Abstract
Noninvasive positive-pressure ventilation (NPPV) might be superior to conventional mechanical ventilation (CMV) in patients with acute exacerbations of chronic obstructive pulmonary disease (AECOPDs). Inefficient clearance of respiratory secretions provokes NPPV failure in patients with hypercapnic encephalopathy (HE). This study compared CMV and NPPV combined with a noninvasive strategy for clearing secretions in HE and AECOPD patients.
The present study is a prospective cohort study of AECOPD and HE patients enrolled between October 2013 and August 2015 in a critical care unit of a major university teaching hospital in China.
A total of 74 patients received NPPV and 90 patients received CMV. Inclusion criteria included the following: physician-diagnosed AECOPD, spontaneous airway clearance of excessive secretions, arterial blood gas analysis requiring intensive care, moderate-to-severe dyspnea, and a Kelly–Matthay scale score of 3 to 5. Exclusion criteria included the following: preexisting psychiatric/neurological disorders unrelated to HE, upper gastrointestinal bleeding, upper airway obstruction, acute coronary syndromes, preadmission tracheostomy or endotracheal intubation, and urgent endotracheal intubation for cardiovascular, psychomotor agitation, or severe hemodynamic conditions.
Intensive care unit participants were managed by NPPV. Participants received standard treatment consisting of controlled oxygen therapy during NPPV-free periods; antibiotics, intravenous doxofylline, corticosteroids (e.g., salbutamol and ambroxol), and subcutaneous low-molecular-weight heparin; and therapy for comorbidities if necessary. Nasogastric tubes were inserted only in participants who developed gastric distension. No pharmacological sedation was administered.
The primary and secondary outcome measures included comparative complication rates, durations of ventilation and hospitalization, number of invasive devices/patient, and in-hospital and 1-year mortality rates.
Arterial blood gases and sensorium levels improved significantly within 2 hours in the NPPV group with lower hospital mortality, fewer complications and invasive devices/patient, and superior weaning off mechanical ventilation. Mechanical ventilation duration, hospital stay, or 1-year mortality was similar between groups.
NPPV combined with a noninvasive strategy to clear secretions during the first 2 hours may offer advantages over CMV in treating AECOPD patients complicated by HE.
Keywords: chronic obstructive pulmonary disease, hospital outcome, hypercapnic encephalopathy, noninvasive positive-pressure ventilation, respiratory secretions, sensorium level
1. Introduction
Chronic obstructive pulmonary disease (COPD) is a serious lung condition with a high incidence and mortality.[1,2] An acute exacerbation of COPD (AECOPD) can lead to life-threatening hypercapnic encephalopathy (HE) that requires emergency intervention with mechanical ventilation. Conventional mechanical ventilation (CMV) is currently recommended as the gold-standard technique for the treatment of patients with AECOPD and HE. CMV is an invasive form of ventilation that utilizes positive pressure to deliver an air/oxygen mixture to the airways via an endotracheal or tracheostomy tube. It can be delivered through one of several modes, including assist-control ventilation, synchronized intermittent mandatory ventilation, and pressure support (PS) ventilation.[3] Despite the undoubted benefits of CMV in some patients with acute respiratory failure, the mortality can be as high as 40%, and a proportion of these deaths may be attributed to complications associated with CMV and its need for an artificial airway, including excessive cuff pressure requirements, self-extubation, stomal infection and hemorrhage, tracheal stenosis, laryngotracheal injury, and ventilation-associated pneumonia.[4–7]
Noninvasive ventilatory support is an alternative approach to CMV that does not require endotracheal intubation (ETI) or the placement of a tracheostomy tube. There is now good evidence from numerous studies that noninvasive positive-pressure ventilation (NPPV) is an effective intervention in patients with acute respiratory failure, including those with COPD and HE, and has advantages over CMV that include no requirement for ETI, fewer complications such as ventilator-associated pneumonia, pneumothorax, and delirium, shorter hospital stay, and reduced mortality.[8–12] NPPV utilizes positive pressure to deliver air/oxygen to the airways via an interface such as a face mask, nasal mask/plugs, or helmet. Various modalities of NPPV exist, including the application of continuous positive airway pressure throughout the respiratory cycle, or the delivery of bilevel positive airway pressure (BiPAP), that is, 2 different pressure levels according to the respiratory cycle.
