Abstract
Rationale:
Postprandial hyperinsulinemic hypoglycemia, known as late dumping syndrome, is a rare but often misdiagnosed complication after gastric surgery. The pathophysiological mechanisms are poorly understood and the treatment of this syndrome is challenging.
Patient concerns:
New-onset postsurgical late dumping syndrome after Toupet fundoplication.
Diagnoses:
Sigstad Score, OGTT, CGM.
Interventions:
Daily subcutaneous injection of liraglutide (0.6 mg and 1.2 mg).
Outcomes:
Reduction in fasting and postprandial peak insulin level with improvement in symptomatic hypoglycemic events.
Lessons:
Liraglutide may be a novel treatment option for postprandial hyperinsulinemic hypoglycemia after gastric surgery.
Keywords: gastric surgery, GLP-1, hyperinsulinemic hypoglycemia, late dumping syndrome, liraglutide
1. Introduction
Gastroesophageal reflux disease (GERD) is the most common upper gastrointestinal condition in Western countries, and its incidence is increasing worldwide because of the obesity epidemic. Laparoscopic posterior fundoplication is the standard surgical treatment for GERD, and the Nissen and Toupet fundoplications have low morbidity and excellent long-term functional outcomes.[1] Moreover Roux-en-Y gastric bypass is getting the new surgical treatment of choice in obese patients with GERD,[2] while gastric bypass surgery shows efficacy similar to that of traditional laparoscopic antireflux surgery in the control of GERD.[3] However, some of the anatomic and functional changes associated with these procedures can cause postoperative complications such as dysphagia, flatulence, distention, and gas-bloat syndrome. Postoperative postprandial hypoglycemia is another complication often reported after fundoplication in children,[4] and a few cases have been reported in adults.[5–9] The apparently high incidence in the pediatric literature suggests that it is an underdiagnosed complication in adults.[5]
Late dumping syndrome is a well-known side effect after gastric surgery and an often-described complication after obesity surgery. It is distinguished from early dumping, which occurs because of the rapid hyperosmolar influx of chyme into the small intestine, and leads to a volume overload and consequent release of gastrointestinal hormones and symptomatic circulatory disturbances. In late dumping syndrome, hyperinsulinemia leads to a hypoglycemic episode 2 to 3 hours after a meal.
The underlying cause of late dumping syndrome is thought to be either that the postoperatively elevated gastric inhibitory polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) levels lead to pancreatic beta cell hypertrophy,[10,11] which increases insulin output and hypoglycemic symptoms, or that changes in the distribution of carbohydrate receptors and transporters in the small intestine contribute to the pathophysiology.[12] These theories are supported by the observation that hyperinsulinemic hypoglycemia most frequently affects patients who have undergone resection of parts of the stomach, in which the gastrojejunostomy bypasses the pylorus. However, none of these theories is proven and none can explain the considerable time delay from surgery to the appearance of symptoms.
Treatment options for late dumping syndrome include dietary changes and medical treatment with glucosidase inhibitors such as acarbose[13] or somatostatin analogs.[14] In cases of treatment failure, partial or even total pancreatectomy is described as the surgical ultima ratio.[15] To address this treatment failure and the side effects of the drugs used currently, Abrahamsson et al[16] described a novel treatment option for postprandial hypoglycemia following gastric bypass surgery with GLP-1 analogs and reported a protective effect of GLP-1 analogs on pronounced symptoms of postprandial hypoglycemic episodes in 5 patients. Prospective randomized trials to define further the role of GLP-1 analogs in treating postprandial hypoglycemia are lacking but are urgently needed.
In this clinical case, we describe the successful use of a GLP-1 analog in treating refractory late dumping syndrome after fundoplication and a possible pathophysiological explanation for the resolution of symptoms.
2. Case presentation and management
A 52-year-old woman who was moderately overweight (BMI 29 kg/m2) and had severe reflux disease underwent a laparoscopic Toupet fundoplication. Twelve months after surgery, she developed episodes of severe symptomatic late dumping syndrome with the full spectrum of hypoglycemic symptoms. About 150 minutes after a meal, she felt palpitations, nausea, sweating, and weakness, which led to collapse. She had no history of diabetes or hypoglycemic symptoms before the operation.
Clinical evaluation included blood tests, functional X-ray examination, upper endoscopy, and magnetic resonance imaging of the upper abdomen. Diagnosis of symptomatic hyperinsulinemic hypoglycemia was made using the Sigstad score questionnaire,[17] an oral glucose tolerance test (OGTT) (Accu-Chek Dextro OGT Roche, Switzerland), and a continuous glucose measurement (CGM) (Dexcom G4 Nintamed, San Diego, California). During the OGTT, the serum insulin level in μU/mL, was quantified at the same time as each glucose measurement.
