Abstract
Helicobacter pylori (H.pylori), a bacterial pathogen, is a causative agent of gastritis and peptic ulcer disease and is a strong risk factor for development of gastric cancer. Environmental conditions, such as poor dietary iron resulting in iron deficiency anemia (IDA), enhance H.pylori virulence and increases risk for gastric cancer. IDA affects billions of people worldwide, and there is considerable overlap between regions of high IDA and high H.pylori prevalence. The primary aims of our study were to evaluate the effect of H.pylori infection on behavior, iron metabolism, red blood cell indices, and behavioral outcomes following comorbid H. pylori infection and dietary iron deficiency in a mouse model. C57BL/6 female mice (n = 40) were used; half were placed on a moderately iron deficient (ID) diet immediately post-weaning, and the other half were maintained on an iron replete (IR) diet. Half were dosed with H.pylori SS1 at 5 weeks of age, and the remaining mice were sham-dosed. There were 4 study groups: a control group (-Hp, IR diet) as well as 3 experimental groups (-Hp, ID diet; +Hp, IR diet; +Hp,ID diet). All mice were tested in an open field apparatus at 8 weeks postinfection. Independent of dietary iron status, H.pylori -infected mice performed fewer exploratory behaviors in the open field chamber than uninfected mice (p<0.001). Hippocampal gene expression of myelination markers and dopamine receptor 1 was significantly downregulated in mice on an ID diet (both p<0.05), independent of infection status. At 12 months postinfection, hematocrit (Hct) and hemoglobin (Hgb) concentration were significantly lower in +Hp, ID diet mice compared to all other study groups. H.pylori infection caused IDA in mice maintained on a marginal iron diet. The mouse model developed in this study is a useful model to study the neurologic, behavioral, and hematologic impact of the common human co-morbidity of H. pylori infection and IDA.
Introduction
The Gram-negative pathogen, Helicobacter pylori, infects over 50% of the world’s population, and was classified as a group I carcinogen in 1994 by the International Agency for Research on Cancer [1]. H. pylori infection causes acute and chronic inflammatory responses in the stomach, which can result in a multitude of disorders including: chronic gastritis, atrophic gastritis, peptic ulcers, gastric cancer, and iron deficiency anemia (IDA) [2–7]. The prevalence of H. pylori infection in developed countries has decreased with improved sanitary conditions and continued therapeutic intervention [5]. In spite of this progress, H. pylori prevalence rates remain as high as >90% in some developing countries, which corresponds to higher risk of gastric cancer in some subpopulations [8]. H. pylori also has been postulated to play a role in chronic neurologic disorders. A prevalent theory as to how H. pylori may contribute to neurologic disease is that H. pylori-induced cytokines and chemokines cause systemic and central nervous system (CNS) inflammation and dysfunction[9].
H. pylori infection and iron deficiency anemia (IDA) are both prevalent in the developing world, and overlapping diseases represent an increased risk for co-morbidity of these two conditions [8,10,11]. Iron is an essential micronutrient with many critical functions in the body, including as an important component of heme compounds that are crucial in the process of oxygen delivery to cells [12]. Iron deficiency (ID) is the most prevalent micronutrient deficiency in many demographic groups worldwide, including children and women of childbearing age. Consumption of a marginal iron diet is one of the most common causes of dietary ID in developing countries [10,13,14] When ID is present during important developmental periods, such as fetal and early postnatal stages, it causes cognitive and socio-emotional deficits in infants and children that persist into adulthood [15–17]. Treatment of IDA during childhood with iron supplementation often fails to prevent the occurrence of deficits, including poor performance IQ scores, visual motor integration problems and gross and fine motor performance deficits, at later developmental stages [18]. The negative neurodevelopmental effects of early-life ID seen in human patients have been substantiated in rodent models [19–21].
Recent basic and clinical research has focused on elucidating the relationship between H. pylori infection and IDA, and how one condition may impact the other. Four recent meta-analyses have concluded that H. pylori infection is a causative factor in the development of IDA in human patients, with proposed mechanisms including: decrease in absorption of dietary iron due to hypochlorhydria caused by H. pylori infection, gastrointestinal blood loss, and enhanced uptake and sequestration of iron by H. pylori [4,22–24]. A previous study conducted in our lab revealed that male INS-GAS/FVB mice infected with H. pylori develop anemia and have significantly lower serum ferritin concentrations than uninfected control mice at 7–8 months postinfection. Interestingly, this occurred in spite of continuous intake of an iron-replete rodent diet. Molecular analysis of whole brain tissue revealed that chronic H. pylori infection of INS-GAS mice resulted in reduced expression of genes involved in dopamine metabolism, myelination, and maintenance of synaptic plasticity; all changes characteristic of brain iron deficiency [25]. These findings prompted the current study in C57BL/6 mice, a strain commonly used as a background strain for behavioral studies. This study is the first to evaluate the effects of H. pylori infection on hippocampal gene expression in mice. The goals of this study were to determine: 1) the effect of H. pylori infection on mouse behavior, 2) the effect of chronic H. pylori infection on expression of genes related to myelination in the hippocampus, 3) how chronic H. pylori infection and concurrent marginal dietary iron deficiency affect dopamine metabolism in the hippocampus, and 4) the impact of chronic H. pylori infection and marginal dietary iron on systemic iron homeostasis and red blood cell indices.
Materials and methods
Animals
The Massachusetts Institute of Technology (MIT) Committee on Animal Care approved the use of mice in this study (Animal Welfare Assurance #A3125-01). All mice were euthanized via carbon dioxide inhalation, in accordance with the American Veterinary Medical Association Euthanasia Guidelines [26]. Female C57BL/6NCrl mice obtained from Charles River Laboratories (Wilmington, MA) were used and maintained on purified diets (either iron-replete or moderately iron-deplete) from immediately post-weaning throughout the duration of the study. Female mice were chosen as a model for this study due to the fact that they develop a more severe proinflammatory Th1-biased response than male mice when infected with H. pylori, and C57BL/6 mice of both sexes provide reliable models for behavioral studies [27,28]. There were a total of 4 treatment groups (1) -Hp, IR; 2)+Hp, IR; 3)-Hp, ID; 4) +Hp, ID), with 7–11 mice per treatment group at study initiation. All mice were maintained in an AAALAC International-accredited facility and were seronegative for: mouse hepatitis virus, Sendai virus, rotavirus (EDIM), pneumonia virus of mice, Theiler’s encephalomyelitis virus, reovirus, lymphocytic choriomeningitis virus, ectromelia virus, polyomavirus, K virus, carbacillus, mouse cytomegalovirus, mouse parvovirus, and minute virus of mice. Mice were also negative for both endo- and ectoparasites, including Syphacia & Aspicularis spp, as well as Salmonella spp, Citrobacter rodentium, & Helicobacter spp. Mice were socially-housed, with two to five mice housed in each standard mouse cage. Mice were housed on heat-treated hardwood bedding (P.J. Murphy Forest Products Corporation; Montville, NJ) with one sterile cotton fiber Nestlet (Ancare) per cage in polycarbonate caging with filter tops, were maintained on a 12:12 light/dark light cycle, and were provided customized purified diet and reverse osmosis chlorinated water ad libitum.
Diet
Mice were allowed ad libitum access to one of two customized purified diets created by Research Diets Inc (New Brunswick, NJ) at approximately 23 days of age (immediately post-weaning). This rodent life stage was selected to model a period of intensive growth in the human brain [29].Dietary iron content was independently verified via ICP Emission Spectrometry performed by Covance Laboratories (Princeton, NJ). The iron concentration of the iron-replete (IR) diet ranged from 47.8–48.7 ppm, and the iron concentration of the moderately iron-deplete (ID) diet ranged from 7.57–8.69 ppm.
Experimental infection
At 5–5.5 weeks of age, female mice were orally gavaged with 1 X 108 colony forming units of H. pylori SS1 in 200 μl of sterile freeze media every other day for a total of 3 doses, or were sham-dosed with sterile freeze media following the same timeline. Dosing at this age was selected for infection since infecting neonatal mice with H. pylori results in the development of tolerance to the pathogen, which precludes development of chronic inflammation [30].
