Abstract
Melanoma is a cutaneous neoplastic growth of melanocytes with great potential to invade and metastasize, especially when not treated early and effectively. Epithelial-mesenchymal transition (EMT) is the process by which melanocytes lose their epithelial characteristics and acquire mesenchymal phenotypes. Mesenchymal protein expression increases the motility, invasiveness, and metastatic potential of melanoma. Many pathways play a role in promotion of mesenchymal protein expression including RAS/RAF/MEK/ERK, PI3K/AKT/mTOR, Wnt/β-catenin, and several others. Downstream effectors of these pathways induce expression of EMT transcription factors including Snail, Slug, Twist, and Zeb that promote repression of epithelial and induction of mesenchymal character. Emerging research has demonstrated that a variety of small molecule inhibitors as well as phytochemicals can influence the progression of EMT and may even reverse the process, inducing re-expression of epithelial markers. Phytochemicals are of particular interest as supplementary treatment options because of their relatively low toxicities and anti-EMT properties. Modulation of EMT signaling pathways using synthetic small molecules and phytochemicals is a potential therapeutic strategy for reducing the aggressive progression of metastatic melanoma. In this review, we discuss the emerging pathways and transcription factor targets that regulate EMT and evaluate potential synthetic small molecules and naturally occurring compounds that may reduce metastatic melanoma progression.
Keywords: Melanoma, Epithelial-mesenchymal transition, Invasion, Signaling pathways, Phytochemicals, Small molecule inhibitors
Introduction
Melanoma is a primary cutaneous tumor of rapidly dividing melanocytes. While melanoma is the least common form of skin cancer, it confers the most serious prognosis when not treated early and effectively. Despite advances in our understanding of melanoma, the incidence of this cancer has risen over the past decade. A recent report by the American Cancer Society estimates that the number of newly diagnosed melanomas for 2016 will be about 76,380. The lifetime risk of developing melanoma for Caucasian men is about 1/34 and about 1/53 for Caucasian women. This disparity has not improved significantly despite advances in treatment and therapy options [1]. Another worrisome trend is the disparity in outcomes for historically disadvantaged social groups; African Americans, Hispanic Americans, and Americans of lower socioeconomic status experience worse outcomes when diagnosed with melanoma [2].
Risk factors for melanoma include family history, fair-colored skin, UV radiation exposure, history of nevi, history of early-childhood sunburns, and a history of a melanoma [3]. Staging and classification of melanoma were heavily revised by the American Joint Committee on Cancer in 2009 [4]. The most important prognostic factor in evaluating melanoma is the depth of invasion. Primary cutaneous melanoma is a highly curable cancer when diagnosed in early stages, and surgical resection is the preferred treatment in most cases [5].
Two distinct patterns of cutaneous melanoma growth have been described: radial and vertical. Radial growth is defined by horizontal advance of dysplastic melanocytes in the epidermis. The prognosis for melanoma identified in the radial growth stage is typically excellent [6,7]. The vertical growth phase of melanoma involves penetration of the superficial cutaneous tumor into deeper tissues. As melanoma penetrates the epidermal basement membrane, it gains access to blood and lymph vessels and potential to metastasize [8]. Transition from radial to vertical growth phase is often accompanied by phenotypic changes enabling greater cell motility and migration.
The epithelial-mesenchymal transition (EMT) is a fundamentally embryologic phenomenon that arises in wound healing and carcinogenesis. In wound healing, damaged epithelium undergoes a host of changes that promote migration of keratinocytes and fibroblasts, loss of keratinocyte polarity, decreased cell-cell adhesion, and induction of angiogenesis [9,10]. In carcinogenesis, analogous processes arise to transform melanoma in situ to invasive, motile melanoma. This transition is similarly characterized by loss of typical epithelial histologic features including apical-basolateral polarization, basement membrane integrity, and cell-cell adhesion [9,11]. Concurrently, cells undergoing EMT gain mesenchymal characteristics via increased expression of mesenchymal proteins and reduced expression of proteins maintaining epithelial integrity [11]. These changes enhance cell migratory capacity, increase invasiveness, and downregulate apoptosis [9,11]. Complex interactions between multiple signaling pathways and the cellular microenvironment enable the transition from melanoma in situ to aggressive, invasive melanoma [12].
Drug treatments for metastatic melanoma have improved significantly over the past decade. Despite limited initial success, synthetic inhibitors targeting commonly mutated proteins in melanoma are beginning to show promise. Recent combination drug approaches have demonstrated growing success. However, these advances have not had significant effect on patient survival or mortality [1,4]. In addition to synthetic agents, phytochemicals have garnered attention as potential preventive or adjuvant treatment options. Phytochemicals have demonstrated significant potential for treating melanoma by inhibiting tumor progression, invasion, and metastasis [13,14]. The low-toxicity of these compounds makes them especially good candidates for use in cancer therapy. Initial studies of phytochemicals for treating melanoma demonstrate the potential for emergence of innovative solutions, especially as supplementary treatment options. In this review, we focused on the pathways leading to expression of EMT transcription factors, action of the transcription factors, and the current synthetic and phytochemical agents that may repress EMT.
Epithelial-mesenchymal transition in invasion and metastasis
During EMT, cells undergo distinct changes in expression of protein markers. EMT is now understood as a continuum along which cells fluctuate between degrees of epithelial, intermediate, and mesenchymal character. Epidermal cells maintain structure and function by continual synthesis and regulation of epithelial proteins. E-cadherin, desmoplakin, collagen IV, claudins, occludins, zonula occludens 1 (ZO-1) and other proteins promote overall integrity of the epidermis [11,15,16]. During EMT, changes in cellular signaling induce epithelial cells to lose their epithelial character by disrupting expression or function of these critical proteins.
Cell-cell adhesion proteins are critical markers of epithelial character. Cells in epithelial tissue are bound via tight junctions, adherens junctions, and desmosomes. The process of EMT accelerates with degradation of epithelial cell-cell adhesion via disruption of adherens junctions and tight junctions. In the epithelial adherens junction, E-cadherin extends extracellularly from each epithelial cell to the next to bind the cytoskeletal structures of adjacent cells together. Intracellularly, E-cadherin has been shown to influence cell signaling via associated proteins including β-catenin [17]. Cytoskeletal changes in conjunction with loss of these junctions allow epithelial cells undergoing EMT to acquire a “spindle-shape” phenotype, which promotes disruption of the basal lamina and greater motility [11,15].
The biochemical hallmark of EMT is loss of E-cadherin expression. This event marks the culmination of dysregulated signaling resulting in loss of cell-cell adhesion and polarity [18]. Activation of multiple signaling pathways promotes loss of E-cadherin expression [9,11,15]. Recent studies have shown that loss of E-cadherin accelerates the EMT process by promoting expression of EMT transcription factors (EMT-TFs) including Twist1 and Zeb1 and subsequently accelerating migration and invasion [19]. Although loss of E-cadherin is a critical step in EMT, this change alone is not necessarily sufficient to drive EMT [20,21]. Expression of mesenchymal proteins is the next step toward increased motility and invasion. Along with loss of epithelial markers, concurrent increased expression of mesenchymal proteins promotes invasive character. This shift includes increased expression of N-cadherin, vimentin, fibronectin, matrix metalloproteinases (MMPs), and A5B1 integrin [11]. Increased N-cadherin expression and decreased E-cadherin expression, or “cadherin switch,” results in a more motile cell that more readily receives and responds to pro-mesenchymal signals [15].
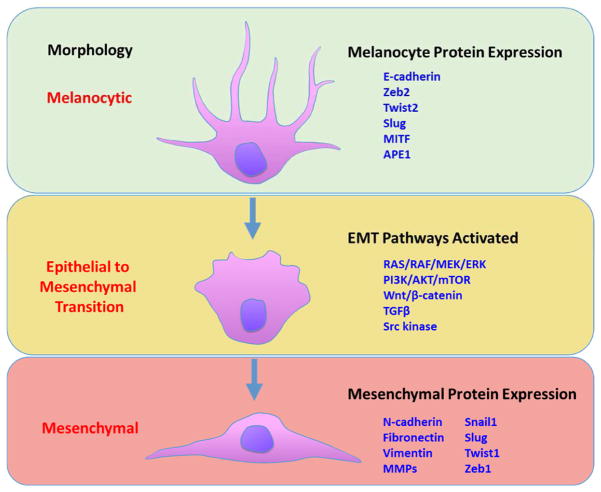
EMT-TFs drive mesenchymal protein expression in melanocytes. Recent evidence suggests that EMT-TFs may be the key to overcoming tumor suppression in primary tumors, permitting subsequent invasion and metastasis [22]. Changes in expression of Snail, Slug, Zeb1, Twist1, and MITF have been correlated with greater migration and invasion of melanoma [23–26]. Pro-mesenchymal mutations may be encoded in the germ-line, acquired during rapid cell proliferation, or induced by signals from the stromal microenvironment. One important exception is Slug, which is expressed in normal melanocytes and plays a role in invasion and metastasis. This characteristic has been attributed to the embryonic melanocyte differentiation regimen from neural crest origin to migration into the epidermis [27]. The invasive front of epithelial tumors contains cells that are most likely to express mesenchymal character [11]. Induction of EMT-TF expression gives rise to malignant stem cell-like phenotypes that are more resistant to known treatments [28]. Figure 1 shows an overview of the EMT process in melanocytes and highlights changes in EMT markers regulated by multiple signaling pathways.
Figure 1.
Epithelial to mesenchymal transition in melanoma involves transformation of melanocyte morphology to a mesenchymal, invasive phenotype. Decreased expression of traditional melanocytic markers suggest loss of “epithelial-like” character. Multiple signaling pathways are involved in these changes, and mesenchymal protein expression facilitates migration, invasion, and metastasis.
Signaling pathways inducing EMT in melanoma
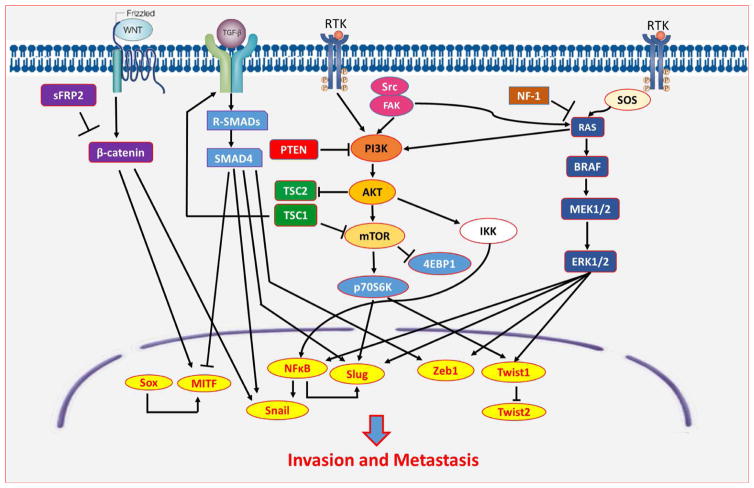
Cellular potential for EMT is influenced by activation of multiple signaling pathways. Figure 2 summarizes the signaling pathways involved in melanoma EMT and their effects on EMT-TF expression, leading to invasion and metastasis. Fundamentally, these signaling pathways mediate the degree of epithelial and mesenchymal character of melanoma cells.
Figure 2.
Signaling pathways govern the switch from epithelial phenotypes to mesenchymal phenotypes by inducing expression of EMT transcription factors (EMT-TFs) favoring migration, invasion, and metastasis.
RAS/RAF/MEK/ERK (MAPK) Pathway
RAS is a small GTPase that regulates downstream activation of signaling pathways including the MAPK and PI3K pathways and is therefore a critical regulator of melanoma progression [29,30]. The NRAS isoform is mutated in approximately 15–25% of melanoma patients [29]. The most common mutations are substitutions of lysine or arginine for glutamine; these mutations result in decreased affinity for GAP proteins and failure to dissociate GTP from the RAS complex resulting in constitutive activation [31]. NRAS overactivation potentiates BRAF and induces expression of downstream effectors [24]. Neurofibromin 1 (NF1) is a tumor suppressor gene coding for a GAP protein that is mutated in approximately 45% of melanomas expressing wild-type BRAF and NRAS [32]. Loss of NF1 function leads to uninhibited activity of NRAS and ultimately proliferation and invasion [29,33]. In nodular melanoma, mutation of BRAF and NRAS were found to accompany each other with one mutated protein typically being active at higher frequency than the other [34].
RAF serine/threonine kinases are the most commonly mutated gene targets in human melanoma. Evidence suggests that approximately 60% of patients diagnosed with melanoma have tumors expressing BRAF mutation [35,36]. The most common mutation of BRAF in melanoma, BRAFV600E, is a substitution of glutamate for valine. This change results in constitutive activation of BRAF and continuous phosphorylation of downstream effectors such as MEK and ERK [12].
Due to its upstream signaling position, BRAF over-activation can induce melanoma cell motility and mesenchymal protein expression through various pathways. BRAF pathway activation has been linked to increased expression of Twist1 and Zeb1 resulting in greater melanoma invasion [24]. In addition, BRAF mutation potentiates the NFκB pathway; NFκB promotes MMP expression, increasing migratory capacity, and induces expression of Snail, a known driver of metastasis [12,37]. Further downstream, BRAF mutation has been linked to compensatory increases in ERK and subsequent overexpression of MITF [38]. In addition, melanocytes expressing BRAFV600E synthesize more MMP-1 and promote greater stromal fibroblast activation than wild-type BRAF melanoma thus promoting greater cell motility [37].
Attempts to therapeutically target BRAF have been relatively unsuccessful. Melanoma cells resist BRAF inhibition therapy via two main mechanisms. First, inhibiting BRAF reactivates MAPK signaling upstream. In BRAF-inhibited melanoma cells, kinase switching is a phenomenon in which other RAF isoforms upregulate in response to BRAF suppression to achieve downstream signaling [39]. Second, upregulation of the PI3K/AKT pathway interferes with the efficacy of BRAF inhibition therapy. This response may be related to complex feedback via RAS-induced pathways [12,40].
Mitogen-activated protein kinase kinase (MEK) and extracellular signal-related kinase (ERK) are downstream effectors of BRAF and potential targets for melanoma therapy. Inhibition of MEK has been shown to inhibit tumor growth by synthetic agents [41]. However, recent evidence suggests that MEK inhibition may actually increase invasive potential in melanoma [42]. One suggested mechanism for this phenomenon is compensatory upregulation of other RTK-mediated pathways such as Src/Fak/STAT3 [43]. ERK has been implicated as a factor in the stabilization of pro-mesenchymal transcription factors. ERK phosphorylation inhibits the degradation of pro-mesenchymal transcription factors such as Zeb, Snail, Slug, and Twist and leads to greater loss of epithelial character [33].
PI3K/AKT/mTOR Pathway
The PI3K/AKT/mTOR axis is known to play a significant role in melanoma EMT. PI3K/AKT constitutive activation results in downstream expression of mesenchymal proteins, repression of E-cadherin, and enhanced migration of melanoma cells [9,44]. Elevated AKT activity is present in approximately 70% of malignant melanomas, and upregulation of the PI3K/AKT pathway plays an important role in melanocyte neoplasia [13,45]. AKT primarily acts as a protein kinase to promote cell survival by activating downstream effectors such as mTOR. However, kinase-independent functions of AKT have recently been found to also induce survival and proliferation of melanoma cells without activation via PIP3 [46]. Activation of AKT is downregulated by phosphatase and tensin homolog (PTEN), a critical inhibitor of PI3K/AKT signal progression. Melanoma tumors expressing PTEN loss-of-function mutations are less responsive to drug therapy and have a poor prognosis [47]. Lack of PTEN function permits uninhibited activation of the PI3K/AKT pathway and is associated with promotion of melanoma invasion when combined with BRAF mutation [48].
