Abstract
We have previously reported that LHCGR expression in the ovary is regulated through a post-transcriptional mechanism involving an mRNA binding protein designated as LRBP, which is regulated, at least in part, by a non-coding RNA, miR-122. Our present study examined the regulatory role of miR-122 in FSH-induced LHCGR expression during follicle development. Treatment of rat granulosa cells concurrently with FSH and 17β estradiol showed, as expected, a time-dependent increase in LHCGR mRNA levels as well as hCG-induced progesterone production. However, miR-122 expression was decreased during the early time periods, which preceded the increased expression of LHCGR mRNA. The role of miR-122 in FSH-induced LHCGR mRNA expression was then examined by overexpressing miR-122 prior to FSH stimulation by infecting granulosa cells with an adenoviral vector containing a miR-122 insert (AdmiR-122). Pretreatment with AdmiR-122 resulted in complete abrogation of FSH- mediated upregulation of LHCGR. AdmiR-122 also blocked FSH-induced decrease in LRBP expression and increased the binding of LHCGR mRNA to LRBP. Based on these results, we conclude that miR-122 plays a regulatory role in LHCGR expression by modulating LRBP levels during FSH-induced follicle growth.
Keywords: Luteinizing hormone receptor, mRNA binding protein, microRNA, miR-122, Follicle stimulating hormone, Estradiol
1. Introduction
Well-synchronized sequential actions of follicle stimulating hormone (FSH) and luteinizing hormone (LH) are critical for follicle growth and preovulatory steroidogenesis leading to successful ovulation. FSH stimulates the formation of large preovulatory follicles with increased levels of luteinizing hormone/choriogonadotropin receptor (LHCGR), making it responsive to a preovulatory LH surge that leads to ovulation and subsequent corpus luteum formation. LHCGR expression undergoes transient changes throughout the ovarian cycle [1–3]. Our previous studies have shown that LHCGR expression is regulated, at least in part, by a post-transcriptional mechanism that is mediated by a LHCGR mRNA binding protein, designated as LRBP [4–9]. Biochemical characterization of LRBP established its identity as being mevalonate kinase (MVK) [8, 9]. LHCGR mRNA and LRBP expression show a reciprocal relationship at different stages of the ovarian cycle since at elevated levels of LHCGR expression, LRBP expression showed a decrease, whereas higher levels of LRBP were seen in response to the preovulatory LH surge, when LHCGR expression is transiently suppressed [12]. Further studies demonstrated that LRBP (MVK), a member of the oxysterol-responsive gene family, is regulated by miR-122, a microRNA that is expressed in the liver as well as in rodent and human ovaries [10, 11]. MiR-122 regulates cholesterol metabolism through its ability to activate the transcription factor, Sterol regulatory element-binding proteins (SREBPs) [10], by controlling the expression of oxysterol-responsive genes including LRBP. In the ovary, we showed that miR-122 expression is increased in response to LH/hCG during LHCGR downregulation [11]. MiR-122, in turn, activates SREBPs, leading to an increase in the expression of LRBP [10]. LRBP then binds to LHCGR mRNA and targets it for degradation in the p bodies by interacting with other cytosolic proteins [12]. Our previous studies have shown that an increase in the expression of miR-122 leads to increased LRBP expression, resulting in LHCGR mRNA downregulation in response to the preovulatory LH surge [10, 11].
The present study examined whether miR-122 plays a role in FSH-mediated induction of LHCGR expression in the granulosa cells. Our results show that a FSH -mediated increase in LHCGR is preceded by decreased levels of miR-122, causing a suppression of LRBP expression. We also show that overexpression of miR-122 blocks FSH-mediated LHCGR mRNA induction by increasing LRBP expression. Taken together, these results demonstrate the role of miR-122 in FSH-mediated LH receptor induction in the growing follicles, a process crucial for ovulation and steroidogenesis.
2. Materials and Methods
2.1. Materials
Ovine FSH (NIDDK-oFSH-20) and purified hCG were purchased from Dr. A. F. Parlow (National Hormone and Peptide Program, Torrance, CA). 17 β-estradiol was from Sigma-Aldrich (St. Louis, MO). EDTA-free protease inhibitor mixture tablets and Quickspin (G-50 Sephadex) columns for radiolabeled RNA purification were purchased from Roche Applied Science (Indianapolis, IN). Real time PCR primers for LHCGR, LRBP (MVK), miR-122, U6 SnRNA, and 18S rRNA (TaqMan Assay-on-Demand Gene Expression Products) were purchased from Applied Biosystems/Life Technologies (Foster City, CA). BCA reagent was purchased from GE Healthcare Life Sciences (Piscataway, NJ). [α-32P]-UTP was obtained from Perkin Elmer Life Sciences (Waltham, MA) and Maxiscript T7 kit was purchased from Ambion (Austin, TX). RNase inhibitor (RNasin) was purchased from Promega (Madison, WI). Ad-miRA-rno-mir-122 (Ad-miR-122) was purchased from ABM (Richmond, Canada) and amplified and titrated (1x1012 pfu) at the University of Michigan vector core. AdGFP (1x1011 pfu) was obtained from the University of Michigan vector core.
2.2. Isolation of rat granulosa cells
Rat granulosa cells were isolated as described previously [13]. Briefly, 23-day-old female rats were injected subcutaneously with 1.5 mg of 17β-estradiol (E2) for three consecutive days. On day 4, rats were sacrificed and their ovaries collected. Ovaries were cleared from the surrounding fat and punctured with 25-gauge needles. Cells were collected in phenol red-free DMEM-F12 containing 0.2% BSA, 10 mM HEPES, and 6.8 mM EGTA; incubated for 15 minutes at 37°C under 95% O2-5% CO2; and centrifuged at 250 × g for 5 minutes. The pellets were suspended in a solution containing 0.5 M sucrose, 0.2% BSA, and 1.8 mM EGTA in DMEM-F12 and incubated for 5 minutes at 37°C. After incubation, the suspension was diluted with 3 vol DMEM-F12, centrifuged at 250 × g, and treated sequentially with trypsin (20 μg/ml) for 1 minute, 300 μg/ml soybean trypsin inhibitor for 5 minutes, and deoxyribonuclease I (100 μg /ml) for 5 minutes at 37°C to remove dead cells. The cells were then rinsed twice with serum-free media and suspended in DMEM-F12, and cell number was determined. Cell viability was examined by the trypan blue exclusion method. Cells were cultured in serum-free DME-F12 media supplemented with 20 mM HEPES (pH 7.4), 4 mM glutamine, 100 IU penicillin/ml, and 100 μg /ml streptomycin. Before seeding, the culture dishes were coated with 10% fetal calf serum for 2 hours at 37°C and washed with DMEM-F12. Animal handling and treatments were conducted in accordance with the accepted standards of humane animal care, as outlined in the Ethical Guidelines of the University of Michigan and were reviewed and approved by the University Committee on Use and Care of Animals (UCUCA).
