1. Introduction
As summarized in many reviews and other articles in this volume, mitochondrial Ca2+ homeostasis plays important roles in cellular physiology. Ca2+ flux across the inner mitochondrial membrane (IMM) regulates bioenergetics, cytoplasmic Ca2+ ([Ca2+]i) signals and activation of cell death pathways [1–11]. Two features are essential for understanding mitochondrial Ca2+ (Ca2+m) uptake. The first was provided by the development of the chemi-osmotic hypothesis and recognition of the notably large voltage across the IMM (ΔΨm) generated by proton pumping by respiratory chain components [12–16]. Although already recognized that Ca2+ uptake is an electrogenic process driven by ΔΨm through a so-called uniporter [17–19], the demonstration that it was mediated by a Ca2+ selective ion channel (MiCa [20] was the second important insight. Whereas it had been previously assumed that the Ca2+ flux through the uniporter was on the order of 104 ions per second, the measurements of MiCa revealed that the flux could be more than two orders of magnitude greater [20]. Electrophysiological characterization of MiCa also revealed another key feature, notably that Ca2+ selectivity of the channel was ensured by an extremely high affinity Ca2+ binding site (apparent Kd < 2 - nM), with high flux through the channel due most likely, as it is in plasma membrane voltage-gated ion channels, to multi-ion occupancy of the pore with electrostatic repulsion between Ca2+ ions. Previously the uniporter was generally found to have a low apparent Ca2+ affinity (10–70 μM) with variable cooperative activation by Ca2+ [21,22]. However, the properties of the uniporter were mostly derived from studies of isolated mitochondria, where the apparent low affinity must reflect, in part, experimental artifact due to an inability to clamp the IMM voltage, and to perhaps the properties of an additional Ca2+ binding site in the channel that enables it to have high Ca2+ conductance over a wide range of [Ca2+]. The notion that the uniporter has intrinsic low Ca2+ affinity nevertheless persisted for the next decade, supported by two basic observations. First, under resting conditions mitochondria have matrix Ca2+ concentration ([Ca2+]m) ~100 nM. At normal ΔΨm ~−180 mV, this concentration is 6 orders of magnitude lower than expected if [Ca2+]m was able to equilibrate across the IMM through the uniporter. It was assumed that the flux through the uniporter was low because low-affinity channel Ca2+ binding sites could not be occupied at resting [Ca2+]i. Second, it was found that agonist- induced [Ca2+]i signals can be rapidly transduced to the mitochondrial matrix [23,24]. The reconciliation of low apparent Ca2+ affinity of the uniporter with this observation led to the proposal that there exist local micro-domains of high [Ca2+]i at sites of close apposition between sites of Ca2+ ER release and mitochondria. These sites were subsequently shown to exist [23–30].
A low Ca2+ affinity of the uniporter was viewed as an intrinsic channel property, whereas the electrophysiological studies suggested that the Ca2+ affinity is in fact high [20]. Furthermore, the open probability of the uniporter channel is nearly unity at normal Δψm [20]. The high open probability and Ca2+ affinity of the uniporter pore suggested that regulatory mechanisms extrinsic to the channel itself must exist to limit its activity to prevent Ca2+m overload in the face of the large thermodynamic driving force for Ca2+ uptake.
MCU was identified as the likely ion-conducting pore of the uniporter [31,32]. Previously, MICU1 was identified as a protein that localized to the IMM and was required for uniporter-mediated Ca2+ uptake [33]. MICU1 and MCU biochemically interact, and their expression patterns are tightly coupled across tissues and species [31]. Additional components have now been identified that suggest that MCU channel is a protein complex, as first suggested by Mallilankaraman et al. [34]. Thus, MCUR1 was identified as a membrane protein that interacted with MCU but not MICU1, and was necessary for MCU-mediated Ca2+ uptake [34]. A homolog of MCU named MCUb was identified as a dominant-negative regulator of the channel [35]. MICU2 was identified as a paralog of MICU1 that was also part of the uniporter complex [36]. Most recently, a single pass membrane protein named EMRE was found to be necessary for MCU-mediated Ca2+ uptake and MiCa currents and to mediate the interaction of MICU proteins with MCU[37]. Here, we focus on MICU proteins and their roles in MCU-mediated Ca2+ uptake into mitochondria, highlighting discrepancies, controversies and recent data.
2. MICU1
It was originally reported that MICU1 was required for MCU-mediated Ca2+ uptake [33]. Some subsequent studies have also found that knockdown of MICU1 expression inhibits mitochondrial Ca2+ uptake [38,39]. However, we found that MICU1 was not required for MCU-mediated mitochondrial Ca2+ uptake. On the contrary, we noted that loss of MICU1 lead to constitutive Ca2+ m accumulation through MCU [40]. We observed that MICU1 expression provided a gatekeeper to limit MCU-mediated Ca2+ uptake, establishing a threshold that prevents Ca2+ uptake in the low [Ca2+]i regime that exists normally in resting cells, and that is experienced by mitochondria only in micro-domains of high [Ca2+]i. Thus, at low [Ca2+]i <3 μM, MICU1 is required to minimize Ca2+ flux through MCU. We found that MICU1 did not confer low apparent Ca2+ affinity or cooperativity of MCU-mediated Ca2+ uptake at higher [Ca2+]i. Thus, we suggested that MICU1 operated only in the low [Ca2+]i regime. The observed gatekeeper function of MICU1 required each of its two Ca2+ binding EF hands, suggesting that they provide a high-affinity [Ca2+] sensing mechanism that enables MICU1 to exert its regulation. We suggested that MICU1 senses matrix [Ca2+] since it inhibited MCU-mediated Ca2+ uptake only when [Ca2+]m was low. We concluded that MICU1 is a gatekeeper to limit MCU-mediated Ca2+ influx to prevent Ca2+ m overload and its associated stress under resting conditions [40]. Undefined were the mechanisms by which higher [Ca2+]i overcame this gatekeeper function (Figs. 1 and 2).
