Abstract
This study aims to investigate the efficacy and safety of neutral macroporous resin hemoperfusion in treating maintenance hemodialysis (MHD) patients with refractory uremic pruritus (RUP).
Ninety patients were enrolled and were randomly divided into 3 groups: control group, experiment 1 group, and experiment 2 group. Clinical symptom scores of skin itching were recorded before and at 4 and 8 weeks after the treatment. In addition, serum parathyroid hormone (PTH), calcium (Ca2+), phosphorus (P3+), and C-reactive protein (CRP) were detected; and the calcium–phosphorus product ([Ca] × [P]) was calculated to compare the curative effect.
VSA score, modified Duo pruritus score, and CRP: these indices decreased to some extent at 4 and 8 weeks after treatment in the 2 experiment groups, compared with pretreatment (P < 0.05); and differences among these 3 groups were statistically significant (P < 0.05). PTH, P3+, and [Ca] × [P]: these indices decreased to some extent at 4 and 8 weeks after treatment in the 2 experiment groups, compared with pretreatment (P < 0.05); and differences between the control and experiment 1 groups, as well as between the control and experiment 2 groups, were statistically significant (P < 0.05). However, the difference between the experiment 1 and experiment 2 groups were not statistically significant (P < 0.05).
The effects of HA330 and HA130 resin hemoperfusion apparatus on secondary hyperparathyroidism and the disorder of calcium and phosphorus metabolism are similar. The mechanism may be related to its strong adsorption effect, and its capacity to widely remove inflammatory mediators, immune mediators, and endotoxins.
Keywords: HA330 type resin hemoperfusion, hemoperfusion, intractable pruritus uremic, maintain the blood purification
1. Introduction
Uremic pruritus (UP) is a common complication in maintenance hemodialysis (MHD) patients, which seriously affects the quality of life of patients and reduces survival rate.[1,2] A study suggested that the degree of UP was not correlated with the length of dialysis, the number of weekly dialysis, primary renal diseases, gender, and age.[3] Most scholars considered that UP was mainly associated with the increase in parathyroid hormone (PTH) and β2-microglobulin (β2-MG).[4] At present, the application of a variety of blood purification methods (such as hemodiafiltration [HDF], hemoperfusion [HP], and HFHD) to improve the symptoms of UP in patients with MHD has achieved certain effects.[5–9] Different blood purification methods are commonly used in clinical practice.[10,11] The order of scavenging the efficiency of moderate- and macromolecular weight uremic toxins is HD + HP > HP > HDF > HF > HD (hemodialysis [HD]).[5] In recent years, HP technology has been widely used in many fields of clinical practice.[12] A number of studies have confirmed that HP can effectively remove PTH, β2-MG, and other moderate- and macromolecular weight uremic toxins,[5] and this technology has achieved good curative effects in improving refractory hypertension, refractory pruritus, and renal osteodystrophy. A study in China[13] revealed that a resin HP apparatus (RHA) can effectively remove iPTH and serum retinol-binding protein, and markedly improve skin itching. HDF can remove moderate-molecular-weight substances in plasma to a certain extent, such as iPTH, but its scavenging effect on the high-molecular complexes formed by retinol-binding protein, retinol, and prealbumin (in blood, retinol-binding protein binds with retinol and prealbumin at a ratio of 1:1:1 in a complex form) is not significant. Hence, it has a certain effect on skin itching. HD has no scavenging effect on serum iPTH and retinol-binding protein. At present, the most widely used RHA in China has the following characteristics:
-
(1)
All of these use neutral macroporous adsorption resin, the specific surface areas of the resin are all >1000 m2/g, the adsorption resin pore size is within 0 to 200 nm, some pore structures are over 10 nm, and there are obvious distribution peaks in the scope >50 nm. Due to the interaction between toxins and the hydrophobic groups, as well as the physical adsorption of the 3-dimensional mesh structures of the resin molecules, these apparatuses have an adsorption specificity that can effectively eliminate pathological molecules in blood, especially moderate- and macromolecular weight molecules.
-
(2)
High mechanical strength, no particles falling off, safe, and reliable.
-
(3)
High adsorption strength and large capacity.
-
(4)
Different types of these apparatuses have relative adsorption specificity and high selectivity to the removal of substances.
-
(5)
Good blood compatibility, few side effects, only a minority of patients had mild allergic reactions, hypotension, chills, shiver, and fever, and decreased white blood cells and platelets.
