Abstract
Background:
Body mass index (BMI) is inconsistently associated with the progression of low bone mass–related fractures. We conducted a systematic review and meta-analysis to summarize the evidence regarding the relationship between BMI and the risk of fracture in men and women separately. Furthermore, we analyzed the association between BMI and fracture risk in women compared with men.
Methods:
PubMed, EmBase, and the Cochrane Library were searched up to November 2015 to identify prospective cohort studies of low bone mass–related fractures. Prospective cohort studies that reported effect estimates of fracture risk for different BMI categories compared to normal weight were included. Relative risk (RR) and the ratio of relative risk (RRR) were calculated using a random-effect model to measure the relationship between BMI and fracture risk.
Results:
We analyzed 37 cohorts (32 articles), which included a total of 506,004 women and 118,372 men; overall, 38,200 incident cases were reported. Overall, a lower BMI was not associated with fracture risk in men (RR: 1.50, 95% confidence interval [CI]: 1.00–2.26; P = 0.051) or women (RR: 1.25, 95% CI: 0.97–1.62; P = 0.083). Although a higher BMI might play a beneficial impact in men (RR: 0.80, 95% CI: 0.69–0.93; P = 0.003), it has little effect in women (RR: 0.91, 95% CI: 0.74–1.11; P = 0.343). In addition, an increase in BMI by 5 kg/m2 decreased the risk of fractures in men (RR: 0.90, 95% CI: 0.83–0.98; P = 0.017) and women (RR: 0.85, 95% CI: 0.81–0.89; P < 0.001). Finally, there was no evidence of a sex difference in the RR for fractures between participants with different BMI categories compared with those with normal BMI. Finally, gender did not affect the risk of fracture for any category of BMI values.
Conclusion:
Higher BMI may affect the risk of fractures regardless of the sex. This association may be due to the interaction between the participants’ BMI and their bone mass density.
Keywords: body mass index, fracture, gender, meta-analysis
1. Introduction
The World Health Organization defines obesity as a body mass index (BMI) ≥30 kg/m2, overweight as a BMI = 25 to 29.9 kg/m2, and underweight as a BMI < 18.5 kg/m2.[1] Guh et al[2] identified a number of comorbidities relating to obesity including osteoarthritis, diabetes, coronary heart disease, and some forms of cancer. The prevalence of overweight and obesity is increasing in the United States.[3] Similarly, the majority of elderly people in European countries and Australia are overweight or obese.[4,5] Although the prevalence of obesity in Asian populations is lower, it is increasing rapidly.[6]
Low BMI increases fracture risk, possibly because low BMI is associated with low bone mineral density (BMD), less soft tissue, and muscle weakness[7]; however, the relationship between high BMI and fracture risk is complex. Therefore, interest in studying the influence of obesity and overweight on the risk of fracture has recently increased. As an independent risk factor, obesity may increase the risk of all osteoporotic and hip fractures.[8,9]
According to a previous meta-analysis,[10] higher BMIs lower the fracture risk in women but not in men. This suggests that BMI affects fracture risk differently in men and women; however, the study was limited by a small sample size. Furthermore, a case–control study suggested that BMI had no effect on hip fracture risk due to a linear associations between body size and adiposity variables.[11] Nevertheless, another meta-analysis evaluated an important nonlinear relationship between BMI and the risk of fracture[9]; however, the meta-analyses[9,10] did not assess and explore the association between BMI and the risk of fracture among the genders in detail. The sex-specific differences in fracture risk associated with BMI are uncertain and need to be evaluated.
As the sex-specific effect of BMI on the rapidly increasing prevalence of obesity[12–14] is unclear,[7] we aim to investigate the association between BMI and fracture risk in men and women separately.
2. Methods
2.1. Data sources, search strategy, and selection criteria
PubMed, EMBASE, and the Cochrane Library databases were searched for articles published since the beginning of studying the relationships between BMI and fractures up to November 2015. The following keywords were used: [“obesity” OR “weight” OR “body weight” OR “body mass” OR “body mass index” OR “anthropometric” OR “anthropometry”] AND [“low bone mass-related fractures” OR “low bone mass” OR “bone fracture” OR “skeletal fracture” OR “osteoporotic fractures” OR “osteoporotic” OR “Osteoporosis” OR “broken bone” OR “low bone density-related fractures”] AND “men” AND “women” AND (“cohort” OR “cohort studies”). The search was limited to articles published in English. Abstracts and virtual meeting presentations from the EMBASE were searched to identify relevant, unpublished clinical trials. We also conducted manual searches of reference lists from all the relevant original and review articles to identify additional eligible studies. The medical subject heading, methods, participant population, design, exposure, and outcome variables of these articles were used to identify the relevant studies. The literature retrieval was performed in duplicate by 2 independent reviewers. This review was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Statement issued in 2009 (Checklist S1).[15] Ethics approval was not necessary for this study, as only deidentified pooled data from individual studies were analyzed.