The tuberous sclerosis tumor suppressor proteins hamartin (TSC1) and tuberin (TSC2) are another important family of PI3K/AKT/mTOR pathway regulators. These proteins have been shown to act as a complex and independently [49]. Active TSC1/2 complex suppresses mTORC1 activity, preventing downstream induction of proliferation and migration [50]. However, activated AKT can decrease mTOR inhibition via decreased TSC2 expression, thereby decreasing TSC1/2 complexing and increasing mTOR activity [49]. mTOR is downstream effector of the PI3K/AKT pathway. The mTOR serine/threonine kinase domain functions as the catalytic unit of two important protein complexes, mTORC1 and mTORC2 [51]. Activation of mTORC1 induces EMT by activating p7026 kinase 1 (S6K1). This change stimulates F-actin reorganization, focal adhesion, and MMP expression. Similar roles have been observed for mTORC2. The mTORC2 complex is induced by PI3K and mTORC1 activity [52]. Interestingly, mTORC2-mediated phosphorylation increases activity of AKT, thereby further potentiating mTORC1 activity [53]. The mTORC2 complex has been implicated in pro-mesenchymal changes. Via phosphorylation of AKT, TGFβ signaling stimulates mTORC2 to induce cytoskeletal reorganization and migration. Evidence suggests that mTORC2 activity is essential to TGFβ-mediated MMP expression and invasion [54]. These characteristics make mTOR a potential focus for melanoma therapies targeting EMT progression.
Wnt Signaling Pathway
Wnt signaling regulates processes involved in embryological development, normal tissue function, and disease. Wnt is a lipid-derived signaling protein that binds to Wnt-pathway G-protein coupled receptors (GPCR), especially Frizzled, to induce transduction extracellular signals via regulation of β-catenin [15,55]. β-catenin plays a major role in cell-cell adhesion by providing structural strength to the epidermis. In the epidermal adherens junctions, β-catenin participates in a structural complex that links epidermal cells together via actin [56]. Recent evidence shows that β-catenin helps the epidermis resist mechanical stress [57].
Wnt signaling has been implicated in EMT induction. Wnt activation induces upregulation of Snail and downregulation of E-cadherin expression [58]. Loss of E-cadherin releases cadherin-bound β-catenin; free β-catenin can then migrate to the nucleus and induce transcription of pro-invasive factors [59]. However, conflicting evidence has emerged regarding the role of β-catenin in invasion. High β-catenin levels have been associated with improved survival and better prognosis in melanoma patients [60]. Low levels of β-catenin expression and nuclear localization have been observed in the invading front of primary melanomas, and β-catenin expression has been correlated with decreased migration of melanoma. One model suggests that β-catenin modulates downstream MITF expression; low levels of β-catenin result in repression of MITF expression and subsequent invasion [61]. Recent study has established that MITF and β-catenin “cross-talk” through a Wnt-signaling positive feedback mechanism that regulates proliferation [62]. Wnt/β-catenin signaling plays a critical role in melanoma EMT and more study is needed to understand the impacts of β-catenin modulation on tumor progression.
Transforming Growth Factor β (TGFβ) Signaling Pathway
Activation of TGFβ pathways induces synthesis of stromal proteins in surrounding fibroblasts. Synthesis of MMP-2 and MMP-9 contributes to degradation of collagen and the basal lamina, increasing melanoma cell motility. In addition, TGFβ signaling represses expression of E-cadherin and increases N-cadherin via greater transcription of Snail, Slug, and Zeb1 [16,63,64]. Recent studies also link greater TGFβ signaling to decreased expression of MITF and subsequent invasion [64]. The effect of TGFβ on melanoma invasive potential has been confirmed by overexpression of SMAD7, a TBR1 inhibitor. SMAD7 prevents activation of SMAD2/3, decreasing invasion in vitro and in vivo [65]. TGFβ signaling induces a variety of effects that synergistically promote a more mesenchymal phenotype.
Src Signaling Pathway
Proto-oncogene tyrosine kinase Src is a cytoplasmic signaling protein with an important role in EMT-induced tumor progression, invasion, angiogenesis, and metastasis. Src has been shown to potentiate known EMT-inducing pathways including MAPKs and PI3K/AKT [66]. Constitutive activation of Src greatly increases melanoma cell motility, promoting invasion and metastasis [67]. In addition, activated Src is associated with changes in the epithelial adherens junction including diminished cell-cell adhesion, reduced E-cadherin expression, increased phosphorylation of N-cadherin, and dissociation of β-catenin [66,68].
Src phosphorylates and stabilizes focal adhesion kinase (FAK), a cytosolic tyrosine kinase that promotes expression of MMP-2 and MMP-9 [66]. Greater phosphorylation of FAK on Tyr397 and Tyr576 was confirmed in more aggressive melanoma cell lines such as metastatic C8161 compared with A375 cells. FAK phosphorylates ERK and induces urokinase, facilitating invasion and migration [69]. However, suppression of FAK in melanoma cells has been shown to increase invasion. Low FAK expression induces invadopodia production in B16F10 melanoma cell, subsequently increasing invasion. [70]. These data suggest that the role of FAK in melanoma EMT remains poorly understood with changes in FAK activity yielding seemingly paradoxical effects.
Transcription factors of EMT
Microphthalmia-associated transcription factor (MITF)
MITF is a key regulator of melanocyte differentiation from the neural crest origin. This potent transcription factor has been shown to induce expression of a variety of gene targets including those coding for melanin. The role of MITF in EMT is complex, and its effect on mesenchymal protein expression remains contentious. Upstream signaling pathways exert multilevel control on the expression and activity of MITF from transcription to post-translation [71]. MITF can combat the progression of malignancy by prompting cell cycle arrest in normal melanocytes, acting as tumor-suppressing factor, and promoting apoptosis [72,73].
The role of MITF in mediation of tumor progression remains complex and disputed. High levels of MITF downregulate pro-invasive pathway activation yet induce proliferation and survival [71,74]. Twist2 and Zeb2 in melanocytes activate MITF to induce pathways that preserve differentiation [24]. BCL2 family anti-apoptotic factor transcription has been associated with high levels of MITF [25,71]. Other evidence suggests that cells exhibiting low MITF expression have greater potential for invasion and that decreasing expression of MITF in vitro promotes greater melanoma invasion [75]. Recently, it was shown that MITF suppresses invasion by reducing intracellular GTP pools by inducing guanosine monophosphate reductase (GMPR); decreased GTP availability results in downregulation of RAC1, RHO-A, and RHO-C [76]. These data have led to a general observation that MITF influences melanoma in a concentration-dependent fashion: high levels of expression are associated with survival and proliferation and low levels of expression with invasion [25]. In order to effectively target MITF in potential treatment regiments, a greater understanding of the role of MITF in melanoma EMT is necessary.
Sex-Determining Region Y-Box (SOX) Family
The SOX family of transcription factors directs the cellular fate of neuroectodermal crest cells during embryogenesis. Due to their critical role in migration during development, SOX protein expression influences tissue migration and invasion and subsequently plays a part in melanoma EMT. SOX2 overexpression induces invasion of tumors from neural crest origins including melanoma [77]. Repression of SOX2 protein expression in A2058 melanoma cells inhibited expression of MMP-3. In melanoma cells infiltrating the dermal stroma, SOX2 expression was greater compared with non-invading cells; similarly, knockdown of SOX2 expression in A375 cells in vitro reduced tumor invasion [78]. The role of SOX proteins in melanoma invasion has been studied much less extensively than other more well-known melanoma EMT-TFs, and further investigation is needed to determine their effects on invasion and metastasis.
Zinc finger protein SNAI1 (Snail)
Snail is a central regulator of both developmental EMT and pathological EMT [79]. Activation of Snail in melanoma cell lines induces the repression of E-cadherin [80]. In pathologic states, high Snail expression drives cadherin switch and promotes melanoma cell motility [23,81]. In melanoma cells, TGFβ signaling upregulates expression of Snail while PI3K signaling represses activity of Snail inhibitors [82]. Snail is a highly labile transcription factor that is sensitive to post-translational controls. Wnt/β-catenin signaling modulates Snail expression via glycogen synthase kinase 3 beta (GSK3β), which phosphorylates Snail to induce degradation while inhibition of GSK3β promotes stability of Snail proteins [58].
Snail expression induces EMT via modulation of epithelial and mesenchymal proteins. In various malignancies, inhibition of Snail has also been shown to decrease cell invasiveness [83]. Snail downregulates desmoplakin and promotes expression of vimentin and fibronectin, increasing cell motility [84]. In addition, Snail expression in tumors has been implicated as a cause of immunosuppression in melanoma patients. Consequently, knockdown of Snail in melanoma cells decreases tumor growth, metastasis, and immunosuppression [85].
Zinc-finger protein SNAI2 (Slug)
Slug is a close relative of Snail. This protein carries out critical homeostatic tasks in normal epidermal cells to mediate inflammation and wound repair [86]. In normal melanocytes, Slug plays a role in differentiation from the neural crest [27]. Like Snail, overexpression of Slug interferes with cell-cell adhesion. Expression of Slug follows similar signaling pathways as those observed in Snail induction [84]. TGFβ and Wnt/β-catenin signaling promote expression of Slug [16,84]. In melanoma cells, Slug expression can be repressed by inhibition of PI3K/AKT/mTOR signaling, suggesting a role for this pathway in Slug induction [44].
Slug plays a similar role to Snail in EMT. High expression of Slug represses expression of desmoplakin and promotes expression of vimentin and fibronectin resulting in less epithelial cell-cell adhesion and greater motility [84,87]. In addition, Slug promotes expression of EMT-TFs that work cooperatively to repress E-cadherin expression [88]. Slug may overactivate early in melanoma progression to degrade epithelial cohesion while Snail may play a later role, especially in the induction of mesenchymal protein expression [86]. Studies have found that Slug concentrations are high during early stages of melanoma but sustained overexpression of Slug is not necessary for progression [89].
Twist-Related Protein (Twist)
The basic helix-loop-helix transcription factor Twist plays a major role in tumor invasion and metastasis. Expression of Twist may be induced by MAPK, PI3K, or GSK3β signaling [90]. Overexpression of Twist1 in normal mammalian epithelial cells induces loss of cell-cell adhesion, loss of cell polarity, and gain of a spindle-shaped, fibroblast-like morphology [91]. Highly invasive, pre-metastatic tumors exhibit high Twist1 expression, and suppression of Twist1 inhibits tumor metastatic potential in vivo [9,92]. Furthermore, greater Twist1 expression independently promotes melanoma invasion via increased expression of MMP-2 [92]. On the other hand, Twist2 is expressed in normal melanocytes and exerts a tumor-suppressing and anti-invasive effect [24].
In addition to increasing expression of pro-mesenchymal proteins, Twist1 expression downregulates Twist2 in response to MEK/ERK overactivation [24]. Regulation of Twist1 and Twist2 influences activation of cadherin switch in melanoma [24,81]. Additionally, silencing of Twist1 in melanoma represses expression of N-cadherin [90]. In effect, molecular “switch” from E-cadherin to N-cadherin occurs downstream of increased MAPK signaling, favoring a mesenchymal phenotype by modulating expression of Twist isoforms [24,92].
Zinc-finger E-box binding homeobox (Zeb)
Zeb transcription factors have recently been shown to modulate epithelial protein expression. Zeb1 is a potent promoter of EMT because it binds directly to CDH1, the promoter domain for E-cadherin [28]. Zeb1 expression may be induced by MAPK and TGFβ signaling as well as expression of NFκB and Slug [24,88,93,94]. In melanoma, expression of Zeb1 is associated with a poor prognosis and represses E-cadherin expression cooperatively with Slug [24,88]. In contrast, Zeb2 seems to have a tumor-suppressing effect in melanoma and is expressed in normal melanocytes; Zeb2 induces greater expression of MITF and greater cell differentiation [24]. In murine models, loss of Zeb2 resulted in decreased MITF expression, increased Zeb1 expression, and greater melanoma invasion [95]. Overactivation of RAS/RAF/MEK/ERK induces a switch from expression of Zeb2 to Zeb1 and greater malignant potential. A direct correlation has been established linking preservation of Zeb2 expression to a positive prognosis [24].
Nuclear factor kappa B (NFκB)
NFκB has a well-established role in promoting EMT in various malignancies and inhibition of NFκB reverses tumor EMT and inhibits metastasis [9]. Upregulation of NFκB has been found in melanomas exhibiting NRAS, BRAF, and PTEN mutations, suggesting a role for both MAPK and PI3K signaling in NFκB activation
Induction of NFκB promotes expression of pro-mesenchymal proteins. NFκB has been shown to induce EMT via induction of vascular endothelial growth factor (VEGF) expression and receptor translation, increase of MMP expression, and promotion of Snail [26,96]. In addition, overexpression of NFκB and increased localization of NFκB to the nucleus have been associated with faster migration of melanoma cells, and miRNA inhibition of targets directly upstream of NFκB has been shown to reduce melanoma cell migration [97,98]. Recent evidence suggests that NFκB promotes invasion by stabilizing favorable transcription factors. Through NFκB-mediated signaling, Snail is stabilized by inhibition of ubiquitination and degradation, leading to greater invasion [96].
Effects of small molecule inhibitors on EMT in melanoma
Various agents have demonstrated efficacy regulating EMT in melanoma in vitro and in vivo. Small molecule inhibitors offer a specific, targeted approach to regulating EMT in melanoma. Table 1 summarizes the effects of small molecule inhibitors on EMT in melanoma.
Table 1.
Effects of small-molecule inhibitors on EMT in melanoma
| Inhibitors | Targets | Effects | Mechanisms | Reference |
|---|---|---|---|---|
|
| ||||
| Tipifarnib (R115777) | RAS | Unknown | ↓pERK, ↓pAKT | [100] |
|
| ||||
| Lonafarnib | RAS | ↓ Invasion | ↓ mTOR effectors | [101] |
|
| ||||
| Vemurafenib (PLX4032) | BRAFV600E BRAFV600k |
↓ Invasion ↓ Migration, ↓ Motility ↓ Fibroblast activation ↓ Metastasis |
↓ pMEK, pERK ↑ Mart1, gp100 ↓ pERK, Twist1 ↓ MMP-1 ↑ Collagen I synthesis |
[37,92,103,104] |
| ↑ Migration ↑ Migration, ↑ cell elongation |
↑ MMPs ↑ pERK ↑ pAKT |
[106–109] | ||
| ↑ Invasion ↑ Metastasis |
↑ pPI3K, Src/STAT3 ↓ PTEN ↑ pEGFR ↓ MITF, ↑ RAC1, RHO-A, RHO-C ↑ NRAS, urokinase, MMP-1 ↑ RTKs |
[76,102,108, 109,111,112] | ||
|
| ||||
| Dabrafenib (GSK 2118436) | BRAFV600E BRAFV600K |
Unknown | ↓ pMEK, ↓pERK ↑ MAPK effectors ↑ NRAS, ↑ MAPK |
[113–116] |
|
| ||||
| Sorafenib | RAFs, VEGFR, PDGFR, TGFβRs | ↓ Migration, Invasion ↓ Metastasis |
↓ pERK ↓ Fibronectin, ↑ E-cadherin ↓ MMPs, ↓MAPK signaling ↓ Zeb1, Snail1, Slug, Twist1 ↓ Vimentin, ↑ E-cadherin |
[118–121] |
|
| ||||
| Trametinib (GSK1120212) | MEK | ↑ Invasion, ↑mesenchymal phenotype | ↓ pMEK, ↓ pERK ↑ pAKT, Rictor ↓ MIG6 |
[42,43,123–125] |
|
| ||||
| Selumetinib (ARRY- 142886) | MEK | ↑ Invasion ↑ Mesenchymal phenotype ↓ Migration |
↓ pERK ↑ MMPs ↓ CDK5R1 |
[42,126] |
|
| ||||
| PD184352 | MEK | ↑ Invasion ↑ Adhesion |
↑ MMPs ↑ β1-integrin ↑ STAT3 |
[42,43] |
|
| ||||
| U0126 | MEK | ↓ Invasion in A375 | ↓ MMP9 ↓ Urokinase |
[127] |
|
| ||||
| PD98059 | MEK | ↓ Invasion ↓ Migration, ↓invasion |
↓ MMP9 ↓ Urokinase |
[127,128] |
|
| ||||
| Vatalinib | VEGFR | ↓ LN metastasis | ↓ Plasma VEGF | [129] |
|
| ||||
| Erlotinib | EGFR | ↓ Invasion, ↓migration | ↓ pAKT | [130] |
|
| ||||
| LY294002 | PI3K | ↓ Migration, invasion | ↓ pAKT ↑ MITF ↓ MMP-2 in TPras cells |
[64,131,132] |
|
| ||||
| Sarcatinib | Src | ↓ Adhesion ↓ Invasion |
↓ pFAK ↓ Integrin activity |
[42] |
|
| ||||
| Dasatinib | Src | ↓ Migration, ↓ invasion | ↓ pFAK, p130CAS, STAT3, MMP-9 ↓ Ephrin A2, pFAK |
[134,135,137] |
| ↓ Metastasis | ↓ HOXC11 interactions with Src ↓ pSTAT3 |
[109, 138] | ||
Farnesyl Transferase Inhibitors
Farnesyl transferase inhibitors decrease RAS signaling activity by interfering with critical post-translational modification. Tipifarnib is a small molecule inhibitor of farnesyl transferase. Tipifarnib has demonstrated potential as an anti-angiogenic agent in other malignancies such as colon cancer, but this effect has not been observed in melanoma [99]. In a recent Phase II study, significant inhibition of ERK and AKT phosphorylation was found in post-tipifarnib treatment tumors. However, these patients did not benefit clinically from tipifarnib treatment [100]. Lonafarnib is another agent that inhibits farnesyl transferase. Evidence suggests that lonafarnib inhibits invasion of BLM melanoma cells in vitro. These effects were associated with decreased activation of mTOR effectors rather than modulation of upstream MAPK or PI3K effectors [101].
