Abstract
Severe alpha-1-antitrypsin (AAT) deficiency (PiZZ) is a risk factor for liver disease, but the prevalence of liver cirrhosis and hepatocellular cancer in PiZZ adults is unknown. The risk of liver disease in adults with moderate AAT deficiency (PiSZ) is also unknown. A cohort of 127 PiZZ, 2 PiZnull, 54 PiSZ, and 1 PiSnull individuals were identified by the Swedish national neonatal AAT screening program between 1972 and 1974, when all 200,000 newborn infants in Sweden were screened for AAT deficiency. The cohort has been followed up since birth. Our aim was to study liver function and signs of liver disease in this cohort at 37 to 40 years of age in comparison with a matched, random sample of control subjects identified from the population registry.
Eighty seven PiZZ, 32 PiSZ, and 92 control subjects (PiMM) answered a questionnaire on medication and alcohol consumption and provided blood samples. Liver stiffness was assessed by Acoustic Radiation Force Impulse (ARFI) elastography in 32 PiZZ, 15 PiSZ, and 51 PiMM subjects.
The median of liver function tests and procollagen-III-peptide were within the normal range in all Pi subgroups. However, the PiZZ men had significantly higher plasma bilirubin than the PiMM men (P = 0.018). Plasma ɣ-glutamyl transferase (GGT) was significantly higher in the PiZZ men (P = 0.009) and the PiSZ men (P = 0.021) compared with the PiMM men. The median of liver stiffness was significantly higher in the PiZZ men (P = 0.037) and the PiSZ men (P = 0.032) compared with the PiMM men. The PiZZ women taking medication influencing liver enzymes had significantly higher GGT than the PiMM women on the corresponding treatment (P = 0.023).
These AAT-deficient individuals identified by neonatal screening have normal plasma levels of liver function tests, and no clinical signs indicating liver disease at the age of 37 to 40 years. However, bilirubin, GGT, and liver stiffness are significantly higher in PiZZ men than PiMM men.
Keywords: alpha-1-antitrypsin deficiency, elastography, liver disease, liver function, screening
1. Introduction
Severe alpha-1-antitrypsin (AAT) deficiency (PiZZ) is a known risk factor for developing liver disease.[1,2] AAT is a 52-kDa protein, a member of the serpin family that inactivates serine proteases such as neutrophil elastase. AAT deficiency is an autosomal codominant hereditary disorder. A mutation in the AAT gene (SERPINA1) leads to the synthesis of defective Z protein with Glu342Lys substitution, which in turn leads to polymerization, accumulation in the endoplasmic reticulum of hepatocytes, impaired secretion, and decreased serum levels.[3–5] The homozygote ZZ genotype may lead to liver cirrhosis and hepatocellular cancer.[6] The defective S protein is not associated with intracellular accumulation of the protein and normally inhibits elastase. The SZ mutation has not been shown to be a risk factor for liver disease in children and the previously published studies in adults are sparse.[6,7] Our gathered knowledge about liver function in AAT deficiency is mostly based on symptoms and disease progression in patients who were first diagnosed with liver affection before they were diagnosed as AAT-deficient. The natural course, early signs, and factors influencing the disease onset are still not clearly understood.
The Swedish national neonatal AAT screening program was carried out between 1972 and 1974, when all 200,000 newborn children were screened for AAT deficiency.[8] The original cohort included 127 PiZZ, 2 PiZnull, 54 PiSZ children, and 1 PiSnull child. The cohort has been followed up regularly.[9–13] Eighteen PiZZ children, but none of the PiSZ children, suffered from liver disease early in life. Five PiZZ children and 1 PiSZ child died before the age of 8 years. On the other hand, 5 new PiZZ individuals born abroad during the screening period have been identified and added to the cohort.
The aim of this study was to investigate liver function and early signs of liver disease at the age of approximately 38 years in this cohort of PiZZ and PiSZ individuals, in comparison with a matched control group randomly selected from the population registry.
2. Methods
2.1. Study population
One PiZZ and 3 PiSZ subjects had died after the previous check-up at the age of 34 years.
All remaining living subjects, 126 PiZZ, 2 PiZnull, 50 PiSZ, and 1 PiSnull individuals, were invited to participate in this follow-up study. A random sample of 300 matched individuals, living in the southern part of Sweden, was identified from the Swedish population registry, and invited to participate as a control group. The control group was the same as that invited at the 2 previous check-ups at 30 and 34 years of age.[12,13]
The study was conducted in accordance with the Helsinki declaration and approved by the Regional Ethical Review Board of Lund, Sweden. All study participants gave their signed, informed consent.
