Abstract
To evaluate the performance of aspartate transaminase-to-platelet ratio index (APRI) and fibrosis index based on four factors (FIB-4) to predict significant fibrosis and cirrhosis in hepatitis B virus e antigen (HBeAg)-negative chronic hepatitis B (CHB) patients with alanine transaminase (ALT) ≤ twice the upper limit of normal (2 ULN).
Histologic and laboratory data of 236 HBeAg-negative CHB patients with ALT ≤ 2 ULN were analyzed. Predicted fibrosis stage, based on established scales and cut-offs for APRI and FIB-4, was compared with METAVIR scores obtained from liver biopsy.
In this study, the areas under the receiver operating characteristic curves (AUROCs) of APRI were lower than that of FIB-4 (0.62 vs 0.69; P = 0.019) for diagnosing significant fibrosis; however APRI and FIB-4 were comparable for diagnosing cirrhosis (0.77 vs 0.81; P = 0.374). When the cut-off proposed by WHO HBV guideline for APRI (>2.0) was used, no cirrhotic patients were correctly predicted. For FIB-4, the WHO proposed cut-off of 3.25 correctly identified significant fibrosis 83% of the time; but for APRI, the WHO proposed cut-off of 1.5 identified significant fibrosis 56%. In ruling out significant fibrosis, the WHO proposed APRI cut-off of 0.5 had a predictive value of 39%, and the FIB-4 cut-off of 1.45 correctly identified lack of significant fibrosis in 47% of the patients. In this study, based on ROC analysis, the optimal cut-offs were 0.46 and 0.65 for APRI, and 1.05 and 1.29 for FIB-4, for diagnosing significant fibrosis and cirrhosis, respectively. When the new cut-off of APRI (>0.65) was used, 82% of the cirrhotic patients were correctly predicted. In ruling out significant fibrosis, the new APRI cut-off (<0.46) had a predictive value of 80%, and new FIB-4 cut-off (<1.05) correctly identified lack of significant fibrosis in 84% of the patients.
The WHO guidelines proposed cut-offs might be higher for HBeAg-negative CHB patients with ALT ≤2 ULN, and might underestimate the proportion of significant fibrosis and cirrhosis. A new set of cut-offs should be used to predict significant fibrosis and cirrhosis in this specific population.
Keywords: aspartate transaminase-to-platelet ratio index, chronic hepatitis B, cirrhosis, fibrosis index based on four factors, significant fibrosis
1. Introduction
Infection with hepatitis B virus (HBV) is a public health problem worldwide, and 240 million people estimated to experience persistent HBV infection.[1] In China, HBV infection is moderately endemic, and chronic hepatitis B (CHB) is the main cause of cirrhosis and hepatocellular carcinoma (HCC).[2] Among CHB patients, those with significant fibrosis and cirrhosis are at increased risk for liver decompensation, HCC, and death.[3]
Antiviral treatment can suppress HBV replication and prevent progression of CHB to cirrhosis, HCC, and death. The indications for antiviral treatment mainly based on the combination of hepatitis B virus e antigen (HBeAg) status, HBV DNA levels, alanine transaminase (ALT) levels, and severity of liver histological changes.[4–6] According to CHB guidelines,[4–6] HBeAg-negative patients with HBV DNA ≥ 2000 IU/mL (104 copies/mL) and ALT > twice the upper limit of normal (2 ULN) should be considered for antiviral treatment. Among HBeAg-negative patients who have ALT ≤ 2 ULN, liver fibrosis assessment can assist the decision of antiviral therapy. Patients with significant fibrosis should be considered for antiviral therapy even if their ALT ≤ 2 ULN. Among HBeAg-negative patients who have ALT > 2 ULN and HBV DNA > 2000 IU/mL, guidelines recommend commencement of antiviral therapy and liver fibrosis assessment may not be necessary. Thus, HBeAg-negative CHB patients with ALT ≤ 2 ULN have more needs for liver fibrosis assessment than the general CHB patients.
