions. Br J Clin Pharmacol. 2012;74:1045–1052. doi: 10.1111/j.1365-2125.2012.04310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronis MJJ, Korourian S, Yoon S, Ingelman-Sundberg M, Albano E, Lindros KO, Badger TM. Lack of sexual dimorphism in alcohol-induced liver damage (ALD) in rats treated chronically with ethanol-containing low carbohydrate diets: the role of ethanol metabolism and endotoxin. Life Sci. 2004;75:469–483. doi: 10.1016/j.lfs.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Rost D, Kopplow K, Gehrke S, Mueller S, Friess H, Ittrich C, Mayer D, Stiehl A. Gender-specific expression of liver organic anion transporters in rat. Eur J Clin Invest. 2005;35:635–643. doi: 10.1111/j.1365-2362.2005.01556.x. [DOI] [PubMed] [Google Scholar]
- Rozman KK, Klaassen CD. Absorption, distribution, and excretion of toxicants. In: Klaassen CD, editor. Casarett & Doull's Toxicology 6th edition. McGraw-Hill; New York: 2001. pp. 107–132. [Google Scholar]
- Ryu J, Lee H, Suh J, Yang M, Kang W, Kim E. Differences between Drug-Induced and Contrast Media-Induced Adverse Reactions Based on Spontaneously Reported Adverse Drug Reactions. PLoS One. 2015;10:e0142418. doi: 10.1371/journal.pone.0142418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saija A, Princi P, D'Amico N, De Pasquale R, Costa G. Aging and sex influence the permeability of the blood-brain barrier in the rat. Life Sciences. 1990;47:2261–2267. doi: 10.1016/0024-3205(90)90157-m. [DOI] [PubMed] [Google Scholar]
- Scandlyn MJ, Stuart EC, Rosengren RJ. Sex-specific differences in CYP450 isoforms in humans. Expert Opin Drug Metab Toxicol. 2008;4:413–424. doi: 10.1517/17425255.4.4.413. [DOI] [PubMed] [Google Scholar]
- Schapira MM, Fletcher KE, Gilligan MA, King TK, Laud PW, Matthews BA, Neuner JM, Hayes E. A framework for health numeracy: how patients use quantitative skills in health care. J Health Comm. 2008;13:501–517. doi: 10.1080/10810730802202169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnermann J. One physiology does not fit all: a path from data variability to “physiogenetics”? Am J Physiol Renal Physiol. 2015;309:F29–32. doi: 10.1152/ajprenal.00125.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz JB. The influence of sex on pharmacokinetics. Clin Pharmacokinet. 2003;42:107–21. doi: 10.2165/00003088-200342020-00001. [DOI] [PubMed] [Google Scholar]
- Seeman MV. Gender differences in the prescribing of antipsychotic drugs. Am J Psychiatry. 2004;161:1324–1333. doi: 10.1176/appi.ajp.161.8.1324. [DOI] [PubMed] [Google Scholar]
- Seker FB, Akgul S, Oztas B. Lifelong consumption of sodium selenite: gender differences on blood-brain barrier permeability in convulsive, hypoglycemic rats. Biol Trace Elem Res. 2008;124:12–19. doi: 10.1007/s12011-008-8101-3. [DOI] [PubMed] [Google Scholar]
- Shalat SL, Donnelly KC, Freeman NC, Calvin JA, Ramesh S, Jimenez M, et al. Nondietary ingestion of pesticides by children in an agricultural community on the US/Mexico border: preliminary results. J Expo Anal Environ Epidemiol. 2003;13:42–50. doi: 10.1038/sj.jea.7500249. [DOI] [PubMed] [Google Scholar]
- Shepherd G, Mohorn P, Yacoub K, May DW. Adverse drug reaction deaths reported in United States vital statistics, 1999-2006. Ann Pharmacother. 2012;46:169–175. doi: 10.1345/aph.1P592. [DOI] [PubMed] [Google Scholar]
- Sogawa N, Sogawa CA, Oda N, Fujioka T, Onodera K, Furuta H. The effects of ovariectomy and female sex hormones on hepatic metallothionein-I gene expression after injection of cadmium chloride in mice. Pharmacol Res. 2001;44:53–57. doi: 10.1006/phrs.2001.0833. [DOI] [PubMed] [Google Scholar]
- Tanaka E. Gender-related differences in pharmacokinetics and their clinical significance. J Clin Pharm Ther. 1999;24:339–346. doi: 10.1046/j.1365-2710.1999.00246.x. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Slitt AL, Leazer TM, Maher JM, Klaassen CD. Tissue distribution and hormonal regulation of the breast cancer resistance protein (BCRP/ABCg2) in rats and mice. Biochem Biophys Res Commun. 2004;326:181–187. doi: 10.1016/j.bbrc.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Tapp AL, Maybery MT, Whitehouse AJ. Evaluating the twin testosterone transfer hypothesis: a review of the empirical evidence. Horm Behav. 2011;60:713–722. doi: 10.1016/j.yhbeh.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Taylor AA, Byrne-Davis LM. Clinician Numeracy: The Development of an Assessment Measure for Doctors. Numeracy. 2016;9:1–27. [Google Scholar]