2.2. Questionnaires
A questionnaire was sent to all study participants. It included questions about occupation and occupational exposure, smoking habits, physical activity, general health, symptoms, and medication specified as prescribed by the medical profession, contraceptive medication, over-the-counter medication, and nutritional supplements.
All prescribed medication and contraceptives were checked against information available in Pharmaceutical Specialties in Sweden (www.fass.se). Over-the-counter medication and nutrition supplements were examined on the basis of information available in PUB-Med and internet searches for known liver affection.
To assess alcohol consumption, the study participants answered the Alcohol Use Disorders Identification Test (AUDIT) questionnaire. It consists of 10 questions designed to measure 3 domains: consumption (AUDIT-C, 3 questions on quantity and frequency), dependency (3 questions), and alcohol-related injuries (4 questions about problems or damage caused by alcohol consumption). Each question has a score ranging from 0 to 4. Thus, the maximum score is 40 points. Results above a cut-off of 6 points for women and 8 points for men are indicative of harmful drinking and a score over 20 points strongly indicates dependency. In the AUDIT-C, risk consumption is defined as a score of ≥3 points for women and a score of ≥4 points for men.[14,15]
2.3. Physical examination and blood samples
AAT-deficient individuals visited either their local hospitals or the Department of Respiratory Medicine, Malmö, Sweden. At this consultation, blood samples were analyzed at the local hospitals. The result of the physical examination, diagnoses, medication, and laboratory tests were reported as a study protocol. All control subjects visited the Department of Respiratory Medicine, Malmö.
The following laboratory analyses were performed: plasma aspartate aminotransferase with reference interval for women 0.25 to 0.60 μkat/L and for men 0.25 to 0.75 μkat/L; plasma alanine aminotransferase with reference interval for women 0.15 to 0.75 μkat/lit and for men 0.15 to 1.1 μkat/L; bilirubin with reference interval 5 to 25 μmol/L; plasma alkaline phosphatase (ALP) with reference interval 0.6 to 1.8 μkat/L; plasma gamma glutamyl transpeptidase (GGT) with reference interval for women 0.15 to 0.75 μkat/L and for men 0.15 to 1.9 μkat/L; plasma prothrombin complex (international normalized ratio [INR]) with reference value <1.2; plasma albumin with reference 36 to 48 g/L; platelet count with reference interval for women 165 to 387 × 109/L and for men 145 to 348 × 109/L; and serum procollagen-III-peptide (PIII-NP) with reference interval 0.3 to 0.8 kU/L.[16,17]
2.4. Ultrasound imaging and acoustic radiation force impulse ARFI imaging elastography
ARFI imaging was used to evaluate the mechanical stiffness of liver parenchyma.[18–24] ARFI elastography was performed with a SIEMENS Acuson S2000 ultrasound system (Siemens AG, Erlangen, Germany) with a 4C1 MHz curved array probe. The study subjects had been fasting for at least 3 hours. The examination was performed in the right liver lobe, through the intercostal space. The ARFI measurements were obtained by measuring a numerical value of shear wave velocity (SWV) implemented by Virtual Touch tissue quantification.[23,24] The results are expressed in meters per second (m/s).
2.5. Statistical analysis
Statistical analyses were performed with the Statistical Package for the Social Sciences (SPSS 22.0) software. Because of skewed distribution, the continuous variables were analyzed with nonparametric tests (the Kruskal–Wallis tests and the Mann–Whitney U test). Categorical values were analyzed by the χ2 test. The correlations were examined by Pearson r and in some cases Spearman rho. A P-value < 0.05 was considered significant.
3. Results
3.1. Study participants
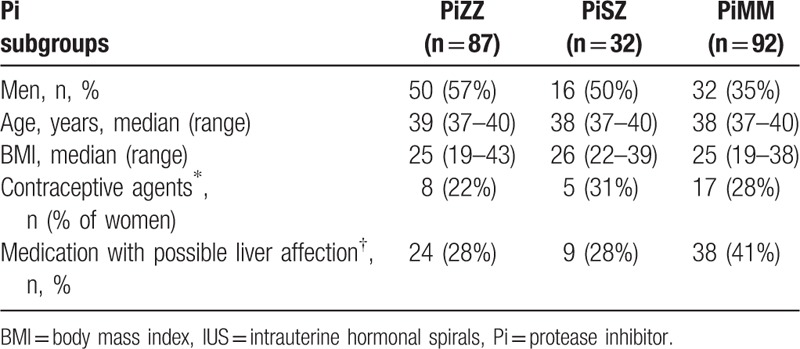
Figure 1 shows a schematic representation of the number of individuals in the various investigations. Table 1 presents demographic data of the study participants. The proportion of women was higher in the control group than in the cohort due to a higher participation rate among the female control subjects than among the female AAT-deficient subjects. The median body mass index (BMI) was somewhat higher in the PiSZ group than in the PiZZ and control group (PiMM) but the differences were not statistically significant (Table 1).