According to the characteristics of the pathogenic substances they can eliminate, these apparatuses can be divided into 5 models: HA130, HA230, HA280, HA330, and HA330-II. These apparatuses can be used in various clinical fields. HA130-RHA is used for the treatment of complications in MHD patients, especially for intractable itching and refractory hypertension.[12]
In the past 10 years, blood purification technology has made great progress and development, and various blood purification methods have been widely used in the treatment of UP. However, the incidence of UP remains very high. The epidemiology of UP has some statistical differences due to the sources of data. But its overall incidence is not optimistic. Narita et al[14] reported that, 50% to 90% of MHD patients had UP at varying degrees. The Japanese Dialysis Outcomes and Practice Patterns Study (JDOPPS) reveals that: the incidence of UP in patients undergoing MHD is 22% to 86% in Japan, among these patients 44% had moderate or severe itching.[2] Data from Israel reveal that, the incidence of UP in HD patients in the area is 66.0% to 74.3%, approximately 3/4 of patients have mild itching, and approximately 2/3 of patients have extensive skin itching.[15,16] The DOPPS I and DOPPS II reveal that, in MHD patients with UP, 42% to 45% patients have moderate to severe itching.[17] Data from Pakistan reveal that, 8% of MHD patients with UP have refractory itching in the area.[18] Risk of death was 17% higher in dialysis patients with pruritus than in other dialysis patients.[17] Despite the full dialysis and various treatment methods of choice, some patients continued to achieve unsatisfactory curative effects.[19] For the mechanism of UP, in recent years, people have considered that itching is not only a local skin disease, but a systemic inflammatory disease; and that this was closely related to the microinflammatory state in uremia patients.[20,14] A study[21] confirmed that itching did not alleviate even when PTH level decreased, and PTH level and mastocyte number did not significantly correlate with the presence of itching and itching degree. Furthermore, the level of serum inflammatory substances such as CXC chemokine receptor 3, CRP, IL-6, TNF-α, and gamma interferon are higher in UP patients than in non-UP patients.[19] In the clinical practice of using HP for the treatment of critically ill patients, the authors found that HA330-RHA can significantly improve the itching symptom in patients. This suggests that the active inflammatory state may play an important role in the pathogenesis of pruritus. By comparing the structural and functional characteristics between HA130-RHA and HA330-RHA, it was found that the former has structural characteristics mainly for the relative specific absorption of moderate- and macromolecular weight toxins produced in uremia, and that the removal of PTH and β2-MG was the most prominent. Furthermore, blood chamber volume was 110 ± 5 mL, and resin adsorption reached a saturation state when the treatment lasted for 2 hours. For the latter, resin gap size and the characteristics of the coating have been improved, its ability to selectively adsorb inflammatory mediators was enhanced, and the efficiency and specificity of removing macromolecular inflammatory mediators and endotoxins were high.[22] Furthermore, blood chamber volume was 185 ± 5 mL, and resin adsorption reached saturation state when the treatment lasted for 2.5 hours. A study has confirmed that this kind of macroporous neutral resin has a definite effect on the removal of inflammatory mediators such as TNF-α, IL-1, IL-6, IL8, and so on.[23] In this study, HA330-RHA was used. For RUP patients who achieved unsatisfactory curative effect after receiving a full HD and a variety of blood purification treatments, the combined treatment of HD and HA330-RHA was performed once every 2 weeks, in order to investigate its therapeutic effect. The authors consider that this is of important clinical significance.
2. Materials and methods
This study was conducted with approval from the Ethics Committee of Yanan Hospital Affiliated to Kunming Medical University in January 2009. Written informed consent was obtained from all participants.
2.1. Inclusion criteria of cases
From January 2009 to January 2016, 90 patients who were treated with MHD for more than 3 months at the blood purification center of Yan’an Hospital Affiliated to Kunming Medical University were included into this study. Inclusion criteria: patients diagnosed with UP;[24,25] patients who have received a variety of blood purification treatments for more than 1 month (including HDF, HFHD, and HA130-HP), and had small improvements on skin itching symptoms or frequent attacks. Exclusion criteria: patients with systemic diseases (the liver, gallbladder disease, allergies, asthma, and tumors), skin diseases (psoriasis and skin tinea diseases), and metabolic diseases experienced itchy skin. Patients who had contraindications to HP.