Open field task
Behavioral testing was conducted at 8 weeks postinfection (13 weeks of age). Locomotor activity and behavioral responses to an unfamiliar environment were measured using the open field test. Mice were individually placed into a Plexiglas testing chamber measuring 40 cm x 40 cm x 30 cm. Mice were placed in the center of the testing chamber initially, and allowed to explore the area for 60 minutes. Motor activity was detected and tracked by infrared photobeam sensors. Individual mouse activity was analyzed by a VersaMax animal activity monitoring system (AccuScan Instruments; Columbus, OH). Parameters measured included: total distance travelled, ambulatory activity, ambulatory episodes, horizontal activity, vertical activity, and total time spent in each area. At the end of the task, all mice were returned to their home cage. Equipment was thoroughly cleaned with Quatricide disinfectant. All behavioral testing took place between 9am and 2pm. In the behavioral testing room, soft lighting provided by a floor lamp containing a single 40W fluorescent light bulb was present during task preparation and chamber cleaning.
Complete blood count
Serial blood samples (< 200μl) were collected at 2, 3, 6, &10 months of age via submandibular venipuncture, and immediately placed into 1 ml EDTA-coated anticoagulant blood collection tubes. Samples were processed by a HemaVet 950 veterinary hematology analyzer (Drew Scientific, Oxford, CT) at the MIT DCM diagnostic laboratory. Whole blood samples (1 mL) were collected at necropsy from anesthetized mice via cardiac puncture, and immediately placed into 1 mL EDTA-coated anticoagulant blood collection tubes. Samples were processed within 4 hours of collection at the Massachusetts General Hospital Center for Comparative Medicine Clinical Pathology lab (Boston, MA). Complete Blood Count (CBC) analysis was performed on a HESKA HemaTrue Veterinary Analyzer (Loveland, CO), using an impedance-based method for counting white blood cells, red blood cells, and platelets. Hemoglobin was measured on the HemaTrue Analyzer using a cyanide-free method and measured by a spectrophotometer set to 535 nm.
Serum ferritin
Serum ferritin concentrations were measured using a mouse specific enzyme-linked immunosorbent assay (ELISA) kit (Kamiya Biomedical, Seattle, WA) according to the instructions provided by the manufacturer. A positive internal control, serum containing a known quantity of ferritin, was included in the commercial kit, and analyzed concurrently with experimental samples. The R2 value of the standard curve was 0.998. All samples were analyzed in duplicate.
Necropsy
Necropsies were performed on all mice at 12 months postinfection. Whole brains were collected immediately post-mortem, and the hippocampus was dissected bilaterally and immediately submerged in liquid nitrogen. All samples were ultimately transferred to and stored at -80°C. Stomach and proximal duodenum were sectioned beginning at the greater curvature, and gastric linear sections were collected for histopathology. Other stomach sections were used for DNA and RNA extraction to evaluate mRNA expression and H. pylori colonization level, as previously described [25]. Stomach tissue samples (linear strips from the squamo-columnar junction through the proximal duodenum) and liver tissue samples collected for DNA and RNA extraction were flash-frozen in liquid nitrogen and stored at -80°C. Sterile razor blades were used for stomach and duodenal sectioning. Sections of stomach and liver were collected, trimmed, and fixed overnight in 10% neutral buffered formalin. All tissues were processed, embedded in paraffin, cut into 4-μ m-thick sections, and stained with hematoxylin and eosin.
Histopathologic evaluation and lesion scoring
All tissues collected at necropsy were evaluated by a board-certified comparative pathologist (V.B.) who was blinded to both treatment groups and sample identity. Gastric lesions were scored on an ascending scale from 0 to 4 using previously established criteria. Lesions related to inflammation, epithelial defects, oxyntic atrophy, epithelial hyperplasia, pseudo-pyloric metaplasia, mucus metaplasia, and dysplasia, were scored. Scores for each individual category were added to generate an overall gastric histopathologic assessment index (GHAI) [31,32].
Real time quantitative PCR
mRNA expression for target genes was assessed in both gastric and liver tissues. Stomachs were incised along the greater curvature, opened, and sectioned into three linear strips. Total RNA from liver and stomach was prepared using Trizol reagent (Life Technologies), according to the manufacturer’s instructions. Two μg of total RNA from each sample was converted to cDNA using a High-Capacity cDNA Reverse Transcriptase Kit (Applied Biosciences). Relative expression of hepcidin antimicrobial peptide (Hamp), bone morphogenic peptide 4(Bmp4), divalent metal transporter (Slc11a2; hereafter referred to as Dmt1), as well as levels of inflammatory cytokines including: tumor necrosis factor alpha (TNFα), interleukin 1 beta (IL1β), interleukin 17 alpha (IL17α), and interferon gamma (IFNγ) mRNA were measured by qPCR using commercially available primer/probes (TaqMan Gene Expression Assays) in the 7500 Fast Sequence Detection System. CT values were normalized to an endogenous control, glyceraldehyde-3-phosphate dehydrogenase mRNA, and were expressed as relative fold-change compared to sham-dosed animals using the comparative CT method. Expression levels of target genes were also analyzed in hippocampal tissue. Total RNA from hippocampal tissue was extracted (RNAqueous kit, Lifetechnologies; Carlsbad, CA) and first strand cDNA was generated using 2 μg of RNA (high capacity RNA-to cDNA kit, Lifetechnologies; Carlsbad, CA). Relative mRNA expression of myelin basic protein (Mbp), proteolipid protein 1 (Plp1) and dopamine receptor 1 (D1r) was evaluated using qPCR [33,34]. Commercially available primer/probes (Lifetechnologies; Carlsbad, CA) and Taqman master-mix (Roche Life Science; Indianapolis, IN) were used on a StratageneMX300P qPCR system (Agilent Technologies; Santa Clara, CA). Samples were assayed in duplicate and normalized against ribosomal protein S18.
Colonization levels of H. pylori SS1
DNA was extracted from stomach tissue using a High Pure PCR Template Preparation Kit (Roche Diagnostics, Indianapolis, IN). Colonization levels of H. pylori SS1 within the gastric mucosa were quantified using H. pylori DNA-specific primer/probes in the 7500 Fast Sequence Detection System (Applied Biosystems) as previously described [35]. To quantify murine DNA in the respective samples, each was probed with commercial 18S rRNA gene-based primer/probes (Life Technologies) as previously published [35]. Copy numbers of H. pylori were measured using per μg of mouse DNA.
Statistical analysis
All statistical analyses were performed using GraphPad Prism Version 7 (GraphPad Software Inc.). Distribution and variance were evaluated for all samples, and statistical analyses were performed using parametric methods after assumptions of normality and equivalence of variance were met. One-way analysis of variance (ANOVA) or Student’s t-tests were used, and multiple comparisons tests (Tukey’s) were performed following ANOVA. Nonparametric tests were used for comparison of mean gastric histopathology scores across groups, and Dunn’s multiple comparisons tests were used for post-hoc analysis. Statistical significance was designated as a p-value < 0.05.