Treatment of late dumping syndrome included dietary changes and administration of acarbose. Dietary changes escalated the meal frequency to 5 to 6 per day, and the meal composition was shifted toward more protein and fiber, and less carbohydrates. Pharmaceutical treatment included administration of acarbose. Acarbose is an oral antidiabetic agent that inhibits the enzyme alpha-glucosidase and reduces the rate of digestion of carbohydrates. Fifty milligrams of acarbose were given before each meal, 3 times a day.
Due to treatment failure the decision to give liraglutide (Victoza, Novo Nordisk Pharma, Clayton, Carolina del Nord) as an off-label drug[16] was established and discussed with the patient, who agreed and signed an informed consent form for the off-label use of liraglutide and data sharing. Liraglutide is a GLP-1 analog that was approved by the European Commission in 2009 for the treatment of type 2 diabetes mellitus in adults and was approved by the US Food and Drug Administration in 2014 as a treatment option for chronic weight management when used with a reduced-calorie diet and physical activity. Liraglutide is known to increase pancreatic insulin production. The drug decelerates stomach emptying, which initiates the central inhibition of hunger feelings.[18] Liraglutide dosage was applied as recommended in the drug information from Liraglutide (Victoza, Novo Nordisk Pharma, USA) in the treatment of type 2 diabetes mellitus: Liraglutide was applied initially as a daily subcutaneous injection at a low dose of 0.6 mg per day, which was increased to 1.2 mg per day after 3 weeks. OGTT and CGM were performed without treatment, with a treatment of 0.6 mg liraglutide per day (at week 2) and with a treatment of 1.2 mg liraglutide per day (at week 4).
All clinical examinations showed normal postoperative findings 1 year after the fundoplication. There was no evidence of a neuroendocrine tumor. Sigstad score was positive for dumping syndrome with a score of 15. Dietary changes provided no improvement of the postprandial hypoglycemic episodes and intensification of treatment by administration of acarbose produced an insufficient therapeutic effect.
The CGM showed that 59% of all glucose values were in the hypoglycemic range. The Homeostatic Model Assessment of Insulin Resistance (HOMA-Index) was 6.6. The HbA1c level was 4.5% (25.7 mmol/mol).
The first baseline OGTT showed a fasting glucose level of 93 mg/dL and a fasting insulin level of 29 μU/mL. After oral carbohydrate stress during the OGTT, the glucose level increased to a maximum of 206 mg/dL at 30 minutes, after which the glucose level declined slowly to 180 mg/dL at 60 minutes and 146 mg/dL at 90 minutes. The insulin peak of 717 μU/mL was observed at 90 minutes. At that time, the blood glucose level had already started decreasing and was 146 mg/dL. This conjunction of an exaggerated, hyperinsulinemic insulin peak of 717 μU/mL with decreasing serum glucose level caused a rebound of considerable symptomatic hypoglycemia with a glucose level of 35 mg/dL at 150 minutes (Fig. 1).
Figure 1.
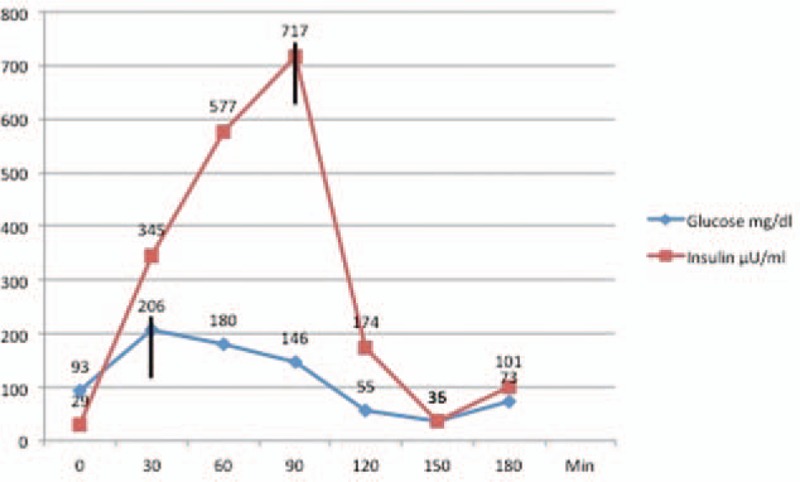
The dynamics of insulin release during OGTT without liraglutide treatment. Note the lack of synchronization of the peak of insulin release to that of serum glucose level.