2.2. Experiment grouping
Ninety patients were randomly divided into 3 groups by sealed letters: control group (regular hemodialysis [RHD]), experiment 1 group (RHD + HA130-HP), and experiment 2 group (RHD + HA330-HP). The specific method was as follows: the words “control group” were written on 30 cards; in the same way, “experiment group 1” and “experiment group 2” were written on 30 cards, respectively; and all the cards were placed into 90 envelopes. Patients who met the inclusion criteria and were willing to attend the trial chose and opened 1 envelope. The patient was assigned into 1 group, according to the card in the envelope. If patients withdrew from the trial (lost rate of follow-up was controlled within 10%), the corresponding number of patients were added into the corresponding group, until 30 patients completed the trial in each group. In the control group, no patient withdrew from the experiment. In experimental group 1, 1 patient withdrew from the experiment (the patient began treatment on May 3, 2009 and withdrew on April 8, 2009); and in experimental group 2, 2 patients withdrew from the experiment (1 patient began treatment on October 7, 2011 and withdrew on October 27, 2011; another patient began treatment on June 25, 2013 and withdrew on July 17, 2013).
2.3. Therapeutic methods
All selected patients provided a signed informed consent on blood purification prior to treatment. Three groups of patients underwent MHD using a German BRAUN Dialog HD machine, a LOPS15 polysulfone membrane low-flux dialyzer (membrane area: 1.5 m2, ultrafiltration coefficient: 9.8 mL/hour mm Hg, Germany BRAUN), bicarbonate dialysate, and ultrapure dialysis water (double reverse osmosis). The frequency of dialysis was 3 times per week, and duration was 4 hours per time. Blood flow volume was 250 to 300 mL/min, and dialysate flow volume was 500 mL/min. Anticoagulation was conducted using low-molecular-weight heparin. Control group: RHD, dialysis frequencies 3 times per week, 4 hours per time, blood flow volume 250 to 300 mL/min, dialysate flow 500 mL/min. Experiment 1 group: on the basis of RHD, patients received HP once every 2 weeks (HP was performed during the last HD in even weeks). The HP apparatus used was the HA130-RHA (Zhuhai Jafron Biotechnology Inc.). The HP apparatus was set before the dialyzer in series, and blood flow volume was set at 180 to 200 mL/min. The RHA was removed after 2 hours of treatment (when resin adsorption reaches a saturation state), and HD was continued for 4 hours. Experiment 2 group: on the basis of RHD, patients received HP once every 2 weeks (HP was performed during the last HD in even weeks). The HP apparatus used was the HA330-RHA (Zhuhai Jafron Biotechnology Inc.). The HP apparatus was set before the dialyzer in series, and blood flow volume was set at 180 to 200 mL/min. The RHA was removed after 2.5 hours of treatment (when resin adsorption reached a saturation state), and HD continued for 4 hours. The experiment foundation treatment for patients in the 3 groups before and after the trial was relatively fixed: PTH >300 pg/L, and oral calcitriol soft capsules 0.25 g at approximately 0.5 g/time, once daily. Pulse therapy: PTH >500 pg/L, and oral calcitriol at 2.0 to 2.5 g/day, twice a week. During the trial, insulin, erythropoietin, calcium supplement, and antihypertensive drug treatments were adjusted according to the condition of the disease and its detection.
2.4. Observation indexes
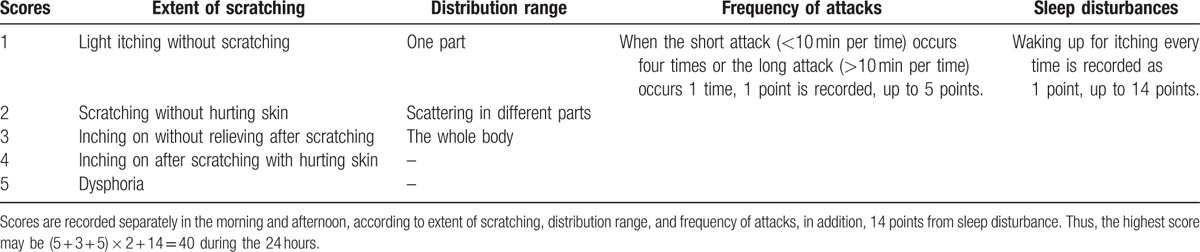
Before and 4 and 8 weeks after treatment, itching was evaluated by visual analogue scale (VAS)[26] and modified Duo pruritus scores.[27] The specific method was as follows: for VAS scores, a vernier of approximately 10 cm in length was used, on which 1 side was marked with 10 scales, and the 2 ends were “0” and “10” ends, respectively. The “0” points represented no itching, and the “10” points represented unbearable itching. Patients faced the side with the scales, and marked their own degrees of itching between 0 and 10 points, according to their own judgement. Scores between 0 and 2 points were excellent, scores between 3 and 5 points were good, scores between 6 and 8 points were medium, and scores >8 were poor (Fig. 1). Modified Duo pruritus scores: doctors evaluated the degree of itching according to the extent of scratching, distribution range, frequency of attacks, and sleep disturbances (Table 1). At the same time, exsanguinate assay for PTH, Ca2+, P3+, and CRP levels was conducted; and [Ca] × [P] was calculated.