Liver biopsy is the gold standard for the detection of liver fibrosis, but has limitations such as invasive procedure, high cost, risk of rare but potentially life-threatening complications, and so on.[7–9] These limitations of liver biopsy promote the development of noninvasive means for assessments of liver fibrosis. FibroScan, which measures liver stiffness, is increasingly being recognized as an excellent tool for the diagnosis of liver fibrosis because of its high diagnostic performance.[10–12] However, the FibroScan device is expensive (€34,000 for the portable machine), and often only accessible in several hospitals in great cities in developing country. Besides FibroScan, serum fibrosis model based on routine laboratory tests might be another noninvasive method for the detection of liver fibrosis. Among serum fibrosis models, aspartate transaminase (AST)-to-platelet ratio index (APRI) and fibrosis index based on four factors (FIB-4) are commonly used for identifying liver fibrosis and cirrhosis in patients with chronic hepatitis C virus (HCV) infection.[13,14] The APRI score was developed in the study of 192 patients with chronic HCV infection, in which APRI got a PPV of 51% for diagnosing significant fibrosis, and 81% for the diagnosis of cirrhosis.[13] The FIB-4 was developed in a study of 592 HCV patients, in which FIB-4 gave a PPV of 82% for prediction of severe fibrosis.[15] APRI and FIB-4 have successfully predicted liver fibrosis in large cohorts of patients with HCV.[15,16]
A number of studies have also described that APRI and FIB-4 are suitable markers for detecting significant fibrosis and cirrhosis in CHB patients.[17,18] Indeed, the recent WHO HBV guidelines recommend APRI as the preferred noninvasive test to assess for the presence of cirrhosis in resource-limited settings.[19] However, recent study by Kim et al[20] found that APRI and FIB-4 scores are not suitable for use in clinical practice in CHB patients for assessment of hepatic fibrosis according to Ishak stage, especially in gauging improvements in liver fibrosis following therapy. Yin et al[21] also found that the cut-offs (>3.25) proposed by WHO HBV guidelines might be higher for CHB patients, and FIB-4 has relatively high diagnostic value for detecting liver fibrosis in CHB patients when the diagnostic threshold value is more than 2.0. In conclusion, the diagnostic performances and cut-offs of APRI and FIB-4 for the diagnosis of significant fibrosis and cirrhosis were controversial, although they have been recommended by the recent WHO HBV guidelines.[19]
At present, there is a lack of data about the performances of APRI and FIB-4 for the diagnosis of significant fibrosis and cirrhosis in HBeAg-negative CHB patients with ALT ≤ 2 ULN. It is unclear whether the cut-offs proposed by the WHO HBV guidelines, which derived from HCV studies, applied to HBeAg-negative CHB patients with ALT ≤ 2 ULN. We evaluated the performances of APRI and FIB-4 in 236 HBeAg-negative CHB patients with ALT ≤ 2 ULN, and verified whether the WHO proposed cut-offs applied to this specific CHB population.
2. Materials and methods
2.1. Study design and patients
Fifteen hundred twenty-one consecutive CHB patients who underwent liver biopsies and routine laboratory tests in Shanghai Public Health Clinical Center between May 2008 and January 2016 were retrospectively screened. CHB was defined as the persistent presence of hepatitis B surface antigen (HBsAg) for more than 6 months.[5] Inclusion criteria were HBeAg-negative, HBV DNA ≥ 500 copies/mL, no anti-HBV treatment, and ALT ≤ 2 ULN (ULN is 40 IU/L).[5] Patients with the following conditions were excluded: alcohol consumption>20 g/day (n = 132); accompanied by nonalcoholic fatty liver disease (NAFLD) (n = 136); coinfection with HCV, hepatitis D virus (HDV), or HIV (n = 106); accompanied by autoimmune liver disease (n = 54). Finally, 236 treatment-naïve HBeAg-negative CHB patients with ALT ≤ 2 ULN were included. Figure 1 summarizes the flow diagram of the study population.
Figure 1.
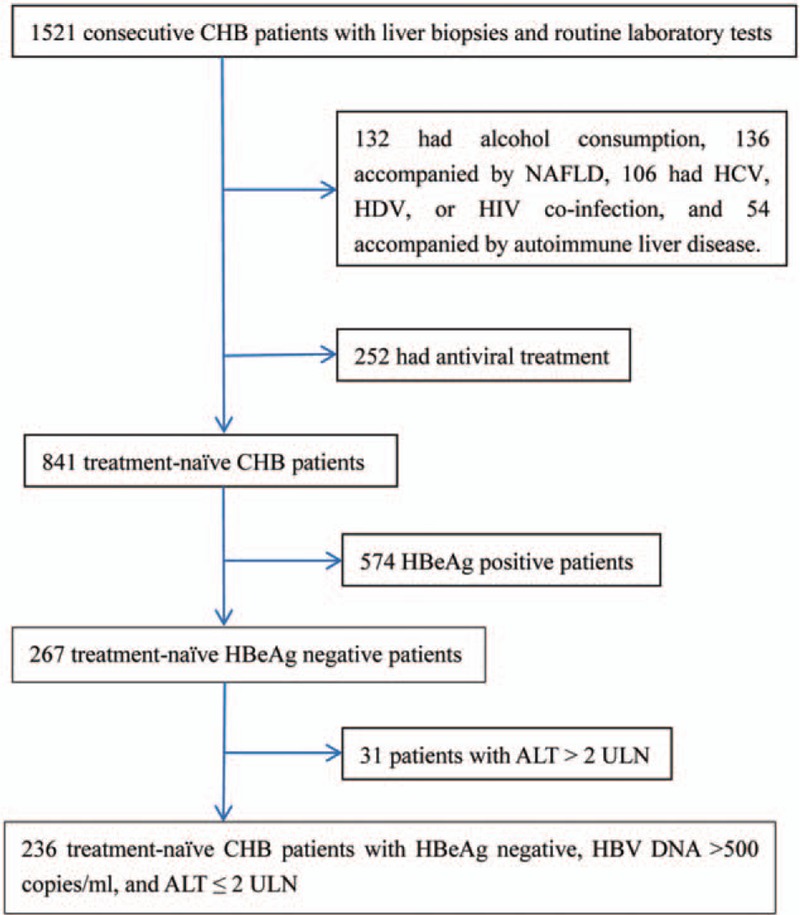
Flow diagram of the study population. ALT = alanine aminotransferase, CHB = chronic hepatitis B, HBeAg = hepatitis B e antigen, HCV = hepatitis C virus, HDV = hepatitis D virus, HIV = human immunodeficiency virus, NAFLD = nonalcoholic fatty liver disease, ULN = upper limit of normal.
