Abstract
Increased cardiovascular morbidity and mortality in patients with type 2 diabetes is well established; diabetes is associated with at least a 2-fold increased risk of coronary heart disease. Approximately two-thirds of deaths among persons with diabetes are related to cardiovascular disease. Previously, diabetes was regarded as a “coronary risk equivalent,” implying a high 10-year cardiovascular risk for every diabetes patient. Following the original study by Haffner et al., multiple studies from different cohorts provided varying conclusions on the validity of the concept of coronary risk equivalency in patients with diabetes. New guidelines have started to acknowledge the heterogeneity in risk and include different treatment recommendations for diabetic patients without other risk factors who are considered to be at lower risk. Furthermore, guidelines have suggested that further risk stratification in patients with diabetes is warranted before universal treatment. The Imaging Council of the American College of Cardiology systematically reviewed all modalities commonly used for risk stratification in persons with diabetes mellitus and summarized the data and recommendations. This document reviews the evidence regarding the use of noninvasive testing to stratify asymptomatic patients with diabetes with regard to coronary heart disease risk and develops an algorithm for screening based on available data.
Keywords: coronary CT, diabetes, echocardiography, exercise testing, nuclear imaging
Diabetes is increasing by epidemic proportions in the United States and throughout the world (1,2). Increased cardiovascular morbidity and mortality in patients with type 2 diabetes is well established; diabetes is associated with at least a 2-fold increased risk of coronary heart disease (CHD) and 2- to 4-fold increased risk of CHD and stroke mortality compared with patients without diabetes (3–5). Currently two-thirds of death among persons with diabetes is related to cardiovascular disease (CVD) (5). Furthermore, management strategies for diabetes have shifted from glucocentric to multi-factorial, to identify and target patients’ cardiovascular risk factors.
Formerly, diabetes was regarded as a “coronary risk equivalent,” implying a 10-year cardiovascular risk of >20% for every diabetes patient (6). This was based on an observational Finnish study by Haffner et al. (6), which showed that people with diabetes without prior myocardial infarction (MI) had a similar risk of CHD to those with MI but without diabetes. Following the original study by Haffner et al. (6), multiple studies from different population cohorts provided varying conclusions on the validity of the concept of coronary risk equivalency in patients with diabetes. Although some large observational studies supported the concept of coronary risk equivalency (7–10), several others did not (11–14). In a systematic review and meta-analysis, Bulugahapitiya et al. (15) included 13 studies involving 45,108 patients. The mean duration of follow-up was 13.4 years (range 5 to 25 years). Patients with diabetes without prior MI had a 43% lower risk of developing CHD compared with patients without diabetes with previous MI (summary odds ratio [OR]: 0.56; 95% confidence interval [CI]: 0.53 to 0.60). The results showed that patients with diabetes were at a lower risk of developing total CHD events compared with patients without diabetes with established CHD (15). New guidelines have started to acknowledge the heterogeneity in risk and include different treatment recommendations for diabetic patients without other risk factors who are considered to be at lower risk (16,17). Furthermore, guidelines have suggested that further risk stratification in patients with diabetes is warranted before universal treatment (18,19). An important clinical question remains: do patients with asymptomatic diabetes need routine screening for CHD, and if so, how? This document reviews the evidence regarding the use of noninvasive testing to stratify asymptomatic patients with diabetes with regard to CHD risk (Central Illustration).
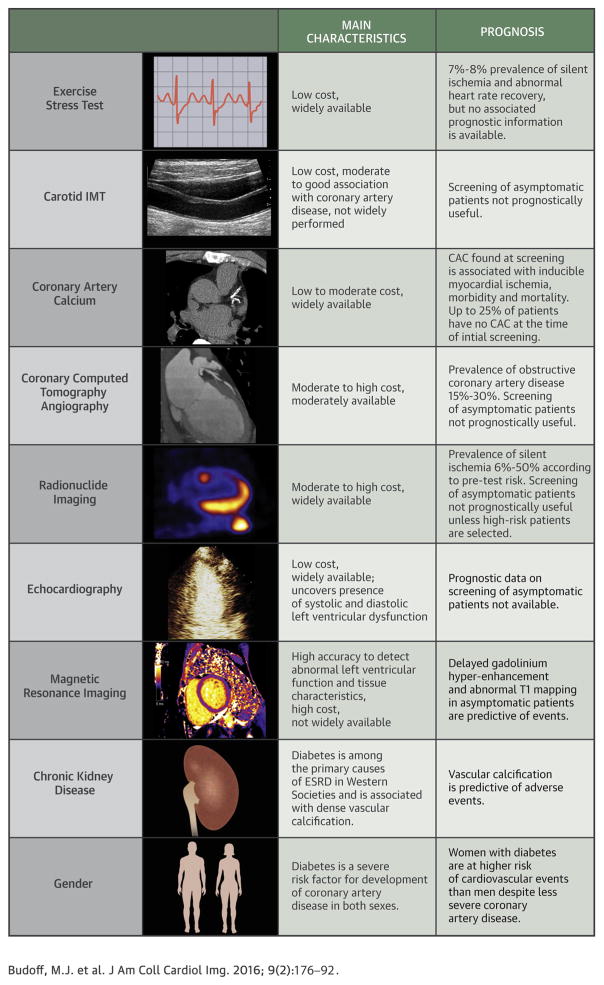
CENTRAL ILLUSTRATION. Approach to Risk Assessment of Diabetic Patients.
As discussed in the text several imaging modalities have been tested to risk stratify asymptomatic diabetic patients but few have provided valuable prognostic information. Diabetic women and diabetic patients with chronic kidney disease are at particularly high risk of cardiovascular complications. ESRD = end-stage renal disease; IMT = intima-media thickness.
1. ROLE OF EXERCISE STRESS TESTING
Exercise testing in patients with diabetes is attractive due to low cost, simplicity, and wide availability. Compared with nondiabetic patients, the goals of exercise testing in a diabetic population are more diverse than mere identification of obstructive coronary artery disease (CAD). In the Look AHEAD (Action for Health in Diabetes) study, Curtis et al. (20) sought to determine the prevalence and correlates of exercise-induced cardiac abnormalities on maximal graded exercise test in 5,783 overweight/obese asymptomatic middle-aged men and women with type 2 diabetes (20). The authors found that exercise-induced abnormalities (including ST-segment depression ≥1.0 mm, ventricular arrhythmia, angina pectoris, poor post-exercise heart rate recovery [<22 beats/min reduction 2 min after exercise], or maximal exercise capacity <5.0 METs) were present in 1,303 (22.5%) participants, of which 693 (12.0%) consisted of impaired exercise capacity. ST-segment depression occurred in 440 (7.6%) and abnormal heart rate recovery in 206 (5.0%). Among the potential predictors, only older age was associated with increased prevalence of all abnormalities. Turrini et al. (21) enrolled 520 moderate- to high-risk asymptomatic diabetic patients to undergo an open-label randomized trial: one-half of the patients were randomized to undergo an exercise stress test aimed at identifying obstructive CAD, and the other one-half were managed with pharmacological and behavioral therapy in the DADDY-D (Does coronary Atherosclerosis Deserve to be Diagnosed and treated early in Diabetics?) trial. They found silent ischemia in 7.6% of patients and concluded that screening and revascularization of silent CAD in patients with diabetes failed to demonstrate a significant reduction in cardiac events and HF episodes (22).
Banthia et al. (23) evaluated autonomic function and found that subjects with diabetes had a delayed heart rate recovery at 1 min after exercise completion (diabetes 18.5 ± 1.9 beats/min, control 27.6 ± 1.5 beats/min, p < 0.001). Similarly, Georgoulias et al. (24) found that diabetes is associated with abnormal heart rate recovery. Cheng et al. (25) found that heart rate recovery was an independent prognostic indicator for CVD and all-cause mortality in 2,333 men with documented diabetes. The adjusted hazard ratios (HRs) for cardiovascular death in men in the slowest quartile of heart rate recovery, compared to the first, second, and third quartiles were 2.0 (95% CI: 1.1 to 3.8), 1.5 (95% CI: 0.8 to 2.7), and 1.5 (95% CI: 0.9 to 2.8), respectively (p for trend <0.001).
Naka et al. (26) showed a prevalence of silent myocardial ischemia 2.2× higher in patients with diabetes mellitus (DM) that in nondiabetic control subjects (p < 0.05). Diabetic patients who received insulin had a 2.6× higher prevalence of silent myocardial ischemia, and patients with diabetic retinopathy had a 2.5× higher prevalence of silent myocardial ischemia (p < 0.05).
Thus, exercise stress testing can identify diabetic patients with silent ischemia; however, whether exercise testing results in improved outcomes in patients with diabetes has not been demonstrated.
2. CAROTID INTIMA-MEDIA THICKNESS
Carotid intima-media thickness (CIMT) has been tested as a surrogate marker of atherosclerosis and has been shown to be associated with incident coronary heart disease (27–30). CIMT had been deemed reasonable for cardiovascular risk assessment in asymptomatic adults who are at intermediate risk per the 2010 American College of Cardiology (ACC)/American Heart Association (AHA) guidelines and 2012 European Society of Cardiology guidelines, but this recommendation was dropped in the 2013 ACC/AHA guidelines due to results from several studies showing lack of a significant relationship with CHD events (17,19,31–33). In an analysis of MESA (Multi-Ethnic Study of Atherosclerosis) participants with metabolic syndrome and diabetes, Malik et al. (34) showed that diabetic patients with a CIMT in the fourth quartile had no significant increase in cardiovascular events (HR: 1.7, 95% CI: 0.7 to 4.3) compared with those in the first quartile.
3. CORONARY ARTERY CALCIUM
Histological studies have shown that the extent of coronary artery calcium (CAC) is closely associated with total coronary artery atherosclerotic plaque burden (35). Furthermore, numerous studies have conclusively proven that CAC scores predict incident CAD in the general population (36,37). Patients affected by type 2 DM harbor larger amounts of CAC than nondiabetic patients of a similar age (38); additionally the extent (39,40) and prevalence (41) of CAC in patients with type 2 diabetes asymptomatic for CAD is similar to that of patients with established CAD but without diabetes. Unlike the general population, women and men with type 2 DM have a similar extent of CAC, confirming the clinical evidence that diabetes negates the well-known advantage of women over men in prevalence and extent of atherosclerosis (39,41).
In the CACTI (Coronary Artery Calcification in Type 1 Diabetes) study (42), 656 adult type 1 DM patients showed a higher prevalence and extent of CAC than 764 age- and sex-matched control subjects with no difference between sexes. Extensive vascular calcification is detectable even in young (17 to 28 years of age) adults with type 1 diabetes (43) and has been associated with factors such as genetic polymorphism for hepatic lipoxygenase (LIPC-480 T) (44), smoking, elevated serum lipoprotein(a) (32), or suboptimal glycemic control (44,45).