Figure 1.
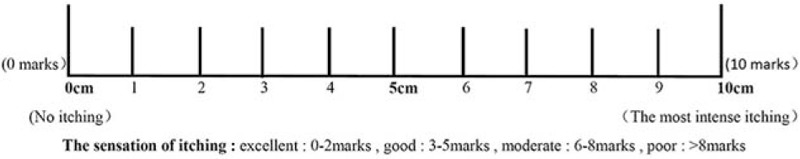
Visual analogue scale.
Table 1.
Modified Duo pruritus score system (0–40 score).
2.5. Statistical methods
Statistics analysis was performed using SPSS 20.0 software. The basic description of data was conducted using mean ± standard deviation, rate, and a correlation graph. Hypothesis testing was performed using 2 independent samples t-test, X2 test, and repeated measures analysis of variance (ANOVA). P < 0.05 was considered statistically significant.
3. Results
3.1. Comparison of the general information of subjects
The comparison was conducted using the variance test of a complete and randomly designed data. Differences in age, urea nitrogen, creatinine, and dialysis age among the 3 groups of patients were not statistically significant (P > 0.05) (Table 2).
Table 2.
Comparison of the basic condition of patients in 3 groups.

3.2. Comparison of clinical test indices among the 3 groups before and 4 and 8 weeks after treatment
Repeated measures ANOVA:
-
(1)
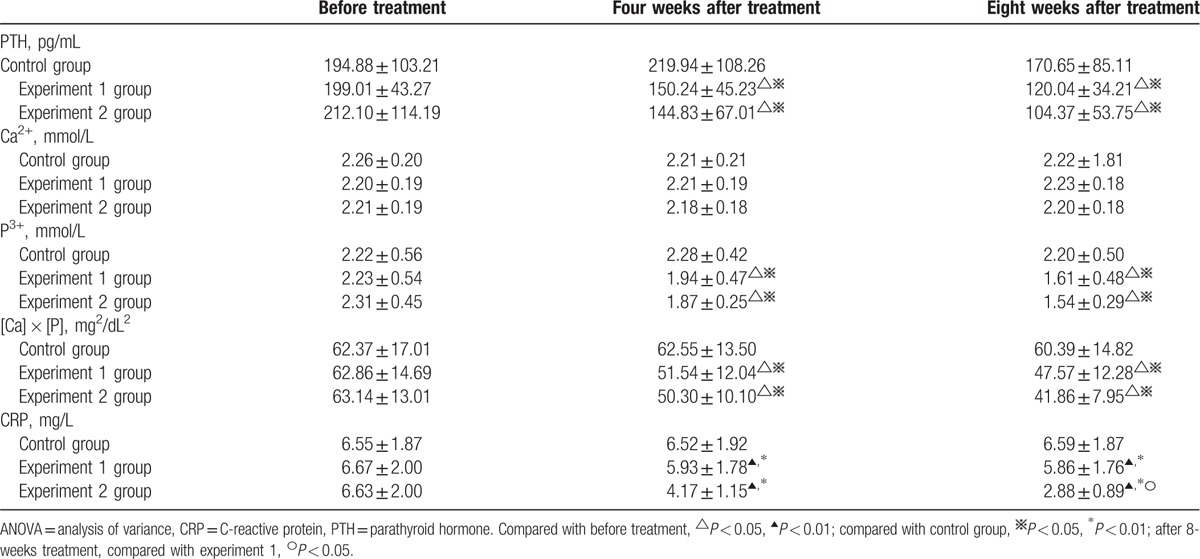
Ca2+, test of within-subject effect: F = 2.625, P = 0.101; test of intersubject effect: F = 0.647, P = 0.424. These results revealed that: with the extension of treatment, the differences between pre- and posttreatment among the 3 groups were not statistically significant (P > 0.05). Hence, it can be considered that differences in the effects of the treatments in the 3 groups on Ca2+ metabolism were not statistically significant.