Two authors conducted independent literature searches using a standardized approach, and any inconsistencies were settled by group consensus. Studies were deemed eligible for inclusion if they met the following criteria: they used a prospective cohort design; they evaluated the relationships between BMI and low bone-related fracture risk neglected of sites; they provided the relative risk (RR), hazard ratio (HR), or odds ratio (OR), and their 95% confidence intervals (CIs), of different BMI categories compared with the normal weight category, or per 5 kg/m2 increment of BMI. For studies without adequate data, we contacted the authors or abstracted data from other relevant articles; if the author or the relevant articles could not provide the necessary data, the study was excluded. In addition, case–control studies or retrospective cohort studies were excluded because various confounding factors could bias the results.
2.2. Data collection and quality assessment
Two authors independently conducted data extraction and assessment. Publication information (B-YX), characteristics of participants (country, gender, assessment of exposure, sample size, age at baseline, and duration of follow-up), and outcomes (fracture incident and effect estimate for the relationship between BMI and fracture) were extracted. Disagreement was resolved by consensus with a third reviewer. Two reviewers independently evaluated the quality of the studies using the Newcastle–Ottawa Scale (NOS).[16] The NOS assesses the selection (4 items), comparability (1 item), and outcome (3 items) of observational studies. The NOS is an 8-point questionnaire that produces a total score ranging from 0 (the worst) to 9 (the best). Disagreements between reviewers were resolved by consensus.
2.3. Statistical analysis
We examined the relationship between BMI and fracture risk by reviewing the effect estimate (RR, HR, or OR) and its 95% CI for each study. We used a random-effects model to calculate summary RRs and 95% CIs for the underweight (<18.5 kg/m2) category and overweight (>25.0 kg/m2) category compared to the normal weight (18.5–24.9 kg/m2) category.[17,18] Furthermore, we combined the RRs for each 5 kg/m2 increase in BMI by using a random-effect meta-analysis.[17,18] Finally, a random-effect model was used to evaluate the ratio of relative risk (RRR) of different BMI categories (compared to the normal weight category) in women compared with men.[17–19] Heterogeneity between studies was investigated by using the Q statistic, and we considered P values <0.10 to be indicative of significant heterogeneity.[20] Subgroup analyses were conducted of adjusted and raw BMD values. We performed a sensitivity analysis by removing each individual study from the meta-analysis.[21] The Egger and Davey Smith[22] and Begg and Mazumdar tests[23] were used to statistically assess publication bias. All reported P values are 2-sided, and P values <0.05 were considered statistically significant. Statistical analyses were performed using STATA software (version 12.0; Stata Corporation; College Station, TX).