ASSOCIATIONS OF CAC WITH ISCHEMIA AND PREDICTION OF CARDIAC EVENTS
The extent of CAC has been shown to be associated with the prevalence of inducible ischemia by single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) (46,47). In nondiabetic patients, the CAC score threshold at which the prevalence of ischemia increases substantially is >400 (46,47). In contrast, in diabetic patients this threshold has been reported to be lower (48). Several studies (Table 1) have demonstrated that increased CAC in persons with metabolic syndrome and diabetes is associated with increased prevalence of ischemia (48), events (34,49,50), and mortality (51). CAC was a better predictor of incident cardiovascular events (85 events after 8.5 years of follow-up) compared with the Framingham risk score and the UKPDS (United Kingdom Prospective Diabetes Study) (area under the curve 0.76, 0.70, and 0.69, respectively; all p < 0.05). Additionally, it improved classification of risk compared with the Framingham risk score (net reclassification index [NRI]: 0.19) and UKPDS (NRI: 0.21). In an observational study of 2,384 patients with diabetes of whom 162 died after a follow-up of 5.6 ± 3 years, Silverman et al. (52) reported that CAC allowed identification of patients at lower risk for whom aspirin preventive treatment might not be beneficial.
TABLE 1.
Studies of CAC Scanning in Asymptomatic Patients With Diabetes
| First Author (Ref. #) | Primary Outcome | Patients | Main Results | Additional Notes |
|---|---|---|---|---|
| Wong et al. (48) | Inducible ischemia on functional stress testing | 1,043 asymptomatic patients submitted to MPI and CAC screening; 140 type 2 diabetes and 173 metabolic syndrome patients | In patients with a CAC score 100–399, the rate of ischemia was 13% vs. 3.6% in the presence or absence of metabolic abnormalities (p < 0.02). In patients with CAC score >400, the rates were 23.4% vs. 13.6% (p = 0.03) | The odds of ischemia in patients with metabolic abnormalities were 2-fold greater per SD increase in log CAC score |
| Anand et al. (49) | Inducible ischemia on functional stress testing and cardiovascular outcomes | 510 asymptomatic type 2 diabetes patients. MPI performed in 127 with CAC score >100 | During 2.2 years of follow-up, there were 20 cardiovascular events, none in patients with CAC score <10 | CAC score and MPI interaction term was statistically significant for prediction of events |
| Raggi et al. (51) | All-cause mortality | 9,474 nondiabetic patients and 903 type 2 diabetes patients followed for 5 years after CAC screening | 44% higher risk of death for diabetic patients compared with control subjects in each category of CAC score (10, 11–100, 101–400, 401–1,000, and >1,000) | Diabetic patients with CAC <10 had the same mortality rate as control subjects with CAC score <10 |
| Malik et al. (34) | Incident coronary heart disease events | 6,603 MESA patients 45–84 years old; 1,686 with the metabolic syndrome and 881 with diabetes mellitus type 2 | Race and risk factors adjusted HR: 2.9–6.2 for type 2 diabetes patients and 3.9–11.9 for metabolic syndrome patients with increasing CAC score categories | CAC had incremental prognostic value over traditional risk factors and carotid IMT |
| Yeboah et al. (50) | Incident coronary heart disease events | 1,343 type 2 diabetes mellitus patients from MESA and Heinz-Nixdorf-Recall studies | 85 events after 8.5 years of follow-up (6.3%). CAC was a better predictor than FRS and UKPDS (AUC: 0.76, 0.70, and 0.69, respectively, all p < 0.05) | CAC improved discrimination of risk compared to the FRS (NRI; 0.19) and UKPDS (NRI: 0.21). |
| Raggi et al. (61) | Retrospective study; occurrence of myocardial infarction and progression of CAC | 157 type 2 diabetes mellitus patients and 1,153 nondiabetic patients followed for 1–3 years | Diabetic patients with and without myocardial infarction during follow-up showed a greater CAC score increase compared with control subjects | Diabetes mellitus and systemic hypertension were the best predictors of CAC score progression. Baseline CAC score and statin therapy were the best predictors of myocardial infarction. |
| Kiramijyan et al. (62) | CAC score progression and all-cause mortality | 296 asymptomatic type 2 diabetic patients and 300 control subjects followed for ~4.5 years | HR of death in diabetic patients compared with nondiabetic patients increased from 1.88 to 6.95 as the annual percent change in CAC score increased from <10% to >30% | Annual %CAC score increase was greater in diabetic patients (29 + 9% vs. 10 + 7%; p = 0.0001) |
| Wong et al. (58) | Incidence and progression of CAC; occurrence of coronary heart disease events | 2,927 MESA subjects without CAC at baseline and 2,735 subjects with CAC at baseline. 1,426 patients had the metabolic syndrome, 198 patients had type 2 diabetes mellitus, and 510 patients had both. Follow-up 4.9 ± 1.3 years | Patients with metabolic disorders had higher incidence and greater progression of CAC score. Patients with metabolic disorders in the highest tertile of CAC progression had a 4- to 4.9-fold higher risk of coronary heart disease events |
AUC = area under the curve; CAC = coronary artery calcium; FRS = Framingham risk score; HR = hazard ratio; IMT = intima-media thickness; MESA = Multi-Ethnic Study of Atherosclerosis; MPI = myocardial perfusion imaging; NRI = net reclassification index; UKPDS = United Kingdom Prospective Diabetes Study.
A high proportion of adults with diabetes have a 0 or very low CAC score, which, along with the excellent prognosis of these patients, provides strong evidence that diabetes per se is not a CHD equivalent. The study by Raggi et al. (51) was the first to document a prevalence of 39% of 0 or very low (<10) CAC in asymptomatic DM patients. In MESA (53), 38% of DM patients were reported to have a CAC score of 0, and in the HNR (Heinz Nixdorf Recall) study, 39.3% of women with diabetes and 13.4% of men with diabetes had a CAC score of 0 (50). Importantly, the absence of CAC predicted a low short-term risk of death (~1% at 5 years) for diabetic patients, which was slightly higher but statistically similar to that of nondiabetic patients.
Sequential CAC imaging has been implemented as a means to assess atherosclerosis progression. The MESA and HNR investigators reported that all traditional risk factors are associated with CAC progression in whites, Asians, Hispanics, and blacks (53–58). However, DM had a stronger association with CAC in blacks than in other races in MESA (50,57). Snell-Bergeon et al. (45) assessed the effect of glycemic control in 109 type 1 DM patients on progression of CAC. On sequential electron beam computed tomography scans performed at an interval of 2.7 years, progression of CAC was associated with baseline hyperglycemia (OR: 7.11; 95% CI: 1.38 to 36.6; p = 0.02). Similarly, Anand et al. (59) followed 392 type 2 diabetic patients and reported that the best predictors of progression were baseline CAC score, statin use, and hemoglobin A1c >7% during follow-up. Table 1 outlines several studies that have demonstrated that progression of CAC is a strong predictor of future MI (57,60–62).
In summary, CAC provides strong risk stratification of patients with diabetes, with an increase in mortality for each increase in CAC score category. The mortality risk is higher for each CAC category in the patients with diabetes than in the patients without diabetes. However, about 40% of adult diabetic patients have a CAC score <10 and a very low mortality rate. Rapid progression of CAC identifies patients at higher risk for CHD events. The overall evidence would support the use of CAC scanning for risk stratification and to guide management in the asymptomatic DM patient, as recommended with a Class IIa indication in the 2010 AHA/ACC guidelines (19).
4. ECHOCARDIOGRAPHY IN PATIENTS WITH DM
Myocardial abnormalities in DM patients at rest and with exercise have been well described in animal and human studies. Many clinical and epidemiological studies have suggested the presence of diabetic cardiomyopathy in humans. The increased incidence of heart failure in diabetic patients is more than what can fully be explained by obstructive CAD and traditional risk factors (63). Damage often occurs at the microvascular level, which then leads to fibrosis and subsequent systolic and diastolic dysfunction (64). Several studies have demonstrated an independent association between diabetes and left ventricular (LV) mass and wall thickness and reduced LV systolic function (65,66). Di Cori et al. (67) evaluated 40 asymptomatic patients with type 1 DM <40 years of age using strain, strain rate, and integrated backscatter and demonstrated subclinical dysfunction (67). Ha et al. (68) showed the value of tissue Doppler indexes at rest and with exercise for unmasking subclinical myocardial dysfunction.
The prevalence of diastolic dysfunction is also greater in diabetic patients than in normal individuals and has been reported to be between 43% to 78% (69–71). The wide range can be explained by differences in age, duration of diabetes, and other comorbidities in the study populations. Left atrial (LA) enlargement and dysfunction (based on speckle tracking–derived strain and Doppler values) have also been reported in diabetic patients. LA fibrosis may subsequently be responsible for abnormal LA function in this population (71).
Several studies have demonstrated the prognostic value of stress echocardiogram in the DM population. Marwick et al. (72) studied 937 DM patients with known or suspected CAD who underwent stress echocardiography or dobutamine stress echocardiography (DSE). Regression analysis showed that the presence and extent of resting LV dysfunction and ischemia were predictive of death. The strongest predictor of death was referral for DSE rather than exercise. The total mortality of persons with DM with a negative test was ~4%/year, which is higher than the 1%/year previously reported for unselected patients. Most of the excess in mortality was seen in the patients undergoing DSE, possibly due to higher comorbidities and risk factor burden in patients referred for pharmacological stress testing. The risk of death in patients with ischemia correlated with the extent of ischemia (72). In 563 DM patients, Elhendy et al. (73) showed similar results. Those with an abnormal stress echocardiography experienced a higher event rate than those with a normal test at 1 year (2% vs. 0%), 3 years (12% vs. 2%), and 5 years (23% vs. 8%). No events were recorded in the first 2 years of follow-up among patients with a normal stress echocardiography. However, at 3 and 5 years, the event rate for the normal group was 2% and 8%, respectively (73). Similarly, Cortigiani et al. (74) evaluated 5,456 patients (749 diabetic) who underwent dipyridamole or dobutamine stress echocardiography. After a median of 31 months, stress-induced ischemia, resting wall motion score index, and age were independent predictors of death and hard events in both diabetic and nondiabetic patients. However, diabetic patients with a normal stress test had a 2-fold greater annual event rate during follow-up compared with nondiabetic patients (74). These 2 studies illustrate the short warranty period for DM in the setting of a normal stress test.
Newer echocardiographic tools such as strain and strain rate now allow us to detect systolic and diastolic dysfunction at the subclinical level. Both pharmacological and exercise stress echocardiography provide excellent prognostic information in the diabetic population. However, the late event rate is higher than the nondiabetic population even in those with a normal stress test, which serves as a reminder of the rapid progression and high-risk nature of diabetic CAD.
5. RADIONUCLIDE IMAGING OF THE HIGH-RISK ASYMPTOMATIC DIABETIC PATIENT
The large volume of data supporting the prognostic value of gated SPECT MPI renders it particularly appealing for screening asymptomatic DM patients (75). The elevated cardiovascular risk in the DM patient alters the post-test risk assessment after SPECT MPI in 2 important ways. First, a normal myocardial perfusion scan in DM patients is associated with a slightly higher risk of adverse cardiac events (1.6%/year) compared with nondiabetic patients (<1%/year) (76). Second, the warranty period of a normal MPI is lower in the DM patient, with event-free survival curves beginning to diverge from nondiabetic populations in the second year after the index normal scan (49,77).