-
(2)
PTH, P3+, and [Ca] × [P], test of within-subject effect: P < 0.05; test of intersubject effect, P < 0.05. These results revealed that: with the extension of treatment, PTH, P3+, and [Ca] × [P] decreased to a certain extent in these 2 experimental groups; pairwise comparison using LSD t test revealed that these decreases were significantly prominent in the 2 experimental groups than in the control group (P < 0.05), and the difference between these 2 experimental groups was not statistically significant (P > 0.05). It can be considered that the removal of PTH and P3+ and the improvement of [Ca] × [P] were better in these 2 experimental group than in the control group, and the effects between these 2 experimental groups were not different.
-
(3)
CRP, test of within-subject effect: F = 53.840, P < 0.001; test of intersubject effect: F = 1211.319, P < 0.001. These results revealed that: with the extension of the treatment, CRP decreased to a certain extent in these 2 experimental groups; pairwise comparison using LSD t test revealed that the differences among these 3 groups were statistically significant (P < 0.05), and the decreases were significantly prominent in the 2 experimental groups than in the control group (P < 0.01).
After 8 weeks of treatment, the decrease was significantly prominent in experimental group 2 than in experimental group 1 (P < 0.05). It can be considered that CRP could be effectively reduced in these 2 experimental groups, and this effect was better in experimental group 2 (Table 3).
Table 3.
ANOVA of repeated data of clinical indicators changes before and after treatment with patients in 3 groups.
3.3. Pruritus score comparison among the 3 groups before and 4 and 8 weeks after treatment
3.3.1. Comparison of VSA scores
Repeated measures ANOVA: test of within-subject effect: F = 393.645, P < 0.001; test of intersubject effect: F = 17.528, P < 0.001. These results revealed that: with the extension of the treatment, VSA scores decreased to a certain extent in these 2 experimental groups. Pairwise comparison using LSD t test revealed that the differences among these 3 groups were statistically significant (P < 0.05), and these decreases were significantly prominent in these 2 experimental groups than in the control group (P < 0.05, P < 0.01). After 8 weeks of treatment, these decreases were significantly prominent in experimental group 2 than in experimental group 1 (P < 0.05). It can be considered that these itching symptoms could be effectively improved in these 2 experimental groups, and this effect was best in experimental group 2 (Fig. 2).
Figure 2.
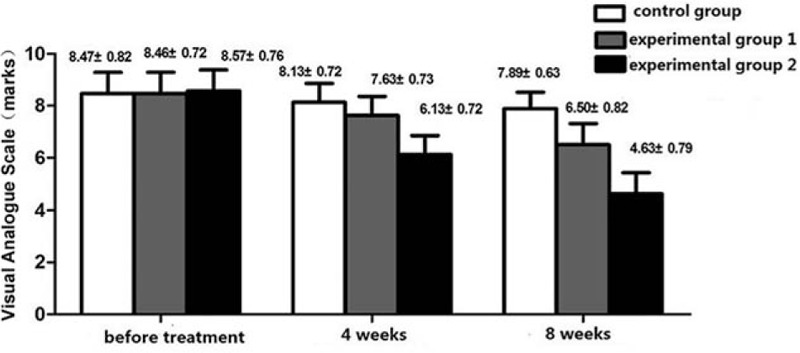
Visual analogue scale (VAS) score before treatment, 4 and 8 weeks of 3 groups (score). Application of duplicate variance analysis: test of within-subject effect, F = 393.645, P < 0.001; test of intersubject effect, F = 17.528, P < 0.001; comparison of each 2 groups, they all have difference (P < 0.05).
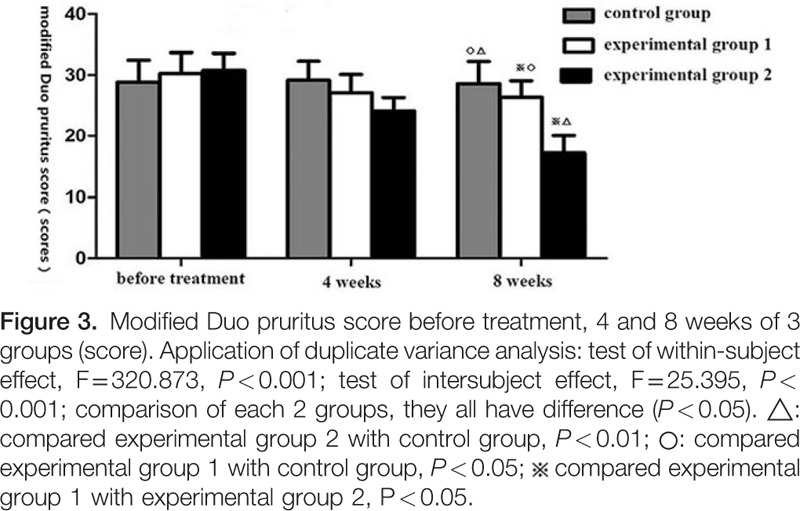
3.3.2. Comparison of modified Duo pruritus scores
Repeated measures ANOVA: test of within-subject effect: F = 320.873, P < 0.001; test of intersubject effect: F = 25.395, P < 0.001. These results revealed that: with the extension of the treatment, modified Duo pruritus scores decreased to a certain extent in these 2 experimental groups. Pairwise comparison using LSD t test revealed that the differences among the 3 groups were statistically significant (P < 0.05), and these decreases were significantly prominent in these 2 experimental groups than in the control group (P < 0.05, P < 0.01). After 8 weeks of treatment, these decreases were significantly prominent in experimental group 2 than in experimental group 1 (P < 0.05). It can be considered that these itching symptoms could be effectively improved in the 2 experimental groups, and this effect was best in experimental group 2 (Fig. 3).