Results
Female mice perform fewer exploratory behaviors in the open field chamber after acute H. pylori infection
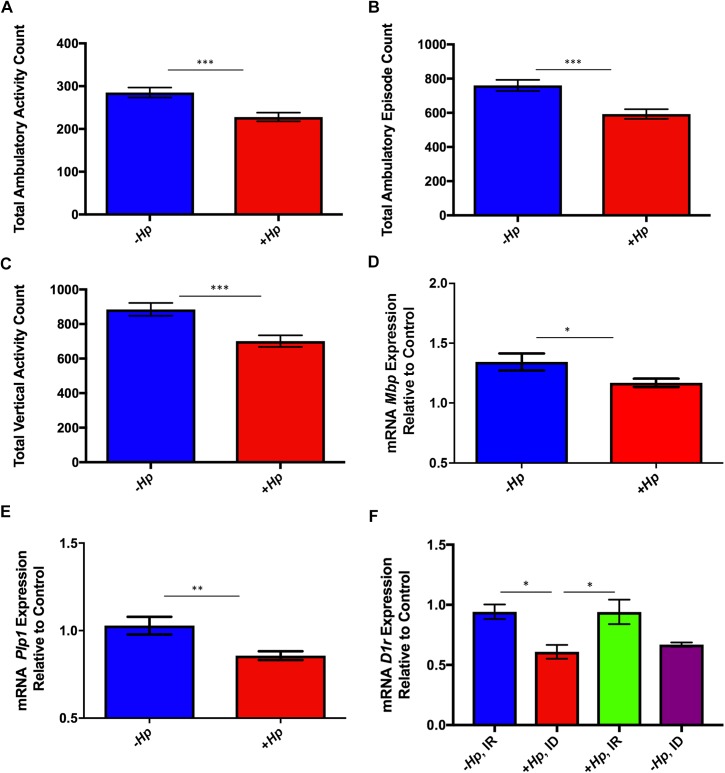
Ambulatory activity and episodes, as well as vertical activity, are considered exploratory behaviors of mice in an open field chamber. Counts of these exploratory behaviors were significantly lower in H. pylori-infected C57BL/6 female mice compared to uninfected mice at 8 weeks postinfection (all p<0.001; Fig 1A–1C). At 8 weeks post-H. pylori infection, no effect of dietary iron status was noted, as evidenced by normal hemogram results at the 8 week postinfection time point.
Fig 1.
(A-F). H. pylori-infected mice performed fewer exploratory behaviors in the open field chamber and hippocampal expression of genes related to myelination and dopamine metabolism was altered by H. pylori infection and dietary iron content. (A-C) H. pylori-infected mice had significantly lower total ambulatory activity, total ambulatory count, and total vertical activity count compared to uninfected mice independent of dietary iron status at 8 weeks postinfection. (D) Expression of hippocampal genes related to myelination was downregulated in H. pylori infected female mice at 12 months postinfection. Expression of both Mbp (D) and Plp1 (E) mRNA was significantly downregulated in H. pylori-infected mice compared with uninfected controls. (F) At 12 months postinfection, expression of D1r was significantly downregulated in H. pylori -infected mice on ID diet compared to all mice on IR diet. All data shown as mean + SEM. (*) = p<0.05, (**) = p<0.01, (***) = p<0.001.
Chronic H. pylori infection and marginal iron diet altered hippocampal gene expression in female mice
Hippocampal gene expression of both myelin basic protein (Mbp) and proteolipid protein 1 (Plp1) was significantly downregulated in H. pylori-infected female mice compared to sham-dosed controls at 12 months postinfection (0.01< p <0.05; Fig 1D & 1E). Both Mbp and Plp1 play a critical role in hippocampal axonal myelination. Dietary iron did not affect expression of Mbp or Plp1. A significant effect of treatment group was found on hippocampal expression of dopamine receptor 1 (D1r), an important component of the CNS dopamine system (p<0.01; Fig 1F). Multiple comparisons analysis revealed that D1r expression was significantly downregulated in adult H. pylori-infected mice on an ID diet compared to both infected and uninfected mice on an IR diet (both p<0.05; Fig 1F).
Concurrent H. pylori infection dietary iron deficiency altered expression of hepatic iron regulatory genes
Expression of divalent metal transporter 1 (Dmt1), an important iron transporter of many types of cells, was significantly upregulated in H. pylori-infected mice on ID diet compared to all other treatment groups (0.001< p<0.01); Fig 2A). Expression of hepcidin (Hamp) was significantly downregulated in H. pylori-infected mice on ID diet compared to all other treatment groups (all p<0.001; Fig 2B). The liver is the primary source of hepcidin antimicrobial peptide, the body’s master iron regulatory protein. No effect of treatment group was noted on hepatic gene expression of bone morphogenic protein 4 (Bmp4) (data not shown).
Fig 2.
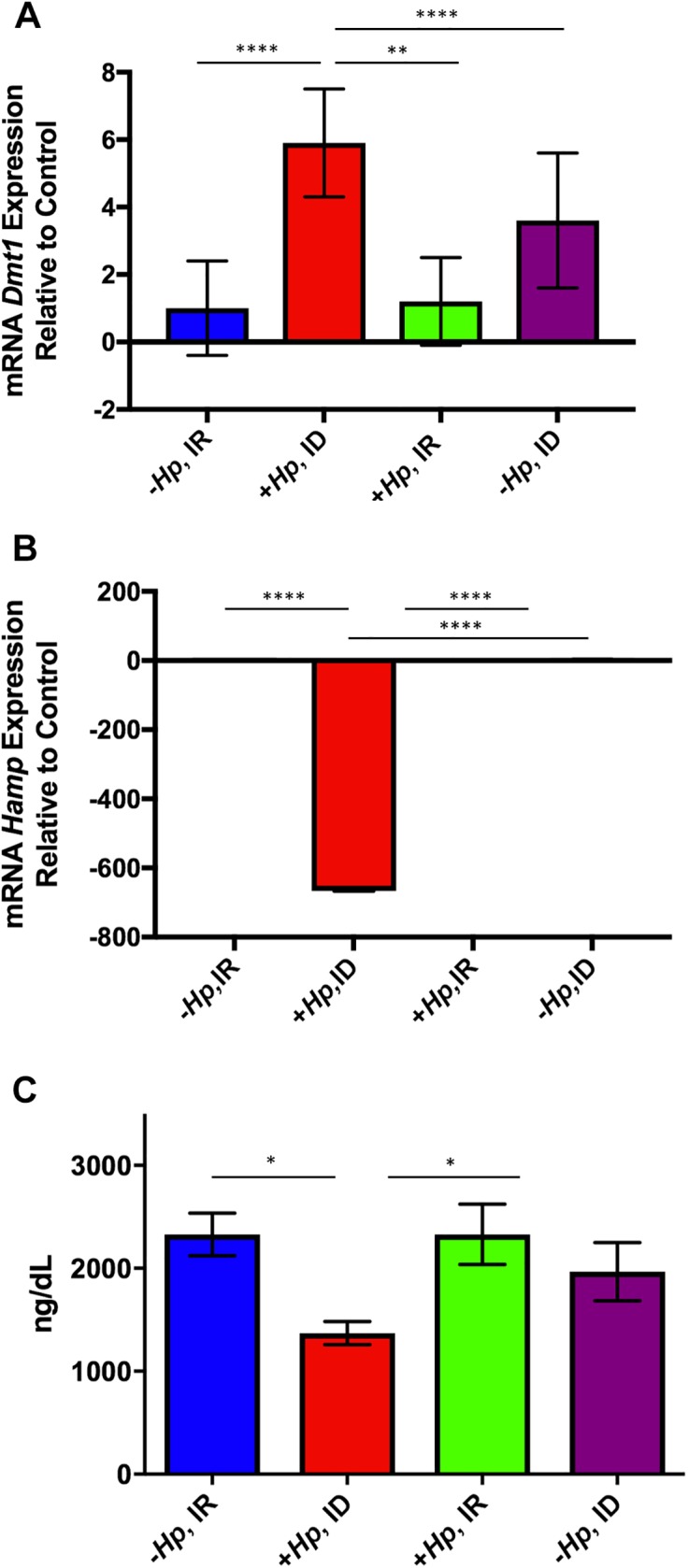
(A-C). Hepatic Dmt1 expression was upregulated while Hamp expression and serum ferritin were downregulated in H. pylori-infected mice on ID diet. Hepatic Dmt1 expression was upregulated and Hamp expression was downregulated in H. pylori-infected mice on ID diet. (A) H. pylori-infected mice had significantly increased expression of Dmt1 compared to all other treatment groups. (B) Expression of Hamp in hepatic tissue was significantly downregulated in H. pylori -infected mice on ID diet compared to all other study groups. (C) Concurrent H. pylori infection and dietary ID decreased serum ferritin levels in female mice. H. pylori-infected mice on ID diet had significantly lower serum ferritin levels than both uninfected mice on IR diet and H. pylori-infected mice on IR diet. All data shown as mean + SEM. (*) = p<0.05, (**) = p<0.01, (****) = p<0.000.