2.3. Treatment of granulosa cells with adenovirus constructs
Granulosa cells were infected with adenoviruses (AdmirR-122 or AdGFP) at a multiplicity of infection 200 and diluted in 3 μl of serum-free DMEM for 24 hours. Initially different doses of the viral constructs were tested out for two different time intervals (24 and 48 time points). Based on the results, we chose the optimum dose of 1x109 pfu (200 moi) and a 24-hour incubation period for our future experiments. After 24 hours of infection, the growth medium was replaced, and the cells were incubated with FSH and 17β–estradiol (E2) in fresh media for different time periods.
2.4. Real Time PCR (qPCR) analysis
Total RNAs were reverse-transcribed and subjected to real time PCR quantitation as per the manufacturer’s instructions (Applied Biosystems). The fold change in gene expression was calculated using the ΔΔCt method with 18S rRNA as the internal control, as described previously [14].
2.5. RNA Electrophoretic Mobility Shift Analysis
REMSA was performed by incubating S100 cytosolic fractions isolated from human granulosa cells with a fixed concentration of [α-32P]UTP-labeled LRBP Binding Sequence (LBS), as described previously by our laboratory [4]. Briefly, the labeled RNA for the binding assay was prepared using the Maxiscript kit and S100 fractions were prepared, as described in detail in our previous publications [4]. The RNA-protein complexes were resolved by 5% native polyacrylamide (70:1) gel electrophoresis and analyzed by autoradiography.
2.6. Measurement of progesterone using Enzyme Immuno assay
Rat granulosa cells were seeded in 35-mm plates (~ 0.5 x 106 cells) and cultured for 24 hours following which they were treated with FSH (50 ng/ml) and beta estradiol (1 nM) for different time periods (4, 6, 24 and 48 h). One set of the above groups was then treated with hCG (50 ng/ml) for 24 hours. At the end of the treatments, media were collected and progesterone was assayed using an EIA kit (Cayman, US) according to the manufacturer’s instructions. Briefly, trace amounts of [3H]-progesterone (10,000 cpm) were added to the media samples (500 μl) to monitor recovery of extraction. The media were then extracted with methylene chloride (3x) and suspended in the ELISA buffer after removing methylene chloride by evaporation under a stream of nitrogen. Fifty μl of the sample or standard(s) were added to the wells of the ELISA plates and treated with tracer (progesterone-acetylcholinesterase conjugate) followed by Ellman’s reagent. After 60-minute incubation in the dark, absorbance was measured at 415 nm using a plate reader. The progesterone values are expressed as pg/ ml of the reconstituted extract after correction for extraction losses. The extraction recovery of the assay was in the range of 70–80%. The assay has a range from 7.8–1000 pg/ml and sensitivity (80% B/B0) of approximately 10 pg/ml. The intra-assay and inter-assay coefficients of variation for progesterone were 7.3–54.5% and 7.7–16.4%, respectively.
2.7. Statistical Analysis
Statistical analysis was carried out using one-way ANOVA followed by the Tukey multiple comparison test. Values were considered statistically significant for p<0.05.
3. Results
3.1. Treatment of rat granulosa cells with FSH and 17β–Estradiol (E2) resulted in a time-dependent increase in LHCGR mRNA expression
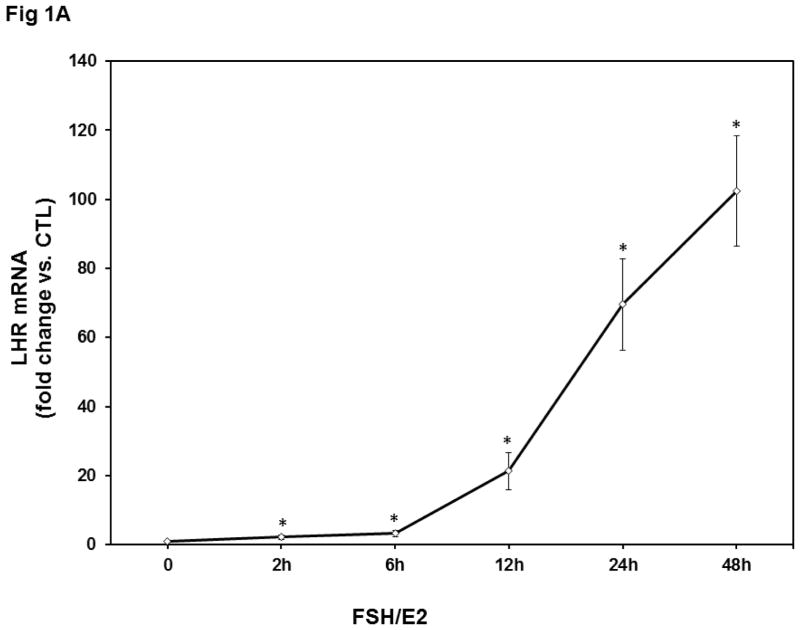
To determine the changes in LHCGR mRNA expression during follicle growth in response to FSH treatment, we isolated rat granulosa cells from 23-day-old female rats treated for three consecutive days with 17β–estradiol. Pretreatment with estradiol has been shown to enhance the responsiveness of the follicles to FSH treatment due to the appearance of large antral follicles and elevated receptor content for both FSH and LH [15]. Granulosa cells were then isolated from the harvested ovaries and cultured for 24 hours. The cultures were treated with a dose of FSH (50 ng/ml) along with 17β–estradiol E2 (1 nM), since E2 has been shown to augment the FSH effect [16]. Cells were lysed after different time points and RNA was isolated. LH receptor levels were measured using a real-time PCR assay. As expected, the mRNA levels of LHCGR showed a time-dependent increase after FSH+E2 treatment (Fig 1A). There was a significant increase by six hours of treatment (FSH+E2- 6h; 3.3±0.89-fold vs. control, p<0.05 vs. control), which then continued at longer incubation periods (fold change, FSH+E2- 12h; 21±5.26, FSH+E2- 24h; 69±13.2, FSH+E2- 48h; 102±15.9 vs. control, p<0.05 vs. control). Since there was an appreciable time-dependent increase in LHCGR expression, we chose this model to test the possible role of miR-122 on the induction of LHCGR mRNA expression during follicle growth.