However, patients with HE and a depressed cough reflex are unable to efficiently clear copious secretions from their airways, and this can cause failure of NPPV.[10,11] A small number of studies have reported that some noninvasive physiotherapeutic techniques can improve mucus clearance in patients with AECOPD managed with NPPV.[11,13–15] Unfortunately, there are currently no data on the use of these techniques in patients with HE and abundant secretions.[16] One recent study demonstrated the safety and effectiveness of early therapeutic fiberoptic bronchoscopy (FBO) during NPPV,[12] raising the possibility that this strategy might be a viable alternative to CMV in the management of selected patients with COPD within expert units. However, the authors acknowledged several limitations of their study (including the invasive nature of the technique and the applicability of the data only to units with a large expertise in NPPV and FBO).[12] Thus, the development of a simpler approach to clearing airway secretions during NPPV is strongly needed to overcome the obstacles of using NPPV in patients with copious respiratory secretions.
An oropharyngeal airway (OPA) helps to establish a patent airway by preventing the tongue from covering the epiglottis.[17] It is used frequently in emergency care for short-term airway management in unconscious subjects with spontaneous respiration,[18] and to facilitate manual ventilation with a face mask.[19] Importantly, the use of an OPA is not only simple but also allows vacuum aspiration of sputum using a noninvasive and established technique.
We hypothesized that a combination of noninvasive techniques, including repeated suctioning of secretions from an OPA, appropriate patient posture, nebulized inhalation of salbutamol and ambroxol, and close monitoring, could be used within an intensive care unit (ICU) to maintain a clear airway during the first 2 hours of NPPV. The aim of this pilot study was to examine the safety and effectiveness of this treatment strategy in the clearing of airways during NPPV. An additional aim was to determine the outcomes of this strategy when administered (within an ICU) to patients with AECOPD and HE who would not typically be considered appropriate candidates for NPPV because of their inability to remove copious secretions.
2. Materials and methods
2.1. Study design
This prospective cohort study was performed between October 2013 and August 2015 in 2 departments: a 6-bed respiratory ICU (RICU) in the Respiratory Division of the Harrison International Peace Hospital, Hengshui, Hebei, China; and a 25-bed general ICU at the same hospital. The study protocol was approved by the ethics committee of Harrison International Peace Hospital, and informed written consent was obtained from the proxy due to the depressed mental status of the study participants. All included participants in the RICU were treated with CMV, while those in the ICU were treated with early 2-hour NPPV combined with noninvasive techniques to clear mucous secretions.
2.2. Patients
The participants were enrolled consecutively using the following inclusion criteria: a diagnosis of AECOPD was made based exclusively on the clinical presentation of the participant, who complained of an acute change in dyspnea, cough, and/or sputum production that was beyond normal day-to-day variation; assessment of the symptoms was based on the subject's medical history, clinical signs of severity, and laboratory tests[20]; the participant was unable to spontaneously clear his/her airways of excessive secretions (highest score of an arbitrary cough efficiency scale)[21]; arterial blood gas (ABG) analysis (PaCO2 >6.0 kPa [45 mm Hg] and/or pH <7.35) pointed toward a life-threatening episode that needed close monitoring or intensive care[20]; the participant had moderate-to-severe dyspnea with use of accessory muscles and paradoxical abdominal motion[20]; and the Kelly–Matthay scale (KMS) score for encephalopathy was between 3 and 5 (3 = lethargic, but rousable and follows simple commands; 4 = stuporous, i.e., only intermittently follows simple commands even with vigorous attempts to rouse the subject; 5 = comatose, brain stem intact).[22]
The exclusion criteria were as follows: preexisting psychiatric and/or neurological disorders unrelated to HE; upper gastrointestinal bleeding; upper airway obstruction; acute coronary syndromes; tracheostomy or ETI before admission; and need for urgent ETI due to cardiac or respiratory arrest, psychomotor agitation, or severe hemodynamic instability.
2.3. NPPV with early 2-hour strategy to clear secretions
Participants in the ICU were managed using NPPV, with airway management and clearance of secretions performed in the initial 2 hours of NPPV.