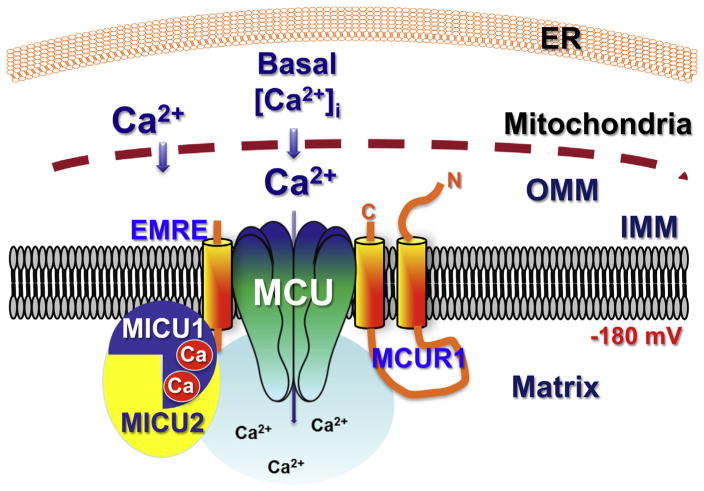
Fig. 1.
Schematic representation of the role of MICU1 in regulating MCU activity in basal conditions, according to [34]. MICU1 is localized to the mitochondrial matrix, constitutively bound to Ca2+ at its two EF-hands in a conformation that suppresses MCU permeability [34,45]. Here and in other figures, MCU refers to a channel composed of MCU and MCUb with unknown stoichiometry. MICU2 is included to account for data that it hetero-dimerizes with MICU1 [36,39,43]. EMRE is included to account for data that it is necessary to mediate the interaction of MICU1 with MCU [37]. MCUR1 shown because it is essential for MCU activity [34].
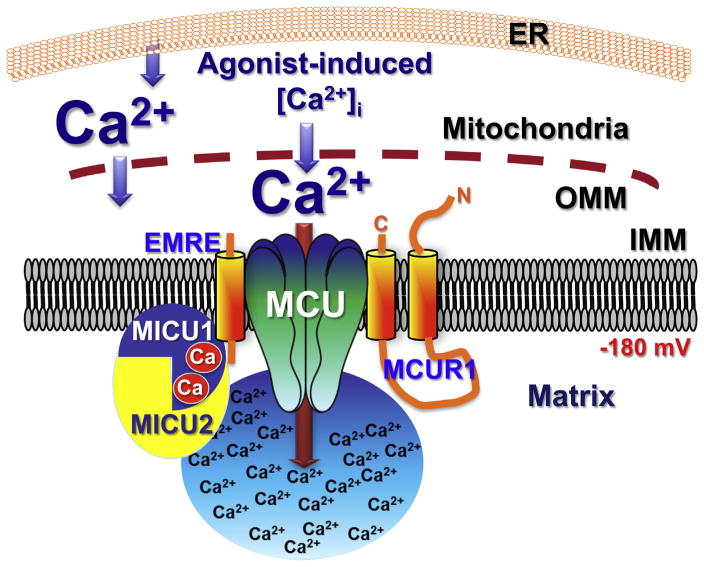
Fig. 2.
Schematic representation of the role of MICU1 in regulating MCU activity during conditions of elevated [Ca2+]i, perhaps in microdomains at ER-mitochondria junctions, according to [34,45]. The mechanisms whereby elevated Ca2+ overcome the gatekeeping function of MICU1 are unknown, but might possibly involve MICU2.
More recent studies have questioned, extended and clarified some of these observations and conclusions. Several groups subsequently confirmed that mitochondria in cells with MICU1 knocked down were able to take up Ca2+ at low [Ca2+]i whereas control mitochondria did not [41–43]. In agreement with Mallilankaraman et al. [40] this was generally observed in the low [Ca2+]i regime <~2–3 μM. Some studies failed to observe constitutively increased basal [Ca2+]m in cells with MICU1 knocked down, in contrast to the observations of Mallilankaraman et al. [33,41,42]. Furthermore, as in the original description of MICU1 [33], several groups noted blunted mitochondrial Ca2+ uptake in response to agonist-induced endoplasmic reticulum (ER)-mediated Ca2+ release in cells with MICU1 knockdown, again in contrast to observations of Mallilankaraman et al. Loss of function mutations in MICU1 cause brain and muscle disorders in affected individuals [44]. In cells from these patients, [Ca2+]m was found to be constitutively elevated, and agonist-induced mitochondrial Ca2+ uptake was not inhibited [44]. Similarly Patron et al. [43] found that mitochondria were constitutively loaded with Ca2+ in cells with MICU1 knocked down. These results from patient cells and the Patron et al. study are in agreement with the original report of Mallilankaraman et al. but contrast with those of others [33,41,42]. What accounts for discrepant results in the various studies is not clear. It is possible that compensatory mechanisms operated in some studies but not in others. Thus, Csordás et al. [41] found that mitochondrial Ca2+ buffering capacity was enhanced in MICU1 knockdown cells, although no effect on buffering was observed in the studies of Mallilankaraman et al. [40]. Furthermore, as discussed below, altered expression in knockdown or over-expression studies can change protein levels of other components of the uniporter channel complex, and the magnitude of these affects may be cell type specific [39].