Concurrent H. pylori infection and dietary iron deficiency depleted serum ferritin stores at 12 months postinfection
At necropsy, mice chronically infected with H. pylori on ID diet had significantly lower serum ferritin than uninfected and H. pylori-infected mice on IR diet (both p<0.05; Fig 2C). Ferritin is critical iron-binding protein, and iron bound to ferritin makes up a large proportion of the total iron storage pool of the body.
Gastric expression of inflammatory cytokines was upregulated in H. pylori-infected female mice
Gastric expression of tumor necrosis factor alpha (TNFα), interleukin one beta (IL1β), interleukin 17 alpha (IL7α), and interferon gamma (IFNγ), was significantly upregulated in H. pylori infected mice compared to both groups of uninfected mice (0.05<p<0.0001; Fig 3A–3D). However, no significant differences in gastric inflammatory cytokine expression were noted between +Hp, ID and +Hp, IR treatment groups (Fig 3A–3D).
Fig 3. Chronic H. pylori infection upregulated gastric expression of inflammatory cytokines in female mice.
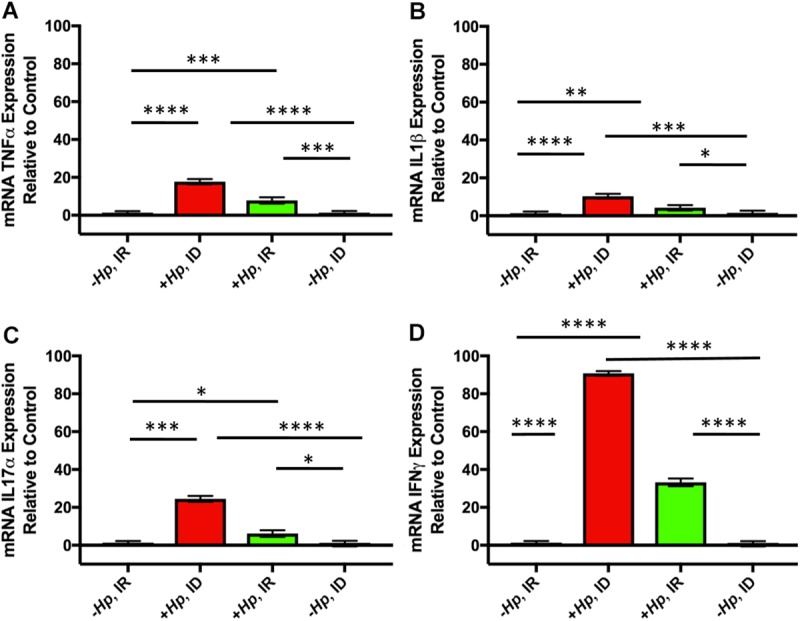
Gastric expression of tumor necrosis factor alpha (TNFα) (A), interleukin one beta (IL1β) (B), interleukin 17 alpha (IL7α) (C), and interferon gamma (IFNγ) (D) was significantly upregulated in H. pylori infected mice compared to uninfected mice. All data shown as mean + SEM. (*) = p<0.05, (**) = p<0.01, (***) = p<0.001, (****) = p<0.0001.
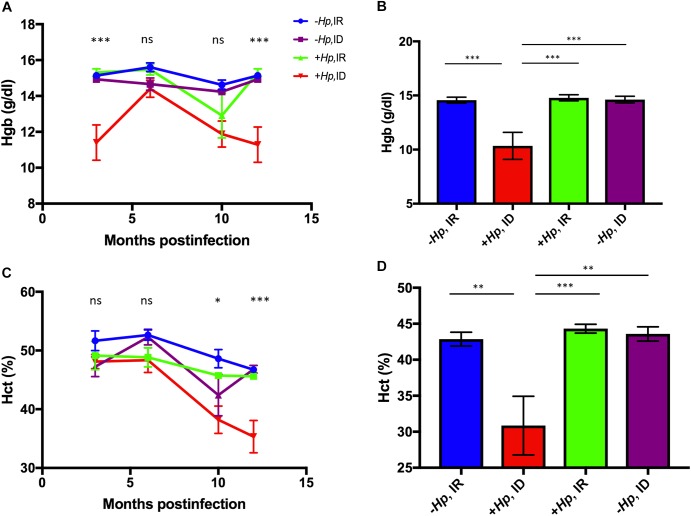
H. pylori-infected female mice on marginal iron diet had lower hemoglobin concentration than control mice by 3 months postinfection
Low hemoglobin (Hgb) concentration and hematocrit (Hct) are commonly used in the clinical diagnosis of anemia. Significant differences in mean Hgb concentration among treatment groups were noted at early and late sampling time points (Fig 4A–4D). A significant treatment effect on mean Hgb concentration was noted at 3 months postinfection (p<0.001; Fig 4A). Post hoc multiple comparisons analysis revealed that the +Hp, ID treatment group had significantly lower mean Hgb concentration than the–Hp, IR (p<0.001), -Hp, ID (p<0.001), and +Hp, IR (p<0.0001) treatment groups at 3 months postinfection (data not shown). No significant treatment effect was noted among groups at 6 or 10 months postinfection. At 12 months postinfection, a significant treatment effect was identified (p<0.0001; Fig 4A); multiple comparison analysis revealed that mean Hgb concentration of H. pylori-infected female mice on ID diet was significantly lower than all other treatment groups (p<0.001; Fig 4B).
Fig 4.
(A-D). Hematocrit and hemoglobin concentration were lower in H. pylori-infected mice on ID diet. (A) Mean hemoglobin (Hgb) differed among treatment groups at multiple sampling time points. A significant treatment effect on Hgb concentration was noted at the first sampling time point (3 months postinfection). At necropsy (B), H. pylori -infected mice on ID diet had lower Hgb than all other treatment groups. (C) Mean Hct was significantly different among treatment groups at both 10 and 12 months postinfection. (D) H. pylori-infected mice on ID diet had significantly lower mean Hct than all other groups at the time of necropsy. All data shown as mean + SEM. (*) = p<0.05, (**) = p<0.01, (***) = p<0.001.
Concurrent H. pylori infection and dietary iron deficiency lowered hematocrit in female mice by 10 months postinfection
No significant differences in mean Hct were noted until 10 months postinfection, at which point mean Hct was significantly lower in the +Hp, ID group than the–Hp, IR group (p<0.05; Fig 4C). At 12 months postinfection, a significant treatment effect was noted among groups (p<0.001; Fig 4C). Mean Hct of the +Hp, ID group was significantly lower than all other treatment groups (0.01<p<0.001; Fig 4D).
Both mean Hgb and mean Hct of mice in the H. pylori-infected mice on ID diet were significantly lower than all other treatment groups after 12 months of infection.
Concurrent H. pylori-infection and moderate dietary ID decreased mean cellular volume of erythrocytes and increased red blood cell distribution width in mice at 12 months postinfection
Mean cellular volume (MCV) is a measurement of RBC size; red cells with reduced MCV are commonly noted on peripheral blood smears and/or complete blood counts as a result of iron deficient erythropoiesis. At 12 months postinfection with H. pylori, mice on an ID diet had significantly lower MCV than all other treatment groups (all p<0.0001; Fig 5A).
Fig 5.