The reported prevalence of silent myocardial ischemia on radionuclide imaging in DM patients has been disparate among studies. Observational studies performed more than a decade ago reported a prevalence ranging from 16% to 59%, with approximately 20% of patients having high-risk findings (78,79). The DIAD (Detection of Ischemia in Asymptomatic Diabetics) study, which was prospective and recruited truly asymptomatic patients, reported a much lower prevalence of any perfusion defect or LV function abnormality (22%) or moderate to large ischemia (6%), and may represent the true prevalence of asymptomatic myocardial ischemia in DM patients (80). A more recent analysis of 1,354 patients (302 with diabetes) without angina or dyspnea who underwent clinically indicated stress testing revealed a low prevalence of any myocardial ischemia (7.2%) or prognostically significant ischemia (4.4%) in the overall study population. The prevalence of asymptomatic ischemia was significantly higher in patients with diabetes (12.5% vs. 5.6%) compared with those without (81).
The yield of stress testing in asymptomatic DM patients can be improved by selecting patients based on the pre-test clinical risk of CAD. The retrospective studies that showed a high prevalence of stress test abnormalities included patients with abnormal electrocardiograms (ECGs) (43% with Q waves) and vascular disease (28%). A high CAC score is predictive of moderate to severe silent myocardial ischemia on SPECT in diabetic patients (48% and 71% ischemia if CAC score >400 and >1,000, respectively) (49). It is noteworthy that depending on age and duration of diabetes, between one-third and one-half of asymptomatic DM patients have no or minimal (<10 AU) CAC (49).
The recent BARDOT (Basel Asymptomatic High-risk Diabetes Outcome) trial (82) prospectively recruited 400 asymptomatic patients with type 2 diabetes. Baseline SPECT MPI was abnormal in 22% (Table 2). Patients randomized to revascularization had similar rates of major adverse cardiovascular events (symptomatic CAD progression), but lower rates of asymptomatic CAD (more ischemia or new scar) progression (54.3% vs. 15.8%; p < 0.001) Thus, in this prospectively recruited cohort of high-risk diabetic patients, almost one-quarter had silent myocardial ischemia, which was associated with a worse outcome.
TABLE 2.
Studies of Nuclear Imaging in Asymptomatic Patients with Diabetes
| First Author (Ref. #) | Primary Outcome | Patients | Main Results | Additional notes |
|---|---|---|---|---|
| Acampa et al. (76) | Nonfatal myocardial infarction and cardiac death in symptomatic and asymptomatic diabetic patients submitted to MPI | Meta-analysis of 14 studies involving 13,493 diabetic patients. Average follow-up 36.2 months | Annual event rate in patients with a negative MPI averaged 1.6% | Reported annual event rate with negative MPI in nondiabetic patients <1% |
| Giri et al. (77) | Death, nonfatal myocardial infarction, and revascularization | Prospective observational study of 929 diabetic and 3,826 nondiabetic patients. Mean follow-up 2.5 years | Event rate in diabetes patients 8.6% vs. 4.5% in nondiabetic patients. Diabetic patients with reversible defects had the highest risk of myocardial infarction, and those with fixed perfusion defects had the highest risk of death | In the presence of a normal MPI, the risk of event started climbing in diabetic patients after 2 years from imaging |
| Wackers et al. (80) (DIAD study) | Prevalence of silent ischemia in asymptomatic type 2 diabetic patients | 522 patients 50–75 years of age submitted to adenosine stress MPI | 113 (22%) had inducible ischemic changes; 33 (6%) had moderate-to-large ischemia on MPI | American Diabetic Association guidelines would have missed 41% of patients with silent ischemia |
| Zellweger et al. (82) | Prevalence, progression, and outcome of silent coronary artery disease in diabetes mellitus | 400 patients with type 2 diabetes mellitus submitted to baseline and repeat MPI after 2 years | Baseline MPI was abnormal in 22% of patients. Patients with abnormal MPI experienced more cardiac deaths, myocardial infarctions, and revascularizations (9.8% vs. 2.9%) than patients with normal MPI. | Ischemia or new scar appeared in 3.2% and 34.2% of patients with normal and abnormal baseline MPI, respectively. Patients randomized to revascularization had similar rates of major adverse cardiovascular events but lower rates of asymptomatic CAD (more ischemia or new scar) progression |
Abbreviations as in Table 1.
In summary, analogous to the general population, the data in DM suggest that routine screening with MPI of all asymptomatic patients is likely to have a low yield and have a limited effect on patient outcome. The yield of MPI can be improved by selecting a higher-risk group of patients with symptoms, peripheral vascular disease, CKD, an abnormal ECG, or a high CAC score (e.g., >400) (83,84). In such patients, intense medical therapy appears to retard progression of asymptomatic and symptomatic CAD (72). Whether coronary revascularization offers additive prognostic benefit to medical therapy when the ischemic burden exceeds any particular threshold is still unclear for the asymptomatic diabetic population.
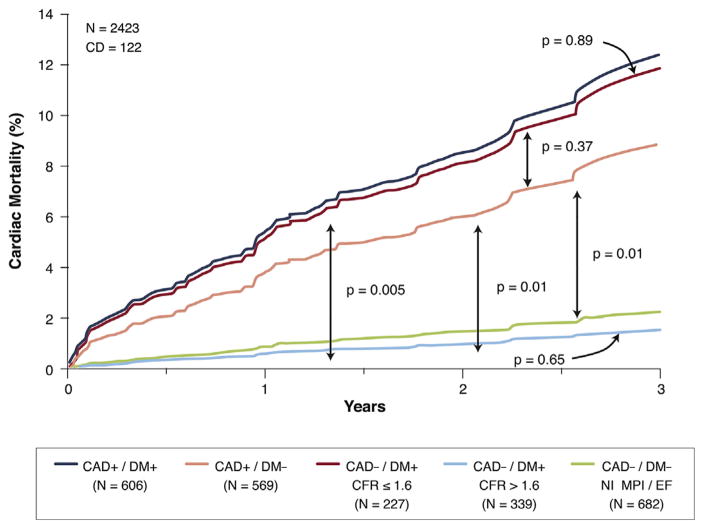
The noninvasive assessment of coronary flow reserve (CFR) using positron-emission tomography is a powerful tool that integrates the effects of focal stenosis, diffuse disease, and coronary microvascular function. A recent study by Murthy et al. (85) addressed the potential added prognostic value of this approach in patients with diabetes. They found an equivalent and low cardiac mortality risk between diabetic patients without known CAD (prior revascularization or MI) but with CFR >1.6 and those without diabetes. In contrast, the subgroup of diabetic patients without known CAD but with CFR <1.6 had essentially the same risk as patients without diabetes but with CAD (Figure 1).
FIGURE 1. Annualized Cardiac Mortality Among Patients With DM or CAD (History of Myocardial Infarction or Coronary Revascularization).
Diabetes mellitus (DM) patients without coronary artery disease (CAD) and with coronary flow reserve (CFR) <1.6 have the same risk as patients without DM or CAD. In contrast, diabetic patients with CFR <1.6 have essentially the same risk as those with CAD. Reprinted with permission from Murthy et al. (85).
6. CORONARY CTA FOR RISK STRATIFICATION OF THE ASYMPTOMATIC DIABETIC PATIENT
The advent of coronary CTA has made possible the noninvasive investigation of atherosclerotic disease. This has piqued the interest of researchers in applying this technology to better define CHD risk in patients with diabetes. The use of coronary CTA in asymptomatic diabetic patients has already been reported in numerous studies. The overall conclusion from these studies is that patients with diabetes have a high prevalence of coronary atherosclerosis and obstructive CAD, as well as a higher prevalence of plaques with features of instability compared with nondiabetic subjects.
PREVALENCE OF CORONARY ATHEROSCLEROSIS AND OBSTRUCTIVE STENOSES
Table 3 summarizes studies published to date that reported the prevalence of plaque and obstructive CAD in asymptomatic patients submitted to coronary CTA. Approximately 25% to 30% of asymptomatic diabetic patients have no demonstrable plaque on coronary CTA. The proportion of patients having ≥50% stenosis generally ranged from 24% to 32%, with 1 outlying study reporting 17%.
TABLE 3.
Coronary CTA Findings in Asymptomatic Patients With Diabetes
| First Author (Ref. #) | Primary Outcome | Patients | Main Results | Additional Notes |
|---|---|---|---|---|
| Kamimura et al. (91) | Prevalence of obstructive CAD and high-risk plaques in patients with a CAC score ≤400 | Asymptomatic diabetic patients (mean age 65 years, 75% men) | A luminal stenosis >50% was present in 30.5% of patients; high-risk plaques in 17% of the patients. | CAC was present in 83% of the patients. Obstructive CAD was seen in 5% of patients with a CAC score = 0 |
| Roos et al. (93) | Prevalence of obstructive CAD and CAC | Cross-sectional analysis of 120 South-Asian and 120 Caucasian diabetic patients (mean age 53 years, 77% men) | South-Asian patients had a higher prevalence of obstructive CAD (41% vs. 28%; p = 0.008) | The prevalence of CAC and the Agatston scores were significantly higher in South-Asian patients |
| Halon et al. (96) | Prevalence of obstructive CAD in asymptomatic type 2 diabetic patients and correlation with increased pulse pressure | 477 patients, age 55–74 years, 58% women | Any coronary atheroma was present in 76.6% of patients, and multivessel coronary atheroma in 55%. Obstructive CAD was present in 22.9% of patients | Pulse pressure correlated with extent of atheroma (p = 0.005). The correlation was independent of Framingham and United Kingdom Prospective Diabetic Study risk scores |
| Park et al. (146) | Composite outcome of cardiac death, nonfatal myocardial infarction, acute coronary syndrome requiring hospitalization, or late revascularization | 577 patients (mean age 62 years, 59% men) submitted to CTA and followed for an average of 34 ± 8 months | 19 cardiac events during follow- up. Patients with significant CAD had more cardiac events (7.1% vs. 0.5%) and lower 3-year event-free survival than those without (99.2% vs. 90.9%; p < 0.001) | Obstructive CAD was detected in 30.5% of patients; 26.7% had obstructive disease of the left main (2%) or proximal left anterior descending coronary artery (24.7%) |
| Muhlestein et al. (102) (FACTOR 64 Study) | Composite outcome of all-cause mortality, nonfatal MI, or unstable angina requiring hospitalization | 900 patients with type 1 or 2 diabetes mellitus for 3 to 5 years randomized to CTA screening or optimal medical management alone; follow-up 4 ± 1.7 years | The primary outcome was not significantly different between the CTA and the control groups (6.2% [28 events] vs 7.6% [34 events]; HR: 0.80 [95% CI: 0.49–1.32]; p = 0.38) | The secondary outcome (composite of CAD death, nonfatal MI, or unstable angina) was also not statistically different (4.4% [20 events] vs. 3.8% [17 events]; HR: 1.15 [95% CI: 0.60–2.19]; p = 0.68) |
| Scholte et al. (147) | Prevalence of CAC, ischemia on MPI, and obstructive CAD on CTA | 100 asymptomatic patients (age 30 to 72 years) with type 2 diabetes mellitus | Obstructive CAD by CTA was found in 24% of patients; however, the correlation between CAC, CTA, and MPI findings was poor | An abnormal MPI was found in 23% of patients, CAC in 60%, and plaque on CTA in 70% of the patients |
These findings further support the concept that diabetes by itself is not a CHD equivalent, because one-third of patients have no demonstrable coronary atherosclerosis (49,51). At the same time, the prevalence of obstructive CAD is nearly 25%, much higher than would be expected in the nondiabetic population (86–100). Halon et al. (96) examined 427 asymptomatic diabetic patients and found coronary plaques in 77% of them and ≥50% stenosis in 1 or more vessels in 23%.