All the patients signed the informed consent before liver biopsy, and all clinical procedures were in accordance with the Helsinki declaration of 1975, as revised in 1983. The study protocol was permitted by the ethics committee of Shanghai Public Health Clinical Center.
2.2. Liver histological score
A minimum of 15 mm of liver tissue with at least 6 portal tracts is considered sufficient for histological scoring, and the METAVIR scoring system was adopted as the histological standard of liver fibrosis.[22] Liver fibrosis was classified into 5 stages: F0, no fibrosis; F1, portal fibrosis without septa; F2, portal fibrosis with rare septa; F3, numerous septa without cirrhosis; and F4, cirrhosis (formation of false lobule).[22] Significant fibrosis was defined as fibrosis stage ≥ F2, and cirrhosis was defined as fibrosis stage = F4.
2.3. APRI and FIB-4 scores
APRI and FIB-4 scores were calculated using laboratory data based on the following formulas:
Note: ULN of AST = 40 IU/L.
2.4. Statistical analysis
Normality tests of all data were performed by Kolmogorov–Smirnov test. The baseline characteristics of patients are presented as follows: normal distribution data as mean ± standard deviation, nonnormal distribution continuous data as median (interquartile range), and categorical variables as number (percentage). The correlations between noninvasive fibrosis scores (APRI or FIB-4) and METAVIR fibrosis scores were analyzed using Spearman test. ROC curve analysis was performed for APRI and FIB-4, respectively, to identify significant fibrosis and cirrhosis in HBeAg-negative CHB patients with ALT ≤ 2 ULN. Three sets of cut-offs were calculated as follows: obtaining a sensitivity of at least 90%, obtaining a specificity of at least 90%, or maximizing Youden index (sensitivity + specificity − 1). All significance tests were 2-tailed, and P < 0.05 was considered statistically significant. All statistical analyses were carried out using the SPSS statistical software version 15.0 (SPSS, Inc., Chicago, IL) and MedCalc Statistical Software version 16.1 (MedCalc Software bvba, Ostend, Belgium).
3. Results
3.1. Baseline data
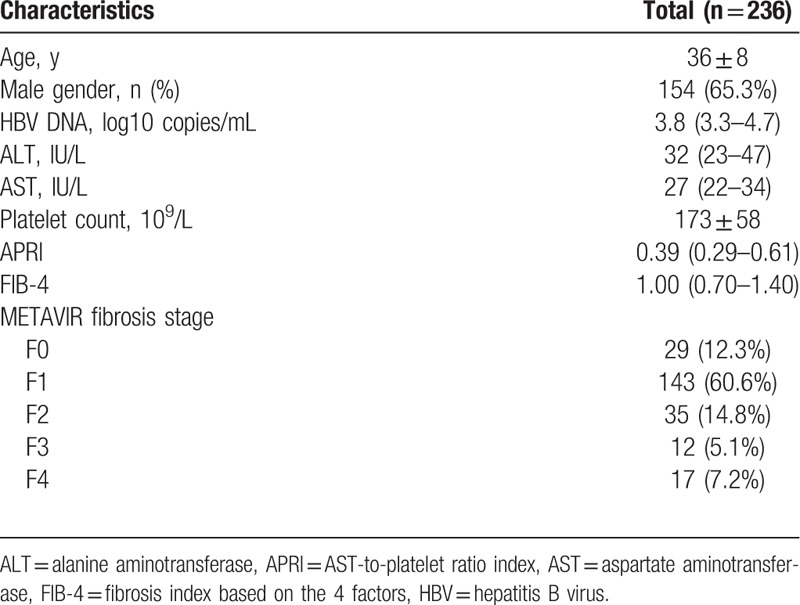
Baseline characteristics of the study population are presented in Table 1. The average age was 36 years, and mostly male (65.3%). Median HBV DNA, ALT, and AST was 3.8 log10 copies/mL (IQR = 3.3–4.7), 32 IU/L (IQR = 23–47), and 27 IU/L (IQR = 22–34), respectively. Median APRI and FIB-4 was 0.39 (IQR = 0.29–0.61) and 1.00 (IQR = 0.70–1.40), respectively. Among 236 enrolled patients, 64 (27.1%) had significant fibrosis, and 17 (7.2%) had cirrhosis.
Table 1.
Baseline characteristics of the study population.