How does MICU1 regulate MCU activity? There have been disagreements and new insights. First, the gatekeeper function of MICU1 was observed by Mallilankaraman et al. [40] in the presence (intact cells) and absence (permeabilized cells) of cytoplasmic Mg2+. In contrast, MICU1 lacked activity in the absence of cytoplasmic Mg2+ in the studies of Csordás et al. [41]. Second, Mallilankaraman et al. concluded that MICU1 operated only in the low [Ca2+]i regime, <3 μM. At higher [Ca2+]i there were no differences observed between control and MICU1 knockdown cells in the rates of mitochondrial Ca2+ uptake or the apparent cooperativity of the dependence of the rate of mitochondrial Ca2+ uptake on [Ca2+]. In contrast, although Csordás et al. [41] also observed no differences in mitochondrial Ca2+ uptake in [Ca2+]i >5 μM, they proposed that MICU1 nevertheless plays a role in the apparent Ca2+ cooperativity of MCU Ca2+ uptake. However, their data do not support such a conclusion. Total steady-state Ca2+ uptake rather than uptake rates were used in some calculations, and where Ca2+ uptake rates were used, they were fitted assuming a single process, whereas two [Ca2+]i regimes should have been considered. Thus, whether MICU1 is involved in cooperative activation by Ca2+ of MCU activity remains to be resolved. Nevertheless, the conclusion that MICU1 may play an activating role at higher [Ca2+] received other support. Thus, de la Fuente et al. [42] observed that MICU1 knockdown suppressed the rate of mitochondrial Ca2+ uptake at [Ca2+] >5 μM. Furthermore, as discussed in more detail below, Patron et al. [43] have recently suggested that MICU1 is a direct activator of MCU.
Originally shown to be strongly associated with the inner mitochondrial membrane [33], MICU1 is predicted to have a transmembrane domain and it is relatively resistant to carbonate extraction [41], suggesting that it might be an integral membrane protein. Nevertheless, it is now generally believed to be a peripheral membrane protein. Recent fluorescence recovery after photo-bleaching (FRAP) measurements of tagged MICU1 showed that it had a higher mobility than that of MCU, although the mobile fraction was only 40% [45]. Thus, in our opinion the environment(s) in which MICU1 resides remain to be further investigated. MICU1 contains two canonical Ca2+ binding EF hands. The roles and location of the EF hands are controversial. In the initial studies of Mallilankaraman et al. [40], mutation of the EF hands phenocopied knockdown of MICU1, suggesting that they play a role in inhibiting MCU activity under basal conditions. The results implied that the EF hands are Ca2+-liganded under resting conditions with high affinity (Fig. 1). In contrast, Csordás et al. [41] claimed that the ability of MICU1 with non-functional EF hands to inhibit Ca2+ uptake was unaffected. However, the data shown (their Fig. 3B) do not appear to support such a conclusion. Furthermore, steady-state Ca2+ uptake was measured, rather than Ca2+ uptake rates, which are necessary to reach such conclusions. Thus, the notion that the EF hands play no role in the gatekeeping function but are important for the cooperative activation by Ca2+ of Ca2+ uptake is in our view not well supported by this particular study. Nevertheless, Kamer and Mootha [39] also reported that the gatekeeper function of MICU1 in the low [Ca2+]i regime was unaffected by mutating the MICU1 EF hands. Remarkably, and in contrast to the other studies, EF hand-mutated MICU1 blocked mitochondrial Ca2+ uptake even in the high [Ca2+]i regime [39]. There is currently no model, including those discussed below, that can account for this observation (see Figs. 1–4). In contrast, Hoffman et al. [45] reported that MiCa currents were elevated in cells with MICU1 knocked down, and this enhancement was phenocopied in cells expressing MICU1 with both EF hands mutated.
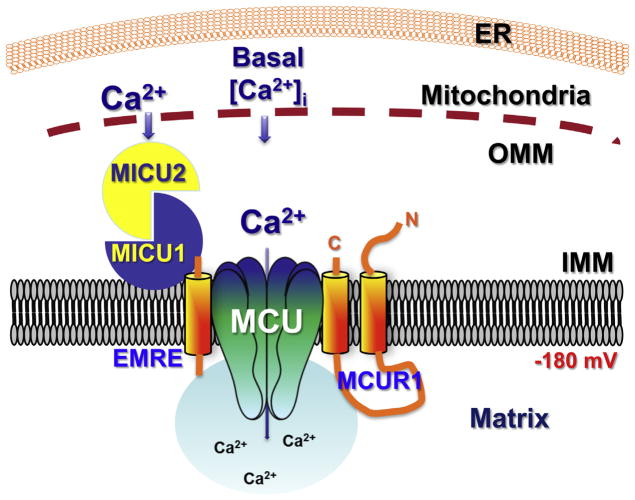
Fig. 3.