Figure 1.
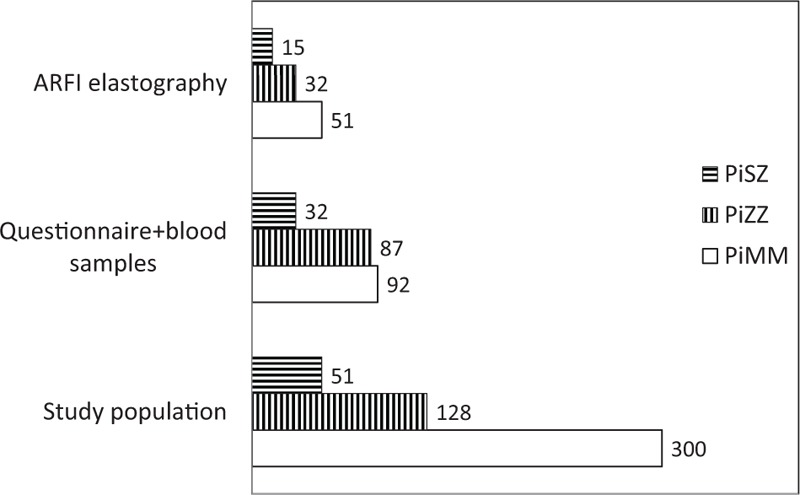
Schematic diagram of the participants in the various investigations in the study. The PiZZ group includes 2 PiZnull subjects and the PiSZ group includes 1 PiSnull subject.
Table 1.
Demographic data of the study participants and their intake of medication with possible effect on liver function.
3.2. Liver function tests
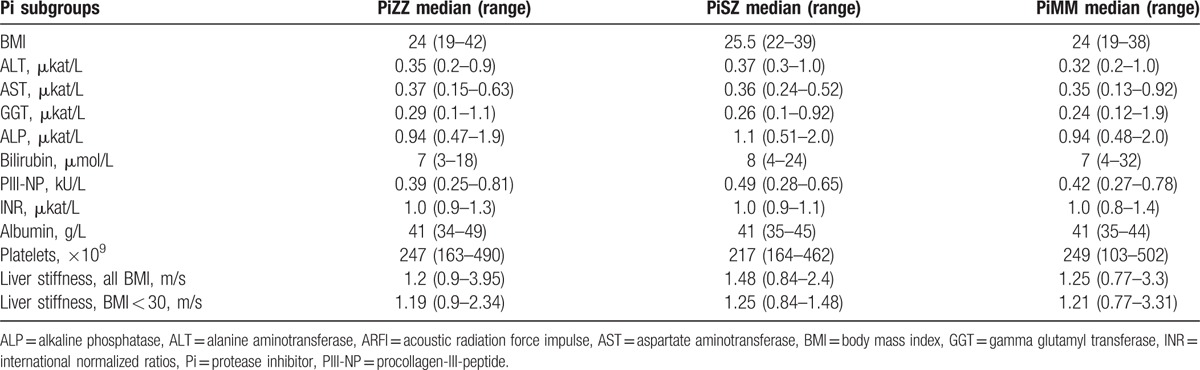
Due to different reference intervals for men and women regarding some of the liver function tests, the results of the liver function tests were analyzed separately in the male and female Pi subgroups. The results are shown in Table 2 for men and in Table 3 for women. A PiSZ man with a known, advanced stage of alcohol abuse had the highest plasma levels of alanine aminotransferase, aspartate aminotransferase, GGT, and bilirubin (Table 2). When the liver function tests with common reference intervals for both genders were analyzed in men and women together, the median bilirubin level was significantly higher in the PiZZ subjects than in the PiMM subjects (9 μmol/L [range 3–23] vs 7 μmol/L [range 4–33]), P = 0.048. Fifteen (17%) of the PiZZ, 6 (19%) of the PiSZ, and 7 (8%) of the PiMM individuals had at least 1 elevated liver enzyme (P = 0.055).
Table 2.