NPPV (Flexo ST30 ventilator, Curative Medical Inc, Santa Clara, CA) was delivered in spontaneous/timed mode with positive end-expiratory pressure (PEEP) via a well-fitting oronasal mask (ZS-MZ-A, Zhongshan, Shanghai, China) with the addition of a single-use heated humidifier (G-314001-0, Vadi Medical Technology Co Ltd, Taiwan, China). PS was initially set at 8 to 10 cm H2O and then titrated to achieve an expiratory tidal volume of 6 to 8 mL/kg up to a maximum of 25 cm H2O, depending on the clinical ABG response and the participant's tolerance. PEEP was always set at 3 to 5 cm H2O. Backup respiratory rate (RR) was set at 14 to 18 breaths/min, lower than the participant's spontaneous RR. The fraction of inspired oxygen (FiO2) was adjusted to keep pulse blood oxygen saturation (SpO2) at about 90% to 94%.[10] As far as possible, the participant was treated in a semirecumbent or sitting position (45°C–90°C) during NPPV in order to minimize the possibility of aspiration.
An OPA (GuedelFix airway, VBM Medizintechnik GmbH, Sulz am Neckar, Germany) of appropriate size was selected so that, from an external viewpoint, it extended from the lower tip of the ear to the corner of the closed mouth (Figs. 1 and 2); choosing the correct size was important to ensure that the tongue was held in place without trauma to the pharynx or airway obstruction. To open the participant's mouth, the thumb and the index finger (crossed-finger method) were pushed against the upper and lower teeth near a corner of the mouth. The tip of the OPA was introduced into the participant's mouth (Fig. 3) with the tube lying on the tongue and the airway tip pointing toward the roof of the mouth (to help to keep the tongue from being pushed toward the back of the throat as the airway was inserted). The airway was advanced, and when its tip reached the back of the tongue (past the soft palate), it was rotated 180° so that the tip pointed down toward the throat. If the airway was difficult to insert or rotate, the participant's tongue was gently pulled forward with the fingertips of the hand not holding the airway. The airway was advanced until the flange rested against the lips, and the tongue was held in place so that it did not slide toward the back of the throat.
Figure 1.
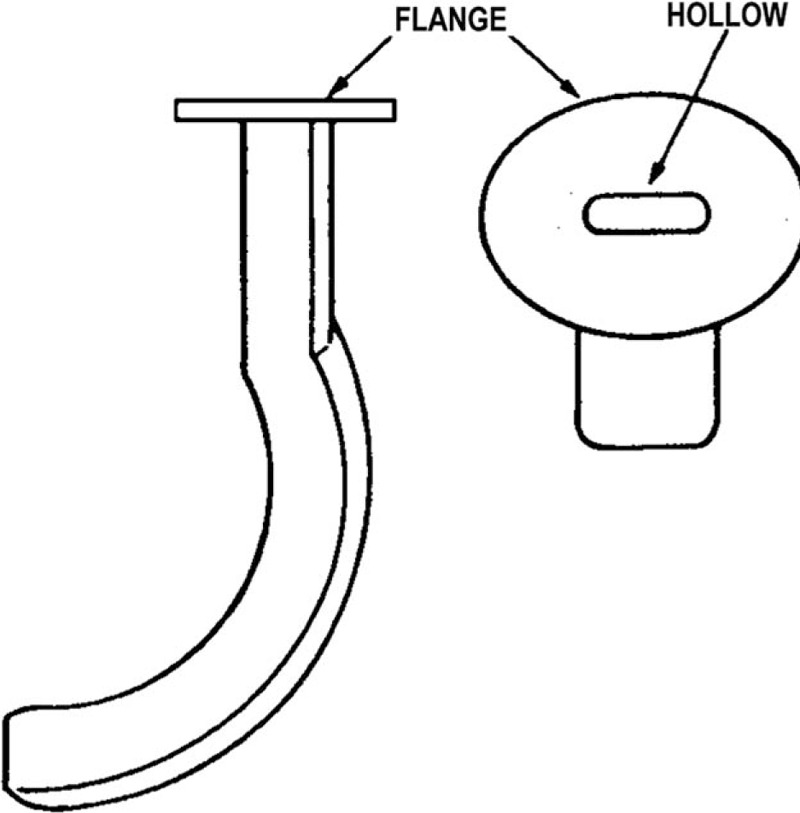
Oropharyngeal airway.