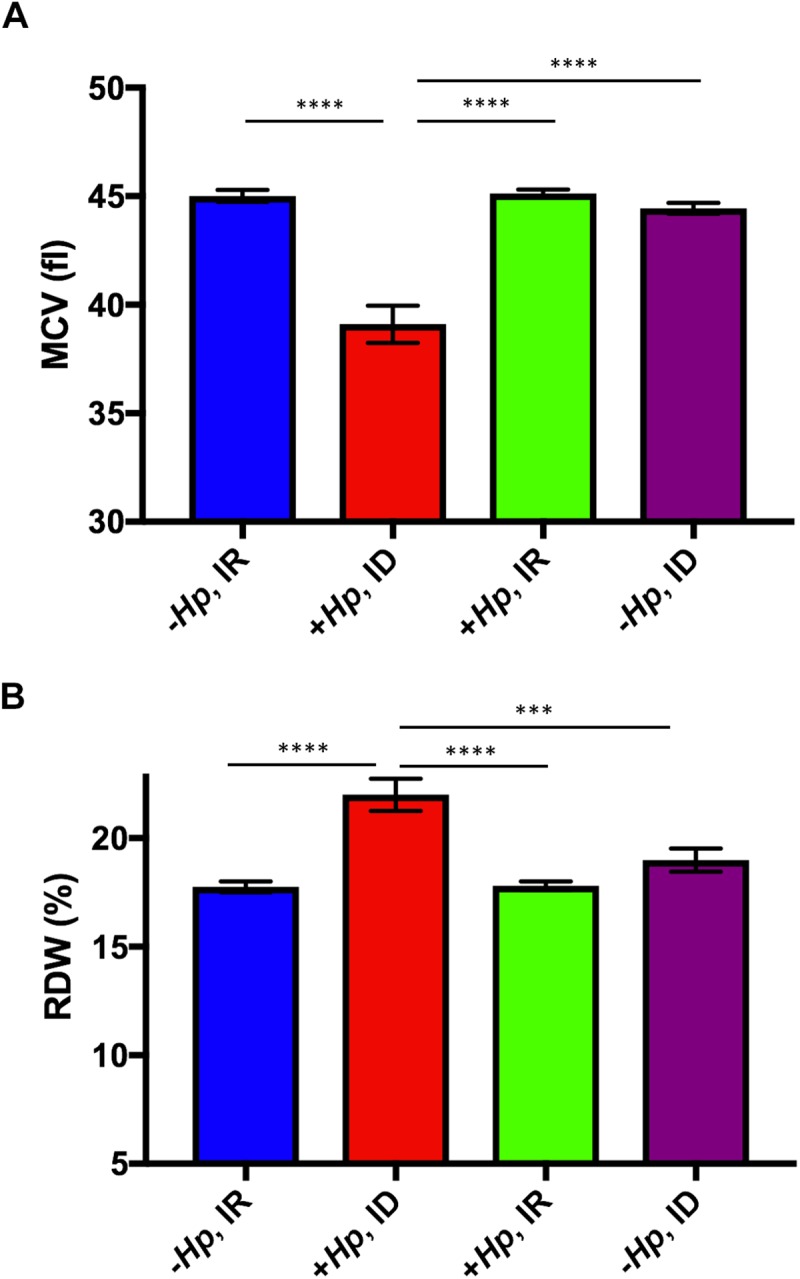
(A&B). Concurrent H. pylori infection and ID diet resulted in lower mean cellular volume (MCV) and higher mean red cell distribution width (RDW) at 12 months postinfection (A) Mean MCV of H. pylori-infected mice on ID diet was significantly lower than mean MCV of all other treatment groups. (B) Mean RDW of H. pylori-infected mice on ID diet was significantly greater than mean RDW of all other treatment groups. All data shown as mean + SEM (***) = p<0.001, (****) = p<0.0001.
Red cell distribution width (RDW) is a parameter that measures variation in red blood cell size [36]. High RDW reflects considerable variation in red blood cell size, and is commonly documented in iron deficient patients. Mice infected with H. pylori for 12 months and maintained on ID diet had significantly higher RDW compared to all other treatment groups (0.0001< p< 0.001; Fig 5B).
Histopathology
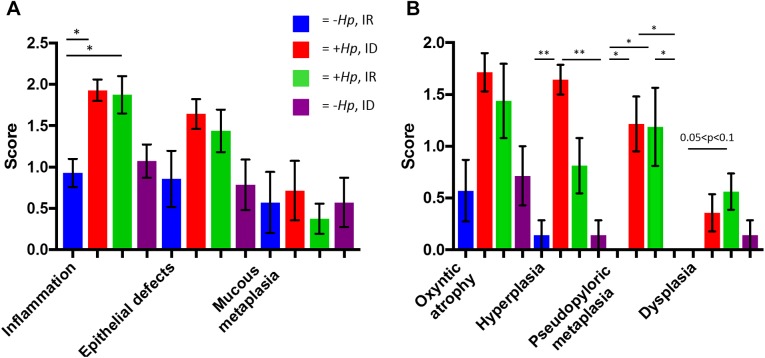
H. pylori SS1-infected mice had significantly higher inflammation, hyperplasia, and pseudopyloric metaplasia scores than uninfected mice (Fig 6A & 6B).
Fig 6.
(A&B). H. pylori infection increased gastric corpus histopathology scores. H. pylori-infected mice had higher histopathology scores for(A) inflammation, (B) hyperplasia, pseudopyloric metaplasia. All data shown as mean + SEM. (*) = p<0.05, (**) = p<0.01, (****) = p<0.0001.
H. pylori SS1 established persistent colonization in gastric tissue at 12 months postinfection
Colonization levels of gastric tissue by H. pylori SS1 were evaluated at necropsy (12 months postinfection). There were two mice in the +Hp, IR treatment group, and one mouse in the +Hp, ID treatment group that had lost colonization at the time of necropsy, and thus were excluded from statistical analysis. Mean relative colonization level of mouse gastric tissue in persistently Hp-infected mice on ID diet was 15503+6834 copies of H. pylori/μg of mouse DNA. Mean relative colonization level of mouse gastric tissue in persistently Hp-infected mice on IR diet was 1234+512.9 copies of H. pylori/μg of mouse DNA (0.05<p<0.1).
Discussion
In this study, behavioral testing was conducted at 8 weeks postinfection, which corresponds to the active phase of inflammation caused by gastric Helicobacter spp. Infections in C57BL/6 mice [7,37] Female C57BL/6 mice infected with H. pylori performed fewer exploratory behaviors in the open field chamber than did sham-dosed mice. This lack of exploratory behaviors is consistent with increased anxiety [38,39]. No hematologic evidence of ID due to consumption of a marginal iron diet was documented at this early timepoint; thus we attributed these behavioral changes to active H. pylori-associated inflammation. While the open field task is used as a measurement of locomotion and anxiety, it is possible that discomfort due to gastric inflammation caused by H. pylori infection influenced behavioral task performance in this study. Multiple hypotheses have been proposed regarding how H. pylori infection affects the brain, including that of a causative relationship between H. pylori-associated inflammation and altered neuronal homeostasis [40]. The behavioral effects of murine GI infections, causing acute inflammation, have been studied previously as models of human disease. Murine Citrobacter rodentium infection used to model inflammatory bowel disease (IBD) produced anxiety-like behaviors in mice [41]. This study found that mice infected with C. rodentium demonstrated more anxiety-like behaviors compared to uninfected controls, as C. rodentium-infected mice spent significantly less time in the center zone of the open field chamber than control mice [41]. Thus, acute H. pylori infection, similar to infections of the lower bowel that cause inflammation, modulated behavioral task performance in mice, and can provide a robust animal model for the study of the effect of GI inflammatory disease on the brain and behavior in humans.
In addition to acute behavioral alterations, hippocampal expression of two genes related to myelination, Mbp and Plp1, was lower in mice chronically infected with H. pylori regardless of dietary iron status when compared to sham-dosed control mice. This indicates that H. pylori infection per se was an independent factor in altering hippocampal gene expression. Mbp and Plp1 are essential to formation of the myelin sheath in the mammalian central nervous system. Previous studies evaluating gene expression in the hippocampus have found that protein levels trend in the same direction as the corresponding gene transcripts [42]. In our previous study, expression of Mbp was significantly lower in brain tissue of H. pylori-infected INS-GAS mice compared to uninfected controls [25]. Protein analysis revealed a parallel decrease in Mbp levels, which were 28% lower in H. pylori-infected mice compared to uninfected control mice. It is possible that dietary iron status in this study did not affect expression of myelination genes due to the use of a post-weaning marginal dietary ID model instead of a gestational dietary ID model.