3. Results
3.1. Literature search and study characteristics
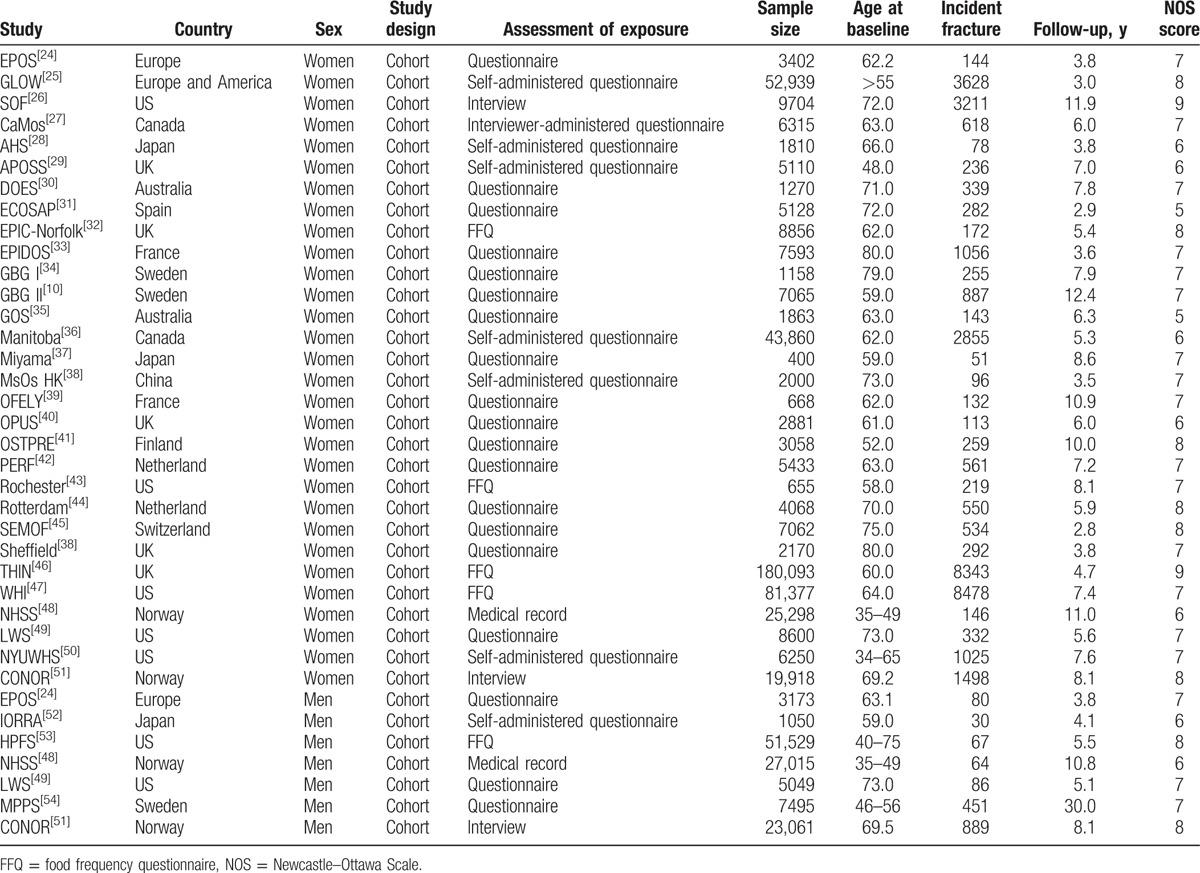
The primary search produced 2842 records. After scanning titles and abstracts, we excluded 2763 irrelevant articles. The remaining 79 full-text articles were reviewed, and 37 prospective cohorts (32 articles)[10,24–54] were selected for this meta-analysis, with a total of 506,004 women and 118,372 men (Fig. 1). A manual search of the studies’ reference lists did not yield any new eligible studies. The general characteristics of the included studies are presented in Table 1. The follow-up period for participants in the included studies ranged from 2.8 to 30.0 years, and the number of participants in each study ranged from 400 to 180,093. Study quality was assessed using the NOS scale (Table 1), and we considered a high-quality study to have an NOS score ≥7. Overall, 2 cohorts[26,46] had a score of 9, 8 cohorts[25,32,41,44,45,51,53] had a score of 8, 18 cohorts had a score of 7,[10,24,27,30,33,34,37–39,42,43,47,49,50,54] 7 cohorts[28,29,36,40,48,52] had a score of 6, and the remaining 2 cohorts[31,35] had a score of 5.
Figure 1.

Flow diagram of the literature search and trials selection process.
Table 1.
Baseline characteristic of studies included in the systematic review and meta-analysis.
3.2. Underweight versus normal weight
The summary RR showed that being underweight was not associated with fracture risk in men (RR: 1.50, 95% CI: 1.00–2.26; P = 0.051) or women (RR: 1.25, 95% CI: 0.97–1.62; P = 0.083), and evidence of significant heterogeneity was seen (Table 2). Furthermore, the RRR (female-to-male) was reduced by 17% (RRR: 0.83, 95% CI: 0.51–1.35; P = 0.458) for underweight participants compared to normal-weight participants, but this reduction was not statistically significant. Finally, the subgroup analysis showed that being underweight had no significant effect on the risk of fractures in men (RR: 0.89, 95% CI: 0.53–1.49; P = 0.658) and women (RR: 0.98, 95% CI: 0.79–1.20; P = 0.816) in BMD-adjusted studies. However, when results were not adjusted for BMD, being underweight increased the risk of fracture in men (RR: 1.89, 95% CI: 1.18–3.15; P = 0.009) and women (RR: 1.51, 95% CI: 1.35–1.68; P < 0.001). Furthermore, although the RRR (female-to-male) increased or decreased, there was no statistical significance for sex-difference in studies adjusted for BMD (RRR: 1.10, 95% CI: 0.63–1.92; P = 0.735) or not (RRR: 0.80; 95% CI: 0.49–1.30; P = 0.367).