3.2. Correlation of APRI and FIB-4 scores with METAVIR fibrosis stages
The METAVIR fibrosis stages obtained from liver biopsy correlated with APRI (Spearman r = 0.16, P = 0.012) and FIB-4 (Spearman r = 0.28, P < 0.001) (Table 2), resulting in higher median APRI and FIB-4 scores with increasing METAVIR fibrosis stages (Fig. 2). It is important to note, however, the correlation coefficients of APRI and FIB-4 were all less than 0.8, which means week correlation despite P-value.
Table 2.
Correlation of APRI and FIB-4 scores with METAVIR scores.
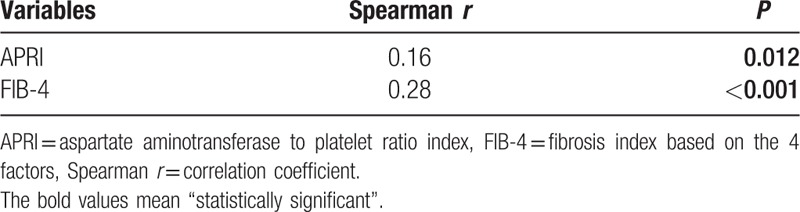
Figure 2.
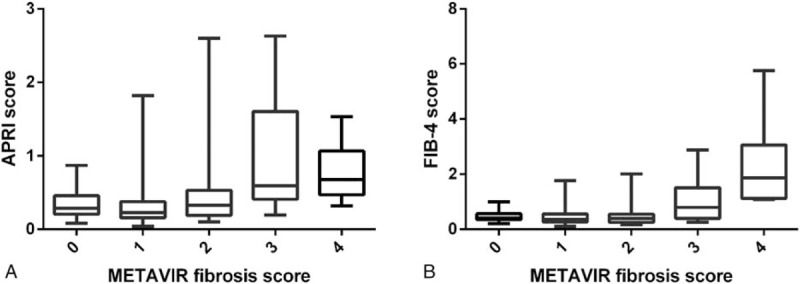
Association between METAVIR scores and (A) APRI and (B) FIB-4 scores. APRI = aspartate aminotransferase to platelet ratio index, FIB-4 = fibrosis index based on the 4 factors; the box represents the interquartile range and the line across the box indicates the median value.
3.3. Diagnostic performances of APRI and FIB-4 for significant fibrosis and cirrhosis at cut-offs proposed by WHO HBV guidelines
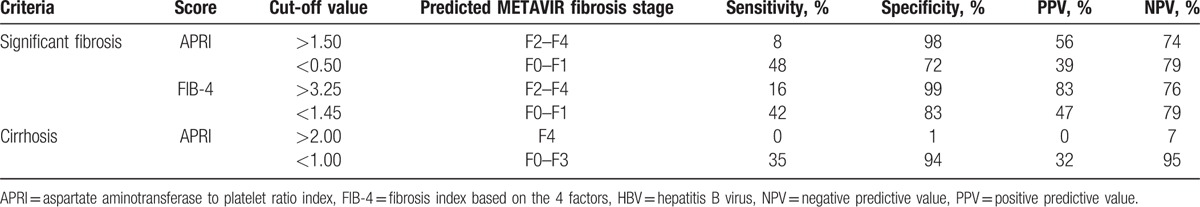
Table 3 presents the summary sensitivities, specificities, positive predictive values (PPVs), and negative predictive values (NPVs) of APRI and FIB-4 for significant fibrosis and cirrhosis at the WHO guideline proposed cut-offs. When APRI > 2.0 was used for the prediction of cirrhosis, no patients were correctly predicted. When the WHO proposed cut-off for FIB-4 (3.25) was used, significant fibrosis could be correctly predicted in 83% of patients; but for APRI, the WHO proposed cut-off of 1.5 just correctly identified significant fibrosis in 56% of patients. In addition, when the WHO proposed cut-offs were used in HBeAg-negative CHB patients with ALT ≤ 2 ULN, many patients with significant fibrosis or cirrhosis would be missed. For example, in this study, all patients with cirrhosis (17/17) had APRI ≤ 2 and similarly, most patients with significant fibrosis (54/64) had FIB-4 ≤ 3.25, and the majority of patients with significant fibrosis (58/64) had APRI ≤ 1.5. In ruling out significant fibrosis, APRI ≤ 0.5 only had a predictive value of 39%, and FIB-4 ≤ 1.45 only had a predictive value of 47%. That is to say, APRI ≤ 0.5 correctly identified nonsignificant fibrosis in 39% of the patients, and FIB-4 ≤ 1.45 correctly identified nonsignificant fibrosis in 47% of the patients. Again, both scores would miss a large proportion of patients without significant fibrosis when the WHO proposed cut-offs were used.
Table 3.
Diagnostic performance of APRI and FIB-4 for significant fibrosis and cirrhosis at cut-off values proposed by the WHO HBV guidelines.