Hippocampal expression of D1r was lower in mice on marginal ID diet than in mice on IR diet, independent of infection status. The impact of ID/IDA on the dopamine system has been studied extensively, and it is well-documented in rat studies that dopamine receptor expression and protein density decrease under ID conditions [39,43] Brain region-specific gene analysis was chosen for this study because the hippocampus plays a crucial role in learning and the formation of spatial and recognition memory. Adequate early life iron is essential for normal neuronal development, sensorimotor function, and dopamine system function in humans and rodents [42,44,45]. The results of the current study were consistent with previously reported findings where mice on ID diet had decreased expression of D1r compared to mice on IR diet. This disruption of dopamine system homeostasis demonstrates that environmental factors that originate early in life, such as marginal dietary ID, may impact nervous system gene expression even in aged animals. Previous studies have shown that long-term dopamine system changes may be induced by dietary ID without brain tissue ID [39]. In this study, no difference in expression of Dmt1, an iron regulator, was noted among treatment groups at 12 months postinfection. This is likely due to lower iron demand in the adult brain compared to the neonatal and juvenile brain.
Mean Hct and Hgb were significantly lower in H. pylori-infected mice on ID diet compared to all other groups in this study, and were also lower than previously established normal hematologic values of C57BL/6 female mice [46]. The combination of low Hct, Hg, and MCV, and concurrent high RDW, is indicative of a microcytic, hypochromic iron deficiency anemia [36]. It is likely that both H. pylori infection and low systemic iron availability contributed to this outcome. H. pylori disrupts host cell polarity and derails host intracellular iron-trafficking in vitro [47]. H. pylori infection causes inflammation, which in turn upregulates expression of hepcidin, an antimicrobial peptide produced by the liver that regulates systemic iron metabolism [48]. Atrophic gastritis caused by H. pylori infection results in a loss of parietal cells, thereby decreasing acid secretion into the gastric lumen, and increasing gastric pH [6,49]. An elevation in pH limits the amount of dietary ferric iron that is reduced to the ferrous form of iron absorbable by enterocytes, which results in impaired iron absorption.
A post-weaning marginal iron diet of 7-8ppm was selected for this study because gestational ID has been associated with high morbidity in mice [50]. The marginal iron diet provided mice with sufficient iron for homeostatic functions, evidenced by the lack of systemic effects of ID for several months after study initiation. Interestingly, mean Hgb concentration was significantly lower in +Hp, ID mice than all other treatment groups at 3 months postinfection, and at 12 months postinfection, but not at 6 and 10 month timepoints. In a previous study that evaluated the effect of low dietary iron (8ppm Fe) in both male and female C57BL/6 mice after 30 weeks of H. pylori infection, mice on the low iron diet had lower serum iron, transferrin saturation, and hemoglobin than uninfected controls on low iron diet [51]. The results of the current study corroborated many of these findings, and extended analysis to a chronic timepoint of 12 months postinfection. Another study of female C57BL/6 mice infected with either H. felis and H. pylori demonstrated that only H. felis, which induces a more robust inflammation than H. pylori in mice, caused an acute drop in serum iron after 4 weeks of infection; however, red blood cell indices and chronic time points were not evaluated [52]. In hypergastrinemic male INS-GAS mice prone to accelerated development of gastric adenocarcinoma, infection with gastric Helicobacter spp. Causes anemia and depleted serum iron stores, as well as altered expression of gastric and liver genes involved in systemic iron homeostasis [25,53]. In the current study, serum ferritin was lower in the H. pylori-infected group on ID diet than both IR treatment groups at 12 months postinfection, demonstrating that chronic infection with H. pylori infection, coupled with dietary ID, impacted serum iron storage as well as red blood cell parameters.
H. pylori colonization persisted for 12 months in mice that were infected at 4–5 weeks of age, and the histopathologic changes caused by a 12-month H. pylori SS1 infection in mice documented in the current study were consistent with previous studies in C57BL/6 mice infected with H. pylori SS1 for 12 months [32]. H. pylori-infected mice had significantly higher scores for inflammation, pseudopyloric metaplasia, and hyperplasia than uninfected mice. Gastric expression of inflammatory cytokines, including TNFα, IL1β, IL7α, and IFNγ, was also significantly upregulated in H. pylori infected mice compared to uninfected mice, which corroborated histopathologic findings; however, no significant differences were found in inflammatory cytokine expression between H. pylori-infected mice on ID vs. IR diet. In a related study, H. pylori-infected gerbils on an ID diet had higher gastric inflammation and dysplasia scores when compared to infected gerbils on an IR diet, and H. pylori strains isolated from infected and iron-depleted gerbils also demonstrated increased virulence and induction of inflammatory factors in vitro when compared to strains isolated from H. pylori infected gerbils on an IR diet [54]. A different gerbil study found that H. pylori-infected gerbils had higher gastric pH, higher incidence of gastric ulceration, and higher incidence of fecal occult blood loss, in addition to lower Hgb and MCV than uninfected animals [55].
Hepatic expression of Hamp, which encodes the body’s master iron regulatory protein, hepcidin, was significantly lower in H. pylori-infected mice on ID diet compared to all other treatment groups. An increase in hepcidin production, often related to inflammation, is a defense against bacterial infection[56,57]. In contrast, Hamp expression is decreased as a compensatory response in instances when there is a need for increased iron uptake, as seen in cases of IDA [58]. Interestingly, a significant upregulation of hepatic expression of an important iron transport receptor, Dmt1, was noted only in the +Hp, ID group. High levels of Dmt1 expression indicate an increased transport of iron into the intracellular space for use in homeostatic functions, and the significant upregulation of expression in the +Hp, ID group suggested a more profound systemic need for iron uptake in that group compared to all other treatment groups. The findings related to gene expression in this study highlight a molecular response to low systemic iron status, as determined by low hematocrit, hemoglobin, and ferritin, and provide a basis for further inquiry into the molecular mechanisms of this important response.
We have reconfirmed the findings of previous mouse studies that have evaluated the relationship between H. pylori infection, ID, and red blood cell homeostasis in mice. H. pylori infection, coupled with dietary ID, caused anemia and lowered serum iron storage through previously established mechanisms, including loss of gastric parietal cells, change in gastric pH, and inflammation causing upregulation of hepcidin, all resulting in decreased iron absorption and transport. After confirming these findings, we further studied the effects of H. pylori infection and ID comorbidity on behavior and neuro-homeostasis. We have previously shown that H. pylori infection in INS-GAS mice altered expression of genes related to iron homeostasis, dopamine metabolism, myelination, and synaptic plasticity in the brain [25]. In the current study, we focused our inquiry on the effects of H. pylori infection on hippocampal gene expression, and found that infection status had both acute effects, seen in the altered behavior of infected mice in the open field task at 8 weeks postinfection, and chronic effects, in the altered hippocampal gene expression levels in infected mice at 12 months postinfection. The current study found that hippocampal expression of genes related to myelination is lower in in mice chronically infected with H. pylori, which demonstrates that early-life infection can result in significant alterations to processes that are critical to the maintenance of neurohomeostasis in the adult brain. This study represents the first documentation of H. pylori-associated inflammation affecting mouse behavioral performances in the open field. Future studies should use gestational and neonatal ID mouse models to more effectively deplete iron during periods of rapid neurodevelopment, which will assist in elucidating the effects of ID and H. pylori infection comorbidity on the developing brain.
Acknowledgments
Thank you to Bailey Clear and Lauren McAllister for help with colony maintenance, sample collection, and assistance with behavioral assays; to Lenzie Cheney and Christian Kaufman for help with necropsy and sample collection; to Zeli Shen for bacterial culture; to Alyssa Pappa for manuscript and figure preparation.