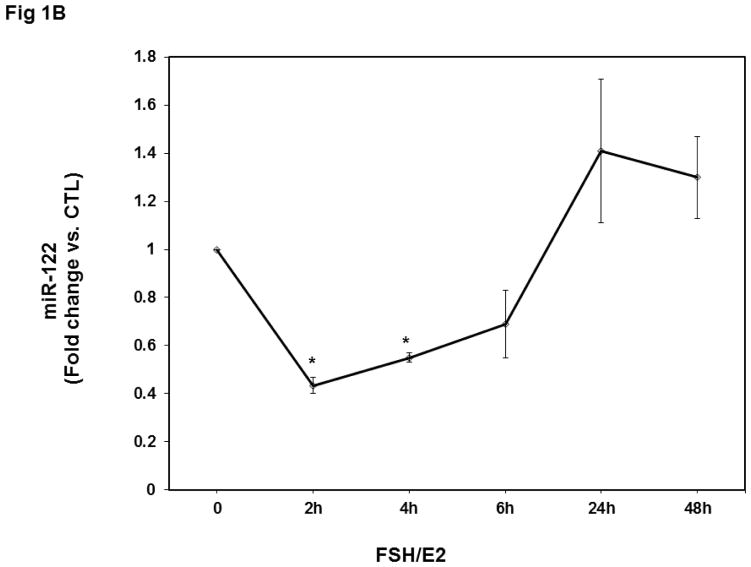
Fig 1. Time-dependent upregulation of LHCGR mRNA and downregulation of miR-122 by FSH and E2 in isolated rat granulosa cells.
Granulosa cells were isolated from immature female rats injected subcutaneously with estradiol (1.5 mg) for three consecutive days. Cells were cultured in serum-free media for 24 hours and then treated with FSH (50 ng/ml) and E2 (1nM) for 2, 4, 6, 12, 24, and 48 hours. Total RNAs were isolated and reverse transcribed, and the resulting cDNAs were subjected to real-time PCR quantitation using specific primers and probes for LHCGR (A) or miR-122 (B). The graph represents changes in mRNA levels normalized to 18S rRNA or U6 SnRNA and shown as fold-change vs. control. Error bars represent mean ±SE. *p<0.05 vs. control; n=4.
3.2. Downregulation of miR-122 prior to the FSH-induced LHCGR upregulation
Since we had previously shown that the miR-122 level in the ovary was increased during hCG-induced downregulation of LHCGR mRNA, we examined whether the increase in LHCGR expression is accompanied by decreased levels of miR-122. Analysis of miR-122 levels using a real-time PCR assay showed that FSH treatment produced a decline in miR-122 expression as predicted, prior to increasing LHCGR mRNA (fold change, FSH+E2-2h; 0.4±0.03 vs. control and FSH+E2-4h; 0.5±0.001 vs. control, p<0.05 vs. control; Fig 1B). However, this increase was not sustained throughout the FSH treatment period. Although there was a slight increase at six hours, it was not statistically significant. After 24 hours of FSH treatment, levels of miR-122 were comparable to that of control (1.41±0.3-fold vs. control, NS). Thus a decrease in miR-122 preceded the induction LHCGR mRNA in response to FSH treatment, suggesting that miR-122 triggers factors that increase LHCGR mRNA expression. Treatment with estradiol alone produced a slight decrease in the levels of miR-122, but the decrease was not statistically significant (data not shown).
3.3. Upregulation of LHCGR is accompanied by increase in hCG-mediated progesterone production by rat granulosa cells
Since FSH treatment produced an increase in LHCGR mRNA expression, we then examined if this is accompanied by corresponding changes in the levels of hCG-stimulated progesterone production under similar experimental conditions as described above. Accordingly, cells were treated with FSH and E2 as described in the earlier experiment, but after different time periods, one set was treated with 50 ng/ml of hCG for 24 hours. The media were collected to determine progesterone secretion by immunoassay. As shown in Table 1, there was a small but significant increase in progesterone production in the FSH/E2 treated cells from latter time periods, without added hCG (pg/ml, FSH+E2- 4h; 9.76±1.3, FSH+E2- 6h; 14.8±0.61, FSH+E2- 24h; 23.8±5.52, FSH+E2- 48h; 43.3±2.9, p<0.05 vs. FSH+E2- 0h). More importantly, as expected, progesterone production in the FSH+E2-treated cells were increased significantly by treatment with hCG (50 ng/ml; 24h) in a time–dependent manner (pg/ml, FSH/E2-4h + hCG; 18.4±0.3, FSH/E2-6h + hCG; 27.08±1.12, FSH/E2-24h + hCG; 61.5±3.99, FSH/E2-48h + hCG; 70.1±6.6, p<0.05 vs. FSH+E2-0H + hCG). These data show that the increased LHCGR mRNA levels generally correlate with increased expression of functional receptors.
Table 1. FSH/E2-mediated upregulation of LHCGR mRNA correlates with increases in progesterone production.
Granulosa cells were isolated from immature female rats treated subcutaneously with estradiol (1.5 mg) for three consecutive days. Cells were cultured in serum-free media for 24 hours and then treated with FSH (50 ng/ml) and E2 (1nM) for 2, 4, 6, 12, 24, and 48 hours. One set of the above groups was then treated with hCG (50 ng/ml) for 24 hours for progesterone measurement. At the end of the treatments, media were collected and progesterone was assayed as described in detail in the Methods.
| Progesterone (pg/ml) | ||
|---|---|---|
| FSH/E2 treatment Time | Control | + HCG (50 ng/ml) |
| 0h | 3.21 ± 0.4 | 5.18 ± 1.2 |
| 4h | 9.76 ± 1.4a | 18.43 ± 0.13bc |
| 6h | 14.82 ± 0.61a | 27.08 ± 1.12bc |
| 24h | 23.79 ± 5.52a | 61.57 ± 4.0bc |
| 48h | 43.29 ± 2.9a | 70.18 ± 6.6bc |
P<.05 vs. 0h–hCG (control)
P<.05 vs. 0h+hCG
P<.05 vs. FSH/E2–hCG, at each time point.