3.4. New cut-offs for APRI and FIB-4 for the diagnosis of significant fibrosis and cirrhosis
We performed a ROC analysis (Fig. 3) to evaluate whether significant fibrosis and cirrhosis could be detected with high sensitivity and specificity using new sets of cut-offs than the WHO proposed cut-offs.[19] The areas under the receiver operating characteristic curves (AUROCs) of APRI were lower than that of FIB-4 (0.62 vs 0.69; P = 0.019) for diagnosing significant fibrosis; however APRI and FIB-4 AUROCs were comparable for diagnosing cirrhosis (0.77 vs 0.81; P = 0.374) (Table 4).
Figure 3.
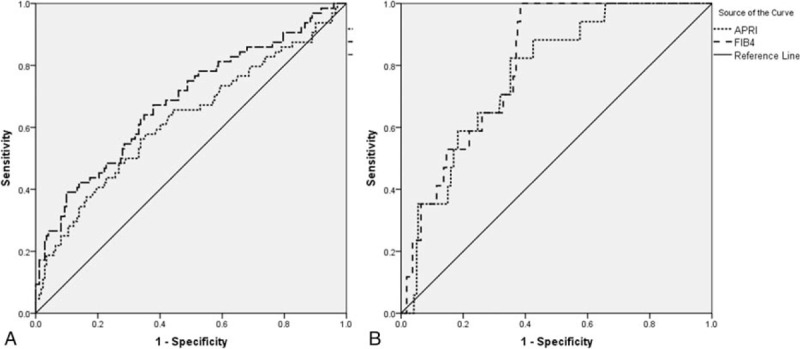
ROC curves of APRI and FIB-4 for significant fibrosis (A) and cirrhosis (B). APRI = aspartate aminotransferase to platelet ratio index, FIB-4 = fibrosis index based on the 4 factors, ROC curves = receiver operating characteristic curves.
Table 4.
The AUROCs of APRI and FIB-4 for diagnosing significant fibrosis and cirrhosis.
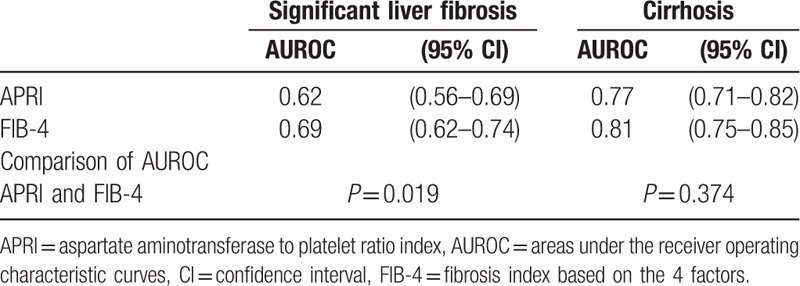
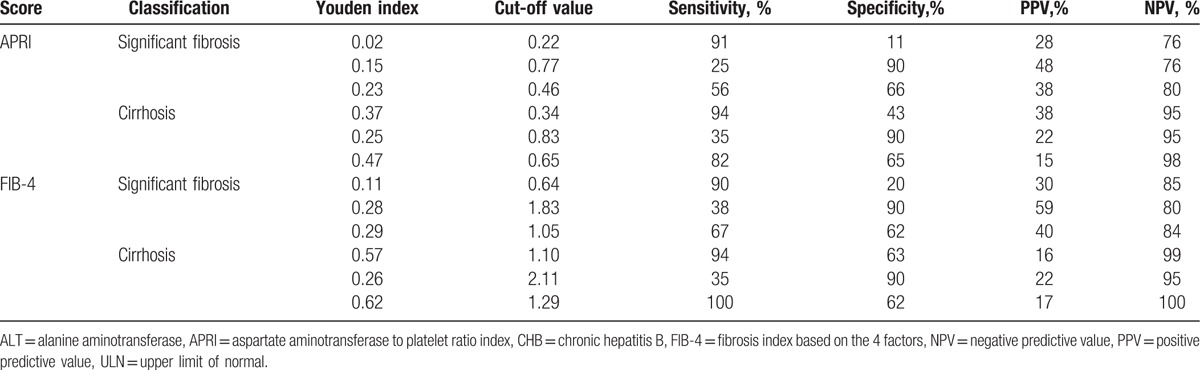
Table 5 presents the new cut-offs of APRI and FIB-4 for the diagnosis of significant fibrosis and cirrhosis in HBeAg-negative CHB patients with ALT ≤ 2 ULN. When cut-offs maximized sensitivity (≥90%), specificity was unsatisfactorily low (11–63%). Cut-offs that maximized specificity (≥90%) were associated with unacceptably low sensitivity (25–38%). Cut-offs that maximized Youden index with a compromise between sensitivity (56–100%) and specificity (62–66%) performed modestly well. According to maximizing Youden index, the optimal cut-offs of APRI were 0.46 and 0.65, respectively, for diagnosing significant fibrosis (the corresponding sensitivity, specificity, PPV, and NPV were 56%, 66%, 38%, and 80%), and cirrhosis (the corresponding sensitivity, specificity, PPV, and NPV were 82%, 65%, 15%, and 98%). The optimal cut-offs of FIB-4 were 1.05 and 1.29, respectively, for diagnosing significant fibrosis (the corresponding sensitivity, specificity, PPV and NPV were 67%, 62%, 40%, and 84%), and cirrhosis (the corresponding sensitivity, specificity, PPV and NPV were 100%, 62%, 17%, and 100%).