Data Availability
All supplemental data can be found at: https://figshare.com/articles/Helicobacter_pylori_infection_and_low_dietary_iron_alter_behavior_induce_iron_deficiency_anemia_and_modulate_hippocampal_gene_expression_in_female_C57BL_6_mice/4584274.
Funding Statement
Research reported in this publication was supported by Office of The Director of the National Institutes of Health under award number T32OD010978, R01CA093405, P01CA028842-23 and National Institute of Environmental Health Sciences under award P30ES0022109. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (www.nih.gov).
References
- 1.Fuchs CS, Mayer RJ (1995) Gastric carcinoma. N Engl J Med 333: 32–41. 10.1056/NEJM199507063330107 [DOI] [PubMed] [Google Scholar]
- 2.Cover TL, Peek RM Jr. (2013) Diet, microbial virulence, and Helicobacter pylori-induced gastric cancer. Gut Microbes 4: 482–493. 10.4161/gmic.26262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansson LE, Nyren O, Hsing AW, Bergstrom R, Josefsson S, Chow WH, et al. (1996) The risk of stomach cancer in patients with gastric or duodenal ulcer disease. N Engl J Med 335: 242–249. 10.1056/NEJM199607253350404 [DOI] [PubMed] [Google Scholar]
- 4.Muhsen K, Cohen D (2008) Helicobacter pylori infection and iron stores: a systematic review and meta-analysis. Helicobacter 13: 323–340. 10.1111/j.1523-5378.2008.00617.x [DOI] [PubMed] [Google Scholar]
- 5.Suerbaum S, Michetti P (2002) Helicobacter pylori infection. N Engl J Med 347: 1175–1186. 10.1056/NEJMra020542 [DOI] [PubMed] [Google Scholar]
- 6.Amieva M, Peek RM Jr. (2016) Pathobiology of Helicobacter pylori-Induced Gastric Cancer. Gastroenterology 150: 64–78. 10.1053/j.gastro.2015.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee A, Fox JG, Otto G, Murphy J (1990) A small animal model of human Helicobacter pylori active chronic gastritis. Gastroenterology 99: 1315–1323. [DOI] [PubMed] [Google Scholar]
- 8.Eusebi LH, Zagari RM, Bazzoli F (2014) Epidemiology of Helicobacter pylori infection. Helicobacter 19 Suppl 1: 1–5. [DOI] [PubMed] [Google Scholar]
- 9.Kountouras J, Zavos C, Polyzos SA, Deretzi G (2015) The gut-brain axis: interactions between Helicobacter pylori and enteric and central nervous systems. Ann Gastroenterol 28: 506. [PMC free article] [PubMed] [Google Scholar]
- 10.McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B (2009) Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr 12: 444–454. 10.1017/S1368980008002401 [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization (WHO) (2001) Iron Deficiency Anaemia: Assessment, Prevention and Control, A guide for program managers. In: World Health Organization, editor.
- 12.Abbaspour N, Hurrell R, Kelishadi R (2014) Review on iron and its importance for human health. J Res Med Sci 19: 164–174. [PMC free article] [PubMed] [Google Scholar]
- 13.(2004) Comparative Quantification of Health Risks Global and Regional Burden of Disease Attributable to Selected Major Risk Factors, Volume 1 World Health Organization. [Google Scholar]
- 14.Beard J (2001) Iron-Deficiency Anemia: Reexamining the Nature and Magnitude of the Public Health Problem: Iron Biology in Immune Function, Muscle Metabolism and Neuronal Functioning. Journal of Nutrition 131: 568S–580S. [DOI] [PubMed] [Google Scholar]
- 15.Lozoff B, Beard J, Connor J, Barbara F, Georgieff M, Schallert T (2006) Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev 64: S34–43; discussion S72-91. 10.1301/nr.2006.may.S34-S43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lukowski AF, Koss M, Burden MJ, Jonides J, Nelson CA, Kaciroti N, et al. (2010) Iron deficiency in infancy and neurocognitive functioning at 19 years: evidence of long-term deficits in executive function and recognition memory. Nutr Neurosci 13: 54–70. 10.1179/147683010X12611460763689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lozoff B (2011) Early iron deficiency has brain and behavior effects consistent with dopaminergic dysfunction. J Nutr 141: 740S–746S. 10.3945/jn.110.131169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lozoff B, Jimenez E, Wolf AW (1991) Long-term developmental outcome of infants with iron deficiency. N Engl J Med 325: 687–694. 10.1056/NEJM199109053251004 [DOI] [PubMed] [Google Scholar]
- 19.Pisansky MT, Wickham RJ, Su J, Fretham S, Yuan LL, Sun M, et al. (2013) Iron deficiency with or without anemia impairs prepulse inhibition of the startle reflex. Hippocampus 23: 952–962. 10.1002/hipo.22151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tran PV, Fretham SJ, Carlson ES, Georgieff MK (2009) Long-term reduction of hippocampal brain-derived neurotrophic factor activity after fetal-neonatal iron deficiency in adult rats. Pediatr Res 65: 493–498. 10.1203/PDR.0b013e31819d90a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwik-Uribe CL, Gietzen D, German JB, Golub MS, Keen CL (2000) Chronic marginal iron intakes during early development in mice result in persistent changes in dopamine metabolism and myelin composition. J Nutr 130: 2821–2830. [DOI] [PubMed] [Google Scholar]
- 22.Yuan W, Li Y, Yang K, Ma B, Guan Q, Wang D, et al. (2010) Iron deficiency anemia in Helicobacter pylori infection: meta-analysis of randomized controlled trials. Scand J Gastroenterol 45: 665–676. 10.3109/00365521003663670 [DOI] [PubMed] [Google Scholar]
- 23.Qu XH, Huang XL, Xiong P, Zhu CY, Huang YL, Lu LG, et al. (2010) Does Helicobacter pylori infection play a role in iron deficiency anemia? A meta-analysis. World J Gastroenterol 16: 886–896. 10.3748/wjg.v16.i7.886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hudak L, Jaraisy A, Haj S, Muhsen K (2017) An updated systematic review and meta-analysis on the association between Helicobacter pylori infection and iron deficiency anemia. Helicobacter 22. [DOI] [PubMed] [Google Scholar]
- 25.Burns M, Muthupalani S, Ge Z, Wang TC, Bakthavatchalu V, Cunningham C, et al. (2015) Helicobacter pylori Infection Induces Anemia, Depletes Serum Iron Storage, and Alters Local Iron-Related and Adult Brain Gene Expression in Male INS-GAS Mice. PLoS One 10: e0142630 10.1371/journal.pone.0142630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.AVMA Panel on Euthanasia (2013) AVMA Guidelines for the Euthanasia of Animals: 2013 Edition Schaumburg, IL: American Veterinary Medical Association. [Google Scholar]
- 27.Romeo RD, Mueller A, Sisti HM, Ogawa S, McEwen BS, Brake WG (2003) Anxiety and fear behaviors in adult male and female C57BL/6 mice are modulated by maternal separation. Horm Behav 43: 561–567. [DOI] [PubMed] [Google Scholar]
- 28.Sheh A, Lee CW, Masumura K, Rickman BH, Nohmi T, Wogan GN, et al. (2010) Mutagenic potency of Helicobacter pylori in the gastric mucosa of mice is determined by sex and duration of infection. Proc Natl Acad Sci U S A 107: 15217–15222. 10.1073/pnas.1009017107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lozoff B, Georgieff MK (2006) Iron deficiency and brain development. Semin Pediatr Neurol 13: 158–165. 10.1016/j.spen.2006.08.004 [DOI] [PubMed] [Google Scholar]
- 30.Arnold IC, Lee JY, Amieva MR, Roers A, Flavell RA, Sparwasser T, et al. (2011) Tolerance rather than immunity protects from Helicobacter pylori-induced gastric preneoplasia. Gastroenterology 140: 199–209. 10.1053/j.gastro.2010.06.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogers AB, Taylor NS, Whary MT, Stefanich ED, Wang TC, Fox JG (2005) Helicobacter pylori but not high salt induces gastric intraepithelial neoplasia in B6129 mice. Cancer Res 65: 10709–10715. 10.1158/0008-5472.CAN-05-1846 [DOI] [PubMed] [Google Scholar]