3.4. Decreased association of LRBP protein with LHCGR mRNA during FSH-induced LHCGR upregulation
In our previous studies, we have shown that an increase in miR-122 is followed by a corresponding increase in LRBP expression, and that this increase in LRBP leads to an increase in its association with LHCGR mRNA and culminates in its accelerated degradation [10, 11]. Based on this increase, it is expected that a decrease in miR-122 levels would lead to a decreased association of LHCGR mRNA with LRBP. To examine this, we analyzed S100 fractions of ovarian cell lysates using an RNA electrophoretic mobility shift assay (REMSA) with a 32P-labeled LHCGR mRNA probe that interacts with LRBP (LBS) [5]. As shown in Fig 2, the results showed a significant decrease in the association of LHCGR mRNA with LRBP during the FSH-mediated induction of LHCGR mRNA. The intensity of the band representing the LRBP-LHCGR mRNP complex in the control lane was significantly higher (fold change, FSH+E2-6h; 0.5±0.04; FSH+E2-12h; 0.49±0.1; FSH+E2-24h; 0.47±0.07 and FSH+E2-48h; 0.43±0.05 vs. control, p<0.05 vs. control) compared to that seen after FSH treatment. This supports our hypothesis that the decrease in miR-122 is accompanied by a decrease in LRBP-LHCGR mRNP complex formation, which inhibits LHCGR mRNA degradation and facilitates increased levels of LHCGR mRNA levels.
Fig 2. Decrease in the association of LRBP and LHCGR mRNA during FSH+E2-mediated LHCGR induction.
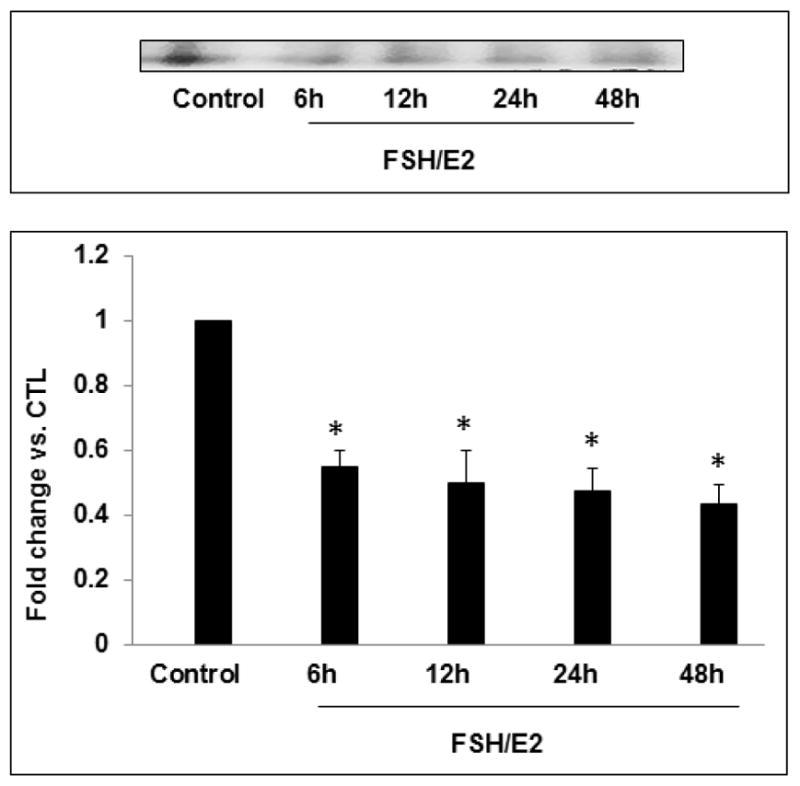
Granulosa cells were isolated from immature female rats injected subcutaneously with estradiol (1.5 mg) for three consecutive days. Cells were cultured in serum-free media for 24 hours and then treated with FSH (50 ng/ml) and E2 (1nM) for different time periods. S100 fractions were isolated as described in Section 2.3. Gel mobility shift analysis was performed using S100 fractions containing equal amounts of total protein after incubating with [32P]-labeled LBS (1.5 x 105 c.p.m). The autoradiogram shown is representative of three independent experiments. Densitometric scanning of the bands is represented in the bar diagram. Error bars represent mean ±SE. *p<0.05 vs. control; n=4.
3.5. Overexpression of miR-122 abrogated FSH-induced upregulation of LHCGR mRNA
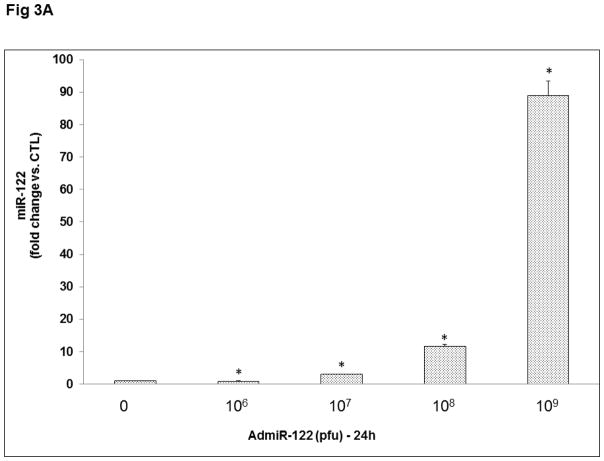
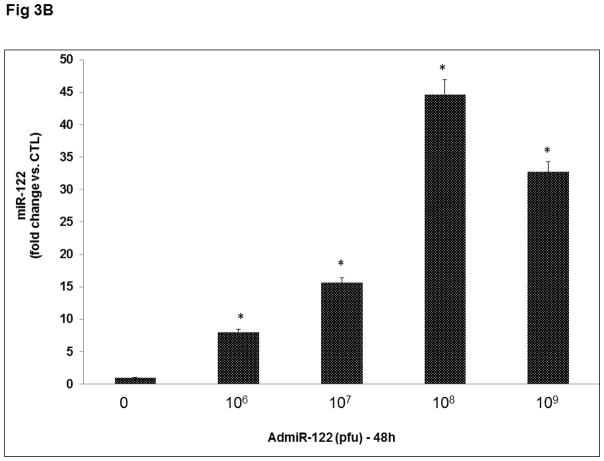
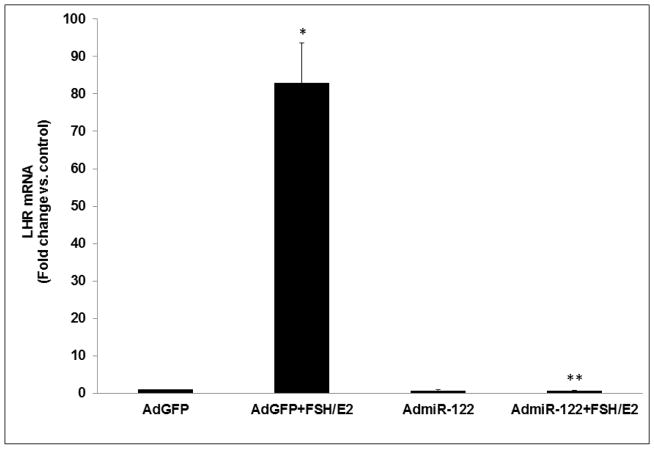
To further establish the role of miR-122 in LHCGR induction during the follicle growth phase, we overexpressed miR-122 using an adenoviral vector as described in detail in the Methods section. Preliminary studies using different doses of GFP labeled AdmiR-122 and two time points (24 and 48 hours; Figs 3A and 3B), followed by fluorescent microscopy and real-time PCR (using miR-122 probe) to determine the optimum dose required for miR-122 overexpression in the rat granulosa cells, showed that the expression of miR-122 increased significantly with an increase in the dose of the AdmiR-122 vector but not with an increase in the infection period. Based on these results, we chose 1x109 pfu and 24 hours of infection as evidenced by maximum expression of miR-122 (Fig 3A). To examine the changes in LHCGR mRNA levels during FSH+E2- treatment in the presence of excess miR-122, cells were pretreated with AdmiR-122 or AdGFP for 24 hours prior to hormone treatment. LHCGR mRNA levels, analyzed by a real-time PCR, is presented in Fig 4. The results showed higher levels of LHCGR expression in the FSH+E2-treated group and complete suppression in the miR-122-infected group (AdGFP+FSH+E2; 82.0±10.79-fold vs. AdGFP, p<0.05; AdmiR-122+FSH+E2; 0.66±0.03-fold vs. AdmiR-122, p<0.05). As is evident from the data, the increase in LHCGR mRNA levels seen in response to FSH and estradiol treatment was not seen in the AdmiR-122 overexpressed group. Since we have previously shown that miR-122 increased SREBP-mediated upregulation of LRBP [10, 11] concomitant with decreased levels of LHCGR mRNA expression, the present results suggest that overexpression of miR-122 initiated a series of events that culminated in accelerated degradation of LHCGR mRNA and prevented FSH-mediated induction of LHCGR.