Table 5.
Feasibility of determining new cut-off values of APRI and FIB-4 for HBeAg-negative CHB patients with ALT ≤ 2 ULN.
4. Discussion
In this study based on 236 HBeAg-negative CHB patients with ALT ≤ 2 ULN, commonly used scores (APRI and FIB-4) to estimate fibrosis in patients with hepatitis C showed the value for diagnosing significant fibrosis and cirrhosis. Previously, some small-scale studies suggest that APRI and FIB-4 scores are higher in CHB patients with significant fibrosis,[23,24] which we also observed in this study. In this study, the AUROC of FIB-4 was higher than that of APRI (0.69 vs 0.62; P = 0.019) for diagnosing significant fibrosis, which indicated FIB-4 might be more reliable than APRI as an indicator of significant fibrosis in HBeAg-negative CHB patients with ALT ≤ 2 ULN. Although the APRI and FIB-4 AUROCs were comparable (0.77 vs 0.81; P = 0.374) for diagnosing cirrhosis, APRI might be superior to FIB-4 as an indicator of cirrhosis in this specific CHB population, considering the more simple calculation.
The cut-offs of APRI (>1.50) and FIB-4 (>3.25) proposed by WHO HBV guidelines provided high specificity (98–99%) for the diagnosis of significant fibrosis, at a cost of very low sensitivity (8–16%) in HBeAg-negative CHB patients with ALT ≤ 2 ULN. This implies that 84% to 92% of patients who had significant fibrosis would be erroneously categorized as patients without significant fibrosis, by the WHO proposed cut-offs. This limits the usefulness of APRI and FIB-4 as screening tests for significant fibrosis and cirrhosis, and selection of candidates for liver biopsy. For the diagnosis of cirrhosis, the WHO proposed cut-off of APRI (>2.0) provided very low sensitivity (0) and specificity (1%) in HBeAg-negative CHB patients with ALT ≤ 2 ULN. All patients with cirrhosis (17/17) had an APRI score of 2 or less, and would be misdiagnosed as patients without cirrhosis, by the WHO proposed cut-off of APRI (>2.0).
To propose a new set of cut-offs with high specificity and sensitivity for use in clinical practice, we evaluated new cut-offs of APRI and FIB-4 specifically for HBeAg-negative CHB patients with ALT ≤ 2 ULN. Based on ROC analysis, the optimal cut-offs were 0.46 and 0.65 for APRI, and 1.05 and 1.29 for FIB-4, for diagnosing significant fibrosis and cirrhosis, respectively, in this study. Compared with the WHO proposed cut-off (APRI > 2.0), the new cut-off of APRI (>0.65) provided higher sensitivity (82% vs 0%) and specificity (65% vs 1%) for diagnosing cirrhosis in HBeAg-negative CHB patients with ALT ≤ 2 ULN. When the new cut-off of APRI (>0.65) was used, 14/17 (82%) cirrhotic patients were correctly predicted in this study. Compared with the WHO proposed cut-off (FIB-4 > 3.25), the new cut-off of FIB-4 (>1.05) provided higher sensitivity (67% vs 16%) but lower specificity (62% vs 99%) for diagnosing significant fibrosis. Obviously, the new cut-off of FIB-4 (>1.05) is more appropriate for screening significant fibrosis and selection of candidates for liver biopsy, and the WHO cut-off (FIB-4 > 3.25) is more appropriate for diagnosing significant fibrosis and avoiding liver biopsy. In ruling out significant fibrosis, the new APRI cut-off (<0.46) had a predictive value of 80%, and new FIB-4 cut-off (<1.05) correctly identified lack of significant fibrosis in 84% of the patients.
The new APRI and FIB-4 cut-offs obtained in this study were lower than the WHO proposed cut-off values, which deriving from HCV studies. Compared with HCV patients, the different magnitude of inflammation and related ALT levels observed in CHB patients, that might render the different cut off values. Two recent studies, showing different pathogenesis and different patterns of fibrosis according to different causes of chronic liver diseases, also justify the need for different cut-points of systems for assessment of fibrosis from different causes.[25,26] In addition, the WHO proposed cut-offs were mainly for general CHB patients including patients with ALT > 2 ULN, and the new cut-offs were especially for HBeAg-negative CHB patients with ALT ≤ 2 ULN, whose AST and ALT levels (components of APRI and FIB-4) were lower than general CHB population.