Figure 2.
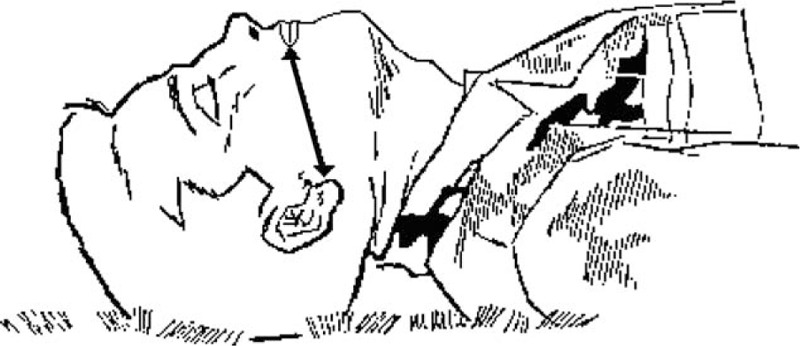
Selecting an oropharyngeal airway of appropriate size.
Figure 3.
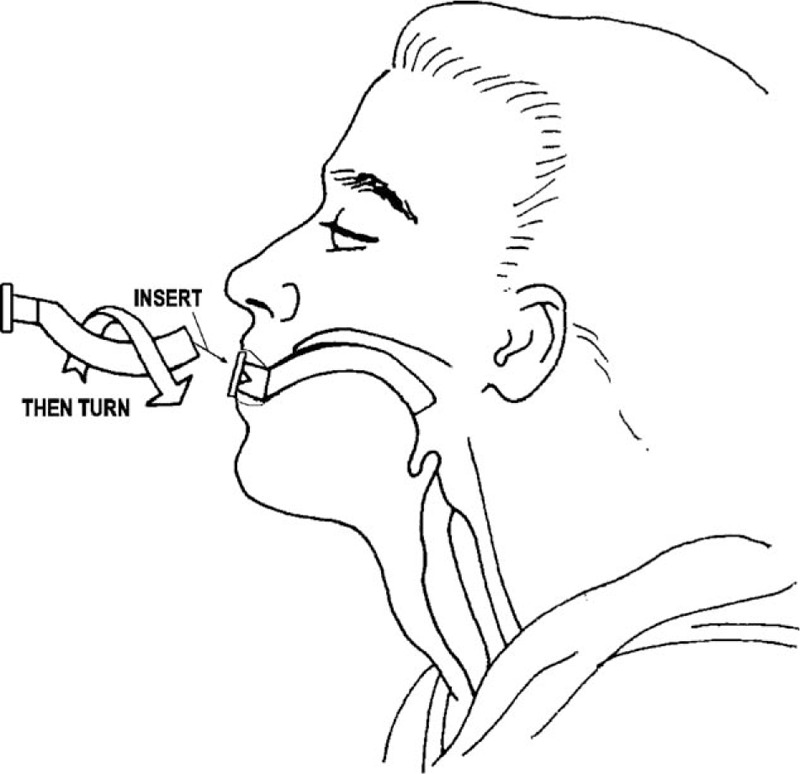
Inserting the oropharyngeal airway.
Respiratory secretions were aspirated every 20 to 30 minutes during the initial 2 hours using a suction catheter inserted as deeply as possible into the trachea via the OPA; the participant's back was slapped before aspiration to facilitate the removal of secretions. Preoxygenation was considered if the participant had a clinically important reduction in oxygen saturation with suctioning; subsequently, the FiO2 was decreased in order to maintain the SpO2 at 90% to 94%.
Salbutamol 5 mg (Salamol, GlaxoSmithKline, Brentford, UK) and ambroxol 15 mg (Mucosolvan, Boehringer Ingelheim, Barcelona, Spain) in 2.5 mL normal saline were administered as a nebulized inhalation for 5 minutes (LCV nebulizer, Pari GmbH, Starnberg, Germany) at an oxygen flow rate of 7 L/min.[23] The nebulizer was placed between the leak port and the connection to the participant, and the nebulizer was maintained in a horizontal position during NPPV (Figs. 4 and 5).
Figure 4.