Table 2.
Summarized results of meta-analyses of relationships between BMI and the risk of fractures.

3.3. Overweight versus normal weight
The pooled analysis results suggested that overweight is associated with lower fracture risk in men (RR: 0.80, 95% CI: 0.69–0.93; P = 0.003), whereas overweight has no significant impact on fracture risk in women (RR: 0.91, 95% CI: 0.74–1.11; P = 0.343). There was no evidence of heterogeneity for men but substantial heterogeneity for women (Table 2). Furthermore, the RRR (female-to-male) increased by 14% for overweight participants compared to normal-weight participants (RRR: 1.14, 95% CI: 0.88–1.46; P = 0.316); however, this increment was not statistically significant.
The subgroups of adjusted BMD values and raw BMD values were analyzed. The summary RR for BMD-adjusted studies suggested that being overweight had no effect on fracture risk in men (RR: 0.74, 95% CI: 0.42–1.30; P = 0.297) or women (RR: 1.05, 95% CI: 0.84–1.30; P = 0.685). Although the RRR (female-to-male) increased by 42% for BMD-adjusted studies, there was no significant gender difference (RRR: 1.42, 95% CI: 0.77–2.60; P = 0.258). In addition, the pooled RR, unadjusted for BMD, indicated that overweight was associated with lower risk of fractures in men (RR: 0.81, 95% CI: 0.69–0.94; P = 0.005) and women (RR: 0.83, 95% CI: 0.74–0.94; P = 0.003). There was no significant difference in the effect of being overweight on the fracture risk between women and men (RRR: 1.02, 95% CI: 0.84–1.25; P = 0.807).
3.4. Per 5 kg/m2 increment in BMI
Data showing the effect of a 5 kg/m2 increment in BMI on the risk of fractures were reported in 3 studies for men and 2 studies for women. All of these studies did not adjust BMD, and the pooled results indicated that increasing BMI in 5 kg/m2 increments decreased fracture risk for men (RR: 0.90, 95% CI: 0.83–0.98; P = 0.017) and women (RR: 0.85, 95% CI: 0.81–0.89; P < 0.001). Furthermore, the RRR (female-to-male) was 0.94, but this reduction was not statistically significant (RRR: 0.94, 95% CI: 0.86–1.04; P = 0.241; Table 2).
3.5. Publication bias
The Egger and Davey Smith[22] and Begg and Mazumdar[23] test results showed no evidence of publication bias for men (underweight vs normal weight [Egger: 0.732, Begg: 0.462] and overweight vs normal weight [Egger: 0.503, Begg: 0.452]) and women (underweight vs normal weight [Egger: 0.112, Begg: 0.462]). Although the Begg test showed no evidence of publication bias for women (overweight vs normal weight [P = 0.806]), the Egger test showed potential evidence of publication bias (P = 0.081). Adjustment for publication bias, performed using the trim and fill method, did not change our conclusions.[55]
4. Discussion
This analysis of prospective studies explored the effect of BMI values on the risk of low bone mass–related fractures. This large quantitative study included 506,004 women and 118,372 men from 37 prospective cohorts with a broad range of study populations. The findings from our meta-analysis suggest that being underweight has no effect on the incidence of fractures in men and women. However, being underweight significantly increased fracture risk in men and women when results were unadjusted for BMD. Furthermore, being overweight decreased fracture risk in men but did not affect fracture risk in women. In addition, the findings of the pooled analysis indicated that as BMI increased by 5 kg/m2, the risk of fracture decreased in men and in women. Finally, the RRR (female-to-male) indicated that there was no significant association between BMI and fracture risk.