BMI, liver function tests, PIII-NP, and median of stiffness in the right liver lobe measured by AFRI elastography in PiZZ, PiSZ, and PiMM men.
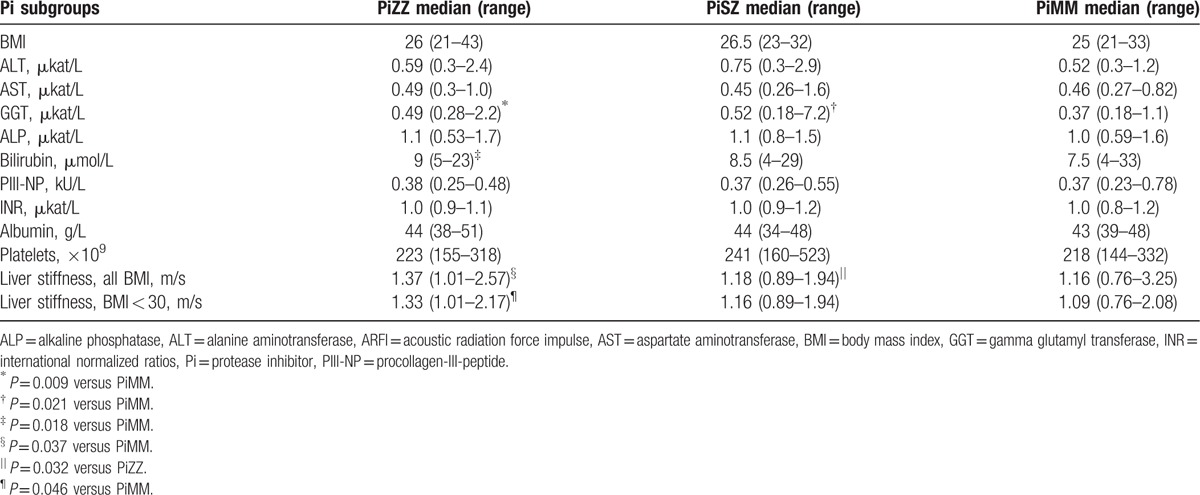
Table 3.
BMI, liver function tests, PIII-NP, and median of stiffness in the right liver lobe measured by AFRI elastography in PiZZ, PiSZ, and PiMM women.
3.3. Medication, contraceptives, nutrition supplements, and diagnoses
Seven women were under treatment with levothyroxine substitution (2 PiSZ and 5 PiMM), 5 individuals were under treatment with immunosuppressive treatment (1 PiZZ woman for pelvospondylitis, 1 PiZZ man for ulcerative colitis, 1 PiMM man for rheumatoid arthritis, 1 PiMM man for Mb Crohn, and 1 PiSZ man for juvenile rheumatoid arthritis). Seventy one individuals (24 PiZZ, 38 PiMM, and 9 PiSZ) were being treated with over-the-counter medication, prescribed medication or contraceptives that may affect liver transaminases. In the PiZZ women on medication which potentially affects liver transaminases, the median GGT was 0.52 μkat/L (range 0.3–1.3) as compared to 0.44 μkat/L (range 0.23–1.1) in the PiMM women on the corresponding treatment (P = 0.023). When only contraceptive treatment was included in the analysis, the difference was still significant (P = 0.047).
3.4. Liver stiffness measured by acoustic radiation force impulse (ARFI) elastography
The results of the ARFI elastography in the male and female subgroups are shown in Tables 2 and 3, respectively. The median stiffness in the right liver lobe was significantly higher in the PiZZ men than in the PiSZ men (P = 0.032) and PiMM men (P = 0.037) (Table 2). When the individuals with BMI ≥30 were excluded from the statistical analysis, the difference was significant only between the PiZZ and PiMM men (P = 0.046, see Table 2).
No significant differences in liver stiffness were found between the female Pi subgroups (Table 3).
The results of liver stiffness were stratified into liver disease stages according to the previously published SWV cut-offs, that is, 1.30 m/s for liver fibrosis and 1.80 m/s for liver cirrhosis, see Table 4.[24] No significant differences were found in the proportion of subjects with values indicating liver fibrosis or liver cirrhosis between the Pi subgroups as a whole, nor in the female or male subgroups, see Table 4.
Table 4.
Liver stiffness stratified into liver disease stages according to the SWV cut-offs of 1.30 m/s for liver fibrosis and 1.80 m/s for liver cirrhosis in the Pi subgroups.