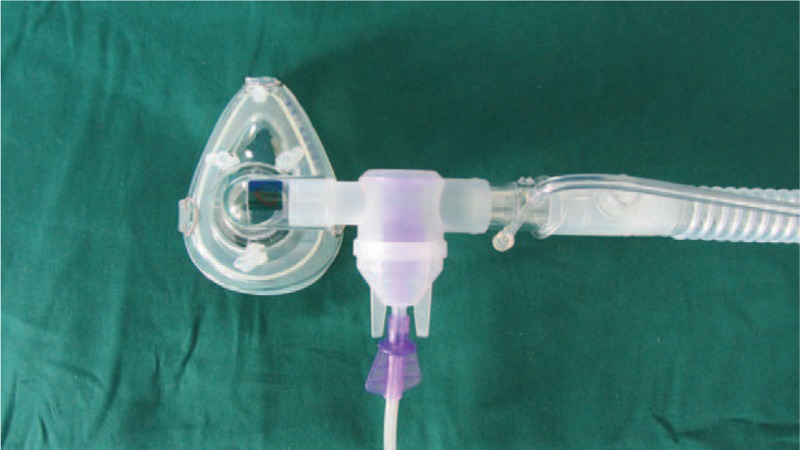
Placement of the nebulizer between the leak port and the subject connection.
Figure 5.
The nebulizer was maintained in a horizontal position during noninvasive positive-pressure ventilation (NPPV).
All participants received standard medical therapy consisting of controlled oxygen therapy during NPPV-free periods; antibiotics, intravenous doxofylline, corticosteroids, and subcutaneous low-molecular-weight heparin; and therapy for comorbidities if necessary. Nasogastric tubes were inserted only in participants who developed gastric distension. No pharmacological sedation was administered.
The 2-hour strategy of NPPV with aspiration of secretions was considered successful if the KMS had declined by at least 1 point and the pH had increased by at least 0.05 to 0.10 by the end of the initial 2-hour period, as compared to baseline. During the following 12 to 24 hours, continuous NPPV was provided with the participant maintained in a semirecumbent position, secretions aspirated hourly via the OPA, and nebulized inhalation of salbutamol/ambroxol administered every 6 hours. The removal of secretions via the OPA was stopped if the participant was able to clear the secretions spontaneously.
NPPV was administered intermittently once the clinical status, KMS score, and ABGs had improved substantially. PS was reduced progressively until a level of ≤8 to 10 cm H2O was reached. NPPV weaning was considered successful when all the following criteria had been met for longer than 24 hours while breathing with oxygen (FiO2 0.28): pH >7.35, SpO2 >90%, RR <30 breaths/min, KMS score 1, and stable hemodynamic status.[24] Noninvasive home mechanical ventilation (HMV) via a facial or nasal mask was considered if the subject remained partially dependent on NPPV (≥8 hours/d) after 10 days.[10]
NPPV was considered to have failed if at least 1 of the following criteria was met and ETI was promptly available: cardiac arrest or severe hemodynamic instability, respiratory arrest or gasping, mask intolerance, difficulty in clearing bronchial secretions, and worsening of ABGs or sensorium level during NPPV.[25]
2.4. Conventional mechanical ventilation
Participants who received CMV were enrolled using the same inclusion criteria as those for patients administered NPPV. Intubation was carried out after admission to the RICU without NPPV being first attempted.
The standard therapeutic protocol was the same as that for the NPPV group except that the participants were sedated at the time of intubation with daily interruption of sedation (2 mg/kg propofol intravenously followed by a continuous infusion at 0.5–3 mg/kg/h, usually for 24–36 hours).[10] CMV was delivered via a ventilator (Puritan Bennett 840, Covidien, Dublin, Ireland) in synchronized intermittent mandatory ventilation + PS mode (target tidal volume 6–8 mL/kg; backup RR 10–15 breaths/min; FiO2 0.30–0.45; PEEP 3–5 cm H2O; PS 8–14 cm H2O, or platform pressure <30 cm H2O).[26]
Extubation was performed if the subject met the same criteria as those used for the NPPV group. If the subject was still intubated and ventilated after 12 days, tracheostomy was performed according to the judgment of the physician in charge. If the subject and/or proxy refused tracheostomy, CMV was considered to have failed. HMV via tracheostomy was considered if the subject was still ventilator-dependent after 30 days.[27]
2.5. Data collection
In addition to KMS score and ABG analysis, the following additional data were collected: age, gender, lung function in the stable phase within the previous 6 months, body mass index, Acute Physiology and Chronic Health Evaluation II score, hospital stay before mechanical ventilation, number of invasive devices, PS, need for de novo long-term oxygen therapy, and HMV.