BRAF Inhibitors
The small molecule inhibitor vemurafenib has shown promise for treating metastatic melanoma. This synthetic agent preferentially targets BRAFV600E and inhibits RAF-RAF dimerization [102]. Targeting of BRAFV600E decreases downstream activation of MEK and ERK and upregulates expression of melanoma antigens such as MART-1 and gp100 [103]. The effect of vemurafenib on tumor growth and apoptosis is fairly well-defined, but its effect on EMT pathways is more controversial [102]. Decreased activation of ERK by vemurafenib has been demonstrated in vitro; subsequently, vemurafenib inhibits expression of Twist1 [92]. Vemurafenib repressed migration and motility of cells expressing BRAFV600E by decreasing MMP-1 expression and reducing activation of adjacent fibroblasts in vitro and in murine models and stimulates collagen I synthesis in vitro and in vivo [37,104]. Phase I clinical trials of vemurafenib have demonstrated treatment reduces phosphorylation of ERK which may decrease downstream invasion by curtailing activation of Twist1 [92]. A recent retrospective review revealed a lower incidence of brain metastases in patients with BRAF-mutated melanoma that were treated with vemurafenib before tumor metastasis than patients who were not treated with vemurafenib [105]. These results suggest that vemurafenib may inhibit migration and metastasis of BRAF-mutated melanoma.
However, other studies have shown that vemurafenib paradoxically increases melanoma cell migration. 3D hydrogel models have demonstrated that vemurafenib-treated melanoma cells exhibited increased MMP activity, greater cell elongation, and augmented cell migration [106]. Increased ERK phosphorylation in wild-type BRAF melanoma cell lines has been observed, reducing cell adherence and increasing migration after vemurafenib treatment [107]. In SK-MEL-24 and MEL-HO cell lines, AKT phosphorylation increased after treatment with vemurafenib, while AKT activation decreased in SK-MEL-28, Colo800, and IPC298 BRAF wild-type cells [108]. One challenge associated with BRAF-targeted treatment is the tendency for melanoma to develop resistance to treatment. Activation of PI3K, upregulation of Src/STAT3, and repression of PTEN have been observed in vemurafenib-treated melanoma [102,109]. Increased EGFR phosphorylation was observed in SK-MEL-5, a BRAFV600E-mutated cell line, after treatment with vemurafenib [108]. Treatment resistance and changes in cell signaling in response to vemurafenib may potentiate expression of mesenchymal phenotypes. Fibroblasts from aged-tumor bearing mice were found to secrete Wnt-antagnoist sFRP2 which was associated with greater vemurafenib resistance and melanoma metastasis [110]. In vemurafenib resistant melanoma cell lines, loss of MITF downregulated expression of guanosine monophosphate reductase resulting in greater activation of RAC1, RHO-A, and RHO-C; these changes conferred greater invasive potential to resistant cell lines [76]. Another study found that inhibition of BRAF caused activation of NRAS, increased expression of MMP1, urokinase, and other proteases, and ultimately increased melanoma invasion and metastasis in vivo [111]. Melanoma tumors from clinical trial patients treated with vemurafenib exhibited upregulated RTKs and NRAS in response to therapy [112].
Dabrafenib is another selective inhibitor of BRAFV600E. Like vemurafenib, dabrafenib downregulates activation of MAPK signaling. In some studies, dabrafenib targeting of BRAFV600E decreased downstream phosphorylation of MEK and ERK [113,114]. However, dabrafenib can upregulate MAPK effector activation in BRAF wild-type melanoma [113]. In addition, melanoma resistance to dabrafenib follows a similar pattern to vemurafenib resistance; surviving tumor cells express NRAS-activating mutations and increased activation of MAPK signaling [115]. This effect has been observed in Phase I clinical trials [116]. More research is necessary to elucidate the effect of dabrafenib on melanoma EMT pathways.
The multikinase inhibitor sorafenib targets RAF kinases and multiple growth factor receptors to modulate EMT in melanoma [117]. Sorafenib preferentially targets constitutively-activated, oncogenic BRAF over the wild-type isoform, allowing sorafenib to inhibit MAPK signaling in melanoma [118]. Sorafenib reduced phosphorylation of ERK in melanoma cells expressing BRAF mutations more avidly than wild-type controls [119]. Previous studies have shown that sorafenib reduces migration and metastasis in various cancers by restoring TGFβ induced repression of E-cadherin and fibronectin, inhibiting MAPK signaling, and repressing MMP expression [120]. Recently, similar effects have been demonstrated in melanoma using sorafenib monotherapy in vitro and in vivo. In SK-MEL-28 and A375 cells, sorafenib monotherapy reduced induction of Zeb1, Snail, Twist1, and Slug resulting in decreased vimentin and increased E-cadherin expression. In athymic nude mice, BRAF-mutated xenograft tumor lung metastases were suppressed by sorafenib [121].
MEK Inhibitors
Small-molecule synthetic MEK inhibitors have an unclear effect on melanoma EMT. Trametinib, a newer generation MEK inhibitor, has been associated with increased progression-free survival that is typically attributed to decreased proliferation and increased apoptosis [122]. However, MEK inhibition in melanoma has been shown to increase invasion and induce motile morphology, especially in BRAF and KRAS mutated cell lines [42,43,123,124]. Trametinib treatment decreased phosphorylation of ERK1/2 yet increased activation of AKT and downstream effectors including Rictor in NRAS-mutated SK-MEL-2 cells. Additionally, decreased expression of mitogen-inducible gene 6 (MIG6) was observed and associated with increased migration and invasion. This study suggested that MEK-inhibitor induced invasion may be a result of EGF-induced signaling [125]. Selumetinib treatment has been shown to induce a mesenchymal pro-invasive morphology in A375, and cells treated with selumetinib invaded much faster than controls. Invasion was facilitated despite decreased phosphorylation of ERK in both A375 and WM266-4 via promotion of MMP-2 and MMP-9 expression. Additionally, selumetinib promoted induction of an actin and myosin-mediated mesenchymal phenotype along with increasing integrin-mediated collagen adhesion [42]. However, in uveal melanoma cells selumetinib may decrease melanoma cell migration. MEK inhibition has been shown to downregulate CDK5R1 which has been implicated in cell migration, and treatment of BRAF and G-protein α-subunit q (GNAQ)-mutated cells with selumetinib inhibited migration. Transfection of uveal melanoma cells with siRNA targeting CDK5R1 decreased migration dramatically, confirming a potential role for this protein in selumetinib therapy [126]. PD184352, another experimental small-molecule inhibitor of MEK, demonstrates a similar pro-invasive effect. This pro-invasive effect was associated with increased expression of MMPs in A375 and WM266-4 melanoma cells. Additionally, melanoma cell adhesion was enhanced via β1-integrin activity in melanoma cells treated with selumetinib [42]. MEK-inhibitor induced invasion may be due to increased RTK or Src activation followed by induction of STAT3 [43]. However, not all MEK inhibitors induce invasion in vitro. U0126 is a potent inhibitor of MEK that has been shown to inhibit invasion of A375 cells in Matrigel models. U0126 decreased expression of urokinase plasminogen and MMP-9. PD98059 also reduces invasion by a similar mechanism, reducing both urokinase and MMP-9 expression [127]. In monolayer cultures, PD98059 reduced rate of migration and invasion of SK-MEL-28 cells [128].
Other RTK Inhibitors
Inhibition of various RTK receptors and their ligands may be a viable strategy for reducing melanoma metastasis. Targeting metastasis via VEGF signaling is one possible approach. Vatalinib, a VEGFR inhibitor, may reduce melanoma metastasis. Vatalinib monotherapy significantly reduced tumor growth and lymph node metastasis in BL6/C57 mice injected with B16/BL6 melanoma cells. Plasma VEGF levels were significantly reduced by vatalanib treatment [129]. Another RTK target for melanoma treatment is EGFR. Erlotinib significantly inhibited melanoma invasion and migration in 518 A2, SK-MEL-28, and M24 melanoma cells, potentially via decreased phosphorylation of AKT [130].
Recent evidence has demonstrated that targeting the PI3K/AKT/mTOR axis reduces melanoma progression. Preliminary studies show that LY294002, an inhibitor of PI3K, may reduce melanoma invasion, migration, and mesenchymal protein expression. Initial evidence suggests that LY294002 reduced migration via decreased phosphorylation of AKT and increased expression of MITF [64]. Migration of human neonatal epidermal melanocytes in collagen I-coated plastic was inhibited by LY294002 in the presence of stem cell factor [131]. Additionally, LY294002 reduced invasion via suppression of MMP-2 expression in TPras melanoma cells. These findings were confirmed in murine models [132].
Src Inhibitors
Src kinases are also emerging as a potential target for reducing melanoma invasion and metastasis. Saracatinib is a synthetic small-molecule inhibitor of Src kinase activity. Treatment of melanoma cells with saracatinib decreased FAK phosphorylation by Src and vigorously repressed cell adhesion and invasion in A375 and WM266-4 melanoma cells. The anti-invasive effect of saracatinib was attributed to inhibition of integrin-mediated collagen adhesion [42]. According to a recent Phase II trial, the anti-invasive benefits of saracatinib observed in vitro have not yet translated successfully to improved patient outcomes [133].
Dasatinib is a broader-specificity tyrosine kinase inhibitor with high affinity for Src that may be useful as an anti-invasive agent. Studies have suggested that dasatinib decreases Src activity and subsequent migration via Src/FAK modulation. Invasion through Matrigel matrices was inhibited in A2058 and Lu1205 cell lines in a dose-dependent manner after dasatinib treatment, and migration was decreased as measured by scratch assays. Additionally, downregulation of Src, FAK, and cellular apoptosis susceptibility protein 130 (p130CAS) were noted and may explain the anti-invasive effects of dasatinib treatment. Decreased expression of MMP-9 was observed along with deactivation of Ephrin A2 kinase activity, which has also been associated with migration and invasion [134]. Dasatinib treatment also inhibited migration of SK-MEL-28 cells via decreased phosphorylation of FAK [135]. Migration of metastatic melanoma cells harvested from lymph nodes (MeWo cells) was decreased by dasatinib-mediated inhibition of homeobox C11 (HOXC11) protein interactions with Src [136]. Dasatinib treatment of uveal melanoma cells injected into zebrafish models has also demonstrated potent anti-migratory effects [137]. Mice inoculated with B16-ovalbumin overexpressing (B16-OVA) cells showed decreased extrapulmonary metastases when treated with high doses of dasatanib, but lung metastases were not inhibited [138]. In vemurafenib-resistant cell lines, dasatinib inhibited melanoma invasion in vitro. Vemurafenib-resistant lines of A375 (A375-VR) and Colo829 (Colo829-VR) demonstrated much greater proclivity for invasion than parental cell lines, and invasion was greatly reduced by treatment with dasatinib in both cell lines. In nude mice subcutaneously injected with A375-VR cells, dasatinib treatment reduced phosphorylation of STAT3 downstream of Src family kinase inhibition. Lymph node and lung metastases were powerfully suppressed in NOD/SCID mice bearing tumor xenografts with resistant cell lines [109].
Phytochemicals with known anti-EMT properties
Phytochemicals are compounds that are naturally expressed by various flora. These agents are of particular interest due to their generally low-toxicity profiles. Multiple phytochemical agents demonstrate promise for potential use in melanoma therapy. Table 2 summarizes the known anti-EMT effects of these phytochemicals.
Table 2.