Schematic representation of the role of MICU1 in regulating MCU activity in basal conditions, based on data from [36,39,41,43]. MICU1 is localized in the intermembrane space with its EF hands unliganded, and bound to MICU2. MICU2 exerts an inhibitory effect on MCU activity [43].
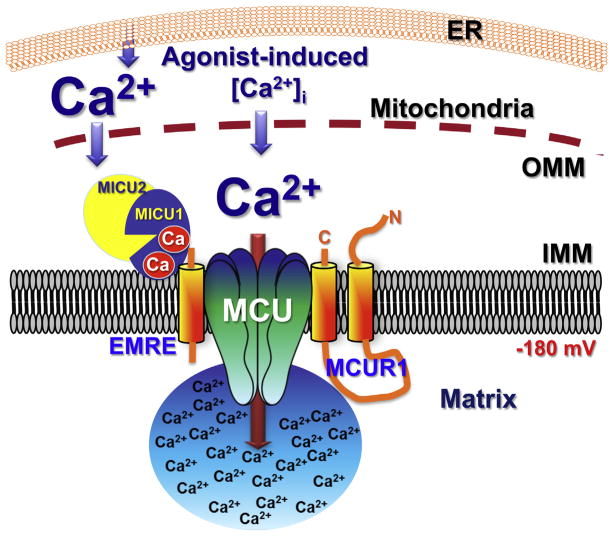
Fig. 4.
Schematic representation of the role of MICU1 in regulating MCU activity in during conditions of elevated [Ca2+]i, based on data from [36,39,41,43]. Ca2+ binding by the EF hands causes a conformational change in MICU1 [46], activating MCU. It is unknown if MICU2 binds Ca2+ and/or undergoes conformational changes in high [Ca2+]i.
Teleologically, one might expect the MICU1 EF hands to be located in the matrix since, first, it interacts with MCU, which has only minimal sequence exposure to the inter-membrane space for protein–protein interactions, and second, because most known Ca2+ channels are associated with mechanisms that sense Ca2+ that has fluxed through the channel to provide feedback regulation. Several approaches have been used to study this issue. Mallilankaraman et al. [40] used a pre-pulse protocol to load the mitochondrial matrix with Ca2+ to explore whether MICU1 sensed Ca2+ in the matrix or in the inter-membrane space. They concluded that MICU1 sensed [Ca2+]m (Fig. 1). Using a different functional protocol, Csordás et al. [41] suggested that inter-membrane space [Ca2+] was being sensed (Fig. 3). However, there are important caveats in both functional assays. Biochemical approaches to this question have nevertheless failed to provide a consensus, although the preponderance of the data suggests an inter-membrane space localization (Figs. 3 and 4). Using protease sensitivity assays in isolated mitochondria or mitoplasts, several groups have concluded that MICU1 is localized in the inter-membrane space [39,41,43,46]. On the other hand, by imaging tagged mitochondrial proteins in plasma membrane-permeabilized cells in response to outer and inner membrane permeabilization, Hoffman et al. [45] concluded that MICU1 behaved as if it was localized in the matrix. Protein interaction studies have also resulted in conflicting conclusions. Hoffman et al. [45] identified regions of protein interaction between MICU1 and MCU that included the matrix-localized domains of MCU. In contrast, Patron et al. [43] found that mutations in the small loop between the two transmembrane domains of MCU (therefore possibly accessible from the inter-membrane space) abolished the interaction with MICU1. Hoffman et al. [45] found that a poly-basic region in the MICU1 amino-terminus was important in the biochemical interaction with MCU, whereas two other studies found that the carboxyl-terminus of MICU1 is critical for the interaction [39,46]. Interpretation of these conflicting studies is complicated by the identification of EMRE and the finding that it is required for the interaction of MCU with MICU1 [37]. Nevertheless, the carboxyl-terminus appears to be functionally important since expression of MICU1 lacking the carboxyl-terminal 31 amino acids distal to the second EF hand abolished its ability to rescue the loss of gatekeeping function in the low [Ca2+] regime [39]. Similarly, the amino-terminal polybasic region also appears to be functionally important, since gatekeeping, as measured by mitochondrial Ca2+ uptake in permeabilized cells and MiCa currents, was absent in cells expressing MICU1 with the region mutated [45].
In summary, the localization of MICU1 is in our view an important unanswered question. On the one hand, one model suggests that MICU1 is localized in the matrix, sensing [Ca2+]m with both EF hands ligated with Ca2+ under resting conditions, establishing a brake on MCU that prevents mitochondrial Ca2+ overload under resting conditions, and not operating at [Ca2+]i >~3 μM (Fig. 1). To further complicate the matter, MICU1 binding to MCU appears to be unaffected by Ca2+ [45]. On the other hand, a majority of the data is consistent with a different model in which MICU1 is localized in the inter-membrane space, with EF hands unliganded, establishing a brake on MCU activity (Fig. 3). The first model does not account for the ability of higher levels of Ca2+ to overcome MICU1 inhibition (Fig. 2), whereas the latter can because it proposes that elevated [Ca2+]i promotes binding of Ca2+ to the EF hands to inhibit the brake function of MICU1 (Fig. 4).