- 32.Fox JG, Rogers AB, Whary MT, Ge Z, Ohtani M, Jones EK, et al. (2007) Accelerated progression of gastritis to dysplasia in the pyloric antrum of TFF2 -/- C57BL6 x Sv129 Helicobacter pylori-infected mice. Am J Pathol 171: 1520–1528. 10.2353/ajpath.2007.070249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siddappa AJ, Rao RB, Wobken JD, Casperson K, Leibold EA, Connor JR, et al. (2003) Iron deficiency alters iron regulatory protein and iron transport protein expression in the perinatal rat brain. Pediatr Res 53: 800–807. 10.1203/01.PDR.0000058922.67035.D5 [DOI] [PubMed] [Google Scholar]
- 34.Jorgenson LA, Wobken JD, Georgieff MK (2003) Perinatal iron deficiency alters apical dendritic growth in hippocampal CA1 pyramidal neurons. Dev Neurosci 25: 412–420. [DOI] [PubMed] [Google Scholar]
- 35.Fox JG, Wang TC, Rogers AB, Poutahidis T, Ge Z, Taylor N, et al. (2003) Host and microbial constituents influence Helicobacter pylori-induced cancer in a murine model of hypergastrinemia. Gastroenterology 124: 1879–1890. [DOI] [PubMed] [Google Scholar]
- 36.Evans TC, Jehle D (1991) The red blood cell distribution width. J Emerg Med 9 Suppl 1: 71–74. [DOI] [PubMed] [Google Scholar]
- 37.Fox JG, Rogers AB, Ihrig M, Taylor NS, Whary MT, Dockray G, et al. (2003) Helicobacter pylori-associated gastric cancer in INS-GAS mice is gender specific. Cancer Res 63: 942–950. [PubMed] [Google Scholar]
- 38.Prut L, Belzung C (2003) The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol 463: 3–33. [DOI] [PubMed] [Google Scholar]
- 39.Felt BT, Beard JL, Schallert T, Shao J, Aldridge JW, Connor JR, et al. (2006) Persistent neurochemical and behavioral abnormalities in adulthood despite early iron supplementation for perinatal iron deficiency anemia in rats. Behav Brain Res 171: 261–270. 10.1016/j.bbr.2006.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alvarez-Arellano L, Maldonado-Bernal C (2014) Helicobacter pylori and neurological diseases: Married by the laws of inflammation. World J Gastrointest Pathophysiol 5: 400–404. 10.4291/wjgp.v5.i4.400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lyte M, Li W, Opitz N, Gaykema RP, Goehler LE (2006) Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia Citrobacter rodentium. Physiol Behav 89: 350–357. 10.1016/j.physbeh.2006.06.019 [DOI] [PubMed] [Google Scholar]
- 42.Carlson ES, Stead JD, Neal CR, Petryk A, Georgieff MK (2007) Perinatal iron deficiency results in altered developmental expression of genes mediating energy metabolism and neuronal morphogenesis in hippocampus. Hippocampus 17: 679–691. 10.1002/hipo.20307 [DOI] [PubMed] [Google Scholar]
- 43.Beard JL, Murray-Kolb LE, Rosales FJ, Solomons NW, Angelilli ML (2006) Interpretation of serum ferritin concentrations as indicators of total-body iron stores in survey populations: the role of biomarkers for the acute phase response. Am J Clin Nutr 84: 1498–1505. [DOI] [PubMed] [Google Scholar]
- 44.Unger EL, Paul T, Murray-Kolb LE, Felt B, Jones BC, Beard JL (2007) Early iron deficiency alters sensorimotor development and brain monoamines in rats. J Nutr 137: 118–124. [DOI] [PubMed] [Google Scholar]
- 45.Carlson ES, Tkac I, Magid R, O'Connor MB, Andrews NC, Schallert T, et al. (2009) Iron is essential for neuron development and memory function in mouse hippocampus. J Nutr 139: 672–679. 10.3945/jn.108.096354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mazzaccara C, Labruna G, Cito G, Scarfo M, De Felice M, Pastore L, et al. (2008) Age-Related Reference Intervals of the Main Biochemical and Hematological Parameters in C57BL/6J, 129SV/EV and C3H/HeJ Mouse Strains. PLoS One 3: e3772 10.1371/journal.pone.0003772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan S, Noto JM, Romero-Gallo J, Peek RM Jr., Amieva MR (2011) Helicobacter pylori perturbs iron trafficking in the epithelium to grow on the cell surface. PLoS Pathog 7: e1002050 10.1371/journal.ppat.1002050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cassat JE, Skaar EP (2013) Iron in infection and immunity. Cell Host Microbe 13: 509–519. 10.1016/j.chom.2013.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Annibale B, Capurso G, Lahner E, Passi S, Ricci R, Maggio F, et al. (2003) Concomitant alterations in intragastric pH and ascorbic acid concentration in patients with Helicobacter pylori gastritis and associated iron deficiency anaemia. Gut 52: 496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hubbard AC, Bandyopadhyay S, Wojczyk BS, Spitalnik SL, Hod EA, Prestia KA (2013) Effect of dietary iron on fetal growth in pregnant mice. Comp Med 63: 127–135. [PMC free article] [PubMed] [Google Scholar]
- 51.Keenan JI, Peterson RA, Fraser R, Frampton CM, Walmsley TA, Allardyce RA, et al. (2004) The effect of Helicobacter pylori infection and dietary iron deficiency on host iron homeostasis: a study in mice. Helicobacter 9: 643–650. 10.1111/j.1083-4389.2004.00278.x [DOI] [PubMed] [Google Scholar]
- 52.Gobel R, Symonds EL, Kritas S, Butler RN, Tran CD (2006) Helicobacter felis infection causes an acute iron deficiency in nonpregnant and pregnant mice. Helicobacter 11: 529–532. 10.1111/j.1523-5378.2006.00455.x [DOI] [PubMed] [Google Scholar]
- 53.Thomson MJ, Pritchard DM, Boxall SA, Abuderman AA, Williams JM, Varro A, et al. (2012) Gastric Helicobacter infection induces iron deficiency in the INS-GAS mouse. PLoS One 7: e50194 10.1371/journal.pone.0050194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Noto JM, Gaddy JA, Lee JY, Piazuelo MB, Friedman DB, Colvin DC, et al. (2013) Iron deficiency accelerates Helicobacter pylori-induced carcinogenesis in rodents and humans. J Clin Invest 123: 479–492. 10.1172/JCI64373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beckett AC, Piazuelo MB, Noto JM, Peek RM, Washington MK, Algood HMS, et al. (2016) Dietary Composition Influences Incidence of Helicobacter pylori-Induced Iron Deficiency Anemia and Gastric Ulceration. Infection and Immunity 84: 3338–3349. 10.1128/IAI.00479-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ganz T (2011) Hepcidin and iron regulation, 10 years later. Blood 117: 4425–4433. 10.1182/blood-2011-01-258467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shanmugam NK, Ellenbogen S, Trebicka E, Wang L, Mukhopadhyay S, Lacy-Hulbert A, et al. (2012) Tumor necrosis factor alpha inhibits expression of the iron regulating hormone hepcidin in murine models of innate colitis. PLoS One 7: e38136 10.1371/journal.pone.0038136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cherian S, Forbes DA, Cook AG, Sanfilippo FM, Kemna EH, Swinkels DW, et al. (2008) An insight into the relationships between hepcidin, anemia, infections and inflammatory cytokines in pediatric refugees: a cross-sectional study. PLoS One 3: e4030 10.1371/journal.pone.0004030 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All supplemental data can be found at: https://figshare.com/articles/Helicobacter_pylori_infection_and_low_dietary_iron_alter_behavior_induce_iron_deficiency_anemia_and_modulate_hippocampal_gene_expression_in_female_C57BL_6_mice/4584274.