A previous meta-analysis[38] of women suggested that being underweight increases the risk of hip and osteoporotic fractures, but may decrease the risk of leg fracture. Furthermore, being overweight may increase the risk of upper arm fracture. The relationship between BMI and low bone mass–related fractures in women might vary after BMD adjustment. Another important meta-analysis[56] of observational studies suggested that lower BMI is associated with higher risk of fractures in men (RR: 1.12, 95% CI: 1.04–1.20). Furthermore, fracture risk increased with every 5 kg/m2 decrease in BMI (RR: 1.30, 95% CI: 1.15–1.47). However, there are some possible limitations of these meta-analyses. First, the studies only evaluated either men or women; consequently, the gender difference was not established. Furthermore, retrospective observational studies were included, which might bias the results. Finally, the interaction between BMI and BMD may modify the relationship between BMI and fracture risk. Therefore, we performed a comprehensive meta-analysis of prospective cohort studies to evaluate any potential correlation between BMI and fractures and included an assessment of the related gender differences.
Most of our findings were in agreement with a previous cohort study conducted in California[49] that included 8600 women and 5049 men. The study found that a high BMI was associated with a strong reduction in hip fracture risk for women, whereas has slight impact for men. Meyer et al[48] suggested that a high BMI is not associated with fracture risk in women, but is strongly associated with a reduced risk of fracture in men. Søgaard et al[51] indicated that being underweight increases the risk of fractures for both men and women. They found that women with a higher BMI had a lower risk of fracture, whereas have a slight impact in men. Our meta-analysis suggests that a low BMI has no effect on fracture risk, whereas overweight men may have a lower risk of fracture. The possible reason for this could be that participants with different BMI levels might play an important role on BMD, which are important interaction confounders for the relationship between BMI and fractures. Furthermore, the waist/hip ratio may be affected by the BMI and also impacts the risk of fracture. Finally, participants with a low BMI are usually tall and underweight. When tall people fall over, they fall from a greater height; consequently, their velocity at impact with the surface may be greater. Moreover, individuals with a different padding effect of the soft tissues may produce diverse energy dissipation after trauma.[57]
There was no significant difference between participants who were underweight and of a normal weight on the risk of low bone mass–related fractures for men and women. However, several studies, including our own, reported inconsistent results. The Leisure World Study[49] suggested that being underweight increases the risk of fracture by 79% in women. Furthermore, the study conducted by Søgaard et al[51] reported similar results in women and men. Finally, Meyer et al[48] suggested that participants with a lower BMI have an increased risk of fracture. The possible reasons for these could be that these studies with longer duration of follow-up periods, and event rates were greater than expected, which always acquired narrow CIs, that is, with statistical significance. In addition, the pooled effect estimate, of nearly 0.05, suggests that being underweight could affect the fracture risk. The reason this association was not significant was perhaps that most trials in our study were designed with other indexes, not for fracture as a primary end point, and their sample sizes were too small to detect potential relationships.
In our study, lower BMI values were associated with a higher fracture risk, and higher BMI values were associated with a lower fracture risk when the results were not adjusted for BMD. These findings were inconsistent with BMD-adjusted studies. This could be because BMD may affect the relationship between BMI and fractures in men and women. Another study concluded that lower BMIs are an important risk factor for low bone mass and increased bone loss in postmenopausal women. The study found that low bone mass and an increased rate of bone loss were associated with an increased risk of postmenopausal osteoporosis.[58] However, as our study included smaller patient cohorts, we are unable to draw such conclusions. Therefore, we gave a relative result and provided a synthetic and comprehensive review.
Three strengths of our study should be highlighted. First, as only prospective studies were included, this should eliminate selection and recall bias, which are possible concerns in retrospective case–control studies. Second, the large sample size allowed us to quantitatively assess the association of BMI with the risk of low bone mass–related fractures, and thus our findings are potentially more robust than the findings of any individual study. Finally, gender difference analyses were conducted to evaluate the relationship between BMI and fracture risk.
Our study had several limitations. First, as the results of the majority of the included studies were only adjusted for age and were not adjusted for any other potential confounders, the fracture risk may have been affected. Second, stratified effect estimates on the basis of mean age, the duration of the follow-up periods, and sites of fracture were not available in individual study, so we could not perform the association between BMI and fractures in specific subsets, and not differentiate this relationship by sites of fracture. Third, in a meta-analysis of published studies, publication bias is an inevitable problem. Finally, our analysis used pooled data (as individual data were not available), which restricted us from performing a more detailed, relevant analysis to obtain results that were more comprehensive.