Phytochemicals with anti-EMT properties
| Phytochemicals | Effects | In vitro studies | In vivo studies | References |
|---|---|---|---|---|
|
| ||||
| Fisetin | ↓ Nuclear localization of β-catenin | MEL-928, WM-35 | [140] | |
| ↓ pMEK1/2, pERK1/2, nuclear localization of NFκB | A375, RPMI-7951 | [141] | ||
| ↓ pAKT, p70SK6, pmTOR | A375, 451Lu | A375, 451Lu melanoma xenografts | [142] | |
| ↓ Invasion, metastasis ↓ N-cadherin, vimentin, fibronectin, MMPs ↑ E-cadherin, desmoglein ↓ Snail1, Zeb1, Twist1, Slug |
A375, SK-MEL-28 | A375, SK-MEL-28 subcutaneously injected in athymic nude mice | [121] | |
| ↓ Radial to vertical transition | A375 | [142] | ||
|
| ||||
| Epigallocatechin gallate (EGCG) | ↓ Migration, ↑ E-cadherin | A375 | [146] | |
| ↓ Migration | B16 | [147] | ||
| ↓ Invasion | B16F10 | [148] | ||
| ↓ Adhesion, α2β1 integrin | A2508 | [149] | ||
| ↓ Adhesion | B16 | [150] | ||
| ↓ Vimentin, fibronectin, N-cadherin ↑ E-cadherin, desmoglein, cytokeratin |
A375, Hs294t | [145] | ||
| ↓ MMP-2, pERK1/2 | M17 | [151] | ||
| ↓ pFAK, MMP-9 | B16 | [152] | ||
| ↓ Metastasis | C57BL/6 mice subcutaneously injected with B16F10 | [148] | ||
| ↓ Lung metastasis | B16F10 and B16- F3m cells intraperitonally inoculated in Balb/c mice | [152] | ||
|
| ||||
| Proanthocyandins | ↓ Migration, COX-2, PGE2 ↑ E-cadherin ↓ N-cadherin, vimentin, fibronectin, Slug ↓ ERK1/2, NFκB |
A375, Hs294t | [156] | |
| ↓ β-catenin expression, MMPs, MITF, ↓ PI3K/AKT signaling ↓ Migration |
A375, Hs294t, MEL-1241 | [157] | ||
| ↓ β-catenin expression, PGE2, MMPs | Athymic nude mice intravenously injected with A375 cells | [157] | ||
|
| ||||
| Apigenin | ↓ Motility, migration ↓ STAT-3 nuclear localization ↓ N-cadherin, fibronectin, MMPs, Twist1 ↑ Keratin-8, E-cadherin |
A375, G361 | [159] | |
| ↓ Integrins, pFAK, pERK1/2 | A2058, A375 | [160] | ||
| ↑ Cadherin-catenin complexing | 518A2 | [161] | ||
| ↓ Lung metastasis, VCAM1 | B16-BL6 melanoma cells injected in lateral tail vein of C57BL/6N mice | [162,163] | ||
|
| ||||
| Quercetin | ↓ Invasion | B16-BL6 | [162] | |
| ↓ Migration, Invasion ↓ pSTAT-3, Src, JAK2 ↑ pAKT, pERK |
A375, A2058 | [164] | ||
| ↓ Migration, invasion, c-MET | BRAF-mutated cell lines | [165] | ||
| ↓ Invasion, MMP-9 | B16-BL6 | [166] | ||
| ↓ Lung metastasis, VCAM1 | C57BL/6N mice injected with B16- BL6 cells in lateral tail vein | [163] | ||
| ↓ Liver metastasis | B16M-F10 cells intravenously injected in C57BL/6J mice | [167] | ||
|
| ||||
| Resveratrol | ↓ Migration, invasion ↓ pAKT |
B16F10 | [170] | |
| ↓ β-catenin and MITF nuclear translocation ↓ MMP-9 |
B16 | [171] | ||
| ↓ MMP-1 | Lu1205 | [172] | ||
| ↓ Lung metastasis | B16-BL6 injected subcutaneously in C57BL6 mice | [170] | ||
| ↓ Liver metastasis, adhesion ↓ NF-κB induced inflammation |
Intrasplenic inject of B16 cells in C57BL/6J | [173] | ||
|
| ||||
| Curcumin | ↓ Migration, MMPs ↓ pJAK-2, pSTAT-3 |
A375, B16F10 A375 |
[175,177] | |
| ↓ Vimentin ↑ E-cadherin |
B16 | [176] | ||
| ↓ Lung metastasis | B16F10 cells subcutaneously injected in C57BL/6 mice | [178] | ||
|
| ||||
| Silymarin | ↓ pMEK1/2, RSK-2, NFκB, STAT3 ↓ β-catenin expression and translocation |
SK-MEL-5, SK-MEL-28 | [180] | |
| ↓ MMP-2, MMP-9 ↓ Migration, β-catenin |
A375, Hs294t, MEL-1241 | [181] | ||
|
| ||||
| Lupeol | ↓ β-catenin, MITF, cyclin D1 | Mel 928, Mel 1241 | [183] | |
| ↓ Motility, migration ↑ Actin depolymerization |
B16 2F2 | [184] | ||
| ↓ Metastasis | Clinical trial on canines with malignant melanoma | [185] | ||
|
| ||||
| Genistein | ↓ MMP-2, MMP-9 | 518A2 | [161] | |
| ↓ Liver metastasis | C57BL/6J mice subcutaneously injected with B164A5 cells | [187] | ||
Fisetin
Fisetin is a flavonoid derived from strawberries, mangoes, apples, grapes, persimmons, onions, tomatoes, and cucumbers [13,139]. Recent studies show that fisetin interferes with key regulators of EMT and may induce MET. Fisetin affects multiple signaling pathways important to EMT. Fisetin decreased expression and nuclear localization of β-catenin in Mel928 and WM-35 cells [140]. Phosphorylation of MEK1/2 and ERK1/2 and nuclear translocation of NFκB were suppressed by fisetin treatment in A375 and RPMI-7951 cells [141]. Treatment with fisetin also decreased activation of AKT in A375 and 451Lu cells in vitro as well as in vivo. Additionally, fisetin dephosphorylated p70S6K and deactivated mTOR in A375 and 451Lu cells by direct-binding [142]. Similarly, fisetin decreased expression of mesenchymal markers N-cadherin, vimentin, and fibronectin and increased expression of epithelial markers E-cadherin and desmoglein in various melanoma cell lines. Fisetin-induced inhibition of MMPs and subsequent repression of invasion has been reported in BRAF-mutated, NRAS-mutated, and BRAF/NRAS wild-type melanoma cells [141,143]. In A375 and SK-MEL-28 cells, fisetin treatment repressed expression of Snail1, Zeb1, Twist1, and Slug. These results were confirmed in vivo in athymic nude mice subcutaneously implanted with A375 and SK-MEL-28 cells [121]. Recently, fisetin was found to prevent transition of melanoma from radial to vertical growth in 3D models using A375 cells [142]. These findings suggest that fisetin may inhibit growth, invasion, and metastasis of melanoma and may induce repression of melanocyte mesenchymal phenotype.
Epigallocatechin Gallate (EGCG)
EGCG a polyphenol derived from green tea, promotes epithelial character in melanoma [144]. EGCG has been reported to inhibit components of MAPK, PI3K, Wnt, and JAK/STAT signaling [145]. In A375, EGCG inhibited migration in matrigel assays via upregulation of E-cadherin expression [146]. Transwell assays indicated reduced migration of B16 cell lines treated with EGCG [147]. Induction of migration and invasion of B16F10 cells via hepatocyte growth factor/scatter factor (HGF/SF) signaling was reduced by EGCG treatment [148]. Adhesion of fibroblasts to matrix proteins including collagen, fibronectin, and fibrinogen cells was attenuated by EGCG and decreased A2508 melanoma cell tube formation when co-cultured via downregulation of α2β1 integrin [149]. EGCG also inhibited laminin adhesion to B16 melanoma cells [150]. Treating A375 and Hs294t cells with EGCG promoted expression of E-cadherin, desmoglein, and cytokeratin while decreasing expression of vimentin, fibronectin, and N-cadherin in a dose-dependent fashion [145]. In M17 uveal melanoma cells, EGCG treatment inhibited MMP-2 secretion, increased expression of TIMP-2 and RECK (MMP-2 inhibitors), and decreased phosphorylation of ERK1/2 [151]. Additionally, phosphorylation of FAK and activity of MMP-9 are repressed by EGCG [152]. Studies have consistently reaffirmed that EGCG decreases NFκB expression and nuclear localization [145,153–155]. In vivo experiments reinforce the promise demonstrated by EGCG as a potential melanoma treatment agent. In C57BL/6 mice inoculated with HGF/SF transfected B16F10 melanoma cells, EGCG treated mice exhibited lesser invasion and metastases of B16F10 cells [148]. Injection of EGCG into B16-F3m melanoma-bearing male Balb/c mice reduced the number of metastatic lung nodules [152].
Proanthocyandins
Proanthocyandins are flavonoid polyphenols found in grapes and wine. Grape seed proanthocyandins (GSPs) induce and promote mesenchymal-epithelial transition. In A375 and Hs294t cells, GSPs potently inhibited migration by decreasing expression of COX-2 and PGE2; this effect was associated with increased E-cadherin and decreased vimentin, fibronectin, and N-cadherin expression. In addition, GSP treatment decreased activation of NFκB and ERK1/2 and reduced expression of Slug [156]. A subsequent study revealed that GSPs reduced intracellular accumulation of β-catenin resulting in downregulation on MMP-2, MMP-9 and MITF expression in A375 and Hs294t cells. PI3K/AKT axis signaling was also suppressed by GSP treatment, and the migratory capacity of these cell lines was reduced in vitro. These results were confirmed in β-catenin activated cells (Mel-1241), while β-catenin inactivated cells (Mel-1011) retained their pro-migratory protein expression profiles. Downregulation of the β-catenin pathway after GSP treatment was confirmed in vivo using athymic nude mice injected with A375 cells. Treated mice expressed lower levels of PGE2, MMP-2, and MMP-9 than control mice [157]. These results demonstrate that proanthocyandins are a good candidate for further study of their effects on melanoma EMT.
Apigenin
Apigenin is a naturally occurring flavone derived from parsley, onions, and chamomile tea [158]. Apigenin has been shown to inhibit melanoma cell migration and invasion. Motility and migration of A375 cells were significantly reduced by apigenin treatment and, migration of G361 cells with constitutively expressed STAT-3 was also impaired. Apigenin downregulated STAT-3 signaling by decreasing STAT-3 nuclear localization. STAT-3 inhibition was associated with downregulation of N-cadherin, fibronectin, MMP-2, MMP-9, and Twist1 and upregulation of keratin-8 and E-cadherin. Overexpression of Twist1 restored the invasive and migratory potential of A375 cells treated with apigenin [159]. Additionally, apigenin treatment decreased integrin expression, inhibited the phosphorylation of FAK, and decreased activation of ERK1/2 in A2058 and A375 cells [160]. Cadherin-catenin complex formation was upregulated by apigenin treatment in 518A2 melanoma cells, suggesting another potential mechanism for migration inhibition [161]. In vivo studies have revealed that apigenin exhibits anti-metastatic properties in melanoma [162]. Lung colonization of B16-BL6 melanoma cells C57BL/6N mice was inhibited by intraperitoneal injection of apigenin. This effect was attributed to decreased TNF-α induced VCAM-1 expression in lung endothelium [163].
Quercetin
Quercetin is an extremely common dietary polyphenolic flavonoid found in onions, grains, and other fruits and vegetables. In B16-BL6 cells, quercetin treatment inhibited invasion more potently than EGCG, resveratrol, or apigenin [162]. Treatment with quercetin inhibited migration and invasion of A375 and A2058; this effect was associated with suppression of STAT-3 phosphorylation via downregulation of Src and JAK2. However, concurrent activation and upregulation AKT and ERK were observed [164]. Additionally, HGF-stimulated migration and invasion of BRAF-mutated melanoma cells was suppressed by quercetin via c-Met downregulation [165]. Quercetin treatment also inhibited invasion in B16-BL6 cells by decreasing expression of MMP-9 via Protein Kinase C pathway signaling without affecting adhesion to laminin, fibronectin, or collagen [166]. Quercetin has been shown to inhibit melanoma metastasis in vivo. Treatment with quercetin decreased lung colonization of B16-BL6 melanoma cells in C57BL/6N mice via decreased TNF-α induced VCAM-1 expression in lung endothelium [163]. Intravenous administration of quercetin inhibited liver metastasis of B16M-F10 cells in C57BL/6J mice [167].
Resveratrol
Resveratrol, a stilbenoid found in grape skins, peanuts and mulberries, exhibits chemopreventive characteristics in skin [168,169]. Evidence suggests that resveratrol inhibits EMT-inducing pathways in melanoma. Treatment with resveratrol decreased migration and invasion by inhibiting activation of AKT in B16F10 cells [170]. Resveratrol decreased invasion of melanoma cells via downregulation of β-catenin and MITF nuclear translocation in murine B16 melanoma cells. Additionally, α-melanocyte stimulating hormone (α-MSH)–induced MMP-9 expression was suppressed [171]. Through inhibition of nitric-oxide mediated tumor progression, resveratrol reduced expression of MMP-1 in Lu1205 cells [172]. In vivo studies have demonstrated that resveratrol inhibits melanoma metastasis. In C57BL6 mice subcutaneously injected with B16-BL6 cells, oral treatment with resveratrol decreased lung metastasis [170]. Inhibition of interleukin-dependent adhesion and NFκB-induced inflammation reduced liver metastasis volume and density in mice injected with B16 cells [173].
Curcumin
Curcumin is a natural phenol present in turmeric, a spice in the ginger family [174]. Curcumin inhibited migration and decreased expression of MMP-2 and MMP-9 in A375 cells, and these effects were associated with decreased phosphorylation of JAK-2 and STAT-3 [175]. Curcumin also blocked TNF-α-induced upregulation of EMT markers in B16 cells; treated cells expressed less vimentin and more E-cadherin than controls [176]. Other studies have confirmed that treatment with curcumin decreased migration of B16F10 cells through collagen matrices via inhibition of MMPs [177]. In murine models, oral administration of curcumin inhibited formation of lung nodules after inoculation with B16F10 melanoma cells, suggesting an anti-metastatic effect [178].
Silymarin
Silymarin is a polyphenolic flavonoid extract of milk thistle that induces pro-epithelial characteristic and represses mesenchymal phenotype. The main active compounds in this extract is silybin also known as silibinin. This compound has demonstrated marked anti-metastatic effects in multiple types of cancer [179]. Silymarin and its constituents decrease activation of EMT-inducing pathway proteins. Silybin directly binds MEK1/2 and ribosomal S6 kinase (RSK)-2 to inhibit kinase activity. Decreased activation of these pathway proteins reduced downstream activation of NFκB and STAT3 in SK-MEL-5 and SK-MEL-28 melanoma cells [180]. Treatment with silymarin inhibited β-catenin expression and β-catenin nuclear translocation in A375 and Hs294t cells. Silymarin also inhibited expression of MMP-2 and MMP-9. Subsequent inhibition of migration in vitro by targeting β-catenin signaling with silymarin was confirmed using MEL-1011 and MEL-1241 cell lines [181].
Lupeol
Lupeol is triterpenoid found in a wide variety of flora including mangos, olives, and various types of berries [182]. Lupeol avidly targets Wnt/β-catenin signaling. In melanoma cell lines expressing constitutive activation of β-catenin, treatment with lupeol decreased β-catenin nuclear localization and repressed expression of Wnt/β-catenin target genes MITF and Cyclin D1 [183]. Furthermore, evidence suggests that lupeol may suppress melanoma motility and migration by promoting actin depolymerization in B16 2F2 cells [184]. Recently, a clinical trial studied the effect of lupeol on metastasis of canine malignant melanoma and found that no canines treated with postoperative lupeol subcutaneous injections developed melanoma metastases [185]. These effects make lupeol a promising subject for future study.
Genistein
Genistein is an isoflavone derived from legumes such as soy and fava beans. Initial evidence suggests that genistein modulates melanoma cell invasion by interfering with tyrosine-motif phosphorylation [186]. In B16F0 cells, genistein significantly impeded melanoma migration in vitro via reduced activity of urokinases. Treatment with genistein also downregulated MMP-2 and MMP-9 expression in 518A2 melanoma cells [161]. Genistein treatment inhibited melanoma liver metastasis in C57BL/6J mice subcutaneously injected with B164A5 [187].
Combination Treatments May Effectively Inhibit Melanoma EMT
Combination drug treatments have demonstrated more promising results than targeted monotherapy because combination treatments offer a potential solution to problems with resistance to targeted therapies. Little research has been conducted to directly study the effects of combination treatments on epithelial to mesenchymal transition pathways in melanoma, but studies have gathered some preliminary evidence. Table 3 summarizes the effects of various combination treatment approaches on EMT in melanoma.
Table 3.
Effects of combination therapies on target molecules, invasion, and metastasis
| Combination | Effects | In vitro studies | In vivo studies | Reference |
|---|---|---|---|---|
|
| ||||
| Vemurafenib + erlotinib | ↓ pEGFR | SK-MEL-5 | [108] | |
|
| ||||
| Vemurafenib + PET16 | ↓ Metastasis | 1205Lu xenograft in NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ mice | [188] | |
|
| ||||
| Sorafenib + lonafarnib | ↓ Invasion | BLM | [101] | |
|
| ||||
| Sorafenib + wortmannin | ↓ Invasion, migration | SK-MEL-28 | [128] | |
|
| ||||
| Sorafenib + fisetin | ↓ pMEK1/2, pERK1/2, PI3K (p110α), pAKT, pmTOR ↑ PTEN |
A375, SK-MEL-28 | A375, SK-MEL-28 subcutaneously implanted in athymic nude mice | [189] |
| ↓ Invasion, migration ↓ MMP-2, MMP-9 ↓ N-cadherin, vimentin, fibronectin ↑ E-cadherin ↓ Snail, Twist1, Zeb1, Slug |
A375, SK-MEL-28 | A375, SK-MEL-28 subcutaneously injected in athymic nude mice | [121] | |
| ↓ Lung metastasis | A375, SK-MEL-28 subcutaneously injected in athymic nude mice | [121] | ||
|
| ||||
| LYS294002 + PD98059 | ↓ Invasion, migration | SK-MEL-28 | [128] | |
|
| ||||
| Selumetinib + MK-8033 | ↓ Migration ↓ pERK1/2, HGF signaling |
Uveal melanoma cell lines | [191] | |
|
| ||||
| Saracatanib + selumetinib | ↓ Invasion, migration, adhesion | A375 | [42] | |
|
| ||||
| CPA7 + UO126 | ↑ Epithelial phenotype | WM983B, WM3918 | [43] | |
|
| ||||
| Vatalinib + everolimus | ↓ Lymph node metastasis ↓ Plasma VEGF |
B16-BL6 injected in the derma of both ears of C57BL/6 mice | [129] | |
|
| ||||
| Vatalinib + fingolimod | ↓ Lymph node metastasis | B16-BL6 injected in C57BL/6 mice | [192] | |
|
| ||||
| Erlotinib + bevacizumab | ↓ Lymph node, lung metastasis ↓ CD31 |
518A2 injected into C.B-17 SCID mice | [130] | |
|
| ||||
| Quercetin + sulforaphane | ↓ Migration ↓ MMP-9 |
B16F10 | [193] | |
|
| ||||
| EGCG + decarbazine | ↓ Lung metastasis | B16-F3m injected in Balb/c mice | [152] | |
Vemurafenib has been studied extensively in combination with various other agents in an attempt to offer a solution to vemurafenib resistance and subsequent monotherapy failure. In vemurafenib-treated SK-MEL-5 cells featuring upregulated EGFR, treatment with erlotinib effectively blocked phosphorylation of EGFR [108]. PET16, an HSP70 inhibitor, synergistically decreased invasion of 1205Lu xenografts injected into the right flank of NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ mice when paired with vemurafenib treatment in vivo [188].