4. Discussion
UP is one of the most common and uncomfortable symptoms in MHD patients.[28] The current situations of research and treatment in MHD patients with UP have been introduced in the introduction section. The combined treatment of HP and HD is the best blood purification method to remove moderate- and macromolecular weight toxins. The Chinese authoritative guide recommends the combined treatment of HD and HP (HA130-RHA) as a relatively ideal blood purification method to treat UP.[12] Despite all the above, the incidence of UP remains high; and no ideal treatment method is currently available. Curative effect and inflammatory response state between the 2 HP apparatuses (HA330-RHA and HA130-RHA) with different design and absorption characteristics on MHD patients with RUP were compared. These results revealed that in these 2 groups of patients who underwent HP treatment, VAS scores, modified Duo scores, PTH, P3+, [Ca] × [P], and CRP decreased to varying degrees with the extension of treatment. These indexes were better compared with before treatment and the control group (RHD group) (P < 0.05, P < 0.01). After 8 weeks of treatment, VAS scores, modified Duo scores, and CRP were significantly lower in experimental group 2 (HA330-RHA group) than in experimental group 1 (HA130-RHA group, P < 0.05). These revealed that HA330-RHA could safely and effectively improve refractory itching symptoms and the inflammation state in MHD patients. With the extension of treatment, its curative effect became better than that of HA130-RHA. The curative effects on secondary hyperparathyroidism and on calcium and phosphorus metabolism disturbance are similar to these. It is known that HA330-RHA can strongly adsorb inflammatory mediators and endotoxins.[22] Compared with HA130-RHA, its better curative effect may be related to its relative specificity; and it is highly active in the removal of macromolecular-weight inflammation mediators and endotoxins. There are many reports on the application of HA130-RHA in MHD patients with UP.[5,13] The application of HA330-RHA was mainly found in critical patients.[22] There was no report on its application in MHD patients with UP. Although a number of studies have revealed a variety of factors related to the occurrence of UP, no specific pathogeny has been found. It is possible that there may be more complex factors participating in the pathogenesis of RUP. Recent studies[29–33] have revealed that a variety of inflammation factors participate in the pathogenesis of UP. A study[34] has revealed that the complex microenvironment network formed by inflammatory factors and the persistent inflammation state play important roles in the pathogenesis of the repeat attacks of pruritus. A study[35] has suggested that in UP patients, stem cell factors were significantly increased, and the elevation of stem cell factors could recruit chemokines and stimulate mastocyte proliferation and activation. These would induce the release of a variety of inflammatory mediators including histamine, and aggravate skin itching. Some foreign scholars have achieved remarkable curative effects in treating recurrent pruritus using molecular adsorbent recirculating system.[36] This suggested that macromolecular-weight inflammatory mediators may be the key factor of RUP. We consider that there are obviously individual differences in the severity of UP, and current various blood purification methods can alleviate these itching symptoms in some patients. However, its curative effect is limited in some patients. This may be related to the limitations of the scope, efficiency, and amount of the itch causing substances that these blood purification methods can remove. This suggests that UP may be caused by a variety of factors. Its refractory feature may be related to the amount and accumulation of macromolecular-weight inflammatory mediators, and the duration and intensity of the inflammatory state. This study did not further explore the relationship between the scavenging effect of macromolecular-weight inflammatory mediators and its curative effect, which requires further exploration.
Footnotes
Abbreviations: ANOVA = analysis of variance, CRP = C-reactive protein, HD = hemodialysis, HDF = hemodiafiltration, HP = hemoperfusion, β2-MG = β2-microglobulin, MHD = maintenance hemodialysis, PTH = parathyroid hormone, RHA = resin HP apparatus, RHD = regular hemodialysis, RUP = refractory uremic pruritus, UP = uremic pruritus.