Fig 3. Adenovirus mediated overexpression of miR-122 in rat granulosa cells.
Granulosa cells were isolated from immature female rats injected subcutaneously with estradiol (1.5 mg) for three consecutive days. Cells were cultured in serum-free media for 24 hours and then pretreated with four different doses of AdmiR-122 (1 x 106, 1 x 107, 1 x 108, 1 x 109 pfu) for 24 (A) or 48 (B) hours. Total RNAs were isolated and reverse transcribed, and the resulting cDNAs were subjected to real-time PCR quantitation using specific primers and probes for miR-122. The graph represents changes in mRNA levels normalized to U6 snRNA and shown as fold-change vs. control. Error bars represent mean ±SE. *p<0.05 vs. control; n=4.
Fig 4. Overexpression of miR-122 inhibited LHCGR upregulation.
Cells were pretreated with AdmiR-122 (1 x 109 pfu) or AdGFP (1 x 109 pfu) for 24 hours. Media was replaced after 24 hours and cells were treated with FSH (50 ng/ml) and E2 (1nM) for six hours. Total RNAs were isolated and reverse transcribed, and the resulting cDNAs were subjected to real-time PCR quantitation using specific primers and probes for LHCGR. The graph represents changes in mRNA levels normalized to 18S rRNA and shown as fold-change vs. control. Error bars represent mean ±SE. *p<0.05 vs. AdGFP; **p<0.05 vs. AdGFP+FSH+E2; n=4.
3.6. Overexpression of miR-122 inhibited the downregulation of LRBP, thereby leading to increased association with LHCGR mRNA
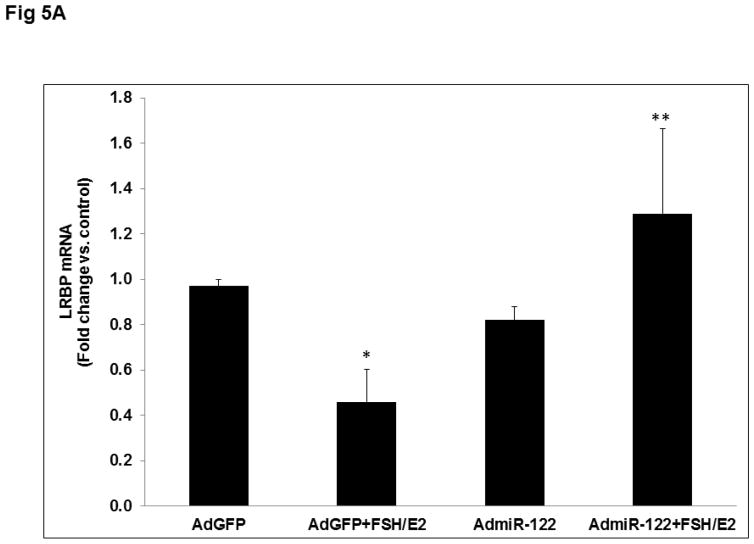
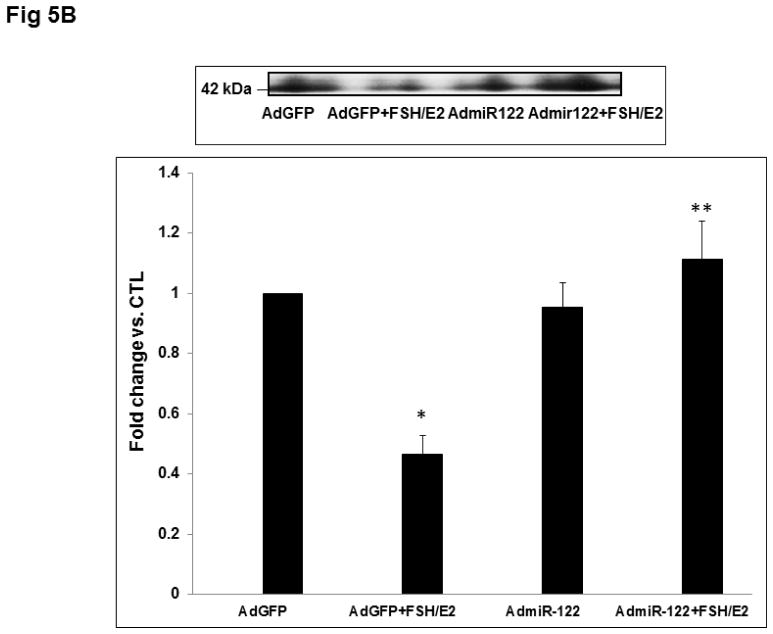
To elucidate the mechanism by which miR-122 overexpression inhibits FSH-mediated LHCGR mRNA induction, we analyzed the expression and binding activity of LRBP. LRBP mRNA was measured using real-time PCR. Results presented in Fig 5A show that while FSH treatment decreased LRBP mRNA expression, overexpression of miR-122 reversed FSH-induced reduction in LRBP gene expression (AdGFP+FSH+E2; 0.46±0.14-fold vs. AdGFP, p<0.05; AdmiR-122+FSH+E2; 1.29±0.37-fold vs. AdmiR-122, p<0.05). Consistent with this data, an RNA electrophoretic mobility gel shift assay also revealed a decrease in the association of LHCGR mRNA with LRBP in the FSH-treated group, but this decrease was abolished when miR-122 was overexpressed prior to FSH treatment (Fig 5B). Densitometric scanning of the bands representing the mRNP complex showed that these changes were significant (AdGFP+FSH+E2; 0.46±0.06-fold vs. AdGFP, p<0.05; AdmiR-122+FSH+E2; 1.1±0.12-fold vs. AdmiR-122, p<0.05). These results confirmed that miR-122 regulates LHCGR levels through its role in regulating LRBP.
Fig 5. Overexpression of miR-122 reversed FSH+E2-induced LRBP downregulation and increased its association with LHCGR mRNA.
Granulosa cells were isolated from immature female rats injected subcutaneously with estradiol (1.5 mg) for three consecutive days. Cells were cultured in serum-free media for 24 hours and then pretreated with AdmiR-122 (1 x 109 pfu) or AdGFP (1 x 109 pfu) for 24 hours. Media was replaced after 24 hours and cells were treated with FSH (50 ng/ml) and E2 (1nM) for six hours. A. Total RNAs were isolated and reverse transcribed, and the resulting cDNAs were subjected to real-time PCR quantitation using specific primers and probes for LRBP (MVK). The graph represents changes in mRNA levels normalized to 18S rRNA and shown as fold-change vs. control. Error bars represent mean ±SE. *p<0.05 vs. AdGFP; **p<0.05 vs. AdGFP+FSH+E2; n=4. B. Gel mobility shift analysis was performed using S100 fractions isolated from different treatment groups containing equal amounts of total protein after incubating with [32P]-labeled LBS (1.5 x 105 c.p.m). The autoradiogram shown is representative of three independent experiments. Densitometric scanning of the bands is represented in the bar diagram. Error bars represent mean ±SE. *p<0.05 vs. AdGFP; **p<0.05 vs. AdGFP+FSH+E2; n=4.