In combination with other synthetic and natural agents, sorafenib has demonstrated noteworthy efficacy in vitro and in vivo. In organotypic culture, combination of lonafarnib with sorafenib completely suppressed melanoma vertical growth while potentiating apoptosis of BLM melanoma cells [101]. Sorafenib in combination with wortmannin, a potent PI3K inhibitor, eliminated migration and invasion of SK-MEL-28 melanoma cells in vitro [128]. In vitro studies using A375 and SK-MEL-28 demonstrated that fisetin combined with sorafenib decreased invasion, migration, pMEK1/2, pERK1/2, PI3K activation (p110α), pAKT, and pmTOR while increasing PTEN. These results were confirmed in athymic nude mice [189]. Combination treatment using sorafenib and fisetin vigorously repressed migration, invasion, and expression of MMP-2 and MMP-9 compared with monotherapy. Similarly, the combination demonstrated potent anti-EMT potential by decreasing N-cadherin, vimentin, and fibronectin expression while increasing E-cadherin expression. EMT-TFs including Zeb1, Snail1, Twist1, and Slug were decreased in A375 and SK-MEL-28 after treatment. This combination also dramatically reduced lung metastases compared with monotherapy using either agent [121]. A phase I study combining sorafenib with temsirolimus found little inhibition of MAPK signaling following combination treatment [190].
Studies have shown that MEK inhibitors combined with MAPK, PI3K, Src, and other pathway targets may reduce invasion and metastasis. Combination of LYS294002 with MEK inhibitors such as PD98059 has been shown to synergistically reduce invasion and migration in SK-MEL-28 melanoma cells [128]. Selumetinib combined with the c-Met inhibitor MK-8033 decreased migration of uveal melanoma cell lines of different genetic backgrounds. These results may be due to repression of HGF signaling along with downregulation of ERK1/2 [191]. Combination of MEK inhibitors with saracatinib yields both cytotoxic and anti-invasive effects. In vitro studies using A375 cells have shown that combining saracatinib with selumetinib potently suppressed melanoma cell growth and invasion in 3D collagen models. Similarly, migration of cells treated with low concentrations of saracatanib in combination with selumetinib was potently inhibited, and saracatanib blocked selumetinib-induced increases in adhesion to collagen [42]. STAT3 inhibitor CPA-7 used in combination with UO126 reversed the induction of invasive phenotypes observed in MEK inhibitor monotherapies using WM983B (BRAFV600E) and WM3918 (BRAF/NRAS wild-type) [43].
Drugs targeting RTKs have demonstrated anti-metastatic potential when used in combinatorial treatments. In combination with vatalanib, everolimus significantly suppressed melanoma lymph node metastasis of B16-BL6 cells injected in the derma of both ears of C57BL/6 mice much more effectively than monotherapy using either agent. VEGF concentrations in plasma and lymph node metastases were significantly reduced by this combination regiment [129]. Inhibition of sphingosine-1-phosphate receptors by fingolimod combined with vatalanib reduced melanoma lymph node metastasis in C57BL/6 mice injected with B16-BL6 melanoma cells [192]. Bevacizumab and erlotinib synergistically suppressed lymph node and lung metastasis via decreased angiogenesis in 518A2 cells injected into C.B-17 SCID mice. The anti-metastatic effect of this combination was attributed to decreased expression of CD31 [130].
Phytochemicals have also been combined with other phytochemicals and classic chemotherapeutic drugs to inhibit melanoma EMT. In assays using B16F10 cells, quercetin combined with sulforaphane reduced migration by decreasing recruitment of MMP-9 [193]. EGCG combined with decarbazine significantly decreased the burden of B16-F3m lung metastases in Balb/c mice compared with decarbazine or EGCG monotherapy [152].
Conclusion and Future Directions
Our understanding of melanoma and EMT continues to evolve rapidly, yet the clinical efficacy of metastatic melanoma treatments has not kept pace. We now understand that many signaling pathways interact to induce tumor progression. Induction of these pathways produces a spectrum of phenotypic changes that can ultimately lead to deadly metastatic disease. The connection of signaling pathways to transcription factors to EMT events has revealed potential targets for limiting or reversing melanoma invasion, migration, and metastasis. Currently, a great deal of resources is being allocated to studying therapies that will inhibit proliferation and induce melanoma apoptosis; however, this approach only addresses one particular portion of the metastatic melanoma equation. Recent preclinical and clinical research has revealed that synthetic compounds targeting pathways activated in melanoma may limit tumor migration and motility. Additionally, new evidence has emerged that supports the role of phytochemicals as potential adjuvant therapies to limit the progression of metastatic melanoma. Preclinical studies show that these natural compounds are flexible due to multifaceted mechanistic effects and demonstrate low toxicity profiles. Future studies should be conducted to carefully elucidate the effect of synthetic and phytochemical agents on melanoma invasion, migration, and metastasis in vitro, in vivo, and eventually in patients. Overall, melanoma is a complex disease, and all potential treatment opportunities must be considered to combat progression.
Highlights.
Signaling dysregulation induces expression of EMT-TFs in melanoma.
EMT-TFs favor loss of epithelial character and induction of invasive phenotypes.
Modulation of EMT signaling pathways is a potential therapeutic strategy for reducing invasion and metastasis of melanoma.
Various phytochemicals inhibit EMT, invasion and metastasis.
Small molecule inhibitors may offer specific approaches to regulating EMT.
Acknowledgments
This work was supported by grants from NIH (1R21CA173043) and ACS-IRG (IRG-60-001-53).
Footnotes
Conflicts of Interest: No potential conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Harvey VM, Patel H, Sandhu S, Wallington SF, Hinds G. Social determinants of racial and ethnic disparities in cutaneous melanoma outcomes. Cancer Control. 2014;21:343–349. doi: 10.1177/107327481402100411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller AJ, Mihm MC. Melanoma. N Engl J Med. 2006;355:51–65. doi: 10.1056/NEJMra052166. [DOI] [PubMed] [Google Scholar]
- 4.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, Eggermont AM, Flaherty KT, Gimotty PA, Kirkwood JM, McMasters KM, Mihm MC, Morton DL, Ross MI, Sober AJ, Sondak VK. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russo AE, Torrisi E, Bevelacqua Y, Perrotta R, Massimo L, McCubrey JA, Spandidos DA, Stivala F, Malaponte G. Melanoma: Molecular pathogenesis and emerging target therapies (review) Int J Oncol. 2009;34:1481–1489. doi: 10.3892/ijo_00000277. [DOI] [PubMed] [Google Scholar]
- 6.Gaggioli C, Sahai E. Melanoma invasion - Current knowledge and future directions. Pigment Cell Res. 2007;20:161–172. doi: 10.1111/j.1600-0749.2007.00378.x. [DOI] [PubMed] [Google Scholar]
- 7.Crowson AN, Magro CM, Mihm MC. Prognosticators of melanoma, the melanoma report, and the sentinel lymph node. Mod Pathol. 2006;19(Suppl 2):S71–S87. doi: 10.1038/modpathol.3800517. [DOI] [PubMed] [Google Scholar]
- 8.Chin L. The genetics of malignant melanoma: lessons from mouse and man. Nat Rev Cancer. 2003;3:559–570. doi: 10.1038/nrc1145. [DOI] [PubMed] [Google Scholar]
- 9.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 10.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 11.Kalluri R, Weinberg RA. The basiscs of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin K, Baritaki S, Militello L, Malaponte G, Bevelacqua Y, Bonavida B. The role of B-RAF mutations in melanoma and the induction of EMT via dysregulation of the NF-κB/Snail/RKIP/PTEN circuit. Genes Cancer. 2010;1:409–420. doi: 10.1177/1947601910373795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strickland LR, Pal HC, Elmets CA, Afaq F. Targeting drivers of melanoma with synthetic small molecules and phytochemicals. Cancer Lett. 2015;359:20–35. doi: 10.1016/j.canlet.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones V, Katiyar SK. Emerging phytochemicals for prevention of melanoma invasion. Cancer Lett. 2013;335:251–258. doi: 10.1016/j.canlet.2013.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–96. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papageorgis P. TGFβ signaling in tumor initiation, epithelial-to-mesenchymal transition, and metastasis. J Oncol. 2015;2015 doi: 10.1155/2015/587193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeanes A, Gottardi CJ, Yap AS. Cadherins and cancer: how does cadherin dysfunction promote tumor progression? Oncogene. 2008;27:6920–6929. doi: 10.1038/onc.2008.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: New insights in signaling, development, and disease. J Cell Biol. 2006;172:973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008;68:3645–3654. doi: 10.1158/0008-5472.CAN-07-2938. [DOI] [PubMed] [Google Scholar]
- 20.Chen A, Beetham H, Black MA, Priya R, Telford BJ, Guest J, Wiggins GAR, Godwin TD, Yap AS, Guilford PJ. E-cadherin loss alters cytoskeletal organization and adhesion in non-malignant breast cells but is insufficient to induce an epithelial-mesenchymal transition. BMC Cancer. 2014;14:552. doi: 10.1186/1471-2407-14-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hollestelle A, Peeters JK, Smid M, Timmermans M, Verhoog LC, Westenend PJ, Heine AA, Chan A, Sieuwerts AM, Wiemer EA, Klijn JG, van der Spek PJ, Foekens JA, Schutte M, den Bakker MA, Martens JW. Loss of E-cadherin is not a necessity for epithelial to mesenchymal transition in human breast cancer. Breast Cancer Res Treat. 2013;138:47–57. doi: 10.1007/s10549-013-2415-3. [DOI] [PubMed] [Google Scholar]
- 22.Puisieux A, Brabletz T, Caramel J. Oncogenic roles of EMT-inducing transcription factors. Nat Cell Biol. 2014;16:488–494. doi: 10.1038/ncb2976. [DOI] [PubMed] [Google Scholar]
- 23.Muqbil I, Wu J, Aboukameel A, Mohammad RM, Azmi AS. Snail nuclear transport: The gateways regulating epithelial-to-mesenchymal transition? Semin Cancer Biol. 2014;27:39–45. doi: 10.1016/j.semcancer.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caramel J, Papadogeorgakis E, Hill L, Browne G, Richard G, Wierinckx A, Saldanha G, sborne J, Hutchinson P, Tse G, Lachuer J, Puisieux A, Pringle JH, Ansieau S, Tulchinsky E. A Switch in the Expression of Embryonic EMT-Inducers Drives the Development of Malignant Melanoma. Cancer Cell. 2013;24:466–480. doi: 10.1016/j.ccr.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 25.Vachtenheim J, Ondrušová L. Microphthalmia-associated transcription factor expression levels in melanoma cells contribute to cell invasion and proliferation. Exp Dermatol. 2015;24:481–484. doi: 10.1111/exd.12724. [DOI] [PubMed] [Google Scholar]
- 26.Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 2013;12:86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta PB, Kuperwasser C, Brunet JP, Ramaswamy S, Kuo WL, Gray JW, Naber SP, Weinberg RA. The melanocyte differentiation program predisposes to metastasis after neoplastic transformation. Nat Genet. 2005;37:1047–1054. doi: 10.1038/ng1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang P, Sun Y, Ma L. ZEB1: At the crossroads of epithelial-mesenchymal transition, metastasis and therapy resistance. Cell Cycle. 2015;14:481–487. doi: 10.1080/15384101.2015.1006048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sullivan RJ, Fisher DE. Understanding the Biology of Melanoma and Therapeutic Implications. Hematol Oncol Clin North Am. 2014;28:437–453. doi: 10.1016/j.hoc.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van ’t Veer LJ, Burgering BM, Versteeg R, Boot AJ, Ruiter DJ, Osanto S, Schrier PI, Bos JL. N-ras mutations in human cutaneous melanoma from sun-exposed body sites. Mol Cell Biol. 1989;9:3114–3116. doi: 10.1128/mcb.9.7.3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Platz A, Egyhazi S, Ringborg U, Hansson J. Human cutaneous melanoma; a review of NRAS and BRAF mutation frequencies in relation to histogenetic subclass and body site. Mol Oncol. 2008;1:395–405. doi: 10.1016/j.molonc.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krauthammer M, Kong Y, Bacchiocchi A, Evans P, Pornputtapong N, Wu C, Mccusker JP, Ma S, Cheng E, Straub R, Serin M, Bosenberg M, Ariyan S, Narayan D, Sznol M, Kluger HM, Mane S, Schlessinger J, Lifton RP, Halaban R. Exome sequencing identifies recurrent mutations in NF1 and RASopathy genes in sun-exposed melanomas. Nat Genet. 2015;47:1–9. doi: 10.1038/ng.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Montalto G, Cervello M, Nicoletti F, Fagone P, Malaponte G, Mazzarino MC, Candido S, Libra M, Bäsecke J, Mijatovic S, Maksimovic-Ivanic D, Milella M, Tafuri A, Cocco L, Evangelisti C, Chiarini F, Martelli AM. Mutations and deregulation of Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR cascades which alter therapy response. Oncotarget. 2012;3:954–987. doi: 10.18632/oncotarget.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiappetta C, Proietti I, Soccodato V, Puggioni C, Zaralli R, Pacini L, Porta N, Skroza N, Petrozza V, Potenza C, Della Rocca C, Di Cristofano C. BRAF and NRAS mutations are heterogeneous and not mutually exclusive in nodular melanoma. Appl Immunohistochem Mol Morphol. 2015;23:172–177. doi: 10.1097/PAI.0000000000000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson Ba, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JWC, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 36.Karasarides M, Chiloeches A, Hayward R, Niculescu-Duvaz D, Scanlon I, Friedlos F, Ogilvie L, Hedley D, Martin J, Marshall CJ, Springer CJ, Marais R. B-RAF is a therapeutic target in melanoma. Oncogene. 2004;23:6292–6298. doi: 10.1038/sj.onc.1207785. [DOI] [PubMed] [Google Scholar]