The primary end points were as follows: the safety (need for urgent ETI) and effectiveness (changes in ABGs and KMS) of the secretion clearing strategy within the first 2 hours of NPPV, and the rate of major complications,[27] especially septic complications and nosocomial pneumonia (including pulmonary aspiration) that were diagnosed using strict criteria.[28,29] The secondary end points were as follows: in-hospital mortality, 1-year mortality, tracheostomy, duration of mechanical ventilation, and length of hospital stay.
2.6. Statistical analysis
The Shapiro–Wilk test was used to verify whether all recorded variables were normally distributed (P > 0.05). Continuous data are expressed as the mean ± standard deviation if distributed normally or as median (interquartile range) if not. Continuous variables were compared with the 2-tailed unpaired Student t test (parametric data) or the Mann–Whitney U test (nonparametric data). Categorical data were compared using the χ2 or, when appropriate, Fisher exact test. The mean ABG data at different time points were compared with repeated measures analysis of variance. The sensorium level between baseline and 2 hours in the NPPV group was compared using the paired Student t test. A P value <0.05 was considered statistically significant. Differences in the probability of remaining on mechanical ventilation over 30 days between the 2 groups were investigated by means of Kaplan–Meier curves.
3. Results
3.1. Baseline characteristics
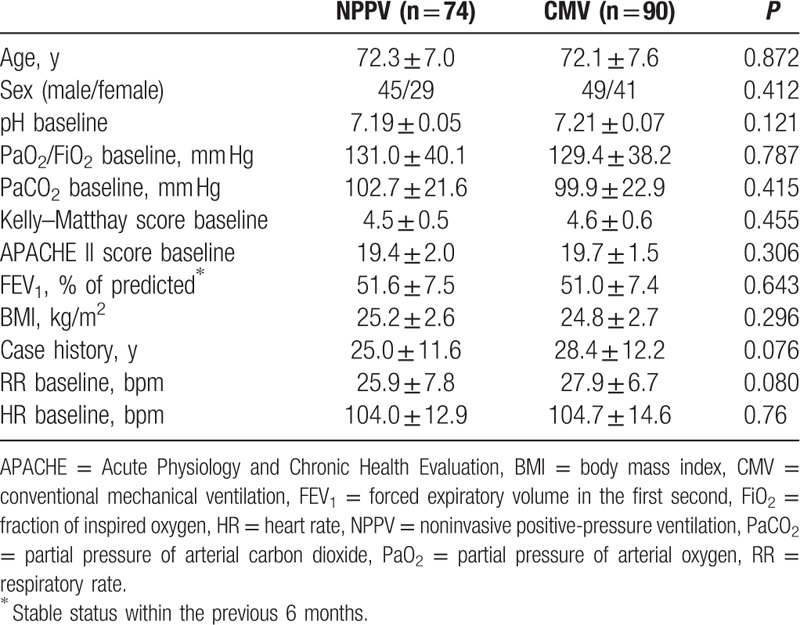
Of 186 patients with AECOPD screened for inclusion in the study, 164 were included in the analysis (90 in the CMV group and 74 in the NPPV group). There were no significant differences between the 2 groups in any of the baseline characteristics (Table 1).
Table 1.
Characteristics of the NPPV and CMV groups.
3.2. ABGs, sensorium level, and other indexes during the first 2 hours of mechanical ventilation
Compared to baseline, the ABGs improved significantly in both groups after 2 hours of mechanical ventilation, but no significant differences were observed in pH, PaO2/FiO2, and PaCO2 between the NPPV and CMV groups within the initial 2-hour period (P = 0.124, 0.095, and 0.740, respectively; Fig. 6). The sensorium level significantly improved within 2 hours in the NPPV group (P < 0.001; Fig. 7), but was not evaluated in the CMV group due to the use of sedation.
Figure 6.