3. MICU2
MICU2 is a paralog of MICU1 with 27% sequence identity that also expresses two EF hands (notably, in humans and rodents neither is as canonical as the two in MICU1), is localized in mitochondria, interacts with MICU1, and whose protein expression levels are dependent upon MICU1 [36,39,43]. Importantly, this latter observation suggests that previous studies in which MICU1 expression was manipulated were associated with, and therefore possibly complicated by, altered expression levels of MICU2. In contrast, knockdown of MICU2 did not affect levels of MICU1 protein [36,39,43] in some cells but it did in others. MICU2 can be immunoprecipitated in a complex with MICU1 and MCU [36,39,43], mediated by EMRE [37]. The presence of MICU2 in the complex depends on the presence of MICU1, but not vice versa [39]. MICU1 and MICU2 can be co-immunoprecipitated in the absence of EMRE [37]. Non-reducing polyacrylamide gel electrophoresis suggested that a MICU1–MICU2 dimer predominates in vivo, although MICU1-homodimers were observed when MICU2 was knocked down [43]. Furthermore, it has been suggested that MICU2 cannot homo-oligomerize [43]. Fluorescence resonance energy transfer (FRET) studies suggested that MICU1and MICU2 physically interact in vivo [43]. The proteinase sensitivity assays used to demonstrate MICU1 localization to the inter-membrane space suggested a similar localization of MICU2 [43]. Of interest, the interaction of MICU2 with MCU (mediated presumably by EMRE) was abolished in the presence of a MICU2 that could not oligomerize with MICU1 [43], suggesting that the dimeric MICU1–MICU2 complex is the functional unit that interacts with EMRE. Thus, a scheme in which MCU binds EMRE, which binds MICU1, which binds MICU2 has been proposed [39] (Figs. 1–4).
As noted, MICU1 has been reported to homo-oligomerize [43,45,46], whereas MICU2 cannot [43]. It was suggested that the oligomerization involves the carboxyl-terminus [46] and conserved cysteine residues in MICU1 and MICU2 [43]. Crystal structures of MICU1 revealed it to be a trimer of dimers in the absence of Ca2+. Ca2+ induced large structural changes and resulted in a mixture of oligomeric forms [46]. In the apo-form, the carboxyl-terminus was packed into the center of the hexameric structure, suggesting that it is important in the assembly of the hexamer. Deletion of the carboxyl-terminus resulted in a predominant dimer in both the Ca2+-bound and apo forms. Biochemical studies of cells suggest that MICU1 exists as a dimer, with no evidence for higher order structures. Thus, the role of the carboxyl-terminus in mediating dimerization is at present unclear. Of note, an oligomeric MICU1 without an amino-terminal polybasic domain, which likely serves as a membrane anchor, is unable to regulate MCU [45]. The observed structures may be influenced not only by the mutations necessary for the crystallization and crystal packing, but also by the absence of normal interaction partners, including EMRE and MICU2. Therefore, the relevance of these structures in vivo is not yet clear, and they raise important issues. First, it should be noted that most studies have not examined direct protein–protein interactions. Second, stoichiometries within the channel complex remain unknown. MICU proteins have been proposed to be dimers; MCU has been proposed to be a tetramer [35], although direct evidence is lacking. The stoichiometry of MCU, EMRE and MICU proteins in the functional uniporter channel is unknown and critical to define. Furthermore, it is important to determine if the stoichiometry is fixed, or if it is regulated or variable among mitochondria from different tissues.
What is the role of MICU2? Knockout of MICU2 inhibited isolated mitochondrial Ca2+ uptake in response to a high [Ca2+] pulse [36], as well as the gatekeeping function observed in response to low [Ca2+] in permeabilized cells [39]. Both effects were smaller than those observed by knocking down MICU1 [36,39], which is expected if MICU1 is required for MICU2 expression and if MICU2 has independent functions. In agreement, knockdown of both MICU1 and MICU2 had an additive effect to inhibit Ca2+ uptake (high [Ca2+] regime) in isolated mitochondria [36]. A similar experiment to evaluate the effects of combined knockdown on the gatekeeping function in the low [Ca2+] regime has not been reported. It was suggested that MICU1 function does not require MICU2, but that the converse was not true [39]. However, the data that purportedly support this conclusion are confusing. MICU1 was unable to rescue the gatekeeping function that was compromised in cells lacking MICU2, whereas EF-hand mutant MICU1 could [39]. This result – a gain of function of the EF-hand mutant - is difficult to reconcile with either of the two models of MICU1 function discussed earlier. Interestingly, Patron et al. [43] also observed a gain-of-function effect of this construct on agonist-induced mitochondrial Ca2+ uptake (high [Ca2+] regime). Expression of EF-hand mutant MICU2 in a wild-type background completely blocked MCU-mediated Ca2+ uptake even in high [Ca2+] [39], suggesting that whereas its function may depend on the presence of MICU1, it can exert a dominant-negative effect on MICU1.