The results of this study suggest that higher BMI values affect the risk of fracture, but lower BMI values do not affect the risk of fracture. According to our sex difference analysis, there was no evidence of a sex difference in the RR for fractures among underweight or overweight participants.
Footnotes
Abbreviations: BMD = bone mineral density, BMI = body mass index, CI = confidence interval, HR = hazard ratio, NOS = Newcastle–Ottawa Scale, OR = odds ratio, RR = relative risk, RRR = ratio of relative risk.
Funding/support: The study was conducted under a grant from Special Fund for Scientific and Technological Cooperation of Zunyi, Guizhou (2015-41). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
References
- [1].WHO. Available at: http://www.who.int/topics/obesity/en/. [Accessed Nov 24, 2015]. [Google Scholar]
- [2].Guh DP, Zhang W, Bansback N, et al. The incidence of comorbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 2009;9:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 2010;303:235–41. [DOI] [PubMed] [Google Scholar]
- [4].Sundquist J, Johnansson S-E, Sundquist K. Levelling off of prevalence of obesity in the adult population of Sweden between 2000/01 and 2004/05. BMC Public Health 2010;10:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Haby MM, Markwick A, Peeters A, et al. Future predictions of body mass index and overweight prevalence in Australia, 2005–2025. Health Promot Int 2012;27:250–60. [DOI] [PubMed] [Google Scholar]
- [6].Finucane MM, Stevens GA, Cowan MJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 2011;377:557–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nielson CM, Srikanth P, Orwoll ES. Obesity and fracture in men and women: an epidemiologic perspective. J Bone Miner Res 2012;27:1–0. [DOI] [PubMed] [Google Scholar]
- [8].Compston J. Obesity and bone. Curr Osteoporos Rep 2013;11:30–5. [DOI] [PubMed] [Google Scholar]
- [9].Johansson H, Kanis J, Oden A, et al. High body mass index, adjusted for BMD, is a risk factor for fracture in women. Paper presented at: American Society for Bone & Mineral Research (ASBMR) 2011 Annual Meeting, San Diego, CA, September 16–20, 2011. [Google Scholar]
- [10].De Laet C, Kanis JA, Oden A, et al. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int 2005;16:1330–8. [DOI] [PubMed] [Google Scholar]
- [11].Nguyen ND, Pongchaiyakul C, Center JR, et al. Abdominal fat and hip fracture risk in the elderly: the Dubbo Osteoporosis Epidemiology Study. BMC Musculoskelet Disord 2005;6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Finucane MM, Stevens GA, Cowan MJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 2011;377:557–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 2010;303:235–41. [DOI] [PubMed] [Google Scholar]
- [14].Haby MM, Markwick A, Peeters A, et al. Future predictions of body mass index and overweight prevalence in Australia, 2005–2025. Health Promot Int 2012;27:250–60. [DOI] [PubMed] [Google Scholar]
- [15].Moher D, Liberati A, Tetzlaff J, et al. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wells G, Shea B, O’Connell D. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2009;Ottawa, ON: Ottawa Hospital Research Institute, Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. [Accessed Nov 24, 2015]. [Google Scholar]
- [17].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [18].Ades AE, Lu G, Higgins JP. The interpretation of random-effects meta-analysis in decision models. Med Decis Making 2005;25:646–54. [DOI] [PubMed] [Google Scholar]
- [19].Huxley RR, Woodward M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: a systematic review and meta-analysis of prospective cohort studies. Lancet 2011;378:1297–305. [DOI] [PubMed] [Google Scholar]
- [20].Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tobias A. Assessing the influence of a single study in meta-analysis. Stata Tech Bull 1999;47:15–7. [Google Scholar]