- Thangavel C, Dhir RN, Volgin DV, Shapiro BH. Sex-dependent expression of CYP2C11 in spleen, thymus and bone marrow regulated by growth hormone. Biochem Pharmacol. 2007;74:1476–1484. doi: 10.1016/j.bcp.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno S, Dalton TP, Sinclair PR, Gorman N, Wang B, Smith AG, Miller ML, Shertzer HG, Nebert DW. Cyp1a1(-/-) male mice: protection against high-dose TCDD-induced lethality and wasting syndrome, and resistance to intrahepatocyte lipid accumulation and uroporphyria. Toxicol Appl Pharmacol. 2004;196:410–421. doi: 10.1016/j.taap.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Vahter M, Berglund M, Åkesson, Liden C. Metals and women's health. Environ Res. 2002;88:145–155. doi: 10.1006/enrs.2002.4338. [DOI] [PubMed] [Google Scholar]
- Vahter M, Gochfeld M, Casati B, Thiruchelvam M, Falk-Filippson A, Kavlock R, Marafante E, Cory-Slechta D. Implications of gender differences for human health risk assessment and toxicology. Environ Res. 2007;104:70–84. doi: 10.1016/j.envres.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Valentin J. International Commission on Radiologic Protection, Annals of the ICRP Publ 89. Pergamon; New York: 2001. Basic Anatomical and Physiological Data for Use in Radiological Protection: Reverence Values. [Google Scholar]
- Van Nas A, Thakurta G, Wang SS, Yehya N, Horvath S, Zhang B, Ingram-Drake L, Chaudhuri G, Schadt EE, Drake TA, Arnold AP, Luisa AJ. Elucidating the Role of Gonadal Hormones in Sexually Dimorphic Gene Coexpression Networks. Endocrinol. 2009;150:1235–1249. doi: 10.1210/en.2008-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Saal F, Bronson F. Sexual characteristics of adult female mice are correlated with their blood testosterone levels during prenatal development. Sci. 1980;208:597–599. doi: 10.1126/science.7367881. [DOI] [PubMed] [Google Scholar]
- Waidyanatha S, Johnson JD, Hong SP, Godfrey-Robinson VD, Graves SW, Cristy T, et al. Toxicokinetics of bis(2-chloroethoxy)methane following intravenous administration and dermal application in male and female F344/N rats and B6C3F1 mice. Toxicol Lett. 2011;205:215–226. doi: 10.1016/j.toxlet.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace L, Gill VJ. Environmental Protection Agency (EPA 600/s4 80-026) Washington, D. C: 1982. Correlations between age-adjusted mortality rates for white males and females in the United States, by county: 1968-1972. [Google Scholar]
- Walsh CT. Toxicokinetics: oral exposure and absorption of toxicants. In: Bond J, editor. Comprehensive toxicology Vol 1: General Principles. Pergamon; New York: 1997. pp. 51–61. [Google Scholar]
- Wang T, Marei HE. Landscape of NAT2 polymorphisms among breast cancer. Biomed Pharmacother. 2016;77:191–196. doi: 10.1016/j.biopha.2015.12.011. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Tanaka M, Tateishi T, et al. Effects of the estrous cycle and gender differences on hepatic drug metabolizing enzyme activities. Pharmacol Res. 1997;35:477–480. doi: 10.1006/phrs.1997.0165. [DOI] [PubMed] [Google Scholar]
- Waxman DJ, O'Connor C. Growth hormone regulation of sex-dependent liver gene expression. Molecular Endocrinol. 2006;20:2613–2629. doi: 10.1210/me.2006-0007. [DOI] [PubMed] [Google Scholar]
- Wenk M, Todesco L, Krahenbuhl S. Effect of St. John's wort on the activities of CYP1A2, CYP3A4, CYP2D6, N-acetyltransferase-2, and xanthine oxidase in healthy males and females. Brit J Clin Pharmacol. 2004;57:495–499. doi: 10.1111/j.1365-2125.2003.02049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wester RC, Maibach HI. Toxicokinetics: dermal exposure and absorption of toxicants. In: Bond J, editor. Comprehensive toxicology Vol 1: General Principles. Pergamon; New York: 1997. pp. 99–114. [Google Scholar]
- Wilson K. Sex-related differences in drug disposition in man. Clin Pharmacokinet. 1984;9:189–202. doi: 10.2165/00003088-198409030-00001. [DOI] [PubMed] [Google Scholar]
- Wizemann TM, Pardue M. Exploring the Biological Contributions to Human Health: Does Sex Matter? Institute of Medicine. National Academy Press; Washington, D.C: 2001. [PubMed] [Google Scholar]
- Worley RR, Fisher J. Application of Physiologically-Based Pharmacokinetic Modeling to Explore the Role of Kidney Transporters in Renal Reabsorption of Perfluorooctanoic Acid in the Rat. Toxicol Applied Pharmacol. 2015;289:428–441. doi: 10.1016/j.taap.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Li Y, Hong H, Chang CW, Guo LW, Lyn-Cook B, et al. Sex differences in the expression of drug-metabolizing and transporter genes in human liver. J Metab Toxicol. 2012;3:1–9. doi: 10.4172/2157-7609.1000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm SH, Fraumeni JF., Jr Racial, ethnic, and gender variations in cancer risk: considerations for future epidemiologic research. Environ Health Perspect. 1995;103:283–286. doi: 10.1289/ehp.95103s8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang EA, Wynder EL. Differences in lung cancer risk between men and women: examination of the evidence. J Natl Cancer Inst. 1996;88:183–192. doi: 10.1093/jnci/88.3-4.183. [DOI] [PubMed] [Google Scholar]