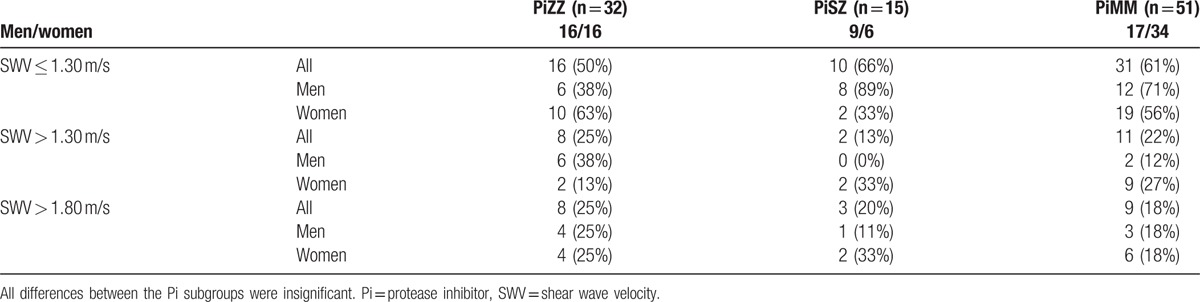
No significant differences were found in the median values of liver function tests or the use of medication influencing liver function between the subjects with SWV above and those with SWV below the cut-offs for liver fibrosis/cirrhosis. None of the study participants had clinical signs of liver fibrosis or cirrhosis by visual assessment of the liver using ultrasound. Three PiZZ (9%), 3 PiSZ (21%), and 3 PiMM subjects (6%) had increased echogenicity indicating liver steatosis as judged by visual assessment of the liver (ns). There was no relationship between liver steatosis and increased values of SWV. The PiSZ man with known alcohol abuse did not participate in the AFRI elastography examination.
There was a significant correlation between liver stiffness and BMI (P < 0.001) and between liver stiffness and ALP (P < 0.001), see Fig. 2. No significant correlations were found between liver stiffness and the other liver enzymes.
Figure 2.

Correlations between liver stiffness and body mass index (BMI) (A), liver stiffness and alkaline phosphatase (B) in 32 PiZZ, 15 PiSZ, and 51 control subjects (PiMM).
3.5. AUDIT questionnaire
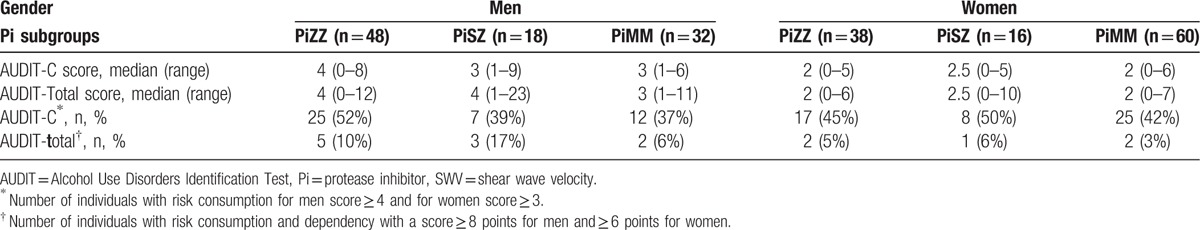
The results of the AUDIT scores are shown in Table 5. Because the risk consumption of alcohol differs between men and women, the results of the AUDIT were analyzed separately. The proportion of subjects with risk consumption was higher among the PiZZ men than among the PiMM men. In contrast, the proportion of PiMM women with risk consumption was higher than that of PiZZ women. However, the differences were not statistically significant. No significant correlations were found between alcohol consumption, liver function tests, BMI, or liver stiffness.
Table 5.
Results of alcohol consumption (AUDIT-C score) and dependency (AUDIT total score) in men and women divided according to the Pi subgroups.
3.6. Results for PiZZ subjects with neonatal liver disease
Ten of the 18 PiZZ individuals with neonatal liver disease participated in the present follow-up. Five individuals had an AUDIT-score indicating risk consumption; 6 individuals had a BMI indicating slight overweight. All of them had essentially normal liver function test results, see Table 6. Because only 2 women with neonatal liver disease participated in the study, the results are presented for men and women together. No significant differences were found in comparison with PiZZ subjects without neonatal liver disease. None of them had any current diagnosis of liver disease.
Table 6.
BMI, liver function tests, and SWV in PiZZ subjects with neonatal liver disease.