PREDICTION OF CARDIAC EVENTS
Despite a prevalence of atherosclerotic plaques in nearly 70% of diabetic patients, the event rate in diabetic patients with no or minimal plaque burden is very low (Table 3).
Min et al. (101) reported the occurrence of all-cause mortality, nonfatal MI, or late revascularization among 400 asymptomatic diabetic patients in the CONFIRM (COronary CT Angiography Evaluation For Clinical Outcomes: An International Multicenter Registry) registry. After a mean follow-up of 2.4 ± 1.1 years, 33 events occurred (3.4% annualized event rate). On multivariable analysis, the presence of obstructive disease on coronary CTA added to the CAC score and age in prediction of adverse events (p < 0.001).
RANDOMIZED TRIAL RESULTS
The FACTOR-64 (For Asymptomatic Obstructive Coronary Artery Disease Among High-Risk Diabetic Patients Using CT Angiography, Following Core 64: A Randomized Control Study) trial was a randomized trial to evaluate whether routine coronary CTA screening in a high-risk population affects changes in treatment (such as pre-emptive coronary revascularization or more aggressive medical therapy) and leads to a reduction in cardiac events (102). High-risk, asymptomatic patients with diabetes were randomized to either screening with coronary CTA with subsequent therapy directed by the imaging results or to standard treatment. The investigators randomized 900 patients to CT screening (n = 452) or standard care (n = 448). CTA showed no CAD in 31%, mild stenosis in 46%, moderate in 12%, and severe stenosis in 11% of the patients. Coronary CTA prompted a stress test in 14% of the cases, and angiography in 8%, of whom 53% underwent subsequent PCI and 19% underwent CABG. There was a 20% lower rate of events in the CTA group: for the primary endpoint of all-cause death, nonfatal MI, and hospitalization for unstable angina, the rate was 6.2% in the CTA arm versus 7.6% in the standard-care group (HR: 0.80 [95% CI: 0.49 to 1.32]; p = 0.38). The authors concluded that coronary CTA screening led to more aggressive risk factor modification in 70% of patients, including improvements in statin use and more aggressive treatment of serum lipids and systemic blood pressure; however, there was no significant reduction in CHD events in this 900-person study. A longer follow-up is underway.
RELATIONSHIP BETWEEN CORONARY CTA AND ISCHEMIA BY SPECT
Choi et al. (88) performed coronary CTA and SPECT in 116 asymptomatic diabetic patients. Interestingly, when comparing the 28 (24%) patients with perfusion defects to the 88 (76%) without defects, they found no difference in prevalence of coronary atherosclerosis, obstructive disease and severe stenosis, plaque composition, or high (>100) CAC score. In this small study, 5 patients had cardiac events during a mean follow-up of 24 months. All had obstructive CAD by coronary CTA, but normal SPECT MPI studies.
Coronary CTA is currently not recommended and is not considered appropriate as a risk stratification tool in the asymptomatic diabetic population (25,103). As noted in Table 3, studies to date have shown clear heterogeneity within this population, with varying degrees of disease prevalence and severity. Hence, a “1 size fits all” approach seems unrefined. Perhaps an approach that would identify a population at higher risk through age, risk factor, or biomarker criteria, coupled with more widespread use of methods that are associated with radiation exposure as low as those associated with CAC scanning, may define a subset of asymptomatic patients with diabetes in whom coronary CTA scanning will become accepted as an appropriate test.
7. CARDIAC MAGNETIC RESONANCE IMAGING
MYOCARDIAL DYSFUNCTION
Diabetic patients are at risk of developing severe cardiomyopathy based both on CAD and mechanisms related to metabolic deregulations even in the absence of CAD. Cardiac magnetic resonance (MR) provides an accurate means to assess myocardial structure and function. With steady-state free precession sequences, investigators have been able to show substantial aberrations of LV volume, mass, and function in patients with insulin resistance and type 2 (104) and type 1 DM (105).
The presence of late gadolinium hyperenhancement as a marker of prior MI in diabetic patients with unsuspected CAD has been linked with a 4-fold increased risk of major adverse cardiovascular events and a 7-fold increased risk of mortality (95). Late gadolinium hyperenhancement was demonstrated in 4.3% of asymptomatic type 1 diabetic patients in the DCCT (Diabetes Control and Complications Trial)/EDIC (Epidemiology of Diabetes Interventions and Complications) trial (105) and in 17% of asymptomatic older diabetic patients in a community-based study conducted in Iceland (106,107).
T1 mapping is a newer technique that takes advantage of enhanced T1 relaxation times induced by gadolinium accumulated in the extracellular space. This allows an assessment of interstitial fibrosis, also described as extracellular volume (ECV) fraction. Employing T1-mapping, Wong et al. (108) demonstrated a higher short-term mortality in patients with increased ECV. Although similar outcome data are not available for diabetic patients, 2 recent publications showed that ECV is increased in these patients (109,110) and inversely related to diastolic function (110).
Although MR spectroscopy is still investigational, it has provided useful insights into the pathophysiology of diabetic cardiomyopathy. Using 1H spectroscopy, Rijzewijk et al. (111) demonstrated a higher myocardial content of triglycerides in type 2 diabetic patients asymptomatic for CAD than in age-, sex-, and BMI-matched healthy volunteers. The increase in triglycerides content was directly correlated with abnormalities of LV diastolic function (111). Ng et al. (112) further demonstrated an association between myocardial triglyceride content in healthy diabetic patients and myocardial strain assessed by echocardiography. Parameters of ventricular function and myocardial steatosis improved after prolonged caloric restriction in obese diabetic patients in 1 prospective study (113).
CAD DETECTION
Adenosine stress MR MPI has been shown to have good to excellent test characteristics for the detection of obstructive CAD (>70% luminal stenosis) in the general population and in diabetic patients (sensitivity 88%; specificity 82%; PPV 90%; and NPV 79%) (114,115). It should be noted that both stress MR studies (114,115) enrolled a small number of diabetic patients (8 of 92 patients: 8.6%; and 96 of 752 patients: 13% of the population); therefore, care should be taken before drawing definitive conclusions based on these preliminary studies.
8. GENDER DIFFERENCES IN PATIENTS WITH DM
DM is a major risk factor and predictor of adverse coronary events in both men and women. Women with DM in particular are at an increased risk of cardiovascular events. The duration of diabetes and presence of prior CAD influences the risk of death. In a study by Hu et al. (9), the relative risk of fatal CHD increased in an incremental fashion according to the duration of disease. The relative risk of cardiovascular death for diabetic versus nondiabetic women for all durations of diabetes (<5, 6 to 10, 11 to 15, 16 to 25, and >25 years) was 2.75, 3.63, 5.51, 6.38, and 11.9, respectively (p < 0.001 for trend). However, a combination of DM >15 years and prior CAD identified the highest-risk group for fatal cardiac events (relative risk: 30.0) (9).
The presence of the metabolic syndrome also appears to increase the risk of cardiovascular events in women (116). In the WISE (Women’s Ischemia Syndrome Evaluation) study, women with angiographically significant CAD and the metabolic syndrome had significantly higher risk of cardiovascular events than those with normal metabolic status (HR: 4.93; 95% CI: 1.02 to 23.76; p < 0.05) (117). This increased risk in women with diabetes and metabolic syndrome may be due, at least in part, to an increased prevalence of other cardiovascular risk factors such as lipid disorders, hypertension, obesity, and physical inactivity, as well as more endothelial dysfunction (118). The metabolic syndrome and diabetes are also associated with systemic inflammation and a hypercoagulable state (117).
Similar to nondiabetic women, diabetic women tend to present with less obstructive coronary disease compared with their male counterparts. The BARI 2D trial showed that there were no sex differences in death, MI, or cerebrovascular accident among patients enrolled in the trial (119).
Cardiac imaging studies in women have further illustrated sex differences in diabetic patients and in those with metabolic syndrome. In an echocardiographic study by Nicolini et al. (120), women with metabolic syndrome had higher posterior wall thickness, relative wall thickness, and concentric LV hypertrophy, and more advanced diastolic abnormalities. Such differences were not seen in men with or without metabolic syndrome (120). Stress testing studies with cardiac imaging in symptomatic and asymptomatic diabetic women have demonstrated less extensive ischemia compared with men (80,121). Despite smaller perfusion defects, the prognosis for women with DM is worse compared with men for similar summed stress scores on adenosine perfusion SPECT imaging (121).
In summary, the risk of cardiovascular events is significantly increased in women with DM and with metabolic syndrome (122). Similar to nondiabetic women, women with DM have a lesser burden of coronary atherosclerosis, thus leading to less ischemia on stress testing. Despite the smaller burden of disease, the symptoms and prognosis are worse than in matched male counterparts.
9. DIABETES AND CKD
DM is a common etiology for CKD, and both are contributors to the development of CVD. CKD is defined by elevated urinary albumin excretion, renal pathology, or an estimated glomerular filtration rate <60 ml/min/1.73 m2 persisting for at least 3 months. Multiple pathways, involving both the microcirculation and macrocirculation, are involved in the association among DM, CKD, and CVD. Diabetes affects renal microcirculation by inducing glomerular capillary hypertension, mesangial, and endothelial cell dysfunction, which can result in a form of CKD, diabetic nephropathy (123–125). Once CKD develops, macrovascular complications of diabetes are further promoted by various mechanisms, including systemic hypertension, dyslipidemia, chronic inflammation, and vascular calcification (126,127). DM and CKD interact with each other to create a milieu that accelerates development of CVD.
Among individuals with end-stage renal disease, CAC is highly prevalent, progresses rapidly, and is associated with an increased risk of death (128–132). CAC is due to the accumulation of calcification in the media of the vessel wall with resultant increased arterial stiffness, increased pulse wave velocity, and LV hypertrophy (133), as well as intimal calcification, more directly related to atherosclerosis as in the general population (134).
Analyses from the CRIC (Chronic Renal Insufficiency Cohort) study show a graded relationship between severity of CKD and CAC, independent of traditional risk factors (135). He et al. (136) further examined the association of risk factors with CAC in CKD patients enrolled in the CRIC study. Among traditional risk factors, patients with CKD and with DM had more than a 3-fold odds of having a high (>100) CAC score (OR: 3.25 [95% CI: 2.44 to 4.34]) compared with those with CKD but without DM. Similarly, earlier analyses from the Dallas Heart Study, using a smaller cohort of CKD patients, demonstrated that patients with diabetes and CKD had a 9-fold greater odds of having a CAC score >10 than patients with diabetes but without CKD (137). Kestenbaum et al. (138) analyzed data from MESA and found that 66% of patients with CKD had prevalent CAC. Incident CAC developed at a rate of 14.8%/year in men and 6.1%/year in women. CAC progression in CKD patients in this study was strongly associated with the presence of diabetes (138,139). Both baseline (140) and progression (141,142) of CAC in patients with CKD have been shown in observational and randomized trials to be associated with increased mortality. Because DM is among the most frequent etiologies of end-stage renal disease and it is associated with rapid progression of CAC, this may become an important marker of risk in patients with advanced stages of CKD.