4. Discussion
It has now been well-established that the sequential actions of FSH and LH play a crucial role in follicle growth and maturation, which prepares them for ovulation. The primary action of FSH in follicular growth is the multiplication of the number of granulosa cells, stimulating estrogen production and increasing the expression of LHCGR. In the growing follicles, LHCGR expression increases, reaching maximum levels prior to ovulation. This allows the ovulatory follicles to respond to a preovulatory LH surge to release the ovum, followed by corpus luteum formation and progesterone production. In addition to FSH, our more recent studies have shown that miR-122 also plays a regulatory role in modulating LHCGR expression in the ovary in response to the preovulatory LH surge [10, 11]. We have now extended these studies to examine its possible role in LHCGR induction during FSH-induced follicle growth. Our results show that by regulating the expression and activity of the RNA-binding protein, LRBP, miR-122 plays a crucial role in the regulation of LHCGR expression during follicle growth.
It is now evident that post-transcriptional gene regulation by microRNAs (miRNAs) plays an important role in target gene expression and mRNA stability. In the ovaries, recent studies have shown that microRNAs can control oocyte maturation, the formation and maintenance of primordial follicles, and corpus luteum formation [17–21]. For example, Fiedler et. al., showed that miR-132 and miR-212, which share the same transcripts, are highly upregulated following LH/hCG induction in mouse mural granulosa cells [22], where they might serve as post-transcriptional regulators. Another study in rodents revealed a bi-phasic regulation of miRNAs by FSH [23]. MiR-122 was not identified in this study. This is not surprising, since this conclusion was based on measurement after 12 hours of FSH treatment. As shown in the present study, miR-122 levels reduced rapidly 2–4 hours after FSH treatment and returned to basal levels by 12 hours (Fig 1B). Thus, it appears that once the downstream signaling events are set in motion, miR-122 expression returns to basal levels. Interestingly, a similar pattern of miR-122 expression was also seen during ligand-induced downregulation of LHCGR as reported previously from our laboratory [10, 11]. The time course of miR-122 expression showed an initial increase after hCG treatment that was followed by an increase in LRBP levels, both of which were not sustained at later time periods [11]. Since LHCGR levels are transiently changing throughout the ovarian cycle in response to changes in the hormonal milieu, it is conceivable that the changes in the expression of miR-122 and LRBP might contribute to these changes. Thus our study suggests that miR-122 expression in the granulosa cells is regulated acutely to fine tune the dynamic changes in LHCGR levels. There have also been other instances where miRNAs have been shown to regulate cellular response to hormones [24, 25]. Thus, evidence from several studies seems to suggest that microRNAs control various aspects of ovarian function by modulating the actions of pituitary hormones.
The striking response of overexpression of miR-122 to abrogate FSH-induced LHCGR expression in granulosa cells (Fig 4) further supports the role of this micro RNA in the growing follicles. The present study also shows that the mechanism by which miR-122 inhibits FSH-stimulated LHCGR expression involves the same post-transcriptional mechanism involving the participation of LRBP that we have previously reported [26]. These results are also supported by the findings of Ikeda et al, who showed that MVK (LRBP) is downregulated during FSH-induced LHCGR induction and that overexpression of MVK inhibits LHCGR upregulation [16]. The present study further shows that miR-122 acts upstream of LRBP in the regulation of LHCGR mRNA expression.