- 37.Whipple CA, Brinckerhoff CE. BRAF(V600E) melanoma cells secrete factors that activate stromal fibroblasts and enhance tumourigenicity. Br J Cancer. 2014;111:1625–1633. doi: 10.1038/bjc.2014.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wellbrock C, Rana S, Paterson H, Pickersgill H, Brummelkamp T, Marais R. Oncogenic BRAF regulates melanoma proliferation through the lineage specific factor MITF. PLoS One. 2008;3 doi: 10.1371/journal.pone.0002734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Villanueva J, Vultur A, Lee JT, Somasundaram R, Cipolla AK, Wubbenhorst B, Xu X, Phyllis A, Kee D, Santiago-walker AE, Letrero R, Andrea KD, Pushparajan A, Hayden JE, Brown KD, Laquerre S, Mcarthur GA, Sosman JA, Nathanson KL, Herlyn M. NIH Public Access. Cancer. 2011;18:683–695. [Google Scholar]
- 40.Fedorenko IV, Gibney GT, Sondak VK, Smalley KSM. Beyond BRAF: where next for melanoma therapy? Br J Cancer. 2015;112:217–226. doi: 10.1038/bjc.2014.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salama AKS, Kim KB. Trametinib (GSK1120212) in the treatment of melanoma. 2013:619–627. doi: 10.1517/14656566.2013.770475. [DOI] [PubMed] [Google Scholar]
- 42.Ferguson J, Arozarena I, Ehrhardt M, Wellbrock C. Combination of MEK and SRC inhibition suppresses melanoma cell growth and invasion. Oncogene. 2013;32:86–96. doi: 10.1038/onc.2012.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vultur A, Villanueva J, Krepler C, Rajan G, Chen Q, Xiao M, Li L, Gimotty PA, Wilson M, Hayden J, Keeney F, Nathanson KL, Herlyn M. MEK inhibition affects STAT3 signaling and invasion in human melanoma cell lines. Oncogene. 2014;33:1850–61. doi: 10.1038/onc.2013.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fenouille N, Tichet M, Dufies M, Pottier A, Mogha A, Soo JK, Rocchi S, Mallavialle A, Galibert MD, Khammari A, Lacour JP, Ballotti R, Deckert M, Tartare-Deckert S. The epithelial-mesenchymal transition (EMT) regulatory factor SLUG (SNAI2) is a downstream target of SPARC and AKT in promoting melanoma cell invasion. PLoS One. 2012;7 doi: 10.1371/journal.pone.0040378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Slipicevic A, Holm R, Nguyen MTP, Bøhler PJ, Davidson B, Flørenes VA. Expression of activated Akt and PTEN in malignant Melanomas: Relationship with clinical outcome. Am J Clin Pathol. 2005;124:528–536. doi: 10.1309/YT58WWMTA6YR1PRV. [DOI] [PubMed] [Google Scholar]
- 46.Vivanco I, Chen ZC, Tanos B, Oldrini B, Hsieh WY, Yannuzzi N, Campos C, Mellinghoff IK. A kinase-independent function of AKT promotes cancer cell survival. Elife. 2014;3:1–13. doi: 10.7554/eLife.03751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nathanson KL, Martin AM, Wubbenhorst B, Greshock J, Letrero R, D’Andrea K, O’Day S, Infante JR, Falchook GS, Arkenau HT, Millward M, Brown MP, Pavlick A, Davies MA, Ma B, Gagnon R, Curtis M, Lebowitz PF, Kefford R, Long GV. Tumor genetic analyses of patients with metastatic melanoma treated with the BRAF inhibitor dabrafenib (GSK2118436) Clin Cancer Res. 2013;19:4868–4878. doi: 10.1158/1078-0432.CCR-13-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dankort D, Curley DP, Cartlidge RA, Nelson B, Anthony N, Damsky WE, Jr, You MJ, Depinho RA, McMahon M, Bosenberg M. BRAF V600E cooperates with PTEN silencing to elicit metastatic melanoma. Nat Genet. 2009;41:544–552. doi: 10.1038/ng.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C. PI3K/Akt and apoptosis: size matters. Oncogene. 2003;22:8983–8998. doi: 10.1038/sj.onc.1207115. [DOI] [PubMed] [Google Scholar]
- 50.Liu L, Parent CA. TOR kinase complexes and cell migration. J Cell Biol. 2011;194:815–824. doi: 10.1083/jcb.201102090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Betz C, Hall MN. Where is mTOR and what is it doing there? J Cell Biol. 2013;203:563–574. doi: 10.1083/jcb.201306041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Masui K, Cavenee WK, Mischel PS. MTORC2 in the center of cancer metabolic reprogramming. Trends Endocrinol Metab. 2014;25:364–373. doi: 10.1016/j.tem.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laplante M, Sabatini DM. An Emerging Role of mTOR in Lipid Biosynthesis. Curr Biol. 2009;19:R1046–R1052. doi: 10.1016/j.cub.2009.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lamouille S, Connolly E, Smyth JW, Akhurst RJ, Derynck R. TGF-beta-induced activation of mTOR complex 2 drives epithelial-mesenchymal transition and cell invasion. J Cell Sci. 2012;125:1259–1273. doi: 10.1242/jcs.095299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 56.MacDonald BT, Tamai K, He X. Wnt/β-catenin signaling:components, mechanisms, and diseases, NIH Public Access. Dev Biol. 2010;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ray S, Foote HP, Lechler T. β-Catenin protects the epidermis from mechanical stresses. J Cell Biol. 2013;202:45–52. doi: 10.1083/jcb.201212140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung MC. Dual regulation of Snail by GSK-3-beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol. 2004;6:931–940. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
- 59.Schmalhofer O, Brabletz S, Brabletz T. E-cadherin, β-catenin, and ZEB1 in malignant progression of cancer. Cancer Metastasis Rev. 2009;28:151–166. doi: 10.1007/s10555-008-9179-y. [DOI] [PubMed] [Google Scholar]
- 60.Chien AJ, Moore EC, Lonsdorf AS, Kulikauskas RM, Rothberg BG, Berger AJ, Major MB, Hwang ST, Rimm DL, Moon RT. Activated Wnt/beta-catenin signaling in melanoma is associated with decreased proliferation in patient tumors and a murine melanoma model. Proc Natl Acad Sci U S A. 2009;106:1193–1198. doi: 10.1073/pnas.0811902106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arozarena I, Bischof H, Gilby D, Belloni B, Dummer R, Wellbrock C. In melanoma, beta-catenin acts as suppressor of invasion through a cell-type specific mechanism. Oncogene. 2011;30:4531–4543. doi: 10.1038/onc.2011.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ploper D, Taelman VF, Robert L, Perez BS, Titz B, Chen HW, Graeber TG, von Euw E, Ribas A, De Robertis EM. MITF drives endolysosomal biogenesis and potentiates Wnt signaling in melanoma cells. Proc Natl Acad Sci U S A. 2015;112:E420–E429. doi: 10.1073/pnas.1424576112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zavadil J, Böttinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–5774. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 64.Schlegel NC, von Planta A, Widmer DS, Dummer R, Christofori G. PI3K signalling is required for a TGFβ-induced epithelial-mesenchymal-like transition (EMT-like) in human melanoma cells. Exp Dermatol. 2015;24:22–28. doi: 10.1111/exd.12580. [DOI] [PubMed] [Google Scholar]
- 65.Javelaud D, Delmas V, Möller M, Sextius P, André J, Menashi S, Larue L, Mauviel A. Stable overexpression of Smad7 in human melanoma cells inhibits their tumorigenicity in vitro and in vivo. Oncogene. 2005;24:7624–7629. doi: 10.1038/sj.onc.1208900. [DOI] [PubMed] [Google Scholar]
- 66.Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004;4:470–480. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- 67.Li X, Regezi J, Ross FP, Blystone S, Ilić D, Leong SP, Ramos DM. Integrin alphavbeta3 mediates K1735 murine melanoma cell motility in vivo and in vitro. J Cell Sci. 2001;114:2665–2672. doi: 10.1242/jcs.114.14.2665. [DOI] [PubMed] [Google Scholar]
- 68.Qi J, Wang J, Romanyuk O, Siu CH. Involvement of Src family kinases in N-cadherin phosphorylation and beta-catenin dissociation during transendothelial migration of melanoma cells. Mol Biol Cell. 2006;17:1261–1272. doi: 10.1091/mbc.E05-10-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hess AR, Postovit LM, Margaryan NV, Seftor EA, Schneider GB, Seftor REB, Nickoloff BJ, Hendrix MJC. Focal adhesion kinase promotes the aggressive melanoma phenotype. Cancer Res. 2005;65:9851–9860. doi: 10.1158/0008-5472.CAN-05-2172. [DOI] [PubMed] [Google Scholar]
- 70.Kolli-Bouhafs K, Sick E, Noulet F, Gies JP, De Mey J, Rondé P. FAK competes for Src to promote migration against invasion in melanoma cells. Cell Death Dis. 2014;5:e1379. doi: 10.1038/cddis.2014.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hartman ML, Czyz M. MITF in melanoma: mechanisms behind its expression and activity. Cell Mol Life Sci. 2014:1249–1260. doi: 10.1007/s00018-014-1791-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Levy C, Khaled M, Fisher DE. MITF: master regulator of melanocyte development and melanoma oncogene. Trends Mol Med. 2006;12:406–414. doi: 10.1016/j.molmed.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 73.Loercher AE, Tank EMH, Delston RB, Harbour JW. MITF links differentiation with cell cycle arrest in melanocytes by transcriptional activation of INK4A. J Cell Biol. 2005;168:35–40. doi: 10.1083/jcb.200410115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thurber AE, Douglas G, Sturm EC, Zabierowski SE, Smit DJ, Ramakrishnan SN, Hacker E, Leonard JH, Herlyn M, Sturm RA. Inverse expression states of the BRN2 and MITF transcription factors in melanoma spheres and tumour xenografts regulate the NOTCH pathway. Oncogene. 2011;30:3036–3048. doi: 10.1038/onc.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eccles MR, He S, Ahn A, Slobbe LJ, Jeffs AR, Yoon HS, Baguley BC. MITF and PAX3 Play Distinct Roles in Melanoma Cell Migration; Outline of a “Genetic Switch” Theory Involving MITF and PAX3 in Proliferative and Invasive Phenotypes of Melanoma. Front Oncol. 2013;3:229. doi: 10.3389/fonc.2013.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bianchi-Smiraglia A, Bagati A, Fink EE, Moparthy S, Wawrzyniak JA, Marvin EK, Battaglia S, Jowdy P, Kolesnikova M. Microphthalmia-associated transcription factor suppresses invasion by reducing intracellular GTP pools. Oncogene. 2016 May 16; doi: 10.1038/onc.2016.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sarkar A, Hochedlinger K. The Sox family of transcription factors: Versatile regulators of stem and progenitor cell fate. Cell Stem Cell. 2013;12:15–30. doi: 10.1016/j.stem.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Girouard SD, Laga AC, Mihm MC, Scolyer RA, Thompson JF, Zhan Q, Widlund HR, Lee CW, Murphy GF. SOX2 contributes to melanoma cell invasion. Lab Invest. 2012;92:362–370. doi: 10.1038/labinvest.2011.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 80.Poser I, Dominguez D, De Herreros AG, Varnai A, Buettner R, Bosserhoff AK. Loss of E-cadherin expression in melanoma cells involves up-regulation of the transcriptional repressor snail. J Biol Chem. 2001;276:24661–24666. doi: 10.1074/jbc.M011224200. [DOI] [PubMed] [Google Scholar]
- 81.Hao L, Ha JR, Kuzel P, Garcia E, Persad S. Cadherin switch from E- to N-cadherin in melanoma progression is regulated by the PI3K/PTEN pathway through Twist and Snail. Br J Dermatol. 2012;166:1184–1197. doi: 10.1111/j.1365-2133.2012.10824.x. [DOI] [PubMed] [Google Scholar]
- 82.Medici D, Hay ED, Olsen BR. Snail and Slug Promote Epithelial-Mesenchymal Transition through β-Catenin – T-Cell Factor-4-dependent Expression of Transforming Growth Factor- β 3. Mol Biol Cell. 2008;19:4875–4887. doi: 10.1091/mbc.E08-05-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Olmeda D, Jordá M, Peinado H, Fabra A, Cano A. Snail silencing effectively suppresses tumour growth and invasiveness. Oncogene. 2007;26:1862–1874. doi: 10.1038/sj.onc.1209997. [DOI] [PubMed] [Google Scholar]
- 84.Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3:155–166. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- 85.Kudo-Saito C, Shirako H, Takeuchi T, Kawakami Y. Cancer metastasis is accelerated through immunosuppression during snail-induced EMT of cancer cells. Cancer Cell. 2009;15:195–206. doi: 10.1016/j.ccr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 86.Shirley SH, Hudson LG, He J, Kusewitt DF. The skinny on slug. Mol Carcinog. 2010;49:851–861. doi: 10.1002/mc.20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Savagner P, Yamada KM, Thiery JP. The zinc-finger protein slug causes desmosome dissociation, an initial and necessary step for growth factor–induced epithelial–mesenchymal transition. 1997;137:1–17. doi: 10.1083/jcb.137.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wels C, Joshi S, Koefinger P, Bergler H, Schaider H. Transcriptional activation of ZEB1 by Slug leads to cooperative regulation of the epithelial-mesenchymal transition-like phenotype in melanoma. J Invest Dermatol. 2011;131:1877–1885. doi: 10.1038/jid.2011.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shirley SH, Greene VR, Duncan LM, Torres Cabala CA, Grimm EA, Kusewitt DF. Slug expression during melanoma progression. Am J Pathol. 2012;180:2479–2489. doi: 10.1016/j.ajpath.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Koefinger P, Wels C, Joshi S, Damm S, Steinbauer E, Beham-Schmid C, Frank S, Bergler H, Schaider H. The cadherin switch in melanoma instigated by HGF is mediated through epithelial-mesenchymal transition regulators. Pigment Cell Melanoma Res. 2011;24:382–385. doi: 10.1111/j.1755-148X.2010.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA, Val C, Lamarque A. Twist, a master regulator of morphogenesis plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 92.Weiss MB, Abel EV, Mayberry MM, Basile KJ, Berger AC, Aplin AE. TWIST1 is an ERK1/2 effector that promotes invasion and regulates MMP-1 expression in human melanoma cells. Cancer Res. 2012;72:6382–6392. doi: 10.1158/0008-5472.CAN-12-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shirakihara T, Saitoh M, Miyazono K. Differential regulation of epithelial and mesenchymal markers by 5EF1 proteins in epithelial mesenchymal transition induced by TGF-β. Mol Biol Cell. 2007;19:308–317. doi: 10.1091/mbc.E07-03-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chua HL, Bhat-Nakshatri P, Clare SE, Morimiya A, Badve S, Nakshatri H. NF-kappaB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2. Oncogene. 2007;26:711–724. doi: 10.1038/sj.onc.1209808. [DOI] [PubMed] [Google Scholar]
- 95.Denecker G, Vandamme N, Akay O, Koludrovic D, Taminau J, Lemeire K, Gheldof A, De Craene B, Van Gele M, Brochez L, Udupi GM, Rafferty M, Balint B, Gallagher WM, Ghanem G, Huylebroeck D, Haigh J, van den Oord J, Larue L, Davidson I, Marine JC, Berx G. Identification of a ZEB2-MITF-ZEB1 transcriptional network that controls melanogenesis and melanoma progression. Cell Death Differ. 2014:1–12. doi: 10.1038/cdd.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu Y, Zhou BP. TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and invasion. Br J Cancer. 2010;102:639–644. doi: 10.1038/sj.bjc.6605530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gao K, Dai DL, Martinka M, Li G. Prognostic significance of nuclear factor-kappaB p105/p50 in human melanoma and its role in cell migration. Cancer Res. 2006;66:8382–8388. doi: 10.1158/0008-5472.CAN-05-4402. [DOI] [PubMed] [Google Scholar]