Ethic statement: complied ethical standards.
Funding/support: This work was supported by Yanan Hospital Affiliated to Kunming Medical University (yyky014–013).
The authors have no conflicts of interest to disclose.
References
- [1].Suseł J, Batycka-Baran A, Reich A, et al. Uraemic pruritus markedly affects the quality of life and depressive symptoms in haemodialysis patients with end-stage renal disease. Acta Derm Venereol 2014;94:276–81. [DOI] [PubMed] [Google Scholar]
- [2].Kimata N, Fuller DS, Saito A, et al. Pruritus in hemodialysis patients: results from the Japanese Dialysis Outcomes and Practice Patterns Study (JDOPPS). Hemodial Int 2014;18:657–67. [DOI] [PubMed] [Google Scholar]
- [3].Khanna D, Singal A, Kalra OP. Comparison of cutaneous manifestations in chronic kidney disease with or without dialysis. Postgrad Med J 2010;86:641–7. [DOI] [PubMed] [Google Scholar]
- [4].Attia EA, Hassan AA. Uremic pruritus pathogenesis, revisited. Arab J Nephrol Transplant 2014;7:91–9. [PubMed] [Google Scholar]
- [5].Chen SJ, Jiang GR, Shan JP, et al. Combination of maintenance hemodialysis with hemoperfusion:a safe and effective model of artificial kidney. Int J Artif Organs 2011;34:339–47. [DOI] [PubMed] [Google Scholar]
- [6].Shinzato T, Maeda K. Push/pull hemodiafiltration. Contrib Nephrol 2007;158:169–76. [DOI] [PubMed] [Google Scholar]
- [7].Wang JD, Li CQ, Chen YL. Observation of curative effect on the way of uremic pruritus of different blood purification. J Clin Nephrol 2012;12:67–9. [Google Scholar]
- [8].Zhao J, Wang MJ, Jiang HW. Blood perfusion flow hemodialysis and curative effect observation of high flux hemodialysis on pruritus in maintenance hemodialysis patients. J Clin Nephrol 2011;11:175–6. [Google Scholar]
- [9].Cai XP, Yang M, You YW. Analysis of curative effect of high flux dialysis combined with low calcium dialysate in uremic pruritus. Chin Blood Purif 2014;1:38–40. [Google Scholar]
- [10].Bammens B, Evenepoel P, Verbeke K, et al. Removal of theprotein-bound solute p-cresol by convective transport: a randomized crossorer study. Am J Kidney Dis 2004;44:278–85. [DOI] [PubMed] [Google Scholar]
- [11].Mandolfo S, Borlandelli S, Imbasciati E. Leptin and beta2-microglobulin kinetics with three different dialysis modalities. Int J Artif Organs 2006;29:949–55. [DOI] [PubMed] [Google Scholar]
- [12].Chen XM. Blood Purification Standard Operating Procedure (SOP) [M], 2010 ed. 2010;Beijing: People's Military Medical Press, 50-70. [Google Scholar]
- [13].Li MX, Lv P, Lei X, et al. Compared the clearance for serum middle molecule substances with different blood purification methods in chronic renal failure. Chin J Blood Purif 2005;12:652–3. [Google Scholar]
- [14].Narita I, Iguchi S, Omori K, et al. Uremic pruritus in chronic hemodialysis patients. J Nephrol 2008;2l:16l–5. [PubMed] [Google Scholar]
- [15].Zucker I, Yosipovitch G, David M, et al. Prevalence and characterization of uremic pruritus in patients undergoing hemodialysis: uremic pruritus is still a major problem for patients with end-stage renal disease. J Am Acad Dermatol 2003;49:842–6. [DOI] [PubMed] [Google Scholar]
- [16].Dyachenko P, Shustak A, Rozenman D. Hemodialysis-related pruritus and associated cutaneous manifestations. Int J Dermatol 2006;45:664–7. [DOI] [PubMed] [Google Scholar]
- [17].Pisoni RL, Wikström B, Elder SJ, et al. Pruritus in haemodialysis patients: International results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 2006;2l:3495–505. [DOI] [PubMed] [Google Scholar]
- [18].Dar NR, Akhter A. Clinical characteristics of uremic pruritus in patients undergoing haemodialysis. J Coll Physicians Surg Pak 2006;16:94–6. [PubMed] [Google Scholar]