After 2 weeks of treatment with 0.6 mg liraglutide per day, another OGTT and CGM were performed. The patient showed a marginal improvement in the subjective hypoglycemic symptoms. The OGTT showed a different dynamic to the previous investigation, and the insulin levels were markedly lower than without treatment. The fasting glucose level was 88 mg/dL, which is in the normal range. The fasting insulin level of 19.3 μU/mL was two-thirds of the value without treatment. The peak insulin level was 311 μU/mL and appeared 30 minutes earlier, at 60 minutes. The peak glucose level occurred 30 minutes after the OGTT and was 155 mg/dL, which was 25% lower than without liraglutide treatment. There was only a 30 minutes time difference between the 2 peaks. A hypoglycemic glucose level was still observed, but this time it occurred after 180 minutes, which was 30 minutes later than without treatment. At 120 minutes, the glucose level was 76 mg/dL and the insulin level was 109 μU/mL. This resulted in hypoglycemia and a glucose concentration of 48 mg/dL 60 minutes later at 180 minutes. The patient still had symptoms, but they were considerably milder.
This was considered to be an insufficient treatment response, and the liraglutide dosage was boosted to 1.2 mg/day. The OGTT and CGM were repeated after 2 weeks of treatment. A further decrease in fasting insulin levels was observed to 10.7 μU/mL, which was one-third of the baseline fasting insulin value of 29 μU/mL. The fasting glucose level was considered normoglycemic at 83 mg/dL. The corresponding OGTT showed a peak in the insulin level of 413 μU/mL at 60 minutes.
The changes in the insulin and glucose courses were remarkable (Fig. 2). The corresponding CGM showed that the glucose level remained above 66 mg/dL and that 84% of all evaluated glucose values reached the target normoglycemia level. With continuing liraglutide treatment, the patient remains free of symptoms.
Figure 2.
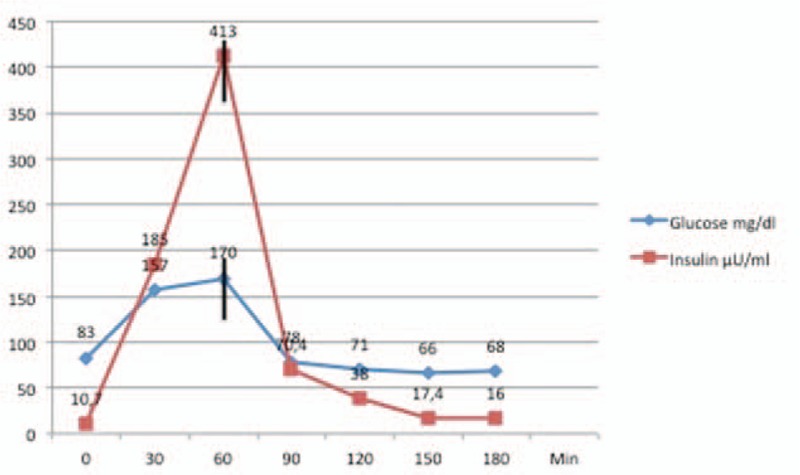
Relationship between the dynamics of insulin release during the OGTT with 1.2 mg liraglutide treatment. Note the optimal synchronization of the peaking of both serum glucose and insulin levels. The rapid decline in insulin output led to a rapid stabilization of serum glucose level and resolution of late dumping symptoms.
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from the patient included in the study.
3. Discussion
Late dumping syndrome is a well-known side effect after gastric surgery, and treatment of this phenomenon is challenging. In our clinical case, late dumping syndrome developed after a Toupet fundoplication, a nonresecting gastric surgery procedure. Hyperinsulinemic hypoglycemia is an often-described postsurgical complication after fundoplication in children,[4] but only a few cases have been described in adults.[5–9]
In up to 40% of patients with GERD, delayed gastric emptying has been described preoperatively and is an independent risk factor for poor outcome after fundoplication in terms of both reflux and symptom control.[19] Fundoplication significantly accelerates gastric emptying.[20] Accelerated gastric emptying may give rise to a larger and earlier increase in plasma glucose, GLP-1, and GIP concentrations, and thus to reactive hypoglycemia.[10] Similar effects have been described after obesity surgery[21,22]; for example, there is a prevalence of up to 50% of postprandial hyperinsulinemic hypoglycemia after Roux-en-Y gastric bypass.[23] The key component is the rapid transit and appearance of nutrients in the small intestine, which causes marked increases in glucose and insulin levels and the GLP-1 peak.[12] Indeed the overshooting and delayed insulin output does not match the time course of the change in serum glucose level. Thus, the insulin level is decoupled and dyssynchronous from the glucose level.