10. GUIDELINES AND APPROPRIATE USE CRITERIA
Current guidelines (18,19,143,144) support risk factor assessment (Class I), coronary artery calcium scanning (Class IIa), and hemoglobin A1c for risk assessment (Class IIb), but not CIMT (Class III) or routine functional testing (Class III) in the asymptomatic DM population.
In 2013, a societal task force developed a multimodality approach to the diagnosis and risk assessment of stable ischemic heart disease (145), which superseded all prior single-modality appropriate use criteria documents and designated DM as having CAD-equivalent status. Hence, asymptomatic patients with diabetes are considered to be in the high global CAD risk category, for which exercise ECG is rated appropriate; stress radionuclide imaging, stress echocardiography, stress cardiac magnetic resonance, calcium scoring, and coronary CTA are all given a “may be appropriate” rating. It must be noted that the document (145) articulates the concept that just because a test is rated “appropriate” or “may be appropriate,” this does not indicate that it must always be performed for that particular clinical scenario, and that physician judgment must finally adjudicate the need, or lack thereof, for diagnostic testing.
11. CLINICAL IMPLICATIONS
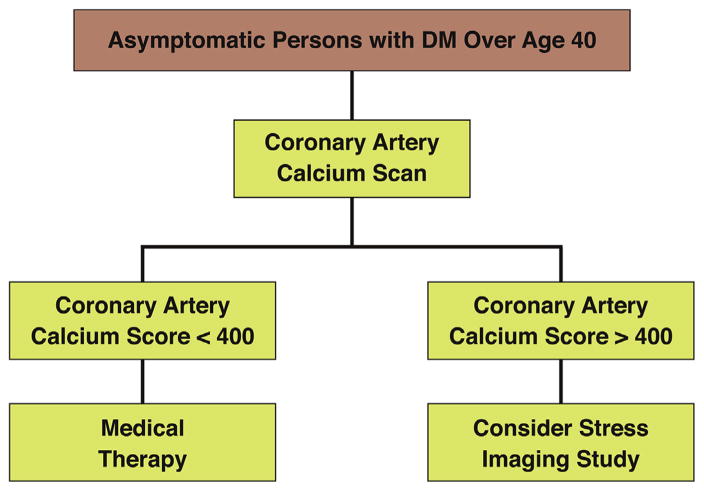
There is great heterogeneity in the global CHD risk of patients with DM. It is clear that DM by itself is not a CHD risk equivalent, based on the sizeable proportion of patients with DM (25% to 30%) who can now be classified as at low risk on the basis of absence of coronary atherosclerosis. The risk of patients with DM ranges from very low in one-third of asymptomatic diabetic patients with no or minimal coronary calcium to high observed in diabetic patients, particularly women, with advanced atherosclerosis (i.e., CAC >400), symptoms, multiple risk factors, and/or CKD, compared with their nondiabetic counterparts. This writing group represents that, at present, CAC screening offers the most sensitive noninvasive risk stratification tool among asymptomatic persons with DM. CAC imaging currently has a Class IIa recommendation for screening in this population from the AHA/ACC guidelines (Figure 2) (19). Functional stress testing may further refine risk estimation in asymptomatic patients with DM who have a high CAC. The available data suggest that this might result in a future recommendation for CAC screening in type 2 diabetes, followed by functional testing for ischemia in patients with a pre-determined CAC threshold. However, although this approach has been shown to improve the stratification of persons with DM, it has not yet been shown to result in improved outcomes. This is a knowledge gap that needs to be addressed with clinical trials.
FIGURE 2. Algorithm for Screening Persons With Diabetes Mellitus.
An algorithm based upon the recommendations of the 2010 ACCF/AHA Guideline for Assessment of Cardiovascular Risk in Asymptomatic Adults (19), with a suggestion to perform stress imaging if the coronary artery calcium score is significantly elevated.
ABBREVIATIONS AND ACRONYMS
- ACC
American College of Cardiology
- AHA
American Heart Association
- CVD
cardiovascular disease
- CIMT
carotid intima-media thickness
- CAC
coronary artery calcium
- CHD
coronary heart disease
- CKD
chronic kidney disease
- DM
diabetes mellitus
- MI
myocardial infarction
- MPI
myocardial perfusion imaging
Footnotes
Dr. Budoff has received grant support from General Electric. Dr. Soman has received grant funding and consultant fees from Astellas. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Engelgau MM, Geiss LS, Saaddine JB, et al. The evolving diabetes burden in the United States. Ann Intern Med. 2004;140:945–50. doi: 10.7326/0003-4819-140-11-200406010-00035. [DOI] [PubMed] [Google Scholar]
- 2.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–7. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 3.Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–22. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kannel WB, McGee DL. Diabetes and glucose tolerance as risk factors for cardiovascular disease: the Framingham study. Diabetes Care. 1979;2:120–6. doi: 10.2337/diacare.2.2.120. [DOI] [PubMed] [Google Scholar]
- 5.Gu K, Cowie CC, Harris MI. Mortality in adults with and without diabetes in a national cohort of the US population, 1971–1993. Diabetes Care. 1998;21:1138–45. doi: 10.2337/diacare.21.7.1138. [DOI] [PubMed] [Google Scholar]
- 6.Haffner SM, Lehto S, Rönnemaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–34. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 7.Natarajan S, Liao Y, Cao G, Lipsitz SR, McGee DL. Sex differences in risk for coronary heart disease mortality associated with diabetes and established coronary heart disease. Arch Intern Med. 2003;163:1735–40. doi: 10.1001/archinte.163.14.1735. [DOI] [PubMed] [Google Scholar]
- 8.Pajunen P, Koukkunen H, Ketonen M, et al. Myocardial infarction in diabetic and non-diabetic persons with and without prior myocardial infarction: the FINAMI Study. Diabetologia. 2005;48:2519–24. doi: 10.1007/s00125-005-0019-0. [DOI] [PubMed] [Google Scholar]
- 9.Hu FB, Stampfer MJ, Solomon CG, et al. The impact of diabetes mellitus on mortality from all causes and coronary heart disease mortality in women: 20 years of follow-up. Arch Intern Med. 2001;161:1717–23. doi: 10.1001/archinte.161.14.1717. [DOI] [PubMed] [Google Scholar]
- 10.Natarajan S, Liao Y, Sinha D, Cao G, McGee DL, Lipsitz SR. Sex differences in the effect of diabetes duration on coronary heart disease mortality. Arch Intern Med. 2005;165:430–5. doi: 10.1001/archinte.165.4.430. [DOI] [PubMed] [Google Scholar]
- 11.Lotufo PA, Gaziano JM, Chae CU, et al. Diabetes and all cause and coronary heart disease mortality among US male physicians. Arch Intern Med. 2001;161:242–7. doi: 10.1001/archinte.161.2.242. [DOI] [PubMed] [Google Scholar]
- 12.Vaccaro O, Eberly LE, Neaton JD, Yang L, Riccardi G, Stamler J. Impact of diabetes and previous myocardial infarction on long-term survival: 25-year mortality follow-up of primary screeners of the Multiple Risk Factor Intervention Trial. Arch Intern Med. 2004;164:1438–43. doi: 10.1001/archinte.164.13.1438. [DOI] [PubMed] [Google Scholar]
- 13.Evans JM, Wang J, Morris AD. Comparison of cardiovascular risk between patients with Type 2 diabetes and those who had myocardial infarction: cross-sectional and cohort studies. Br Med J. 2002;324:939–42. doi: 10.1136/bmj.324.7343.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu G, Jousilahti P, Qiao Q, Katoh S, Tuomilehto J. Sex differences in cardiovascular and total mortality among diabetic and nondiabetic individuals with and without history of myocardial infarction. Diabetologia. 2005;48:656–61. doi: 10.1007/s00125-005-1730-6. [DOI] [PubMed] [Google Scholar]
- 15.Bulugahapitiya U, Siyambalapitiya S, Sithole J, Idris I. Is diabetes a coronary risk equivalent? Systematic review and meta-analysis. Diabet Med. 2009;26:142–8. doi: 10.1111/j.1464-5491.2008.02640.x. [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(Suppl 1):S11–66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perk J, De Backer G, Gohlke H, et al. European guidelines on cardiovascular disease prevention in clinical practice (version 2012): The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J. 2012;33:1635–701. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 18.Greenland P, Bonow RO, Brundage BH, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) J Am Coll Cardiol. 2007;49:378–402. doi: 10.1016/j.jacc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:50–103. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Curtis JM, Horton ES, Bahnson J, et al. Prevalence and predictors of abnormal cardiovascular responses to exercise testing among individuals with type 2 diabetes: the Look AHEAD (Action for Health in Diabetes) study. Diabetes Care. 2010;33:901–7. doi: 10.2337/dc09-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turrini F, Messora R, Giovanardi P, et al. Screening asymptomatic patients with diabetes for unknown coronary artery disease: does it reduce risk? An open-label randomized trial comparing a strategy based on exercise testing aimed at revascularization with management based on pharmacological/behavioural treatment of traditional risk factors. DADDY-D Trial (Does coronary Atherosclerosis Deserve to be Diagnosed and treated early in Diabetics?) Trials. 2009;10:119. doi: 10.1186/1745-6215-10-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turrini F, Scarlini S, Mannucci C, et al. Does coronary atherosclerosis deserve to be diagnosed and treated early in diabetic patients? The DADDY-D Trial. Screening diabetic patients for unknown coronary disease. Eur J Intern Med. 2015;26:407–13. doi: 10.1016/j.ejim.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Banthia S, Bergner DW, Chicos AB, et al. Detection of cardiovascular autonomic neuropathy using exercise testing in patients with type 2 diabetes mellitus. J Diabetes Complications. 2013;27:64–9. doi: 10.1016/j.jdiacomp.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Georgoulias P, Demakopoulos N, Orfanakis A, et al. Evaluation of abnormal heart-rate recovery after exercise testing in patients with diabetes mellitus: correlation with myocardial SPECT and chronotropic parameters. Nucl Med Commun. 2007;28:165–71. doi: 10.1097/MNM.0b013e328013ebd7. [DOI] [PubMed] [Google Scholar]
- 25.Cheng YJ, Lauer MS, Earnest CP, et al. Heart rate recovery following maximal exercise testing as a predictor of cardiovascular disease and all-cause mortality in men with diabetes. Diabetes Care. 2003;26:2052–7. doi: 10.2337/diacare.26.7.2052. [DOI] [PubMed] [Google Scholar]
- 26.Naka M, Hiramatsu K, Aizawa T, et al. Silent myocardial ischemia in patients with non-insulin-dependent diabetes mellitus as judged by treadmill exercise testing and coronary angiography. Am Heart J. 1992;123:46–53. doi: 10.1016/0002-8703(92)90745-h. [DOI] [PubMed] [Google Scholar]
- 27.Chambless LE, Heiss G, Folsom AR, et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987–1993. Am J Epidemiol. 1997;146:483–94. doi: 10.1093/oxfordjournals.aje.a009302. [DOI] [PubMed] [Google Scholar]
- 28.Taylor AJ, Kent SM, Flaherty PJ, Coyle LC, Markwood TT, Vernalis MN. ARBITER: Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol: a randomized trial comparing the effects of atorvastatin and pravastatin on carotid intima medial thickness. Circulation. 2002;106:2055–60. doi: 10.1161/01.cir.0000034508.55617.65. [DOI] [PubMed] [Google Scholar]