3.7. Causes of death in the deceased AAT-deficient subjects
One PiZZ woman died due to acute bacterial meningitis at the age of 36 years. One PiSZ man with known drug and alcohol abuse died at the age of 37 years after a drug overdose. The autopsy in both of these cases revealed a normal liver configuration. Of the other 2 PiSZ cases, 1 woman suffered from liver cirrhosis due to alcohol overconsumption and died secondary to acute hepato-renal syndrome at the age of 37, and 1 woman died at the age of 38 due to acute myocardial inflammation and the autopsy showed an enlarged liver (3050 gr).
4. Discussion
Our results show that at the age of 37 to 40 years, the PiZZ men have significantly higher levels of GGT and bilirubin, and higher liver stiffness assessed by ARFI elastography than the PiMM men. No significant differences in liver function test results and ARFI were found between PiZZ and PiMM women.
GGT is considered to be a sensitive marker for detecting and monitoring liver affection.[25] Contraceptives, some of the prescribed medications, some of the over-the-counter medications, and dietary supplements may cause atoxic elevation of liver transaminases. In the present study, the PiZZ women who were being treated with medication influencing liver enzymes, including oral contraceptives, had higher plasma GGT than the PiMM women with corresponding treatment. Similar results were found at the age of 30, but not at the age of 34.[12,13] However, until the age of 37 to 40, none of the PiZZ women has shown any clinical signs of liver affection. Furthermore, no differences were found in other liver function tests or in ARFI elastography. It remains unclear whether these differences predict future liver disease.
At the 30-year follow-up, significantly higher levels of AST were found in both PiZZ and PiSZ subjects in comparison with the control group.[12] At the 34-year follow-up, the PiZZ individuals had significantly higher mean GGT and albumin than the control group.[13] In both of these previous check-ups, the results of liver function tests were analyzed in men and women together. In the present study, we analyzed the results separately in men and women because of the different reference intervals, and because the gender proportion was not the same among the AAT-deficient and control groups.
PIII-NP is a marker of collagen turnover, and its serum level is considered to be a reliable indicator of hepatic fibrosis in both alcohol and drug-induced liver damage.[26] We did not find any significant differences in PIII-NP between the Pi subgroups (see Tables 2 and 3). Boffa et al[27] have repeated measurements of PIII-NP in patients with psoriasis who were being treated with Methotrexate. They found that a single PIII-NP test could not be considered as a predictor of the development of abnormal histopathology of the liver, but repeated normal levels indicated a low risk of significant liver damage.
In this cohort, PIII-NP has previously only been analyzed at the age of 8, 12, and 16 years. At the age of 12, both PiZZ and PiSZ children had significantly higher PIII-NP than the age matched controls, but at the age of 16, no significant differences were found.[9,10] In adulthood, PIII-NP has not been analyzed in this cohort, and therefore no conclusions can be drawn from our results with normal levels at the present follow-up. It is important to repeat the analysis of PIII-NP at the future follow-ups of the cohort.
ARFI elastography is a new, noninvasive method for detecting and monitoring fibrosis and cirrhosis of the liver.[18,19] We found increased liver stiffness in the PiZZ men in comparison with PiMM men, but no significant differences were found in the female subgroups. Unexpectedly, a high proportion of the study subjects had elevated values of SWV that may indicate liver fibrosis and cirrhosis according to the previously published cut-offs (Table 4).[23] However, the possible presence of liver fibrosis and cirrhosis was similar in all Pi subgroups, both among the men and the women. These results were an isolated finding, because no other clinical or laboratory parameters differed significantly between the subjects with elevated SWV and those with SWV below the cut-off for liver fibrosis/cirrhosis. Furthermore, visual assessment of the liver parenchyma by ultrasound did not reveal any liver disease, and none of the subjects with elevated SWV values shown any signs of liver steatosis. It remains unclear, why we found elevated values of liver stiffness in these asymptomatic, AAT-deficient and healthy control subjects.
Previously published studies have shown good correlations between the result of ARFI measurements and stages of fibrosis determined by biopsy,[21] which is considered to be the most reliable method for assessing hepatic fibrosis and cirrhosis. However, liver biopsy is an invasive procedure with potentially serious complications. In these asymptomatic, AAT-deficient subjects, we did not find that liver biopsy would be ethically justified. This decision was made before the study start, that is, before we had knowledge of the results of elevated liver stiffness in a high proportion of both AAT-deficient and control subjects.
The ARFI result showed a strong correlation with BMI and was also correlated with ALP, but not with other liver function parameters (Fig. 2A, B). To our knowledge, this is the first study in which this method is used to assess liver stiffness in asymptomatic, AAT-deficient individuals.