In our patient, late dumping syndrome triggered by hyperinsulinemia was associated with overshooting of insulin output, which was delayed and did not match the time course of the change in serum glucose level. Thus, the insulin level was decoupled and dyssynchronous from the glucose level without treatment. After treatment with liraglutide, the patient's fasting insulin level decreased markedly.
We suggest that this glucose–insulin mismatch triggered by accelerated gastric emptying and larger and earlier increases in plasma glucose, GLP-1, and GIP concentrations is the cause of reactive hypoglycemia.[10] Abrahamsson et al[16] proposed a glucose-stabilizing mechanism to explain the positive effect of GLP-1 analogs. The outcome of the case study presented here is consistent with this proposal and suggests that the GLP-1 analog liraglutide used to treat this patient may have provided a stable concentration of GLP-1 and thus resolution of the mismatch. Abrahamsson et al also proposed that endogenous GLP-1 has a short window of activity, whereas exogenous GLP-1 analogs promote persistent GLP-1 receptor activation. This could explain the lower total insulin level after treatment with liraglutide and the resolution of symptoms.
Laferrère et al[21] studied the incretin effect after Roux-en-Y gastric bypass and reported decreases in fasting glucose levels from 7.95 ± 1.74 before gastric bypass to 6.42 ± 0.80 mmol/L after gastric bypass (P = 0.957), in peak glucose levels from 14.24 ± 3.23 before gastric bypass to 12.02 ± 1.39 mmol/L after gastric bypass (P = 0.514), and fasting insulin levels from 172 ± 69 before gastric bypass to 127 ± 51 pmol/L after gastric bypass (P = 0.195), but an increase in peak insulin levels from 492 ± 288 before gastric bypass to 769 ± 335 pmol/L after gastric bypass (P = 0.286).
The higher and mismatched peak insulin level may be one reason for postprandial hyperinsulinemic hypoglycemia. Treatment with liraglutide reduced the peak insulin level, which may explain the improvement in the symptoms of dumping syndrome experienced by our patient.
Finally, in our case report liraglutide had a dose-dependent effect on the insulin course. After liraglutide treatment, the insulin peak during the OGTT was lower, occurred sooner, and was matched more closely to the change in glucose level. This change in insulin release was probably directly related to the resolution of the patient's symptoms. Thus, exogenous GLP-1 analogs seem to synchronize the pancreatic insulin output and blood glucose level, and may even decrease insulin output.
In this patient, liraglutide was effective in treating hyperinsulinemic hypoglycemia associated with late dumping syndrome. The OGTT and CGM showed considerable effects of the drug on serum insulin level and on the glucose–insulin mismatches, which were probably triggered by accelerated gastric emptying and a larger and earlier increase in plasma glucose, GLP-1, and GIP concentrations. Daily use of the GLP-1 analog liraglutide caused this mismatch to disappear, and the matching effect occurred through the rapid decrease in the insulin level.
4. Conclusion
The analysis of our case study suggests a novel treatment approach to treat patients with late dumping syndrome that fail standard medical treatment. Additional studies are warranted to address the overall effect size and safety of GLP-1 analogs in treatment of late dumping syndrome.
Footnotes
Abbreviations: CGM = continuous glucose measurement, GERD = gastroesophageal reflux disease, GIP = gastric inhibitory polypeptide, GLP-1 = glucagon-like peptide-1, OGTT = oral glucose tolerance test.
Authors’ contribution: All authors performed substantial contributions to conception and design of the article and to acquisition, analysis and interpretation of data. All authors reviewed the manuscript for important intellectual content and approved the final version for publication.
Informed consent: Informed consent was obtained from the patient included in the study.
Ethical approval: All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
The authors have no conflicts of interest to disclose.