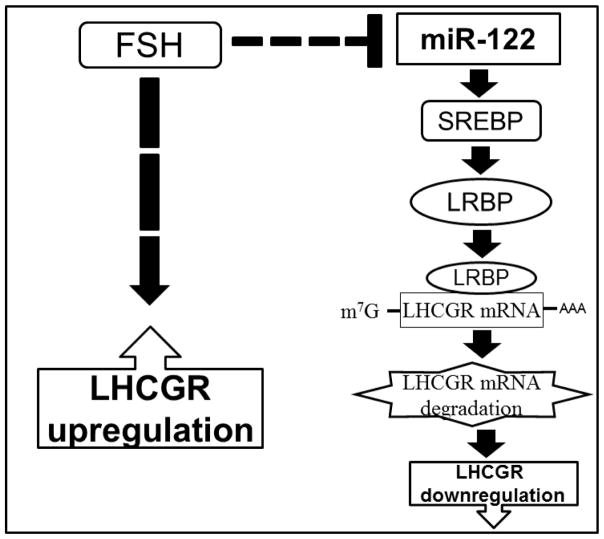
We have previously shown that miR-122 regulates the LHCGR pathway [10] by activating the transcription factor, SREBP, during LHCGR downregulation. SREBPs belong to a large class of transcription factors whose target genes include enzymes involved in cholesterol biosynthesis (reviewed in [27]). The inactive SREBP binds to SREBP cleavage–activating protein (SCAP) in an endoplasmic reticulum (ER), which is an escort for SREBPs as well as a sensor of sterols. When cells become depleted in cholesterol, SCAP escorts SREBP from the ER to the Golgi apparatus, where two proteases cleave SREBP in a two-step process to release the active form. The active SREBP translocates to the nucleus and activates transcription of target genes, including LRBP, by binding to sterol response elements (SREs) in the promoter/enhancer regions of multiple target genes. LRBP then binds LHCGR mRNA and targets it for degradation [12]. Based on the present results combined with those of previous studies, a schematic model that depicts the molecular mechanism illustrating the role of miR-122 in FSH-induced LHCGR mRNA expression is presented in Fig 6. As shown in the figure, miR-122 levels are downregulated in granulosa cells following FSH and estradiol treatment. This leads to a decrease in the activation of SREBPs and, in turn, causes a decrease in LRBP level. Decreased levels of LRBP would allow increased expression of LHCGR mRNA by preventing LHCGR mRNA degradation. This notion is supported by the finding that overexpression of miR-122 leads to an increase in LRBP levels and accelerates LHCGR mRNA degradation. This leads to the abolishment of FSH-mediated increases in LHCGR expression. In summary, miR-122 modulates the FSH effect in granulosa cells by suppressing LRBP levels—leading to increased levels of LHCGR, which are crucial for mediating LH action in growing follicles, including stimulation of steroidogenesis, ovulation, and corpus luteum formation
Fig 6. Schematic model depicting the role of miR-122 in the regulation of LHCGR in granulosa cells.
The model depicted in the figure represents how miR-122 regulates LHCGR expression by modulating the levels of its binding protein LRBP in the ovaries. The dashed line indicates that several steps might be involved in this process.
Highlights.
LH receptor (LHCGR) is regulated by the microRNA, miR-122 during follicle development.
miR-122 is downregulated during FSH-induced LHCGR upregulation in rat granulosa cells.
Overexpression of miR-122 inhibits FSH-induced LHCGR upregulation by increasing the expression of LHCGR mRNA binding protein (LRBP) and increased binding of LHCGR mRNA.
Acknowledgments
This work was supported by National Institutes of Health Grant R01 HD06656-40.
Footnotes
DISCLOSURE STATEMENT: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hoffman YM, Peegel H, Sprock MJ, Zhang QY, Menon KMJ. Evidence that human chorionic gonadotropin/luteinizing hormone receptor down-regulation involves decreased levels of receptor messenger ribonucleic acid. Endocrinology. 1991;128:388–93. doi: 10.1210/endo-128-1-388. [DOI] [PubMed] [Google Scholar]
- 2.LaPolt PS, Oikawa M, Jia XC, Dargan C, Hsueh AJ. Gonadotropin-induced up- and down-regulation of rat ovarian LH receptor message levels during follicular growth, ovulation and luteinization. Endocrinology. 1990;126:3277–9. doi: 10.1210/endo-126-6-3277. [DOI] [PubMed] [Google Scholar]
- 3.Segaloff DL, Wang HY, Richards JS. Hormonal regulation of luteinizing hormone/chorionic gonadotropin receptor mRNA in rat ovarian cells during follicular development and luteinization. Mol Endocrinol. 1990;4:1856–65. doi: 10.1210/mend-4-12-1856. [DOI] [PubMed] [Google Scholar]
- 4.Kash JC, Menon KMJ. Identification of a hormonally regulated luteinizing hormone/human chorionic gonadotropin receptor mRNA binding protein. Increased mrna binding during receptor down-regulation. J Biol Chem. 1998;273:10658–64. doi: 10.1074/jbc.273.17.10658. [DOI] [PubMed] [Google Scholar]
- 5.Kash JC, Menon KMJ. Sequence-specific binding of a hormonally regulated mRNA binding protein to cytidine-rich sequences in the lutropin receptor open reading frame. Biochemistry. 1999;38:16889–97. doi: 10.1021/bi9915770. bi9915770 [pii] [DOI] [PubMed] [Google Scholar]
- 6.Menon KMJ, Clouser CL, Nair AK. Gonadotropin receptors: role of post-translational modifications and post-transcriptional regulation. Endocrine. 2005;26:249–57. doi: 10.1385/ENDO:26:3:249. ENDO:26:3:249 [pii] [DOI] [PubMed] [Google Scholar]
- 7.Menon KMJ, Munshi UM, Clouser CL, Nair AK. Regulation of luteinizing hormone/human chorionic gonadotropin receptor expression: a perspective. Biol Reprod. 2004;70:861–6. doi: 10.1095/biolreprod.103.024471. biolreprod.103.024471 [pii] [DOI] [PubMed] [Google Scholar]
- 8.Nair AK, Kash JC, Peegel H, Menon KMJ. Post-transcriptional regulation of luteinizing hormone receptor mRNA in the ovary by a novel mRNA-binding protein. J Biol Chem. 2002;277:21468–73. doi: 10.1074/jbc.M111653200. M111653200 [pii] [DOI] [PubMed] [Google Scholar]
- 9.Nair AK, Menon KMJ. Isolation and characterization of a novel trans-factor for luteinizing hormone receptor mRNA from ovary. J Biol Chem. 2004;279:14937–44. doi: 10.1074/jbc.M309484200. M309484200 [pii] [DOI] [PubMed] [Google Scholar]