- [22].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [24].Roy DK, O’Neill TW, Finn JD, et al. Determinants of incident vertebral fracture in men and women: results from the European Prospective Osteoporosis Study (EPOS). Osteoporos Int 2003;14:19–26. [DOI] [PubMed] [Google Scholar]
- [25].Compston JE, Flahive J, Hosmer DW, et al. Relationship of weight, height, and body mass index with fracture risk at different sites in postmenopausal women: the global longitudinal study of osteoporosis in women (GLOW). J Bone Miner Res 2014;29:487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chapurlat RD, Bauer DC, Nevitt M, et al. Incidence and risk factors for a second hip fracture in elderly women. The Study of Osteoporotic Fractures. Osteoporos Int 2003;14:130–6. [DOI] [PubMed] [Google Scholar]
- [27].Papaioannou A, Joseph L, Berger GIC, et al. Risk factors associated with incident clinical vertebral and nonvertebral fractures in postmenopausal women: the Canadian Multicentre Osteoporosis Study (CaMos). Osteoporos Int 2005;16:568–78. [DOI] [PubMed] [Google Scholar]
- [28].Fujiwara S, Kasagi F, Yamada M, et al. Risk factors for hip fracture in a Japanese cohort. J Bone Miner Res 1997;12:998–1004. [DOI] [PubMed] [Google Scholar]
- [29].Barr R, Macdonald H, Stewart A, et al. Association between vitamin D receptor gene polymorphisms, falls, balance and muscle power: results from two independent studies (APOSS and OPUS). Osteoporos Int 2010;21:457–66. [DOI] [PubMed] [Google Scholar]
- [30].Wang CY, Nguyen ND, Morrison NA, et al. β3-adrenergic receptor gene, body mass index, bone mineral density and fracture risk in elderly men and women: the Dubbo Osteoporosis Epidemiology Study (DOES). BMC Med Genet 2006;7:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Díez-Pérez A, González-Macías J, Marín F, et al. Prediction of absolute risk of non-spinal fractures using clinical risk factors and heel quantitative ultrasound. Osteoporos Int 2007;18:629–39. [DOI] [PubMed] [Google Scholar]
- [32].Moayyeri A, Luben RN, Bingham SA, et al. Measured height loss predicts fractures in middle-aged and older men and women: the EPIC-Norfolk prospective population study. J Bone Miner Res 2008;23:425–32. [DOI] [PubMed] [Google Scholar]
- [33].Levasseur R, Dargent-Molina P, Sabatier JP, et al. Beta-blocker use, bone mineral density, and fracture risk in older women: results from the Epidemiologie del’Osteoporose prospective study. J Am Geriatr Soc 2005;53:550–2. [DOI] [PubMed] [Google Scholar]
- [34].Jonasson G, Sundh V, Ahlqwist M, et al. A prospective study of mandibular trabecular bone to predict fracture incidence in women: a low-cost screening tool in the dental clinic. Bone 2011;49:873–9. [DOI] [PubMed] [Google Scholar]
- [35].Henry MJ, Pasco JA, Seeman E, et al. Assessment of fracture risk: value of random population-based samples—the Geelong Osteoporosis Study. J Clin Densitom 2001;4:283–9. [DOI] [PubMed] [Google Scholar]
- [36].Leslie WD, Anderson WA, Metge CJ, et al. Clinical risk factors for fracture in postmenopausal Canadian women: a population-based prevalence study. Bone 2007;40:991–6. [DOI] [PubMed] [Google Scholar]
- [37].Tanaka S, Kuroda T, Yamazaki Y, et al. Serum 25-hydroxyvitamin D below 25 ng/mL is a risk factor for long bone fracture comparable to bone mineral density in Japanese postmenopausal women. J Bone Miner Metab 2014;32:514–23. [DOI] [PubMed] [Google Scholar]
- [38].Johansson H, Kanis JA, Odén A, et al. A meta-analysis of the association of fracture risk and body mass index in women. J Bone Miner Res 2014;29:223–33. [DOI] [PubMed] [Google Scholar]
- [39].Sornay-Rendu E, Boutroy S, Vilayphiou N, et al. In obese postmenopausal women, bone microarchitecture and strength are not commensurate to greater body weight: the Os des FemmesdeLyon (OFELY) study. J Bone Miner Res 2013;28:1679–87. [DOI] [PubMed] [Google Scholar]
- [40].Finigan J, Gossiel F, Glüer CC, et al. Endogenous estradiol and the risk of incident fracture in postmenopausal women: the OPUS study. Calcif Tissue Int 2012;91:59–68. [DOI] [PubMed] [Google Scholar]