References
- [1].Dallemagne B, Weerts J, Markiewicz S, et al. Clinical results of laparoscopic fundoplication at ten years after surgery. Surg Endosc 2006;20:159–65. [DOI] [PubMed] [Google Scholar]
- [2].Perry Y, Courcoulas AP, Fernando HC, et al. Laparoscopic Roux-en-Y gastric bypass for recalcitrant gastroesophageal reflux disease in morbidly obese patients. JSLS 2004;8:19–23. [PMC free article] [PubMed] [Google Scholar]
- [3].De Luca M, Angrisani L, Himpens J, et al. Indications for surgery for obesity and weight-related diseases: position statements from the International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO). Obes Surg 2016;26:1659–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Calabria AC, Gallagher PR, Simmons R, et al. Postoperative surveillance and detection of postprandial hypoglycemia after fundoplasty in children. J Pediatr 2011;159:597–601.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kreckler S, Dowson H, Willson P. Dumping syndrome as a complication of laparoscopic Nissen fundoplication in an adult. JSLS 2006;10:94–6. [PMC free article] [PubMed] [Google Scholar]
- [6].Roldán Baños S, Ruiz de Angulo Martín D, Munítiz Ruiz V, et al. Dumping syndrome with severe hipoglycemia after Nissen fundoplication in adults. Case report and literature review. Endocrinol Nutr 2014;61:550–1. [DOI] [PubMed] [Google Scholar]
- [7].Mizrahi M, Almogy G, Adar T, et al. Dumping syndrome following Nissen fundoplication in an adult patient diagnosed by continuous online 13C/12C monitoring of 13C-octanoic acid breath test “a case report”. BMC Gastroenterol 2011;11:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bernad B, Kline GA, Service FJ. Hypoglycaemia following gastrointestinal surgery: case report and review of the literature. BMC Gastroenterol 2010;10:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zaloga GP, Chernow B. Postprandial hypoglycemia after Nissen fundoplication for reflux esophagitis. Gastroenterology 1983;84:840–2. [PubMed] [Google Scholar]
- [10].Miholic J, Hoffmann M, Holst JJ, et al. Gastric emptying of glucose solution and associated plasma concentrations of GLP-1, GIP, and PYY before and after fundoplication. Surg Endosc 2007;21:309–14. [DOI] [PubMed] [Google Scholar]
- [11].Dirksen C, Bojsen-Møller KN, Jørgensen NB, et al. Exaggerated release and preserved insulinotropic action of glucagon-like peptide-1 underlie insulin hypersecretion in glucose-tolerant individuals after Roux-en-Y gastric bypass. Diabetologia 2013;56:2679–87. [DOI] [PubMed] [Google Scholar]
- [12].Dyer J, Daly K, Salmon KS, et al. Intestinal glucose sensing and regulation of intestinal glucose absorption. Biochem Soc Trans 2007;35(Pt 5):1191–4. [DOI] [PubMed] [Google Scholar]
- [13].Valderas JP, Ahuad J, Rubio L, et al. Acarbose improves hypoglycaemia following gastric bypass surgery without increasing glucagon-like peptide 1 levels. Obes Surg 2012;22:582–6. [DOI] [PubMed] [Google Scholar]
- [14].Hopman WP, Wolberink RG, Lamers CB, et al. Treatment of the dumping syndrome with the somatostatin analogue SMS 201-995. Ann Surg 1988;207:155–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Clancy TE, Moore FD, Jr, Zinner MJ. Post-gastric bypass hyperinsulinism with nesidioblastosis: subtotal or total pancreatectomy may be needed to prevent recurrent hypoglycemia. J Gastrointest Surg 2006;10:1116–9. [DOI] [PubMed] [Google Scholar]
- [16].Abrahamsson N, Engström BE, Sundbom M, et al. GLP1 analogs as treatment of postprandial hypoglycemia following gastric bypass surgery: a potential new indication? Eur J Endocrinol 2013;169:885–9. [DOI] [PubMed] [Google Scholar]
- [17].Sigstad H. Clinical diagnostic index in the diagnosis of the dumping syndrome. Acta Med Scand 1970;188:479–86. [PubMed] [Google Scholar]
- [18].Campbell JE, Ducker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab 2013;17:819–37. [DOI] [PubMed] [Google Scholar]
- [19].Lindeboom MYA, Ringers J, van Rijin PJJ, et al. Gastric emptying and vagus nerve function after laparoscopic partial fundoplication. Ann Surg 2004;240:785–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rebecchi F, Allaix ME, Giaccone C, et al. Gastric emptying as a prognostic factor for long-term results of total laparoscopic fundoplication for weakly acidic or mixed reflux. Ann Surg 2013;258:831–6. discussion 836–837. [DOI] [PubMed] [Google Scholar]
- [21].Laferrère B, Teixeira J, McGinty J, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab 2008;93:2479–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Vidal J, Nicolau J, Romero F, et al. Long-term effects of Roux-en-Y gastric bypass surgery on plasma glucagon-like peptide-1 and islet function in morbidly obese subjects. J Clin Endocrinol Metab 2009;94:884–91. [DOI] [PubMed] [Google Scholar]
- [23].Banerjee A, Ding Y, Mikami DJ, et al. The role of dumping syndrome in weight loss after gastric bypass surgery. Surg Endosc 2013;27:1573–8. [DOI] [PubMed] [Google Scholar]