- 29.Chambless LE, Folsom AR, Davis V, et al. Risk factors for progression of common carotid atherosclerosis: the Atherosclerosis Risk in Communities Study, 1987–1998. Am J Epidemiol. 2002;155:38–47. doi: 10.1093/aje/155.1.38. [DOI] [PubMed] [Google Scholar]
- 30.Burke GL, Evans GW, Riley WA, et al. Arterial wall thickness is associated with prevalent cardiovascular disease in middle-aged adults. The Atherosclerosis Risk in Communities (ARIC) Study. Stroke. 1995;26:386–91. doi: 10.1161/01.str.26.3.386. [DOI] [PubMed] [Google Scholar]
- 31.Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2935–59. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeboah J, McClelland RL, Polonsky TS, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308:788–95. doi: 10.1001/jama.2012.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Den Ruijter HM, Peters SA, Anderson TJ, et al. Common carotid intima-media thickness measurements in cardiovascular risk prediction: a meta-analysis. JAMA. 2012;308:796–803. doi: 10.1001/jama.2012.9630. [DOI] [PubMed] [Google Scholar]
- 34.Malik S, Budoff MJ, Katz R, et al. Impact of subclinical atherosclerosis on cardiovascular disease events in individuals with metabolic syndrome and diabetes: the Multi-Ethnic Study of Atherosclerosis. Diabetes Care. 2011;34:2285–90. doi: 10.2337/dc11-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary artery calcium area by electron beam computed tomography and coronary atherosclerotic plaque area: a histopathologic correlative study. Circulation. 1995;92:2157–62. doi: 10.1161/01.cir.92.8.2157. [DOI] [PubMed] [Google Scholar]
- 36.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–45. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 37.Budoff MJ, Möhlenkamp S, McClelland R, et al. A comparison of outcomes with coronary artery calcium scanning in unselected populations: the Multi-Ethnic Study of Atherosclerosis (MESA) and Heinz Nixdorf RECALL study (HNR) J Cardiovasc Comput Tomogr. 2013;7:182–91. doi: 10.1016/j.jcct.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong ND, Sciammarella MG, Polk D, et al. The metabolic syndrome, diabetes, and subclinical atherosclerosis assessed by coronary calcium. J Am Coll Cardiol. 2003;41:1547–53. doi: 10.1016/s0735-1097(03)00193-1. [DOI] [PubMed] [Google Scholar]
- 39.Mielke CH, Shields JP, Broemeling LD. Coronary artery calcium, coronary artery disease, and diabetes. Diabetes Res Clin Pract. 2001;53:55–61. doi: 10.1016/s0168-8227(01)00239-x. [DOI] [PubMed] [Google Scholar]
- 40.Schurgin S, Rich S, Mazzone T. Increased prevalence of significant coronary artery calcification in patients with diabetes. Diabetes Care. 2001;24:335–8. doi: 10.2337/diacare.24.2.335. [DOI] [PubMed] [Google Scholar]
- 41.Khaleeli E, Peters SR, Bobrowsky K, Oudiz RJ, Ko JY, Budoff MJ. Diabetes and the associated incidence of subclinical atherosclerosis and coronary artery disease: implications for management. Am Heart J. 2001;141:637–44. doi: 10.1067/mhj.2001.113224. [DOI] [PubMed] [Google Scholar]
- 42.Dabelea D, Kinney G, Snell-Bergeon JK, et al. Effect of type 1 diabetes on the gender difference in coronary artery calcification: a role for insulin resistance? The Coronary Artery Calcification in Type 1 Diabetes (CACTI) Study. Diabetes. 2003;52:2833–9. doi: 10.2337/diabetes.52.11.2833. [DOI] [PubMed] [Google Scholar]
- 43.Starkman HS, Cable G, Hala V, Hecht H, Donnelly CM. Delineation of prevalence and risk factors for early coronary artery disease by electron beam computed tomography in young adults with type 1 diabetes. Diabetes Care. 2003;26:433–6. doi: 10.2337/diacare.26.2.433. [DOI] [PubMed] [Google Scholar]
- 44.Hokanson JE, Cheng S, Snell-Bergeon JK, et al. A common promoter polymorphism in the hepatic lipase gene (LIPC-480C_T) is associated with an increase in coronary calcification in type 1 diabetes. Diabetes. 2002;51:1208–13. doi: 10.2337/diabetes.51.4.1208. [DOI] [PubMed] [Google Scholar]
- 45.Snell-Bergeon JK, Hokanson JE, Jensen L, et al. Progression of coronary artery calcification in type 1 diabetes: the importance of glycemic control. Diabetes Care. 2003;26:2923–8. doi: 10.2337/diacare.26.10.2923. [DOI] [PubMed] [Google Scholar]
- 46.Berman DS, Wong ND, Gransar H, et al. Relationship between stress-induced myocardial ischemia and atherosclerosis measured by coronary calcium tomography. J Am Coll Cardiol. 2004;44:923–30. doi: 10.1016/j.jacc.2004.06.042. [DOI] [PubMed] [Google Scholar]
- 47.He ZX, Hedrick TD, Pratt CM, et al. Severity of coronary artery calcification by electron beam computed tomography predicts silent myocardial ischemia. Circulation. 2000;101:244–51. doi: 10.1161/01.cir.101.3.244. [DOI] [PubMed] [Google Scholar]
- 48.Wong ND, Rozanski A, Gransar H, et al. Metabolic syndrome and diabetes are associated with an increased likelihood of inducible myocardial ischemia among patients with subclinical atherosclerosis. Diabetes Care. 2005;28:1445–50. doi: 10.2337/diacare.28.6.1445. [DOI] [PubMed] [Google Scholar]
- 49.Anand DV, Lim E, Hopkins D, et al. Risk stratification in uncomplicated type 2 diabetes: prospective evaluation of the combined use of coronary artery calcium imaging and selective myocardial perfusion scintigraphy. Eur Heart J. 2006;27:713–21. doi: 10.1093/eurheartj/ehi808. [DOI] [PubMed] [Google Scholar]
- 50.Yeboah J, Erbel R, Delaney JC, et al. Development of a new diabetes risk prediction tool for incident coronary heart disease events: The Multi-Ethnic Study of Atherosclerosis and the Heinz Nixdorf Recall Study. Atherosclerosis. 2014;236:411–7. doi: 10.1016/j.atherosclerosis.2014.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raggi P, Shaw LJ, Berman DS, Callister TQ. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. J Am Coll Cardiol. 2004;43:1663–9. doi: 10.1016/j.jacc.2003.09.068. [DOI] [PubMed] [Google Scholar]
- 52.Moebus S, Stang A, Möhlenkamp S, et al. for the Heinz Nixdorf Recall Study Group. Association of impaired fasting glucose and coronary artery calcification as a marker of subclinical atherosclerosis in a population-based cohort—results of the Heinz Nixdorf Recall Study. Diabetologia. 2009;52:81–9. doi: 10.1007/s00125-008-1173-y. [DOI] [PubMed] [Google Scholar]
- 53.Lehmann N, Möhlenkamp S, Mahabadi AA, et al. Effect of smoking and other traditional risk factors on the onset of coronary artery calcification: results of the Heinz Nixdorf Recall study. Atherosclerosis. 2014;232:339–45. doi: 10.1016/j.atherosclerosis.2013.11.045. [DOI] [PubMed] [Google Scholar]
- 54.Silverman MG, Blaha MJ, Budoff MJ, et al. Potential implications of coronary artery calcium testing for guiding aspirin use among asymptomatic individuals with diabetes. Diabetes Care. 2012;35:624–6. doi: 10.2337/dc11-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kronmal RA, McClelland RL, Detrano R, et al. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2007;115:2722–30. doi: 10.1161/CIRCULATIONAHA.106.674143. [DOI] [PubMed] [Google Scholar]
- 56.Erbel R, Lehmann N, Churzidse S, et al. on behalf of the Heinz Nixdorf Recall Study Investigators. Progression of coronary artery calcification seems to be inevitable, but predictable—results of the Heinz Nixdorf Recall (HNR) study. Eur Heart J. 2014;35:2960–71. doi: 10.1093/eurheartj/ehu288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pandey AK, Blaha MJ, Sharma K, et al. Family history of coronary heart disease and the incidence and progression of coronary artery calcification: Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2014;232:369–76. doi: 10.1016/j.atherosclerosis.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wong ND, Nelson JC, Granston T, et al. Metabolic syndrome, diabetes, and incidence and progression of coronary calcium: the Multi-Ethnic Study of Atherosclerosis study. J Am Coll Cardiol Img. 2012;5:358–66. doi: 10.1016/j.jcmg.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anand DV, Lim E, Darko D, et al. Determinants of progression of coronary artery calcification in type 2 diabetes: role of glycemic control and inflammatory/vascular calcification markers. J Am Coll Cardiol. 2007;50:2218–25. doi: 10.1016/j.jacc.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 60.Budoff MJ, Hokanson JE, Nasir K, et al. Progression of coronary artery calcium predicts all-cause mortality. J Am Coll Cardiol Img. 2010;3:1229–36. doi: 10.1016/j.jcmg.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 61.Raggi P, Cooil B, Ratti C, Callister TQ, Budoff M. Progression of coronary calcification and occurrence of myocardial infarction in patients with and without diabetes mellitus. Hypertension. 2005;46:238–43. doi: 10.1161/01.HYP.0000164575.16609.02. [DOI] [PubMed] [Google Scholar]
- 62.Kiramijyan S, Ahmadi N, Isma’eel H, et al. Impact of coronary artery calcium progression and statin therapy on clinical outcome in subjects with and without diabetes mellitus. Am J Cardiol. 2013;111:356–61. doi: 10.1016/j.amjcard.2012.09.033. [DOI] [PubMed] [Google Scholar]
- 63.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–23. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 64.Galderisi M. Diastolic dysfunction and diabetic cardiomyopathy: evaluation by Doppler echocardiography. J Am Coll Cardiol. 2006;48:1548–51. doi: 10.1016/j.jacc.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 65.Galderisi M, Anderson KM, Wilson PW, Levy D. Echocardiographic evidence for the existence of a distinct diabetic cardiomyopathy (The Framingham Heart Study) Am J Cardiol. 1991;68:85–9. doi: 10.1016/0002-9149(91)90716-x. [DOI] [PubMed] [Google Scholar]
- 66.Devereux RB, Roman MJ, Paranicas M, et al. Impact of diabetes on cardiac structure and function: the Strong Heart Study. Circulation. 2000;101:2271–6. doi: 10.1161/01.cir.101.19.2271. [DOI] [PubMed] [Google Scholar]
- 67.Di Cori A, Di Bello V, Miccoli R, et al. Left ventricular function in normotensive young adults with well-controlled type 1 diabetes mellitus. Am J Cardiol. 2007;99:84–90. doi: 10.1016/j.amjcard.2006.07.063. [DOI] [PubMed] [Google Scholar]