- 10.Menon B, Gulappa T, Menon KMJ. miR-122 Regulates LH Receptor Expression by Activating Sterol Response Element Binding Protein in Rat Ovaries. Endocrinology. 2015;156:3370–80. doi: 10.1210/en.2015-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menon B, Sinden J, Franzo-Romain M, Botta RB, Menon KMJ. Regulation of LH receptor mRNA binding protein by miR-122 in rat ovaries. Endocrinology. 2013;154:4826–34. doi: 10.1210/en.2013-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menon B, Sinden J, Menon KMJ. Association of luteinizing hormone receptor (LHR) mRNA with its binding protein leads to decapping and degradation of the mRNA in the p bodies. Biochim Biophys Acta. 2013;1833:1173–9. doi: 10.1016/j.bbamcr.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pradeep PK, Li X, Peegel H, Menon KMJ. Dihydrotestosterone inhibits granulosa cell proliferation by decreasing the cyclin D2 mRNA expression and cell cycle arrest at G1 phase. Endocrinology. 2002;143:2930–5. doi: 10.1210/endo.143.8.8961. [DOI] [PubMed] [Google Scholar]
- 14.Menon B, Franzo-Romain M, Damanpour S, Menon KMJ. Luteinizing hormone receptor mRNA down-regulation is mediated through ERK-dependent induction of RNA binding protein. Mol Endocrinol. 2011;25:282–90. doi: 10.1210/me.2010-0366. me.2010-0366 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richards JS, Ireland JJ, Rao MC, Bernath GA, Midgley AR, Jr, Reichert LE., Jr Ovarian follicular development in the rat: hormone receptor regulation by estradiol, follicle stimulating hormone and luteinizing hormone. Endocrinology. 1976;99:1562–70. doi: 10.1210/endo-99-6-1562. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda S, Nakamura K, Kogure K, Omori Y, Yamashita S, Kubota K, Mizutani T, Miyamoto K, Minegishi T. Effect of estrogen on the expression of luteinizing hormone-human chorionic gonadotropin receptor messenger ribonucleic acid in cultured rat granulosa cells. Endocrinology. 2008;149:1524–33. doi: 10.1210/en.2007-1163. [DOI] [PubMed] [Google Scholar]
- 17.Pan B, Toms D, Shen W, Li J. MicroRNA-378 regulates oocyte maturation via the suppression of aromatase in porcine cumulus cells. Am J Physiol Endocrinol Metab. 2015;308:E525–34. doi: 10.1152/ajpendo.00480.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H, Jiang X, Zhang Y, Xu B, Hua J, Ma T, Zheng W, Sun R, Shen W, Cooke HJ, Hao Q, Qiao J, Shi Q. microRNA 376a regulates follicle assembly by targeting Pcna in fetal and neonatal mouse ovaries. Reproduction. 2014;148:43–54. doi: 10.1530/REP-13-0508. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Ji X, Zhou D, Li Y, Lin J, Liu J, Luo H, Cui S. miR-143 is critical for the formation of primordial follicles in mice. Front Biosci (Landmark Ed) 2013;18:588–97. doi: 10.2741/4122. [DOI] [PubMed] [Google Scholar]
- 20.Yang S, Wang S, Luo A, Ding T, Lai Z, Shen W, Ma X, Cao C, Shi L, Jiang J, Rong F, Ma L, Tian Y, Du X, Lu Y, Li Y, Wang S. Expression patterns and regulatory functions of microRNAs during the initiation of primordial follicle development in the neonatal mouse ovary. Biol Reprod. 2013;89:126. doi: 10.1095/biolreprod.113.107730. [DOI] [PubMed] [Google Scholar]
- 21.Xu B, Hua J, Zhang Y, Jiang X, Zhang H, Ma T, Zheng W, Sun R, Shen W, Sha J, Cooke HJ, Shi Q. Proliferating cell nuclear antigen (PCNA) regulates primordial follicle assembly by promoting apoptosis of oocytes in fetal and neonatal mouse ovaries. PLoS One. 2011;6:e16046. doi: 10.1371/journal.pone.0016046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiedler SD, Carletti MZ, Hong X, Christenson LK. Hormonal regulation of MicroRNA expression in periovulatory mouse mural granulosa cells. Biol Reprod. 2008;79:1030–7. doi: 10.1095/biolreprod.108.069690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao N, Yang BQ, Liu Y, Tan XY, Lu CL, Yuan XH, Ma X. Follicle-stimulating hormone regulation of microRNA expression on progesterone production in cultured rat granulosa cells. Endocrine. 2010;38:158–66. doi: 10.1007/s12020-010-9345-1. [DOI] [PubMed] [Google Scholar]
- 24.Sirotkin AV, Kisova G, Brenaut P, Ovcharenko D, Grossmann R, Mlyncek M. Involvement of microRNA Mir15a in control of human ovarian granulosa cell proliferation, apoptosis, steroidogenesis, and response to FSH. Microrna. 2014;3:29–36. doi: 10.2174/2211536603666140227232824. [DOI] [PubMed] [Google Scholar]
- 25.Lannes J, L’Hote D, Garrel G, Laverriere JN, Cohen-Tannoudji J, Querat B. Rapid communication: A microRNA-132/212 pathway mediates GnRH activation of FSH expression. Mol Endocrinol. 2015;29:364–72. doi: 10.1210/me.2014-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nair AK, Menon KMJ. Regulation of luteinizing hormone receptor expression: evidence of translational suppression in vitro by a hormonally regulated mRNA-binding protein and its endogenous association with luteinizing hormone receptor mRNA in the ovary. J Biol Chem. 2005;280:42809–16. doi: 10.1074/jbc.M503154200. M503154200 [pii] [DOI] [PubMed] [Google Scholar]
- 27.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–31. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]