The PiSZ phenotype is not considered to be a risk factor for liver disease, and none of the PiSZ children in this cohort had clinical signs of liver disease early in life.[8] However, 2 of the 3 PiSZ individuals who had died before the age of 38 years had pathological changes in the liver as seen in the autopsy. They had known alcohol abuse and both had liver cirrhosis, and 1 was on the waiting list for liver transplantation. Whether the liver pathology was caused only by alcohol or whether these individuals are more vulnerable to alcohol than the general population remains unclear. At the present check-up, 1 PiSZ man had an advanced stage of alcohol abuse. He also had elevated liver function tests. Nevertheless, except for a significantly higher GGT in PiSZ men compared with the PiMM men, no other significant differences were found in liver function tests or AFRI elastography between the PiSZ and PiMM subjects.
Only 1 PiZZ woman has died after the age of 8 years. She was 36 years of age, and the cause of death was acute meningitis. All remaining PiZZ individuals are alive, and none of those participating in the present follow-up suffered from liver disease. Their liver function tests were essentially within the normal range (Tables 2 and 3). Also the PiZZ subjects who suffered from liver disease early in life had normal liver function test results (Table 6). Thus, our results show that the prognosis of liver function in the PiZZ individuals identified by neonatal screening seems to be good up to 37 to 40 years of age.
There are some limitations in our study. Due to long distances or their social situation, not all AAT-deficient subjects were able to visit Malmö. A common reason for not participating was well-being. Despite the fact that many of them visited their local hospitals where the liver function tests were analyzed, the participation rate was relatively low, which may have influenced the results. The ARFI elastography was performed in the afternoon, and thus the subjects were not in correct fasting condition, only having fasted for a minimum of 3 hours. The diagnoses were self-reported, and therefore we cannot exclude that some subjects had undiagnosed liver disease.
In conclusion, at the age of 37 to 40 years the PiZZ and PiSZ individuals identified by neonatal screening have normal plasma levels of liver function tests and PIII-NP. However, bilirubin, GGT, and liver stiffness, as assessed by AFRI elastography, differ significantly between the PiZZ and the PiMM men.
Acknowledgments
The authors thank Ewa Ringdal Szemberg, Isabella Björk and Helene Johansson Kvist for technical and secretarial support. The authors also thank all Swedish colleges for reporting data; and Tomas Sveger and Olle Ekberg for their contribution and constructive criticism.
Footnotes
Abbreviations: AAT = alpha-1-antitrypsin, ALP = alkaline phosphatase, ARFI = acoustic radiation force impulse, AUDIT = Alcohol Use Disorders Identification Test, BMI = body mass index, GGT = gamma glutamyl transferase, Pi = protease inhibitor, PIII-NP = procollagen-III-peptide, SWV = shear wave velocity.
Funding/support: This study was supported by Swedish Heart-Lung Foundation, Skåne University Hospital Grant.
The authors have no conflicts of interest to disclose.
References
- [1].American Thoracic Society. European respiratory society statement: standards for the diagnosis and management of individuals with alpha-1 antitrypsin deficiency. Am J Respir Crit Care Med 2003;168:818–900. [DOI] [PubMed] [Google Scholar]
- [2].Stoller JK, Aboussouan LS. A review of alpha-1-antitrypsin deficiency. Am J Respir Crit Care Med 2012;185:246–59. [DOI] [PubMed] [Google Scholar]
- [3].Gettins PG. Serpin structure, mechanism, and function. Chem Rev 2002;102:4751–804. [DOI] [PubMed] [Google Scholar]
- [4].Laurell CB, Eriksson S. The electrophoretic alpha-1-globulin pattern of serum in alpha-1-antitrypsin deficiency. Scand J Clin Lab Invest 1963;15:132–40. [Google Scholar]
- [5].Gooptu B, Dickens JA, Lomas DA. The molecular and cellular pathology of α1-antitrypsin deficiency. Trends 2014;20:116–27. [DOI] [PubMed] [Google Scholar]
- [6].Sharp HL, Bridges RA, Krivit W, et al. Cirrhosis associated with alpha-1-antitrypsin deficiency: a previously unrecognized inherited disorder. J Lab Clin Med 1969;73:934–9. [PubMed] [Google Scholar]