- [41].Valtola A, Honkanen R, Kröger H, et al. Lifestyle and other factors predict ankle fractures in perimenopausal women: a population-based prospective cohort study. Bone 2002;30:238–42. [DOI] [PubMed] [Google Scholar]
- [42].Oei L, Estrada K, Duncan EL, et al. Genome-wide Association Study for radiographic vertebral fractures: a potential role for the 16q24 BMD locus versus lessons learned from challenging phenotype definition. Bone 2013;pii: S8756-3282(13)00425-0 [Epub ahead of print]. [PMC free article] [PubMed] [Google Scholar]
- [43].Hoehner CM, Greenlund KJ, Rith-Najarian S, et al. Association of the insulin resistance syndrome and microalbuminuria among nondiabetic native Americans. The Inter-Tribal Heart Project. J Am Soc Nephrol 2002;13:1626–34. [DOI] [PubMed] [Google Scholar]
- [44].Hofman A, Grobbee DE, de Jong PT, et al. Determinants of disease and disability in the elderly: the Rotterdam Elderly Study. Eur J Epidemiol 1991;7:403–22. [DOI] [PubMed] [Google Scholar]
- [45].Krieg MA, Cornuz J, Hartl F, et al. Quality controls for two heel bone ultrasounds used in the Swiss evaluation of the methods of measurement of osteoporotic fracture risk study. J Clin Densitom 2002;5:335–41. [DOI] [PubMed] [Google Scholar]
- [46].Weber DR, Haynes K, Leonard MB, et al. Type 1 diabetes is associated with an increased risk of fracture across the life span: a population-based cohort study using the health improvement network (THIN). Diabetes Care 2015;38:1913–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Crandall CJ, Yildiz VO, Wactawski-Wende J, et al. Postmenopausal weight change and incidence of fracture: post hoc findings from Women's Health Initiative Observational Study and Clinical Trials. BMJ 2015;350:h25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Meyer HE, Tverdal A, Falch JA, et al. Risk factors for hip fracture in middle-aged Norwegian women and men. Am J Epidemiol 1993;137:1203–11. [DOI] [PubMed] [Google Scholar]
- [49].Paganin-Hill A, Chao A, Ross RK, et al. Exercise and other factors in the prevention of hip fracture: the leisure world study. Epidemiology 1991;2:16–25. [DOI] [PubMed] [Google Scholar]
- [50].Kato I, Toniolo P, Zeleniuch-Jacquotte A, et al. Diet, smoking and anthropometric indices and postmenopausal bone fractures: a prospective study. Int J Epidemiol 2000;29:85–92. [DOI] [PubMed] [Google Scholar]
- [51].Søgaard AJ, Holvik K, Omsland TK, et al. Abdominal obesity increases the risk of hip fracture. A population-based study of 43,000 women and men aged 60–79 years followed for 8 years. Cohort of Norway. J Intern Med 2015;277:306–17. [DOI] [PubMed] [Google Scholar]
- [52].Furuya T, Kotake S, Inoue E, et al. Risk factors associated with incident fractures in Japanese men with rheumatoid arthritis: a prospective observational cohort study. J Bone Miner Metab 2008;26:499–505. [DOI] [PubMed] [Google Scholar]
- [53].Hemenway D, Azrael DR, Rimm EB, et al. Risk factors for hip fracture in US men aged 40 through 75 years. Am J Public Health 1994;84:1843–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Trimpou P, Landin-Wilhelmsen K, Odén A, et al. Male risk factors for hip fracture-a 30-year follow-up study in 7,495 men. Osteoporos Int 2010;21:409–16. [DOI] [PubMed] [Google Scholar]
- [55].Duvall S, Tweedie R. A nonparametric “trim and fill” method for assessing publication bias in meta-analysis. J Am Stat Assoc 2000;95:89–98. [Google Scholar]
- [56].Drake MT, Murad MH, Mauck KF, et al. Risk factors for low bone mass-related fractures in men: a systematic review and meta-analysis. J Clin Endocrinol Metab 2012;97:1861–70. [DOI] [PubMed] [Google Scholar]
- [57].Greenspan SL, Myers ER, Maitland LA, et al. Fall severity and bone mineral density as risk factors for hip fracture in ambulatory elderly. JAMA 1994;271:128–33. [PubMed] [Google Scholar]
- [58].Ravn P, Cizza G, Bjarnason NH, et al. Low body mass index is an important risk factor for low bone mass and increased bone loss in early post menopausal women. Early Postmenopausal Intervention Cohort (EPIC) study group. J Bone Miner Res 1999;14:1622–7. [DOI] [PubMed] [Google Scholar]