Nearly all studies that have examined the roles of MICU proteins have employed knockdown strategies. Notably, overexpression of MICU1 revealed a remarkably similar phenotype to that of MICU1 knockdown. Thus, the [Ca2+]m in response to strong agonist stimulation was similarly elevated whether MICU1 was overexpressed or knocked down [43]. A similar phenotype was observed when MICU2 was knocked down, whereas its overexpression did not phenocopy the MICU1 knockdown effect [43]. This result suggested that MICU1 is an activator of MCU, whereas MICU2 is the gatekeeper. In this scheme, overexpression of MICU1, by generating MICU1-homodimers in lieu of MICU1-2 heterodimers, relieves MICU2-mediated inhibition, providing additional stimulation and accounting for enhanced mitochondrial Ca2+ uptake in the high [Ca2+]i regime. In agreement, overexpression of EF-hand mutated MICU1 did not potentiate agonist-induced [Ca2+]m. In the low [Ca2+] regime, the absence of MICU2 in complex with MICU1 in MICU1-overexpressing cells abolishes the gatekeeping function conferred by MICU2, therefore causing elevated agonist-induced [Ca2+]m in MICU1 knockdown as well as overexpressing mitochondria [43]. Electrophysiological studies provided support for such a model [43]. Purified MCU was incorporated into planar bilayer membranes, and purified MICU1 or MICU2 was added. Addition of MICU1 protein (to which side of the channel was not determined) had no effect on the channel open probably in the absence of Ca2+. In contrast, it stimulated channel open probability three-fold in the presence (which side not specified) of 1 μM free Ca2+. Addition of MICU2 protein (to which side of the channel was not determined) in 0-Ca2+ completely inhibited channel activity.
4. Conclusions
We [40] discovered that expression of MICU1 provided a critical gatekeeping function that prevents Ca2+ influx through MCU despite the presence of a considerable thermodynamic driving force. This gatekeeping function establishes a threshold that prevents mitochondrial Ca2+ uptake in the low [Ca2+]i regime that exists normally in resting cells, and that is experienced by mitochondria only in micro-domains of high [Ca2+]i. This function prevents mitochondrial Ca2+ overload, which when absent, in vitro or in patients, has detrimental consequences. Subsequent studies have confirmed this finding, but the mechanism has become more clarified and complicated. Thus, MICU2 has been proposed to be the true inhibitor of the MCU channel, with MICU1 playing a gatekeeper role indirectly, by hetero-dimerizing with MICU2 to, presumably, bring it into proximity to MCU to exert its inhibitory effects [43]. This scheme will be strengthened by data that address the mechanism by which MICU2 interacts with the channel and inhibits its activity. The inhibitory action does not appear to require the EF hands of MICU2. This is interesting since, as noted, the human and rodent MICU2 EF hands are not canonical: the critical glycine at position 6 is replaced by a glutamic acid residue in both EF hands. This may suggest that they do not bind Ca2+. Future studies are required to determine the Ca2+ binding and structural features of MICU2. In addition, biochemical, structural and functional studies are required to understand how EMRE mediates the interactions between the MICU proteins and MCU in different [Ca2+] regimes. It is notable that EMRE is necessary for MCU activity in intact mitochondria, whereas purified MCU in bilayers is nevertheless functional [32,43]. This raises the question of whether the effects of purified MICU proteins on MCU activity observed in vitro fully recapitulate those in situ. Although most of the published data suggest that MICU1 and MICU2 are localized specifically to the inter-membrane space, in our view, the pool of Ca2+ that is being sensed by MICU1 is not yet fully resolved, and may require approaches different from those employed to date.
Acknowledgments
This work was supported by NIH Grants GM56328 (J.K.F.) and HL086699 and HL119306 (M.M).