- 98.Zehavi L, Schayek H, Jacob-Hirsch J, Sidi Y, Leibowitz-Amit R, Avni D. MiR-377 targets E2F3 and alters the NF-kB signaling pathway through MAP3K7 in malignant melanoma. Mol Cancer. 2015;14:68. doi: 10.1186/s12943-015-0338-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.End DW, Smets G, Todd AV, Applegate TL, Fuery CJ, Angibaud P, Venet M, Sanz G, Poignet H, Skrzat S, Devine A, Wouters W, Bowden C. Characterization of the antitumor effects of the selective farnesyl protein transferase inhibitor R115777 in vivo and in vitro. Cancer Res. 2001;61:131–137. [PubMed] [Google Scholar]
- 100.Gajewski TF, Salama AKS, Niedzwiecki D, Johnson J, Linette G, Bucher C, Blaskovich MA, Sebti SM, Haluska F. Phase II study of the farnesyltransferase inhibitor R115777 in advanced melanoma (CALGB 500104) J Transl Med. 2012;10:1–8. doi: 10.1186/1479-5876-10-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Niessner H, Beck D, Sinnberg T, Lasithiotakis K, Maczey E, Gogel J, Venturelli S, Berger A, Mauthe M, Toulany M, Flaherty K, Schaller M, Schadendorf D, Proikas-Cezanne T, Schittek B, Garbe C, Kulms D, Meier F. The farnesyl transferase inhibitor lonafarnib inhibits mTOR signaling and enforces sorafenib-induced apoptosis in melanoma cells. J Invest Dermatol. 2011;131:468–479. doi: 10.1038/jid.2010.297. [DOI] [PubMed] [Google Scholar]
- 102.Bollag G, Tsai J, Zhang J, Zhang C, Ibrahim P, Nolop K, Hirth P. Vemurafenib: the first drug approved for BRAF-mutant cancer. Nat Rev Drug Discov. 2012;11:873–886. doi: 10.1038/nrd3847. [DOI] [PubMed] [Google Scholar]
- 103.George AL, Suriano R, Rajoria S, Osso MC, Tuli N, Hanly E, Geliebter J, Arnold AN, Wallack M, Tiwari RK. PLX4032 mediated melanoma associated antigen potentiation in patient derived primary melanoma cells. J Cancer. 2015;6:1320–1330. doi: 10.7150/jca.11126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jenkins MH, Croteau W, Mullins DW, Brinckerhoff CE. The BRAFV600E inhibitor, PLX4032, increases type I collagen synthesis in melanoma cells. Matrix Biol. 2015;48:66–77. doi: 10.1016/j.matbio.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gummadi T, Zhang BY, Valpione S, Kim C, Kottschade LA, Mittapalli RK, Chiarion-Sileni V, Pigozzo J, Elmquist WF, Dudek AZ. Impact of BRAF mutation and BRAF inhibition on melanoma brain metastases. Melanoma Res. 2014:75–79. doi: 10.1097/CMR.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 106.Leight JL, Tokuda EY, Jones CE, Lin AJ, Anseth KS. Multifunctional bioscaffolds for 3D culture of melanoma cells reveal increased MMP activity and migration with BRAF kinase inhibition. Proc Natl Acad Sci U S A. 2015;112:5366–5371. doi: 10.1073/pnas.1505662112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Halaban R, Zhang W, Bacchiocchi A, Cheng E, Parisi F, Ariyan S, Krauthammer M, McCusker JP, Kluger Y, Sznol M. PLX4032, a selective BRAFV600E kinase inhibitor, activates the ERK pathway and enhances cell migration and proliferation of BRAFWT melanoma cells. Pigment Cell Melanoma Res. 2010;23:190–200. doi: 10.1111/j.1755-148X.2010.00685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gross A, Niemetz-Rahn A, Nonnenmacher A, Tucholski J, Keilholz U, Fusi A. Expression and activity of EGFR in human cutaneous melanoma cell lines and influence of vemurafenib on the EGFR pathway. Target Oncol. 2015;10:77–84. doi: 10.1007/s11523-014-0318-9. [DOI] [PubMed] [Google Scholar]
- 109.Girotti MR, Pedersen M, Sanchez-Laorden B, Viros A, Turajlic S, Niculescu-Duvaz D, Zambon A, Sinclair J, Hayes A, Gore M, Lorigan P, Springer C, Larkin J, Jorgensen C, Marais R. Inhibiting EGF receptor or SRC family kinase signaling overcomes BRAF inhibitor resistance in melanoma. Cancer Discov. 2013;3:158–167. doi: 10.1158/2159-8290.CD-12-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kaur A, Webster MR, Marchbank K, Behera R, Ndoye A, Kugel CH, Dang VM, Appleton J, O’Connell MP, Cheng P, Valiga AA, Morissette R, McDonnell NB, Ferrucci L, Kossenkov AV, Meeth K, Tang HY, Yin X, Wood WH, Lehrmann E, Becker KG, Flaherty KT, Frederick DT, Wargo JA, Cooper ZA, Tetzlaff MT, Hudgens C, Aird KM, Zhang R, Xu X, Liu Q, Bartlett E, Karakousis G, Eroglu Z, Lo RS, Chan M, Menzies AM, Long GV, Johnson DB, Sosman J, Schilling B, Schadendorf D, Speicher DW, Bosenberg M, Ribas A, Weeraratna AT. sFRP2 in the aged microenvironment drives melanoma metastasis and therapy resistance. Nature. 2016;532:250–254. doi: 10.1038/nature17392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sanchez-Laorden B, Viros A, Girotti MR, Pedersen M, Saturno G, Zambon A, Niculescu-Duvaz D, Turajlic S, Hayes A, Gore M, Larkin J, Lorigan P, Cook M, Springer C, Marais R. BRAF inhibitors induce metastasis in RAS mutant or inhibitor-resistant melanoma cells by reactivating MEK and ERK signaling. Sci Signal. 2014;7:ra30. doi: 10.1126/scisignal.2004815. [DOI] [PubMed] [Google Scholar]
- 112.Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, Chen Z, Lee MK, Attar N, Sazegar H, Chodon T, Nelson SF, McArthur G, Sosman JA, Ribas A, Lo RS. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973–977. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.King AJ, Arnone MR, Bleam MR, Moss KG, Yang J, Fedorowicz KE, Smitheman KN, Erhardt JA, Hughes-Earle A, Kane-Carson LS, Sinnamon RH, Qi H, Rheault TR, Uehling DE, Laquerre SG. Dabrafenib; Preclinical Characterization, Increased Efficacy when Combined with Trametinib, while BRAF/MEK Tool Combination Reduced Skin Lesions. PLoS One. 2013;8 doi: 10.1371/journal.pone.0067583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Greger JG, Eastman SD, Zhang V, Bleam MR, Hughes AM, Smitheman KN, Dickerson SH, Laquerre SG, Liu L, Gilmer TM. Combinations of BRAF, MEK, and PI3K/mTOR inhibitors overcome acquired resistance to the BRAF inhibitor GSK2118436 dabrafenib, mediated by NRAS or MEK mutations. Mol Cancer Ther. 2012;11:909–920. doi: 10.1158/1535-7163.MCT-11-0989. [DOI] [PubMed] [Google Scholar]
- 115.Gowrishankar K, Snoyman S, Pupo GM, Becker TM, Kefford RF, Rizos H. Acquired resistance to BRAF inhibition can confer cross-resistance to combined BRAF/MEK inhibition. J Invest Dermatol. 2012;132:1850–1859. doi: 10.1038/jid.2012.63. [DOI] [PubMed] [Google Scholar]
- 116.Falchook GS, Long GV, Kurzrock R, Kim KB, Arkenau TH, Brown MP, Hamid O, Infante JR, Millward M, Pavlick AC, O’Day SJ, Blackman SC, Curtis CM, Lebowitz P, Ma B, Ouellet D, Kefford RF. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: A phase 1 dose-escalation trial. Lancet. 2012;379:1893–1901. doi: 10.1016/S0140-6736(12)60398-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Velho TR. Metastatic melanoma - A review of current and future drugs. Drugs Context. 2012:1–17. doi: 10.7573/dic.212242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129–3140. doi: 10.1158/1535-7163.MCT-08-0013. [DOI] [PubMed] [Google Scholar]
- 119.Augustine CK, Toshimitsu H, Jung SH, Zipfel PA, Yoo JS, Yoshimoto Y, Selim MA, Burchette J, Beasley GM, McMahon N, Padussis J, Pruitt SK, Ali-Osman F, Tyler DS. Sorafenib, a multikinase inhibitor, enhances the response of melanoma to regional chemotherapy. Mol Cancer Ther. 2010;9:2090–2101. doi: 10.1158/1535-7163.MCT-10-0073. [DOI] [PubMed] [Google Scholar]
- 120.Nagai T, Arao T, Furuta K, Sakai K, Kudo K, Kaneda H, Tamura D, Aomatsu K, Kimura H, Fujita Y, Matsumoto K, Saijo N, Kudo M, Nishio K. Sorafenib inhibits the hepatocyte growth factor-mediated epithelial mesenchymal transition in hepatocellular carcinoma. Mol Cancer Ther. 2011;10:169–177. doi: 10.1158/1535-7163.MCT-10-0544. [DOI] [PubMed] [Google Scholar]
- 121.Pal HC, Diamond AC, Strickland LR, Kappes JC, Katiyar SK, Elmets CA, Athar M, Afaq F. Fisetin, a dietary flavonoid, augments the anti-invasive and anti-metastatic potential of sorafenib in melanoma. Oncotarget. 2016;7:1227–1241. doi: 10.18632/oncotarget.6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chung C, Reilly S. Trametinib: A novel signal transduction inhibitor for the treatment of metastatic cutaneous melanoma. Am J Heal Pharm. 2015;72:101–110. doi: 10.2146/ajhp140045. [DOI] [PubMed] [Google Scholar]
- 123.Solit DB, Garraway LA, Pratilas CA, Sawai A, Getz G, Basso A, Ye Q, Lobo JM, She Y, Osman I, Golub TR, Sebolt-Leopold J, Sellers WR, Rosen N. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006;439:358–362. doi: 10.1038/nature04304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gilmartin AG, Bleam MR, Groy A, Moss KG, Minthorn EA, Kulkarni SG, Rominger CM, Erskine S, Fisher KE, Yang J, Zappacosta F, Annan R, Sutton D, Laquerre SG. GSK1120212 (JTP-74057) is an inhibitor of MEK activity and activation with favorable pharmacokinetic properties for sustained in vivo pathway inhibition. Clin Cancer Res. 2011;17:989–1000. doi: 10.1158/1078-0432.CCR-10-2200. [DOI] [PubMed] [Google Scholar]
- 125.Vu HL, Rosenbaum S, Capparelli C, Purwin TJ, Davies MA, Berger AC, Aplin AE. MIG6 Is MEK Regulated and Affects EGF-Induced Migration in Mutant NRAS Melanoma. J Invest Dermatol. 2015;136:453–463. doi: 10.1016/j.jid.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ambrosini G, Pratilas CA, Qin LX, Tadi M, Surriga O, Carvajal RD, Schwartz GK. Identification of unique MEK-dependent genes in GNAQ mutant uveal melanoma involved in cell growth, tumor cell invasion, and MEK resistance. Clin Cancer Res. 2012;18:3552–3561. doi: 10.1158/1078-0432.CCR-11-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ge X, Fu YM, Meadows GG. U0126, a mitogen-activated protein kinase kinase inhibitor, inhibits the invasion of human A375 melanoma cells. Cancer Lett. 2002;179:133–140. doi: 10.1016/s0304-3835(02)00004-6. [DOI] [PubMed] [Google Scholar]
- 128.Meier F, Busch S, Lasithiotakis K, Kulms D, Garbe C, Maczey E, Herlyn M, Schittek B. Combined targeting of MAPK and AKT signalling pathways is a promising strategy for melanoma treatment. Br J Dermatol. 2007;156:1204–1213. doi: 10.1111/j.1365-2133.2007.07821.x. [DOI] [PubMed] [Google Scholar]
- 129.O’Reilly T, Lane HA, Wood JM, Schnell C, Littlewood-Evans A, Brueggen J, McSheehy PMJ. Everolimus and PTK/ZK show synergistic growth inhibition in the orthotopic BL16/BL6 murine melanoma model. Cancer Chemother Pharmacol. 2011;67:193–200. doi: 10.1007/s00280-010-1307-z. [DOI] [PubMed] [Google Scholar]
- 130.Schicher N, Paulitschke V, Swoboda A, Kunstfeld R, Loewe R, Pilarski P, Pehamberger H, Hoeller C. Erlotinib and bevacizumab have synergistic activity against melanoma. Clin Cancer Res. 2009;15:3495–3502. doi: 10.1158/1078-0432.CCR-08-2407. [DOI] [PubMed] [Google Scholar]
- 131.Todd JR, Scurr LL, Becker TM, Kefford RF, Rizos H. The MAPK pathway functions as a redundant survival signal that reinforces the PI3K cascade in c-Kit mutant melanoma. Oncogene. 2014;33:236–245. doi: 10.1038/onc.2012.562. [DOI] [PubMed] [Google Scholar]
- 132.Bedogni B, O’Neill MS, Welford SM, Bouley DM, Giaccia AJ, Denko NC, Powell MB. Topical treatment with inhibitors of the phosphatidylinositol 3′-kinase/Akt and Raf/mitogen-activated protein kinase kinase/extracellular signal-regulated kinase pathways reduces melanoma development in severe combined immunodeficient mice. Cancer Res. 2004;64:2552–2560. doi: 10.1158/0008-5472.can-03-3327. [DOI] [PubMed] [Google Scholar]
- 133.Gangadhar TC, Clark JI, Karrison T, Gajewski TF. Phase II study of the Src kinase inhibitor saracatinib (AZD0530) in metastatic melanoma. Invest New Drugs. 2013;31:769–773. doi: 10.1007/s10637-012-9897-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Buettner R, Mesa T, Vultur A, Lee F, Jove R. Inhibition of SRC family kinases with dasatinib blocks migration and invasion of human melanoma cells. Mol Cancer Res. 2008;6:1766–1774. doi: 10.1158/1541-7786.MCR-08-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Eustace AJ, Crown J, Clynes M, O’Donovan N. Preclinical evaluation of dasatinib, a potent Src kinase inhibitor, in melanoma cell lines. J Transl Med. 2008;6:53. doi: 10.1186/1479-5876-6-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.deBlacam C, Byrne C, Hughes E, McIlroy M, Bane F, Hill aDK, Young LS. HOXC11-SRC-1 regulation of S100beta in cutaneous melanoma: new targets for the kinase inhibitor dasatinib. Br J Cancer. 2011;105:118–123. doi: 10.1038/bjc.2011.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Snaar-Jagalska BE. Modeling of human uveal melanoma in zebrafish xenograft embryos. Invest Ophthalmol Vis Sci. 2014;55:6612–6622. doi: 10.1167/iovs.14-15202. [DOI] [PubMed] [Google Scholar]
- 138.Fraser CK, Lousberg EL, Guerin LR, Hughes TP, Brown MP, Diener KR, Hayball JD. Dasatinib alters the metastatic phenotype of B16-OVA melanoma in vivo. Cancer Biol Ther. 2010;10:715–727. doi: 10.4161/cbt.10.7.12926. [DOI] [PubMed] [Google Scholar]
- 139.Khan N, Syed DN, Ahmad N, Mukhtar H. Fisetin: a dietary antioxidant for health promotion. Antioxid Redox Signal. 2013;19:151–162. doi: 10.1089/ars.2012.4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Syed DN, Afaq F, Maddodi N, Johnson JJ, Sarfaraz S, Ahmad A, Setaluri V, Mukhtar H. Inhibition of human melanoma cell growth by the dietary flavonoid fisetin is associated with disruption of Wnt/β-catenin signaling and decreased Mitf levels. J Invest Dermatol. 2011;131:1291–1299. doi: 10.1038/jid.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pal HC, Sharma S, Strickland LR, Katiyar SK, Ballestas ME, Athar M, Elmets CA, Afaq F. Fisetin inhibits human melanoma cell invasion through promotion of mesenchymal to epithelial transition and by targeting MAPK and NFκB signaling pathways. PLoS One. 2014;9 doi: 10.1371/journal.pone.0086338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Syed DN, Chamcheu JC, Khan MI, Sechi M, Lall RK, Adhami VM, Mukhtar H. Fisetin inhibits human melanoma cell growth through direct binding to p70S6K and mTOR: Findings from 3-D melanoma skin equivalents and computational modeling. Biochem Pharmacol. 2014;89:349–360. doi: 10.1016/j.bcp.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Park JH, Jang YJ, Choi YJ, Jang JW, Kim JH, Rho YK, Kim IJ, Kim HJ, Leem MJ, Lee ST. Fisetin inhibits matrix metalloproteinases and reduces tumor cell invasiveness and endothelial cell tube formation. Nutr Cancer. 2013;65:1192–1199. doi: 10.1080/01635581.2013.828090. [DOI] [PubMed] [Google Scholar]