APRI and FIB-4 were used mainly in resource-limited areas to predict liver fibrosis and cirrhosis.[27] Indeed, the WHO guidelines on the treatment of CHB recommended APRI as a noninvasive tool to detect cirrhosis in resource-limited settings, based on the following advantages: comprising only inexpensive laboratory tests; available in primary care; and noninvasive procedure.[19] However, our data suggested that the WHO guidelines proposed cut-offs might be higher for HBeAg-negative CHB patients with ALT ≤ 2 ULN. The WHO proposed cut-offs performed poorly in identifying significant fibrosis in HBeAg-negative CHB patients with ALT ≤ 2 ULN in that most patients (84–92%) had lower scores than WHO proposed higher cut-offs. In addition, APRI and FIB-4 were also limited by the WHO proposed cut-offs in making treatment decisions by identifying significant fibrosis, because 52% to 58% of patients without significant fibrosis would have higher scores than WHO proposed lower cut-offs, in HBeAg-negative CHB patients with ALT ≤ 2 ULN. This could result in large numbers of patients receiving unnecessary antiviral therapy. Therefore, the new cut-offs obtained in this study were necessary for the diagnosis of significant fibrosis and cirrhosis in HBeAg-negative CHB patients with ALT ≤ 2 ULN.
It is important to note, however, the rates of PPV of new APRI or FIB-4 cut-off for predicting significant fibrosis or cirrhosis were very low and the rates of specificity were not high. This is an important issue to be discussed. Note that the low PPV was a common problem with serum fibrosis models. According to the WHO HBV guideline, the PPV was low (less than 50%) for all noninvasive tests for the diagnosis of liver fibrosis and cirrhosis, and FibroScan had a relatively higher PPV (42%) than APRI using either a high or low cut-off (26% and 22%).[19] In this study, the rates of specificity were not high (62–66%) when cut-offs obtained by maximizing the sum of sensitivity and specificity. To overcome this, a set of cut-offs were established by obtaining a specificity of at least 90% for APRI and FIB-4, for the diagnosis of significant fibrosis and cirrhosis, respectively. In the clinical work, the cut-offs with high specificity (i.e., fewer false-positive results) could be used to diagnose persons with significant fibrosis and cirrhosis, and the cut-offs with high sensitivity (i.e., fewer false-negative results) could be used to rule out the presence of significant fibrosis and cirrhosis.
This study has limitations. First, the retrospective design of this study might have caused selective bias resulting in underestimated sensitivity and overestimated specificity of APRI and FIB-4.[28] Second, in this study, the case number of cirrhosis was limited (17, 7.2%), which could have resulted in statistical bias when we evaluated and compared the performances of APRI and FIB-4 for the diagnosis of cirrhosis. Therefore, larger sample, prospective, multicentre studies will be necessary to validate the new cut-offs of APRI and FIB-4.
In summary, these data derived from 236 HBeAg-negative CHB patients with ALT ≤ 2 ULN demonstrate that the WHO HBV guidelines proposed cut-offs might be higher for this specific CHB population, and might underestimate the proportion of significant fibrosis and cirrhosis. A new set of cut-offs should be used to predict significant fibrosis and cirrhosis in HBeAg-negative CHB patients with ALT ≤ 2 ULN.
Footnotes
Abbreviations: ALT = alanine transaminase, APRI = aspartate transaminase-to-platelet ratio index, AST = aspartate transaminase, AUROC = area under the receiver operating characteristic curve, CHB = chronic hepatitis B, CI = confidence interval, FIB-4 = fibrosis index based on the 4 factors, HBeAg = hepatitis B virus e antigen, HBsAg = hepatitis B virus surface antigen, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, HCV = hepatitis C virus, HDV = hepatitis D virus, NAFLD = nonalcoholic fatty liver disease, NPV = negative predictive value, PPV = positive predictive value, ROC curve = receiver operating characteristic curve, ULN = upper limit of normal.
Funding: This study was supported by grant no. SHDC12015129 from the ShenKang Development Center of Shanghai and grant no. 13401902100 from the Science and Technology Commission of Shanghai.
Role of the sponsor: The funding organizations are public institutions and had no role in the design and conduct of the study; collection, management, and analysis of the data; or preparation, review, and approval of the manuscript.
Authors’ contributions: Study concept and design: QL and XR; analysis and interpretation of data: LC, XR, CL, WL, YH, and QL; and drafting of the manuscript: QL; critical revision of the manuscript for important intellectual content: LC.
QL and XR contributed equally to this study.
The authors have no conflicts of interest to disclose.