- 68.Ha JW, Lee HC, Kang ES, et al. Abnormal left ventricular longitudinal functional reserve in patients with diabetes mellitus: implications for detecting subclinical myocardial dysfunction using exercise tissue Doppler echocardiography. Heart. 2007;93:1571–6. doi: 10.1136/hrt.2006.101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boyer JK, Thanigaraj S, Schechtman KB, Perez JE. Prevalence of ventricular diastolic dysfunction in asymptomatic, normotensive patients with diabetes mellitus. Am J Cardiol. 2004;93:870–5. doi: 10.1016/j.amjcard.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 70.Zabalgoitia M, Ismaeil MF, Anderson L, Maklady FA. Prevalence of diastolic dysfunction in normotensive, asymptomatic patients with well controlled type 2 diabetes mellitus. Am J Cardiol. 2001;87:320–3. doi: 10.1016/s0002-9149(00)01366-7. [DOI] [PubMed] [Google Scholar]
- 71.Kadappu KK, Boyd A, Eshoo S, et al. Changes in left atrial volume in diabetes mellitus: more than diastolic dysfunction? Eur Heart J Cardiovasc Imaging. 2012;13:1016–23. doi: 10.1093/ehjci/jes084. [DOI] [PubMed] [Google Scholar]
- 72.Marwick TH, Case C, Sawada S, Vasey C, Short L, Lauer M. Use of stress echocardiography to predict mortality in patients with diabetes and known or suspected coronary artery disease. Diabetes Care. 2002;25:1042–8. doi: 10.2337/diacare.25.6.1042. [DOI] [PubMed] [Google Scholar]
- 73.Elhendy A, Adelaide AM, Mahoney DW, Pellikka PA. Prognostic stratification of diabetic patients by exercise echocardiography. J Am Coll Cardiol. 2001;37:1551–7. doi: 10.1016/s0735-1097(01)01199-8. [DOI] [PubMed] [Google Scholar]
- 74.Cortigiani L, Bigi R, Sicari R, Landi P, Bovenzi F, Picano E. Prognostic value of pharmacological stress echocardiography in diabetic and nondiabetic patients with known or suspected coronary artery disease. J Am Coll Cardiol. 2006;47:605–10. doi: 10.1016/j.jacc.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 75.Hachamovitch R, Kang X, Amanullah AM, et al. Prognostic implications of myocardial perfusion single-photon emission computed tomography in the elderly. Circulation. 2009;120:2197–206. doi: 10.1161/CIRCULATIONAHA.108.817387. [DOI] [PubMed] [Google Scholar]
- 76.Acampa W, Cantoni V, Green R, et al. Prognostic value of normal stress myocardial perfusion imaging in diabetic patients: a meta-analysis. J Nucl Cardiol. 2014;21:893–902. doi: 10.1007/s12350-014-9918-0. [DOI] [PubMed] [Google Scholar]
- 77.Giri S, Shaw LJ, Murthy DR, et al. Impact of diabetes on the risk stratification using stress single-photon emission computed tomography myocardial perfusion imaging in patients with symptoms suggestive of coronary artery disease. Circulation. 2002;105:32–40. doi: 10.1161/hc5001.100528. [DOI] [PubMed] [Google Scholar]
- 78.Miller TD, Rajagopalan N, Hodge DO, Frye RL, Gibbons RJ. Yield of stress single-photon emission computed tomography in asymptomatic patients with diabetes. Am Heart J. 2004;147:890–6. doi: 10.1016/j.ahj.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 79.Rajagopalan N, Miller TD, Hodge DO, Frye RL, Gibbons RJ. Identifying high-risk asymptomatic diabetic patients who are candidates for screening stress single-photon emission computed tomography imaging. J Am Coll Cardiol. 2005;45:43–9. doi: 10.1016/j.jacc.2004.06.078. [DOI] [PubMed] [Google Scholar]
- 80.Wackers FJ, Young LH, Inzucchi SE, et al. for the Detection of Ischemia in Asymptomatic Diabetics Investigators. Detection of silent myocardial ischemia in asymptomatic diabetic subjects: the DIAD study. Diabetes Care. 2004;27:1954–61. doi: 10.2337/diacare.27.8.1954. [DOI] [PubMed] [Google Scholar]
- 81.Malhotra S, Sharma R, Kliner DE, Follansbee WP, Soman P. Relationship between silent myocardial ischemia and coronary artery disease risk factors. J Nucl Cardiol. 2013;20:731–8. doi: 10.1007/s12350-013-9708-0. [DOI] [PubMed] [Google Scholar]
- 82.Zellweger MJ, Maraun M, Osterhues HH, et al. Progression to overt or silent CAD in asymptomatic patients with diabetes mellitus at high coronary risk. Main findings of the prospective multicenter BARDOT trial with a pilot randomized treatment substudy. J Am Coll Cardiol Img. 2014;7:1001–10. doi: 10.1016/j.jcmg.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 83.Hachamovitch R, Rozanski A, Shaw LJ, et al. Impact of ischaemia and scar on the therapeutic benefit derived from myocardial revascularization vs. medical therapy among patients undergoing stress-rest myocardial perfusion scintigraphy. Eur Heart J. 2011;32:1012–24. doi: 10.1093/eurheartj/ehq500. [DOI] [PubMed] [Google Scholar]
- 84.Navare SM, Mather JF, Shaw LJ, Fowler MS, Heller GV. Comparison of risk stratification with pharmacologic and exercise stress myocardial perfusion imaging: a meta-analysis. J Nucl Cardiol. 2004;11:551–61. doi: 10.1016/j.nuclcard.2004.06.128. [DOI] [PubMed] [Google Scholar]
- 85.Murthy VL, Masano N, Foster CR, et al. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation. 2012;126:1858–68. doi: 10.1161/CIRCULATIONAHA.112.120402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sassa S, Shimada K, Yoshida K, Tanaka H, Jissho S, Yoshikawa J. Comparison of 64-slice multi-detector computed tomography coronary angiography between asymptomatic, type 2 diabetes mellitus and impaired glucose tolerance patients. J Cardiol. 2008;52:133–9. doi: 10.1016/j.jjcc.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 87.Loffroy R, Bernard S, Serusclat A, et al. Noninvasive assessment of the prevalence and characteristics of coronary atherosclerotic plaques by multidetector computed tomography in asymptomatic type 2 diabetic patients at high risk of significant coronary artery disease: a preliminary study. Arch Cardiovasc Dis. 2009;102:607–15. doi: 10.1016/j.acvd.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 88.Choi EK, Chun EJ, Choi SI, et al. Assessment of subclinical coronary atherosclerosis in asymptomatic patients with type 2 diabetes mellitus with single photon emission computed tomography and coronary computed tomography angiography. Am J Cardiol. 2009;104:890–6. doi: 10.1016/j.amjcard.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 89.Rivera JJ, Nasir K, Choi EK, et al. Detection of occult coronary artery disease in asymptomatic individuals with diabetes mellitus using non-invasive cardiac angiography. Atherosclerosis. 2009;203:442–8. doi: 10.1016/j.atherosclerosis.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 90.Silva JD, Mota P, Coelho A, Catarino R, Leitao-Marques A. Incidence of subclinical atherosclerosis in asymptomatic type-2 diabetic patients: the potential of multi-slice computed tomography coronary angiography. Coron Artery Dis. 2011;22:26–31. doi: 10.1097/MCA.0b013e328340233b. [DOI] [PubMed] [Google Scholar]
- 91.Kamimura M, Moroi M, Isobe M, Hiroe M. Role of coronary CT angiography in asymptomatic patients with type 2 diabetes mellitus. Int Heart J. 2012;53:23–8. doi: 10.1536/ihj.53.23. [DOI] [PubMed] [Google Scholar]
- 92.Nasti R, Carbonara O, di Santo Stefano ML, et al. Coronary artery disease is detectable by multi-slice computed tomography in most asymptomatic type 2 diabetic patients at high cardiovascular risk. Diab Vasc Dis Res. 2012;9:10–7. doi: 10.1177/1479164111426439. [DOI] [PubMed] [Google Scholar]
- 93.Roos CJ, Kharagjitsingh AV, Jukema JW, Bax JJ, Scholte AJ. Comparison by computed tomographic angiography—the presence and extent of coronary arterial atherosclerosis in South Asians versus Caucasians with diabetes mellitus. Am J Cardiol. 2014;113:1782–7. doi: 10.1016/j.amjcard.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 94.Zeina AR, Odeh M, Rosenschein U, Zaid G, Barmeir E. Coronary artery disease among asymptomatic diabetic and nondiabetic patients undergoing coronary computed tomography angiography. Coron Artery Dis. 2008;19:37–41. doi: 10.1097/MCA.0b013e3282f2f19e. [DOI] [PubMed] [Google Scholar]
- 95.Manfrini O, Russo V, Ciavarella A, Ceroni L, Montalti M, Fattori R. Coronary plaque quantification and composition in asymptomatic patients with type II diabetes mellitus. J Cardiovasc Med. 2012;13:423–31. doi: 10.2459/JCM.0b013e32835593f9. [DOI] [PubMed] [Google Scholar]
- 96.Halon DA, Dobrecky-Mery I, Gaspar T, et al. Pulse pressure and coronary atherosclerosis in asymptomatic type 2 diabetes mellitus: a 64 channel cardiac computed tomography analysis. Int J Cardiol. 2010;143:63–71. doi: 10.1016/j.ijcard.2009.01.066. [DOI] [PubMed] [Google Scholar]
- 97.Leem J, Hee Koh E, Jeong E, et al. Prevalence of angiographically defined obstructive coronary artery disease in asymptomatic patients with type 2 diabetes according to the coronary calcium score. Intern Med. 2012;51:3017–23. doi: 10.2169/internalmedicine.51.8221. [DOI] [PubMed] [Google Scholar]
- 98.Kwan AC, May HT, Cater G, et al. Coronary artery plaque volume and obesity in patients with diabetes: the FACTOR-64 study. Radiology. 2014;272:690–9. doi: 10.1148/radiol.14140611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Choo EH, Kim JJ, Hwang BH, et al. Status of hypertension and coronary stenosis in asymptomatic type 2 diabetic patients: analysis from Coronary Computed Tomographic Angiography Registry. Int J Cardiol. 2014;174:282–7. doi: 10.1016/j.ijcard.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 100.Jeong HC, Kim I, Park KH, et al. New strategy for detection of subclinical coronary atherosclerosis in asymptomatic patients with type 2 diabetes based on cardiac multi-detector computed tomography and treadmill test. Circ J. 2014;78:671–8. doi: 10.1253/circj.cj-13-1038. [DOI] [PubMed] [Google Scholar]
- 101.Min JK, Dunning A, Lin FY, et al. for the CONFIRM Investigators. Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the international multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol. 2011;58:849–60. doi: 10.1016/j.jacc.2011.02.074. [DOI] [PubMed] [Google Scholar]
- 102.Muhlestein JB, Lappe DL, Lima JA, et al. Effect of screening for coronary artery disease using CT angiography on mortality and cardiac events in high-risk patients with diabetes: the FACTOR-64 randomized clinical trial. JAMA. 2014;312:2234–43. doi: 10.1001/jama.2014.15825. [DOI] [PubMed] [Google Scholar]