- 144.Yang CS, Wang H. Mechanistic issues concerning cancer prevention by tea catechins. Mol Nutr Food Res. 2011;55:819–831. doi: 10.1002/mnfr.201100036. [DOI] [PubMed] [Google Scholar]
- 145.Singh T, Katiyar SK. Green tea catechins reduce invasive potential of human melanoma cells by targeting COX-2, PGE2 receptors and epithelial-to-mesenchymal transition. PLoS One. 2011;6:e25224. doi: 10.1371/journal.pone.0025224. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 146.Wu Y, Lin Y, Liu H, Li J. Inhibition of invasion and up-regulation of E-cadherin expression in human malignant melanoma cell line A375 by (−)-epigallocatechin-3-gallate. J Huazhong Univ Sci Technolog Med Sci. 2008;28:356–359. doi: 10.1007/s11596-008-0330-3. [DOI] [PubMed] [Google Scholar]
- 147.Watanabe T, Kuramochi H, Takahashi A, Imai K, Katsuta N, Nakayama T, Fujiki H, Suganuma M. Higher cell stiffness indicating lower metastatic potential in B16 melanoma cell variants and in (2)-epigallocatechin gallate-treated cells. J Cancer Res Clin Oncol. 2012;138:859–866. doi: 10.1007/s00432-012-1159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kwak I, Shin YH, Kim M, Cha HY, Nam HJ, Lee BS, Chaudhary SC, Pai KS, Lee JH. Epigallocatechin-3-gallate inhibits paracrine and autocrine hepatocyte growth factor/scatter factor-induced tumor cell migration and invasion. Exp Mol Med. 2011;43:111–120. doi: 10.3858/emm.2011.43.2.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hung CF, Huang TF, Chiang HS, Bin Wu W. (−)-Epigallocatechin-3-gallate, a polyphenolic compound from green tea, inhibits fibroblast adhesion and migration through multiple mechanisms. J Cell Biochem. 2005;96:183–197. doi: 10.1002/jcb.20509. [DOI] [PubMed] [Google Scholar]
- 150.Suzuki Y, Isemura M. Inhibitory effect of epigallocatechin gallate on adhesion of murine melanoma cells to laminin. Cancer Lett. 2001;173:15–20. doi: 10.1016/s0304-3835(01)00685-1. [DOI] [PubMed] [Google Scholar]
- 151.Chang C, Hsieh Y, Yang W, Yang S, Chen Y, Hu D. Epigallocatechingallate inhibits migration of human uveal melanoma cells via downregulation of matrix metalloproteinase-2 activity and ERK1/2 pathway. Biomed Res Int. 2014;2014 doi: 10.1155/2014/141582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liu JD, Chen SH, Lin CL, Tsai SH, Liang YC. Inhibition of melanoma growth and metastasis by combination with (−)-epigallocatechin-3-gallate and dacarbazine in mice. J Cell Biochem. 2001;83:631–642. doi: 10.1002/jcb.1261. [DOI] [PubMed] [Google Scholar]
- 153.Nihal M, Ahsan H, Siddiqui Ia, Mukhtar H, Ahmad N, Wood GS. (−)-Epigallocatechin-3-gallate (EGCG) sensitizes melanoma cells to interferon induced growth inhibition in a mouse model of human melanoma. Cell Cycle. 2009;8:2057–2063. doi: 10.4161/cc.8.13.8862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tucker KB, Makey KL, Chinchar E, Huang M, Sheehan N, Vijayakumar S, Gu JW. EGCG suppresses melanoma tumor angiogenesis and growth without affecting angiogenesis and VEGF expression in the heart and skeletal muscles in mice. J Can Res Updates. 2014;3:19–29. [Google Scholar]
- 155.Ellis LZ, Liu W, Luo Y, Okamoto M, Qu D, Dunn JH, Fujita M. Green tea polyphenol epigallocatechin-3-gallate suppresses melanoma growth by inhibiting inflammasome and IL-1βsecretion. Biochem Biophys Res Commun. 2011;414:551–556. doi: 10.1016/j.bbrc.2011.09.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Vaid M, Singh T, Katiyar SK. Grape seed Proanthocyanidins inhibit Melanoma cell invasiveness by reduction of PGE2 synthesis and reversal of epithelial-to-mesenchymal transition. PLoS One. 2011;6 doi: 10.1371/journal.pone.0021539. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 157.Vaid M, Singh T, Prasad R, Kappes JC, Katiyar SK. Therapeutic intervention of proanthocyanidins on the migration capacity of melanoma cells is mediated through PGE 2 receptors and β-catenin signaling molecules. 2015;5:3325–3338. [PMC free article] [PubMed] [Google Scholar]
- 158.Patel D, Shukla S, Gupta S. Apigenin and cancer chemoprevention: Progress, potential and promise. Int J Oncol. 2007;30:233–245. [PubMed] [Google Scholar]
- 159.Cao HH, Chu JH, Kwan HY, Su T, Yu H, Cheng CY, Fu XQ, Guo H, Li T, Tse AKW, Chou GX, Mo HB, Yu ZL. Inhibition of the STAT3 signaling pathway contributes to apigenin-mediated anti-metastatic effect in melanoma. Sci Rep. 2016;6:21731. doi: 10.1038/srep21731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hasnat MA, Pervin M, Lim JH, Lim BO. Apigenin attenuates melanoma cell migration by inducing anoikis through integrin and focal adhesion kinase inhibition. Molecules. 2015;20:21157–21166. doi: 10.3390/molecules201219752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Spoerlein C, Mahal K, Schmidt H, Schobert R. Effects of chrysin, apigenin, genistein and their homoleptic copper(II) complexes on the growth and metastatic potential of cancer cells. J Inorg Biochem. 2013;127:107–115. doi: 10.1016/j.jinorgbio.2013.07.038. [DOI] [PubMed] [Google Scholar]
- 162.Caltagirone S, Rossi C, Poggi A, Ranelletti FO, Natali PG, Brunetti M, Aiello FB, Piantelli M. Flavonoids apigenin and quercetin inhibit melanoma growth and metastatic potential. Int J Cancer. 2000;87:595–600. doi: 10.1002/1097-0215(20000815)87:4<595::aid-ijc21>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 163.Piantelli M, Rossi C, Iezzi M, La Sorda R, Iacobelli S, Alberti S, Natali PG. Flavonoids inhibit melanoma lung metastasis by impairing tumor cells endothelium interactions. J Cell Physiol. 2006;207:23–29. doi: 10.1002/jcp.20510. [DOI] [PubMed] [Google Scholar]
- 164.Cao HH, Tse AKW, Kwan HY, Yu H, Cheng CY, Su T, Fong WF, Yu ZL. Quercetin exerts anti-melanoma activities and inhibits STAT3 signaling. Biochem Pharmacol. 2014;87:424–434. doi: 10.1016/j.bcp.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 165.Cao HH, Cheng CY, Su T, Fu XQ, Guo H, Li T, Tse AKW, Kwan HY, Yu H, Yu ZL. Quercetin inhibits HGF/c-Met signaling and HGF-stimulated melanoma cell migration and invasion. Mol Cancer. 2015;14:1–12. doi: 10.1186/s12943-015-0367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang XM, Huang SP, Xu Q. Quercetin inhibits the invasion of murine melanoma B16-BL6 cells by decreasing pro-MMP-9 via the PKC pathway. Cancer Chemother Pharmacol. 2004;53:82–88. doi: 10.1007/s00280-003-0702-0. [DOI] [PubMed] [Google Scholar]
- 167.Ferrer P, Asensi M, Segarra R, Ortega A, Benlloch M, Obrador E, Varea MT, Asensio G, Jordá L, Estrela JM. Association between pterostilbene and quercetin inhibits metastatic activity of B16 melanoma. Neoplasia. 2005;7:37–47. doi: 10.1593/neo.04337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tong LX, Young LC. Nutrition: The future of melanoma prevention? J Am Acad Dermatol. 2014;71:151–160. doi: 10.1016/j.jaad.2014.01.910. [DOI] [PubMed] [Google Scholar]
- 169.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 170.Bhattacharya S, Darjatmoko SR, Polans AS. Resveratrol modulates the malignant properties of cutaneous melanoma through changes in the activation and attenuation of the antiapoptotic protooncogenic protein Akt/PKB. Melanoma Res. 2011;21:180–187. doi: 10.1097/CMR.0b013e3283456dfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chen YJ, Chen YY, Lin YF, Hu HY, Liao HF. Resveratrol inhibits alpha-melanocyte-stimulating hormone signaling, viability, and invasiveness in melanoma cells. Evidence-Based Complement Altern Med. 2013;2013 doi: 10.1155/2013/632121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yang Z, Yang S, Misner BJ, Chiu R, Liu F, Meyskens FL. Nitric oxide initiates progression of human melanoma via a feedback loop mediated by apurinic/apyrimidinic endonuclease-1/redox factor-1, which is inhibited by resveratrol. Mol Cancer Ther. 2008;7:3751–3760. doi: 10.1158/1535-7163.MCT-08-0562. [DOI] [PubMed] [Google Scholar]
- 173.Salado C, Olaso E, Gallot N, Valcarcel M, Egilegor E, Mendoza L, Vidal-Vanaclocha F. Resveratrol prevents inflammation-dependent hepatic melanoma metastasis by inhibiting the secretion and effects of interleukin-18. J Transl Med. 2011;9:59. doi: 10.1186/1479-5876-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Scarpa ES, Ninfali P. Phytochemicals as innovative therapeutic tools against cancer stem cells. Int J Mol Sci. 2015;16:15727–15742. doi: 10.3390/ijms160715727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhang YP, Li YQ, Lv YT, Wang JM. Effect of curcumin on the proliferation, apoptosis, migration, and invasion of human melanoma A375 cells. Genet Mol Res. 2015;14:1056–1067. doi: 10.4238/2015.February.6.9. [DOI] [PubMed] [Google Scholar]
- 176.Jiang GM, Xie WY, Wang HS, Du J, Wu BP, Xu W, Liu HF, Xiao P, Liu ZG, Li HY, Liu SQ, Yin WJ, Zhang QG, Liang JP, Huang HJ. Curcumin combined with FAPαc vaccine elicits effective antitumor response by targeting indolamine-2,3-dioxygenase and inhibiting EMT induced by TNF-α in melanoma. Oncotarget. 2015;6:25932–25942. doi: 10.18632/oncotarget.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Menon LG, Kuttan R, Kuttan G. Anti-metastatic activity of curcumin and catechin. Cancer Lett. 1999;141:159–165. doi: 10.1016/s0304-3835(99)00098-1. [DOI] [PubMed] [Google Scholar]
- 178.Menon LG, Kuttan R, Kuttan G. Inhibition of lung metastasis in mice induced by B16F10 melanoma cells by polyphenolic compounds. Cancer Lett. 1995;95:221–225. doi: 10.1016/0304-3835(95)03887-3. [DOI] [PubMed] [Google Scholar]
- 179.Deep G, Agarwal R. Antimetastatic efficacy of silibinin: molecular mechanisms and therapeutic potential against cancer. Cancer Metastasis Rev. 2010;29:447–463. doi: 10.1007/s10555-010-9237-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lee MH, Huang Z, Kim DJ, Kim SH, Kim MO, Lee SY, Xie H, Park SJ, Kim JY, Kundu JK, Bode AM, Surh YJ, Dong Z. Direct targeting of MEK1/2 and RSK2 by silybin induces cell-cycle arrest and inhibits melanoma cell growth. Cancer Prev Res. 2013;6:455–465. doi: 10.1158/1940-6207.CAPR-12-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Vaid M, Prasad R, Sun Q, Katiyar SK. Silymarin targets β-Catenin signaling in blocking migration/invasion of human melanoma cells. PLoS One. 2011;6 doi: 10.1371/journal.pone.0023000. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 182.Siddique HR, Saleem M. Beneficial health effects of lupeol triterpene: A review of preclinical studies. Life Sci. 2011;88:302–306. doi: 10.1016/j.lfs.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 183.Tarapore RS, Siddiqui IA, Saleem M, Adhami VM, Spiegelman VS, Mukhtar H. Specific targeting of wnt/β-catenin signaling in human melanoma cells by a dietary triterpene lupeol. Carcinogenesis. 2010;31:1844–1853. doi: 10.1093/carcin/bgq169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hata K, Mukaiyama T, Tsujimura N, Sato Y, Kosaka Y, Sakamoto K, Hori K. Differentiation-inducing activity of lupane triterpenes on a mouse melanoma cell line. Cytotechnology. 2006;52:151–158. doi: 10.1007/s10616-007-9069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yokoe I, Azuma K, Hata K, Mukaiyama T, Goto T, Tsuka T, Imagawa T, Itoh N, Murahata Y, Osaki T, Minami S, Okamoto Y. Clinical systemic lupeol administration for canine oral malignant melanoma. Mol Clin Oncol. 2014;3:89–92. doi: 10.3892/mco.2014.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yan C, Han R. Genistein suppresses adhesion-induced protein tyrosine phosphorylation and invasion of B16-BL6 melanoma cells. Cancer Lett. 1998;129:117–124. doi: 10.1016/s0304-3835(98)00093-7. [DOI] [PubMed] [Google Scholar]
- 187.Danciu C, Borcan F, Bojin F, Zupko I, Dehelean C. Effect of the isoflavone genistein on tumor size, metastasis potential and melanization in a B16 mouse model of murine melanoma. Nat Prod Commun. 2013;8:343–346. [PubMed] [Google Scholar]
- 188.Budina-Kolomets A, Webster MR, Leu JIJ, Jennis M, Krepler C, Guerrini a, Kossenkov AV, Xu W, Karakousis GC, Schuchter LM, Amaravadi RK, Wu H, Yin X, Liu Q, Lu Y, Mills GB, Xu X, George DL, Weeraratna AT, Murphy ME. HSP70 inhibition limits FAK-dependent invasion and enhances the response to melanoma treatment with BRAF inhibitors. Cancer Res. 2016;76:2720–2731. doi: 10.1158/0008-5472.CAN-15-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Pal HC, Baxter RD, Hunt KM, Agarwal J, Elmets CA, Athar M, Afaq F. Fisetin, a phytochemical, potentiates sorafenib-induced apoptosis and abrogates tumor growth in athymic nude mice implanted with BRAF-mutated melanoma cells. Oncotarget. 2015;6:28296–28311. doi: 10.18632/oncotarget.5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Davies MA, Fox PS, Papadopoulos NE, Bedikian AY, Hwu WJ, Lazar AJ, Prieto VG, Culotta KS, Madden TL, Xu Q, Huang S, Deng W, Ng CS, Gupta S, Liu W, Dancey JE, Wright JJ, Bassett RL, Hwu P, Kim KB. Phase I study of the combination of sorafenib and temsirolimus in patients with metastatic melanoma. Clin Cancer Res. 2012;18:1120–1128. doi: 10.1158/1078-0432.CCR-11-2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chattopadhyay C, Grimm EA, Woodman SE. Simultaneous inhibition of the HGF/MET and Erk1/2 pathways affect uveal melanoma cell growth and migration. PLoS One. 2014;9 doi: 10.1371/journal.pone.0083957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.LaMontagne K, Littiewood-Evans A, Schnell C, O’Reilly T, Wyder L, Sanchez T, Probst B, Butler J, Wood A, Liau G, Billy E, Theuer A, Hla T, Wood J. Antagonism of sphingosine-1-phosphate receptors by FTY720 inhibits angiogenesis and tumor vascularization. Cancer Res. 2006;66:221–231. doi: 10.1158/0008-5472.CAN-05-2001. [DOI] [PubMed] [Google Scholar]
- 193.Pradhan SJ, Mishra R, Sharma P, Kundu GC. Quercetin and sulforaphane in combination suppress the progression of melanoma through the down-regulation of matrix metalloproteinase-9. Exp Ther Med. 2010;1:915–920. doi: 10.3892/etm.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]