References
- 1.Hajnoczky G, Robb-Gaspers LD, Seitz MB, Thomas AP. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995;82:415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- 2.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 3.Denton RM, McCormack JG. The role of calcium in the regulation of mitochondrial metabolism. Biochem Soc Trans. 1980;8:266–268. doi: 10.1042/bst0080266. [DOI] [PubMed] [Google Scholar]
- 4.Balaban RS. The role of Ca2+ signaling in the coordination of mitochondrial ATP production with cardiac work. Biochim Biophys Acta. 2009;1787:1334–1341. doi: 10.1016/j.bbabio.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gunter KK, Gunter TE. Transport of calcium by mitochondria. J Bioenerg Biomembr. 1994;26:471–485. doi: 10.1007/BF00762732. [DOI] [PubMed] [Google Scholar]
- 6.Duchen MR, Verkhratsky A, Muallem S. Mitochondria and calcium in health and disease. Cell Calcium. 2008;44:1–5. doi: 10.1016/j.ceca.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 7.McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev. 1990;70:391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- 8.Lemasters JJ, Theruvath TP, Zhong Z, Nieminen AL. Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta. 2009;1787:1395–1401. doi: 10.1016/j.bbabio.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szalai G, Krishnamurthy R, Hajnoczky G. Apoptosis driven by IP(3)-linked mitochondrial calcium signals. EMBO J. 1999;18:6349–6361. doi: 10.1093/emboj/18.22.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansford RG. Physiological role of mitochondrial Ca2+ transport. J Bioenerg Biomembr. 1994;26:495–508. doi: 10.1007/BF00762734. [DOI] [PubMed] [Google Scholar]
- 11.Herrington J, Park YB, Babcock DF, Hille B. Dominant role of mitochondria in clearance of large Ca2+ loads from rat adrenal chromaffin cells. Neuron. 1996;16:219–228. doi: 10.1016/s0896-6273(00)80038-0. [DOI] [PubMed] [Google Scholar]
- 12.O’Rourke B. Mitochondrial ion channels. Annu Rev Physiol. 2007;69:19–49. doi: 10.1146/annurev.physiol.69.031905.163804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rottenberg H, Scarpa A. Calcium uptake and membrane potential in mitochondria. Biochemistry. 1974;13:4811–4817. doi: 10.1021/bi00720a020. [DOI] [PubMed] [Google Scholar]
- 14.Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- 15.Nicholls DG. Mitochondria and calcium signaling. Cell Calcium. 2005;38:311–317. doi: 10.1016/j.ceca.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Drago I, Pizzo P, Pozzan T. After half a century mitochondrial calcium in- and efflux machineries reveal themselves. EMBO J. 2011;30:4119–4125. doi: 10.1038/emboj.2011.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santo-Domingo J, Demaurex N. Calcium uptake mechanisms of mitochondria. Biochim Biophys Acta. 2010;1797:907–912. doi: 10.1016/j.bbabio.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Igbavboa U, Pfeiffer DR. EGTA inhibits reverse uniport-dependent Ca2+ release from uncoupled mitochondria. Possible regulation of the Ca2+ uniporter by a Ca2+ binding site on the cytoplasmic side of the inner membrane. J Biol Chem. 1988;263:1405–1412. [PubMed] [Google Scholar]
- 19.Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev. 1999;79:1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- 20.Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 21.Bragadin M, Pozzan T, Azzone GF. Kinetics of Ca2+ carrier in rat liver mitochondria. Biochemistry. 1979;18:5972–5978. doi: 10.1021/bi00593a033. [DOI] [PubMed] [Google Scholar]
- 22.Gunter TE, Gunter KK, Sheu SS, Gavin CE. Mitochondrial calcium transport: physiological and pathological relevance. Am J Physiol. 1994;267:C313–C339. doi: 10.1152/ajpcell.1994.267.2.C313. [DOI] [PubMed] [Google Scholar]
- 23.Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 24.Rizzuto R, Duchen MR, Pozzan T. Flirting in little space: the ER/mitochondria Ca2+ liaison. Sci STKE. 2004;2004:re1. doi: 10.1126/stke.2152004re1. [DOI] [PubMed] [Google Scholar]
- 25.Filippin L, Magalhaes PJ, Di Benedetto G, Colella M, Pozzan T. Stable interactions between mitochondria and endoplasmic reticulum allow rapid accumulation of calcium in a subpopulation of mitochondria. J Biol Chem. 2003;278:39224–39234. doi: 10.1074/jbc.M302301200. [DOI] [PubMed] [Google Scholar]
- 26.Collins TJ, Lipp P, Berridge MJ, Bootman MD. Mitochondrial Ca2+ uptake depends on the spatial and temporal profile of cytosolic Ca2+ signals. J Biol Chem. 2001;276:26411–26420. doi: 10.1074/jbc.M101101200. [DOI] [PubMed] [Google Scholar]