- 103.Taylor AJ, Cerqueira M, Hodgson JM, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2010;56:1864–94. doi: 10.1016/j.jacc.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 104.Velagaleti RS, Gona P, Chuang ML, et al. Relations of insulin resistance and glycemic abnormalities to cardiovascular magnetic resonance measures of cardiac structure and function: the Framingham Heart Study. Circ Cardiovasc Imaging. 2010;3:257–63. doi: 10.1161/CIRCIMAGING.109.911438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Turkbey EB, Backlund JY, Genuth S, et al. Myocardial structure, function, and scar in patients with type 1 diabetes mellitus. Circulation. 2011;124:1737–46. doi: 10.1161/CIRCULATIONAHA.111.022327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kwong RY, Sattar H, Wu H, et al. Incidence and prognostic implication of unrecognized myocardial scar characterized by cardiac magnetic resonance in diabetic patients without clinical evidence of myocardial infarction. Circulation. 2008;118:1011–20. doi: 10.1161/CIRCULATIONAHA.107.727826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schelbert EB, Cao JJ, Sigurdsson S, et al. Prevalence and prognosis of unrecognized myocardial infarction determined by cardiac magnetic resonance in older adults. JAMA. 2012;308:890–6. doi: 10.1001/2012.jama.11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wong TC, Piehler K, Meier CG, et al. Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation. 2012;126:1206–16. doi: 10.1161/CIRCULATIONAHA.111.089409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ng AC, Auger D, Delgado V, et al. Association between diffuse myocardial fibrosis by cardiac magnetic resonance contrast-enhanced T(1) mapping and subclinical myocardial dysfunction in diabetic patients: a pilot study. Circ Cardiovasc Imaging. 2012;5:51–9. doi: 10.1161/CIRCIMAGING.111.965608. [DOI] [PubMed] [Google Scholar]
- 110.Jellis C, Wright J, Kennedy D, et al. Association of imaging markers of myocardial fibrosis with metabolic and functional disturbances in early diabetic cardiomyopathy. Circ Cardiovasc Imaging. 2011;4:693–702. doi: 10.1161/CIRCIMAGING.111.963587. [DOI] [PubMed] [Google Scholar]
- 111.Rijzewijk LJ, van der Meer RW, Smit JW, et al. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J Am Coll Cardiol. 2008;52:1793–9. doi: 10.1016/j.jacc.2008.07.062. [DOI] [PubMed] [Google Scholar]
- 112.Ng AC, Delgado V, Bertini M, et al. Myocardial steatosis and biventricular strain and strain rate imaging in patients with type 2 diabetes mellitus. Circulation. 2010;122:2538–44. doi: 10.1161/CIRCULATIONAHA.110.955542. [DOI] [PubMed] [Google Scholar]
- 113.Hammer S, Snel M, Lamb HJ, et al. Prolonged caloric restriction in obese patients with type 2 diabetes mellitus decreases myocardial triglyceride content and improves myocardial function. J Am Coll Cardiol. 2008;52:1006–12. doi: 10.1016/j.jacc.2008.04.068. [DOI] [PubMed] [Google Scholar]
- 114.Plein S, Radjenovic A, Ridgway JP, et al. Coronary artery disease: myocardial perfusion MR imaging with sensitivity encoding versus conventional angiography. Radiology. 2005;235:423–30. doi: 10.1148/radiol.2352040454. [DOI] [PubMed] [Google Scholar]
- 115.Greenwood JP, Maredia N, Younger JF, et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet. 2012;379:453–60. doi: 10.1016/S0140-6736(11)61335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kannel WB. Metabolic risk factors for coronary heart disease in women: perspectives from the Framingham study. Am Heart J. 1987;114:413–29. doi: 10.1016/0002-8703(87)90511-4. [DOI] [PubMed] [Google Scholar]
- 117.Marroquin OC, Kip KE, Kelley DE, et al. Metabolic syndrome modifies the cardiovascular risk associated with angiographic coronary artery disease in women. Circulation. 2004;109:714–21. doi: 10.1161/01.CIR.0000115517.26897.A7. [DOI] [PubMed] [Google Scholar]
- 118.Helmut HO, Paradisi G, Cronin J, et al. Type II diabetes abrogates sex differences in endothelial function in premenopausal women. Circulation. 2000;101:2040–6. doi: 10.1161/01.cir.101.17.2040. [DOI] [PubMed] [Google Scholar]
- 119.Tamis-Holland JET, Lu J, Korykowski M, et al. Sex differences in presentation and outcome among patients with type 2 diabetes and coronary artery disease treated with contemporary medical therapy with or without prompt revascularization. J Am Coll Cardiol. 2013;61:1767–76. doi: 10.1016/j.jacc.2013.01.062. [DOI] [PubMed] [Google Scholar]
- 120.Nicolini E, Martegani G, Maresca AM, et al. Left ventricular remodeling in patients with metabolic syndrome: influence of gender. Nutr Metab Cardiovasc Dis. 2013;23:771–5. doi: 10.1016/j.numecd.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 121.Berman D, Kang X, Hayes SW, et al. Adenosine myocardial perfusion single-photon emission computed tomography in women compared to men. J Am Coll Cardiol. 2003;41:1125–33. doi: 10.1016/s0735-1097(03)00085-8. [DOI] [PubMed] [Google Scholar]
- 122.Budoff MJ, Achenbach S, Blumenthal RS, et al. Assessment of coronary artery disease by cardiac computed tomography, a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation. 2006;114:1761–91. doi: 10.1161/CIRCULATIONAHA.106.178458. [DOI] [PubMed] [Google Scholar]
- 123.Harjutsalo V, Groop PH. Epidemiology and risk factors for diabetic kidney disease. Adv Chronic Kidney Dis. 2014;21:260–6. doi: 10.1053/j.ackd.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 124.Parving H-H, Mauer M, Fioretto P, Rossing P, Ritz E. Diabetic nephropathy. In: Taal M, Chertow G, Marsden P, Skoreki K, Yu ASL, Brenner BM, editors. The Kidney. 9. Philadelphia: Elsevier-Saunders; 2012. pp. 1411–54. [Google Scholar]
- 125.Jindal A, Garcia-Touza M, Jindal N, Whaley-Connell A, Sowers JR. Diabetic kidney disease and the cardiorenal syndrome: old disease, new perspectives. Endocrinol Metab Clin North Am. 2013;42:789–808. doi: 10.1016/j.ecl.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Peev V, Nayer A, Contreras G. Dyslipidemia, malnutrition, inflammation, cardiovascular disease and mortality in chronic kidney disease. Curr Opin Lipidol. 2014;25:54–60. doi: 10.1097/MOL.0000000000000045. [DOI] [PubMed] [Google Scholar]
- 127.Halter JB, Musi N, McFarland Horne F, et al. Diabetes and cardiovascular disease in older adults: current status and future directions. Diabetes. 2014;63:2578–89. doi: 10.2337/db14-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Blacher J, Guerin AP, Pannier B, et al. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38:938–42. doi: 10.1161/hy1001.096358. [DOI] [PubMed] [Google Scholar]
- 129.Raggi P, Boulay A, Chasan-Taber S, et al. Cardiac calcification in adult hemodialysis patients: a link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol. 2002;39:695–701. doi: 10.1016/s0735-1097(01)01781-8. [DOI] [PubMed] [Google Scholar]
- 130.Block GA, Spiegel DM, Ehrlich J, et al. Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int. 2005;68:1815–24. doi: 10.1111/j.1523-1755.2005.00600.x. [DOI] [PubMed] [Google Scholar]
- 131.Qunibi WY, Abouzahr F, Mizani MR, et al. Cardiovascular calcification in Hispanic Americans (HA) with chronic kidney disease (CKD) due to type 2 diabetes. Kidney Int. 2005;68:271–7. doi: 10.1111/j.1523-1755.2005.00402.x. [DOI] [PubMed] [Google Scholar]
- 132.Russo D, Palmiero G, De Blasio AP, et al. Coronary artery calcification in patients with CRF not undergoing dialysis. Am J Kidney Dis. 2004;44:1024–30. doi: 10.1053/j.ajkd.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 133.London GM, Guerin AP, Marchais SJ, Metivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731–40. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- 134.Nakamura S, Ishibashi-Ueda H, Niizuma S, Yoshihara F, Horio T, Kawano Y. Coronary calcification in patients with chronic kidney disease and coronary artery disease. Clin J Am Soc Nephrol. 2009;4:1892–900. doi: 10.2215/CJN.04320709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Budoff MJ, Rader DJ, Reilly MP, et al. Relationship of estimated GFR and coronary artery calcification in the CRIC (Chronic Renal Insufficiency Cohort) Study. Am J Kidney Dis. 2011;58:519–26. doi: 10.1053/j.ajkd.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.He J, Reilly M, Yang W, et al. for the CRIC Investigators. Risk factors for coronary artery calcium among patients with chronic kidney disease (from the Chronic Renal Insufficiency Cohort Study) Am J Cardiol. 2012;110:1735–41. doi: 10.1016/j.amjcard.2012.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kramer H, Toto R, Peshock R, Cooper R, Victor R. Association between chronic kidney disease and coronary artery calcification: the Dallas Heart Study. J Am Soc Nephrol. 2005;16:507–13. doi: 10.1681/ASN.2004070610. [DOI] [PubMed] [Google Scholar]
- 138.Kestenbaum B, Adeney K, de Boer IH, Ix J, Shlipak M, Siscovick D. Incidence and progression of coronary calcification in chronic kidney disease: the Multi-Ethnic Study of Atherosclerosis. Kidney International. 2009;76:991–8. doi: 10.1038/ki.2009.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Adeseun GA, Xie D, Wang X, et al. Carotid plaque, carotid intima-media thickness, and coronary calcification equally discriminate prevalent cardiovascular disease in kidney disease. Am J Nephrol. 2012;36:342–7. doi: 10.1159/000342794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shantouf RS, Budoff MJ, Ahmadi N, et al. Total and individual coronary artery calcium scores as independent predictors of mortality in hemodialysis patients. Am J Nephrol. 2010;31:419–25. doi: 10.1159/000294405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Block GA, Raggi P, Bellasi A, Kooniega L, Spiegel DM. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int. 2007;71:438–41. doi: 10.1038/sj.ki.5002059. [DOI] [PubMed] [Google Scholar]
- 142.Di Iorio B, Bellasi A, Russo D for the INDEPENDENT Study Investigators. Mortality in kidney disease patients treated with phosphate binders: a randomized study. Clin J Am Soc Nephrol. 2012;7:487–93. doi: 10.2215/CJN.03820411. [DOI] [PubMed] [Google Scholar]
- 143.Hendel RC, Berman DS, Di Carli MF, et al. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 appropriate use criteria for cardiac radionuclide imaging: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine. J Am Coll Cardiol. 2009;53:2201–29. doi: 10.1016/j.jacc.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 144.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2889–934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 145.Wolk MJ, Bailey SR, Doherty JU, et al. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;63:380–406. doi: 10.1016/j.jacc.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 146.Park GM, Lee SW, Cho YR, et al. Coronary computed tomographic angiographic findings in asymptomatic patients with type 2 diabetes mellitus. Am J Cardiol. 2014;113:765–71. doi: 10.1016/j.amjcard.2013.11.028. [DOI] [PubMed] [Google Scholar]
- 147.Scholte AJ, Schuijf JD, Kharagjitsingh AV, et al. Different manifestations of coronary artery disease by stress SPECT myocardial perfusion imaging, coronary calcium scoring, and multislice CT coronary angiography in asymptomatic patients with type 2 diabetes mellitus. J Nucl Cardiol. 2008;15:503–9. doi: 10.1016/j.nuclcard.2008.02.015. [DOI] [PubMed] [Google Scholar]