References
- [1].Raptopoulou M, Papatheodoridis G, Antoniou A, et al. Epidemiology, course and disease burden of chronic hepatitis B virus infection. HEPNET study for chronic hepatitis B: a multicentre Greek study. J Viral Hepat 2009;16:195–202. [DOI] [PubMed] [Google Scholar]
- [2].Liang P, Zu J, Yin J, et al. The independent impact of newborn hepatitis B vaccination on reducing HBV prevalence in China, 1992–2006: a mathematical model analysis. J Theor Biol 2015;386:115–21. [DOI] [PubMed] [Google Scholar]
- [3].Wu JF, Chang MH. Natural history of chronic hepatitis B virus infection from infancy to adult life—the mechanism of inflammation triggering and long-term impacts. J Biomed Sci 2015;22:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Terrault NA, Bzowej NH, Chang KM, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 2016;63:261–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016;10:1–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].European Association for the Study of the Liver. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol 2012; 57:167–185. [DOI] [PubMed] [Google Scholar]
- [7].Cadranel JF, Rufat P, Degos F. Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF). Hepatology 2000;32:477–81. [DOI] [PubMed] [Google Scholar]
- [8].Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol 2002;97:2614–8. [DOI] [PubMed] [Google Scholar]
- [9].Westin J, Lagging LM, Wejstal R, et al. Interobserver study of liver histopathology using the Ishak score in patients with chronic hepatitis C virus infection. Liver 1999;19:183–7. [DOI] [PubMed] [Google Scholar]
- [10].Meng F, Zheng Y, Zhang Q, et al. Noninvasive evaluation of liver fibrosis using real-time tissue elastography and transient elastography (FibroScan). J Ultrasound Med 2015;34:403–10. [DOI] [PubMed] [Google Scholar]
- [11].Dong DR, Hao MN, Li C, et al. Acoustic radiation force impulse elastography, FibroScan(R), Forns’ index and their combination in the assessment of liver fibrosis in patients with chronic hepatitis B, and the impact of inflammatory activity and steatosis on these diagnostic methods. Mol Med Rep 2015;11:4174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jia J, Hou J, Ding H, et al. Transient elastography compared to serum markers to predict liver fibrosis in a cohort of Chinese patients with chronic hepatitis B. J Gastroenterol Hepatol 2015;30:756–62. [DOI] [PubMed] [Google Scholar]
- [13].Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003;38:518–26. [DOI] [PubMed] [Google Scholar]
- [14].Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317–25. [DOI] [PubMed] [Google Scholar]
- [15].Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007;46:32–6. [DOI] [PubMed] [Google Scholar]
- [16].Holmberg SD, Lu M, Rupp LB, et al. Noninvasive serum fibrosis markers for screening and staging chronic hepatitis C virus patients in a large US cohort. Clin Infect Dis 2013;57:240–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Li J, Gordon SC, Rupp LB, et al. The validity of serum markers for fibrosis staging in chronic hepatitis B and C. J Viral Hepat 2014;21:930–7. [DOI] [PubMed] [Google Scholar]
- [18].Teshale E, Lu M, Rupp LB, et al. APRI and FIB-4 are good predictors of the stage of liver fibrosis in chronic hepatitis B: the Chronic Hepatitis Cohort Study (CHeCS). J Viral Hepat 2014;21:917–20. [DOI] [PubMed] [Google Scholar]
- [19].World Health Organization. Guidelines for the Prevention, Care and Treatment of Persons with Chronic Hepatitis B Infection. Geneva: World Health Organization; 2015. [PubMed] [Google Scholar]
- [20].Kim WR, Berg T, Asselah T, et al. Evaluation of APRI and FIB-4 scoring systems for non-invasive assessment of hepatic fibrosis in chronic hepatitis B patients. J Hepatol 2016;64:773–80. [DOI] [PubMed] [Google Scholar]
- [21].Yin Z, Zou J, Li Q, et al. Diagnostic value of FIB-4 for liver fibrosis in patients with hepatitis B: a meta-analysis of diagnostic test. Oncotarget 2017;DOI: 10.18632/oncotarget.14430. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 1996;24:289–93. [DOI] [PubMed] [Google Scholar]
- [23].Ucar F, Sezer S, Ginis Z, et al. APRI, the FIB-4 score, and Forn's index have noninvasive diagnostic value for liver fibrosis in patients with chronic hepatitis B. Eur J Gastroenterol Hepatol 2013;25:1076–81. [DOI] [PubMed] [Google Scholar]
- [24].Shin WG, Park SH, Jang MK, et al. Aspartate aminotransferase to platelet ratio index (APRI) can predict liver fibrosis in chronic hepatitis B. Dig Liver Dis 2008;40:267–74. [DOI] [PubMed] [Google Scholar]
- [25].Mason WS, Gill US, Litwin S, et al. HBV DNA integration and clonal hepatocyte expansion in chronic hepatitis B patients considered immune tolerant. Gastroenterology 2016;151:986–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Quaglia A, Alves VA, Balabaud C, et al. Role of aetiology in the progression, regression, and parenchymal remodelling of liver disease: implications for liver biopsy interpretation. Histopathology 2016;68:953–67. [DOI] [PubMed] [Google Scholar]
- [27].Xiao G, Yang J, Yan L. Comparison of diagnostic accuracy of aspartate aminotransferase to platelet ratio index and fibrosis-4 index for detecting liver fibrosis in adult patients with chronic hepatitis B virus infection: a systemic review and meta-analysis. Hepatology 2015;61:292–302. [DOI] [PubMed] [Google Scholar]
- [28].Choi BC. Sensitivity and specificity of a single diagnostic test in the presence of work-up bias. J Clin Epidemiol 1992;45:581–6. [DOI] [PubMed] [Google Scholar]