- [7].Nelson DR, Teckman J, Di Biseglie AM, et al. Diagnosis and management of patients with α1-antitrypsin (A1AT) deficiency. Clin Gastroenterol Hepatol 2012;10:575–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sveger T. Liver disease in α1-antitrypsin deficiency detected by screening of 200,000 infants. N Engl J Med 1976;294:1316–21. [DOI] [PubMed] [Google Scholar]
- [9].Sveger T, Eriksson S. The liver in adolescents with α1-antitrypsin deficiency. Hepatology 1995;22:513–7. [DOI] [PubMed] [Google Scholar]
- [10].Eriksson S, Sveger T. Procollagen type III peptide in asymptomatic children with alpha 1-antitrypsin deficiency. J Pediatr Gastroenterol Nutr 1998;7:938–9. [DOI] [PubMed] [Google Scholar]
- [11].Sveger T, Ohlsson K, Piitulainen E. Adolescents with α1-antitrypsin deficiency have high α2-macroglobulin and low neutrophil lipocalin and elastase levels in plasma. Pediatr Res 1998;44:939–41. [DOI] [PubMed] [Google Scholar]
- [12].Bernspång E, Carlson J, Piitulainen E. The liver in 30-year-old individuals with alpha(1)-antitrypsin deficiency. Scand J Gastroenterol 2009;44:1349–55. [DOI] [PubMed] [Google Scholar]
- [13].Tanash HA, Nystedt-Düzakin M, Cano Montero L, et al. The Swedish alpha 1-antitrypsin screening study: health status, lung and liver function at age 34. Ann Am Thorac Soc 2015;12:817–2. [DOI] [PubMed] [Google Scholar]
- [14].WHO, Babor T, Higgins-Biddle JC, Aaunders JB, et al. AUDIT. The Alcohol Disorders Identification Test. Guidelines for Use in Primary Care. 2nd ed.2001. [Google Scholar]
- [15].Reinert DF, Allen JP. The alcohol use disorders identification test: an update of research findings. Alcohol Clin Exp Res 2007;31:185–99. [DOI] [PubMed] [Google Scholar]
- [16].Rustad P, Felding P, Franzson L, et al. The Nordic Reference Interval Project 2000: recommended reference intervals for 25 common biochemical properties. Scand J Clin Lab Invest 2004;64:271–84. [DOI] [PubMed] [Google Scholar]
- [17].Lund Student Litteratur, Nilsson-Ehle P., red Laurells Klinisk Kemi i Praktisk Medicin 9:e upplagan. 2012. [Google Scholar]
- [18].Wildner D, Strobel D, Konturek PC, et al. Impact of acoustic radiation force impulse imaging in clinical practice of patients after orthotopic liver transplantation. Med Sci Monit 2014;20:2027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sporea I, Bota S, Grădinaru-Taşcău O, et al. Comparative study between two point shear wave elastographic techniques: acoustic radiation force impulse (ARFI) elastography and ElastPQ. Med Ultrason 2014;16:309–14. [DOI] [PubMed] [Google Scholar]
- [20].Bamber J, Cosgrove D, Dietrich CF, et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: basic principles and technology. Ultraschall Med 2013;34:169–84. [DOI] [PubMed] [Google Scholar]
- [21].Kircheis G, Sagir A, Vogt C, et al. Evaluation of acoustic radiation force impulse imaging for determination of liver stiffness using transient elastography as a reference. World J Gastroenterol 2012;18:1077–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ferraioli G, Filice C, Castera L, et al. WFUMB Guidelines and recommendations for clinical use of ultrasound elastography: Part 3: liver. Ultrasound Med Biol 2015;41:1161–79. [DOI] [PubMed] [Google Scholar]
- [23].Bota S, Herkner H, Sporea I, et al. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int 2013;33:1138–47. [DOI] [PubMed] [Google Scholar]
- [24].Ferraioli G, Tinelli C, Zicchetti M, et al. Reproducibility of real-time shear wave elastography in the evaluation of liver elasticity. Eur J Radiol 2012;81:3102–6. [DOI] [PubMed] [Google Scholar]
- [25].Elsevier, Burits CA, Ashwood ER, Bruns DE., red Tietz Textbook of Clinical Chemistry and Molecular Diagnostics, 5th ed. 2012. [Google Scholar]
- [26].Torres-Salinas M, Parés A, Caballería J, et al. Serum procollagen type III peptide as a marker of hepatic fibrogenesis in alcoholic hepatitis. Gastroenterology 1986;90:1241–6. [DOI] [PubMed] [Google Scholar]
- [27].Boffa MJ, Smith A, Chalmers RJ, et al. Serum type III procollagen aminopeptide for assessing liver damage in methotrexate-treated psoriatic patients. Br J Dermatol 1996;135:538–44. [PubMed] [Google Scholar]