- 27.Carafoli E, Lehninger AL. A survey of the interaction of calcium ions with mitochondria from different tissues and species. Biochem J. 1971;122:681–690. doi: 10.1042/bj1220681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Contreras L, Drago I, Zampese E, Pozzan T. Mitochondria: the calcium connection. Biochim Biophys Acta. 2010;1797:607–618. doi: 10.1016/j.bbabio.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 29.Csordas G, Varnai P, Golenar T, Roy S, Purkins G, Schneider TG, Balla T, Hajnoczky G. Imaging interorganelle contacts and local calcium dynamics at the ER–mitochondrial interface. Mol Cell. 2010;39:121–132. doi: 10.1016/j.molcel.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giacomello M, Drago I, Bortolozzi M, Scorzeto M, Gianelle A, Pizzo P, Pozzan T. Ca2+ hot spots on the mitochondrial surface are generated by Ca2+ mobilization from stores, but not by activation of store-operated Ca2+ channels. Mol Cell. 2010;38:280–290. doi: 10.1016/j.molcel.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, Koteliansky V, Mootha VK. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perocchi F, Gohil VM, Girgis HS, Bao XR, McCombs JE, Palmer AE, Mootha VK. MICU1 encodes a mitochondrial EF hand protein required for Ca2+ uptake. Nature. 2010;467:291–296. doi: 10.1038/nature09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mallilankaraman K, Cardenas C, Doonan PJ, Chandramoorthy HC, Irrinki KM, Golenar T, Csordas G, Madireddi P, Yang J, Muller M, Miller R, Kolesar JE, Molgo J, Kaufman B, Hajnoczky G, Foskett JK, Madesh M. MCUR1 is an essential component of mitochondrial Ca2+ uptake that regulates cellular metabolism. Nat Cell Biol. 2012;15:123. doi: 10.1038/ncb2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raffaello A, De Stefani D, Sabbadin D, Teardo E, Merli G, Picard A, Checchetto V, Moro S, Szabo I, Rizzuto R. The mitochondrial calcium uniporter is a multimer that can include a dominant-negative pore-forming subunit. EMBO J. 2013;32:2362–2376. doi: 10.1038/emboj.2013.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plovanich M, Bogorad RL, Sancak Y, Kamer KJ, Strittmatter L, Li AA, Girgis HS, Kuchimanchi S, De Groot J, Speciner L, Taneja N, Oshea J, Koteliansky V, Mootha VK. MICU2, a paralog of MICU1, resides within the mitochondrial uniporter complex to regulate calcium handling. PLoS One. 2013;8:e55785. doi: 10.1371/journal.pone.0055785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sancak Y, Markhard AL, Kitami T, Kovacs-Bogdan E, Kamer KJ, Udeshi ND, Carr SA, Chaudhuri D, Clapham DE, Li AA, Calvo SE, Goldberger O, Mootha VK. EMRE is an essential component of the mitochondrial calcium uniporter complex. Science. 2013;342:1379–1382. doi: 10.1126/science.1242993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alam MR, Groschner LN, Parichatikanond W, Kuo L, Bondarenko AI, Rost R, Waldeck-Weiermair M, Malli R, Graier WF. Mitochondrial Ca2+ uptake 1 (MICU1) and mitochondrial Ca2+ uniporter (MCU) contribute to metabolism–secretion coupling in clonal pancreatic beta-cells. J Biol Chem. 2012;287:34445–34454. doi: 10.1074/jbc.M112.392084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kamer KJ, Mootha VK. MICU1 and MICU2 play nonredundant roles in the regulation of the mitochondrial calcium uniporter. EMBO Rep. 2014;15:299–307. doi: 10.1002/embr.201337946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mallilankaraman K, Doonan P, Cardenas C, Chandramoorthy HC, Muller M, Miller R, Hoffman NE, Gandhirajan RK, Molgo J, Birnbaum MJ, Rothberg BS, Mak DO, Foskett JK, Madesh M. MICU1 is an essential gatekeeper for MCU-mediated mitochondrial Ca2+ uptake that regulates cell survival. Cell. 2012;151:630–644. doi: 10.1016/j.cell.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Csordas G, Golenar T, Seifert EL, Kamer KJ, Sancak Y, Perocchi F, Moffat C, Weaver D, de la Fuente Perez S, Bogorad R, Koteliansky V, Adijanto J, Mootha VK, Hajnoczky G. MICU1 controls both the threshold and cooperative activation of the mitochondrial Ca2+ uniporter. Cell Metab. 2013;17:976–987. doi: 10.1016/j.cmet.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de la Fuente S, Matesanz-Isabel J, Fonteriz RI, Montero M, Alvarez J. Dynamics of mitochondrial Ca2+ uptake in MICU1-knockdown cells. Biochem J. 2014;458:33–40. doi: 10.1042/BJ20131025. [DOI] [PubMed] [Google Scholar]
- 43.Patron M, Checchetto V, Raffaello A, Teardo E, Vecellio Reane D, Mantoan M, Granatiero V, Szabo I, De Stefani D, Rizzuto R. MICU1 and MICU2 finely tune the mitochondrial Ca2+ uniporter by exerting opposite effects on MCU activity. Mol Cell. 2014;53:726–737. doi: 10.1016/j.molcel.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Logan CV, Szabadkai G, Sharpe JA, Parry DA, Torelli S, Childs AM, Kriek M, Phadke R, Johnson CA, Roberts NY, Bonthron DT, Pysden KA, Whyte T, Munteanu I, Foley AR, Wheway G, Szymanska K, Natarajan S, Abdelhamed ZA, Morgan JE, Roper H, Santen GW, Niks EH, van der Pol WL, Lindhout D, Raffaello A, De Stefani D, den Dunnen JT, Sun Y, Ginjaar I, Sewry CA, Hurles M, Rizzuto R, Duchen MR, Muntoni F, Sheridan E Consortium UK. Loss-of-function mutations in MICU1 cause a brain and muscle disorder linked to primary alterations in mitochondrial calcium signaling. Nat Genet. 2014;46:188–193. doi: 10.1038/ng.2851. [DOI] [PubMed] [Google Scholar]
- 45.Hoffman NE, Chandramoorthy HC, Shamugapriya S, Zhang X, Rajan S, Mallilankaraman K, Gandhirajan RK, Vagnozzi RJ, Ferrer LM, Sreekrishnanilayam K, Natarajaseenivasan K, Vallem S, Force T, Choi ET, Cheung JY, Madesh M. MICU1 motifs define mitochondrial calcium uniporter binding and activity. Cell Rep. 2013;5:1576–1588. doi: 10.1016/j.celrep.2013.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang L, Yang X, Li S, Wang Z, Liu Y, Feng J, Zhu Y, Shen Y. Structural and mechanistic insights into MICU1 regulation of mitochondrial calcium uptake. EMBO J. 2014;33:594–604. doi: 10.1002/embj.201386523. [DOI] [PMC free article] [PubMed] [Google Scholar]