- [19].Mistik S, Utas S, Ferahbas A, et al. An epidemiology study of patients with uremic pruritus. J Eur Acad Dermatol Venereol 2006;20:672–8. [DOI] [PubMed] [Google Scholar]
- [20].Kimmel M, Alscher DM, Dunst R, et al. The role of micro-inflammation in the pathogenesis of uraemic pruritus in haemodialysis patients. Nephrol Dial Transplant 2006;21:749–55. [DOI] [PubMed] [Google Scholar]
- [21].Chou FF, Ho JC, Huang SC, et al. A study on pruritus after parathyroidectomy for secondary hyperparathyroidism. J Am Coll Surg 2000;190:65–70. [DOI] [PubMed] [Google Scholar]
- [22].Huang Z, Wang SR, Su W, et al. Removal of humoral mediators and the effect on the survival of septic patients by hemoperfusion with neutral microporous resin column. Ther Apher Dial 2010;14:596–602. [DOI] [PubMed] [Google Scholar]
- [23].Tian S, Li T, Luo LQ, et al. Study on the adsorption of endotoxin and proinflammatory cytokines with HB-H-7 resin in vitro. Chin Criti Care Med 2009;21:179–82. [PubMed] [Google Scholar]
- [24].Urbonas A, Schwartz RA, Szepietowski JC. Uremic pruritus-an update. Am J Nephrol 2001;21:343–50. [DOI] [PubMed] [Google Scholar]
- [25].Wang HY. Wang HY<Eds>. Nephrology. Beijing: People's Medical Publishing House; 2008. 1872–3. [Google Scholar]
- [26].Reich A, Heisig M, Phan NQ, et al. Visual analogue scale: evaluation of the instrument for the assessment of pruritus. Acta Derm Venereol 2012;92:497–501. [DOI] [PubMed] [Google Scholar]
- [27].Mapar MA, Pazyar N, Siahpoosh A, et al. Comparison of the efficacy and safety of zinc sulfate vs placebo in the treatment of pruritus of hemodialytic patients: a pilot randomized, triple-blind study Giornale Italiano di Dermatologia e Venereologia: organo ufficiale. Società Italiana di Dermatologia e Sifilografia 2015;150:351–5. [PubMed] [Google Scholar]
- [28].Ko MJ, Yang JY, Wu HY, et al. Narrowband ultraviolet B phototherapy for patients with refractory uraemic pruritus: a randomized controlled trial. Br J Dermatol 2011;165:633–9. [DOI] [PubMed] [Google Scholar]
- [29].Ko MJ, Peng YS, Chen HY, et al. Interleukin-31 is associated with uremic pruritus in patients receiving hemodialysis. J Am Acad Dermatol 2014;71: 1151.el-1159.e1. [DOI] [PubMed] [Google Scholar]
- [30].Azim AA, Farag AS, El-Maleek Hassan DA, et al. Role of interleukin-2 in uremic pruritus among attendants of AL-Zahraa Hospital Dialysis Unit. Indian J Dermatol Venereol Leprol 2015. 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lin HH, Liu YL, Liu JH, et al. Uremic pruritus, cytokines, and polymethylmethacrylate artificial kidney. Artif Organs 2008;32:468–72. [DOI] [PubMed] [Google Scholar]
- [32].Zhang N, Liao WH, Zeng R. Maintaining blood relationship between inflammation in dialysis patients with pruritus. Chin Blood Purif 2012;8:429–32. [Google Scholar]
- [33].Qiu FX, Song L, Su J. Maintain the relationship between the serum of hemodialysis patients with stem cell factor and skin pruritus. Study Tissue Eng China 2014;18:3111–6. [Google Scholar]
- [34].Malekmakan L, Malekmakan A, Sayadi M, et al. Association of high-sensitive C-reactive protein and dialysis adequacy with uremic pruritus. Saudi J Kidney Dis Transpl 2015;26:890–5. [DOI] [PubMed] [Google Scholar]
- [35].Dugas-Breit S, Schöpf P, Dugas M, et al. Baseline serum-levels of mast cell tryptaxc are raised in hemodialysis patients and associated with severity of pruitus. J Dtsch Dermatol Ges 2005;3:343–7. [DOI] [PubMed] [Google Scholar]
- [36].Mullhaupt B, Ambuhl PM, Renner EL. Successful use of the Molecular Adsorbent Recirculating System(MARS) in a patient with primary biliary cirrhosis (PBC) and treatment refractory pruritus. Hepatol Res 2003;25:442–6. [DOI] [PubMed] [Google Scholar]