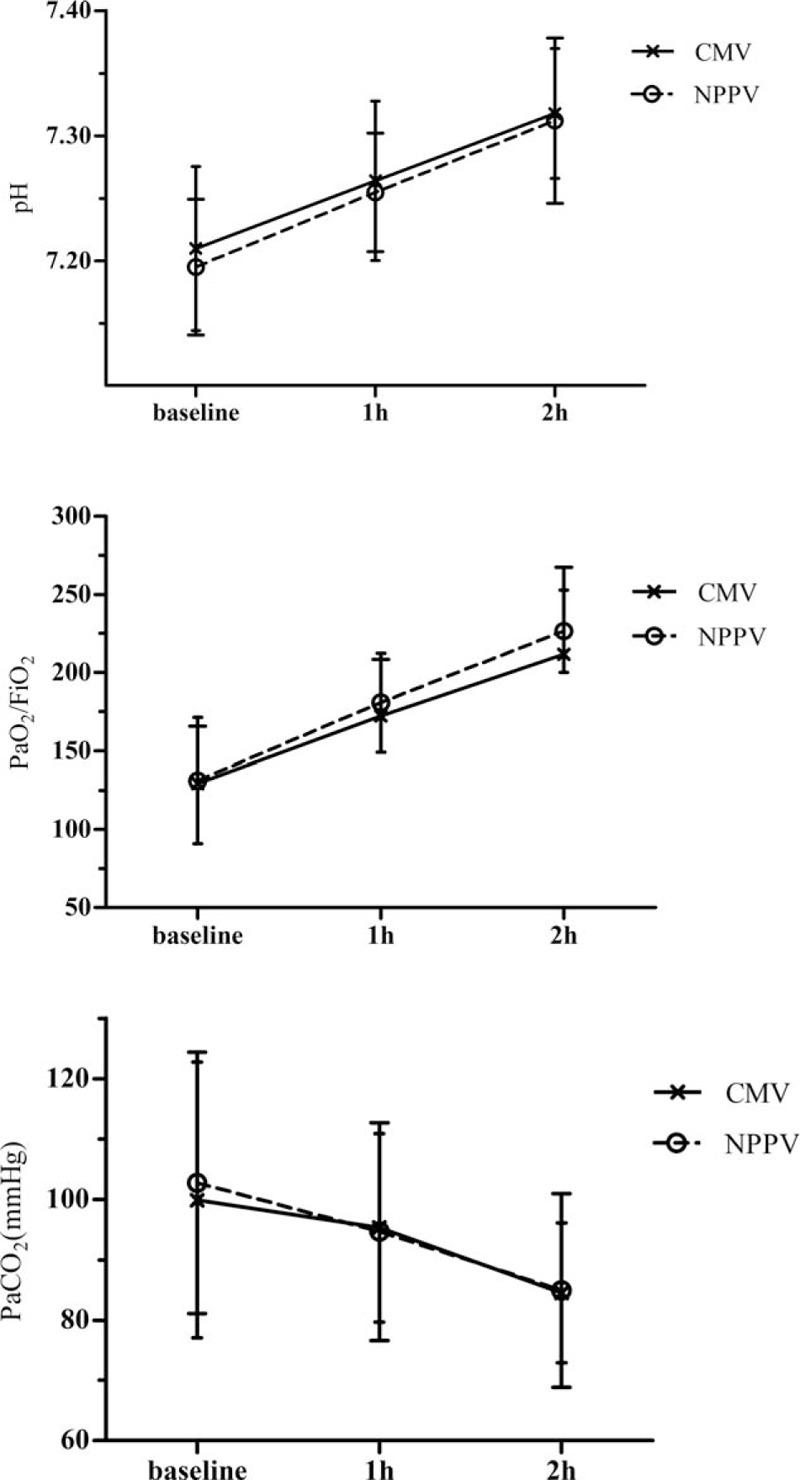
Comparisons of the pH, PaO2/FiO2, and PaCO2 values between the noninvasive positive-pressure ventilation (NPPV) and conventional mechanical ventilation (CMV) groups within the initial 2 hours of ventilation (P = 0.124, 0.095, and 0.740, respectively; repeated measures analysis). Values are expressed as the mean ± SD. FiO2 = fraction of inspired oxygen, PaCO2 = partial pressure of arterial carbon dioxide, PaO2 = partial pressure of arterial oxygen; SD = standard deviation.
Figure 7.
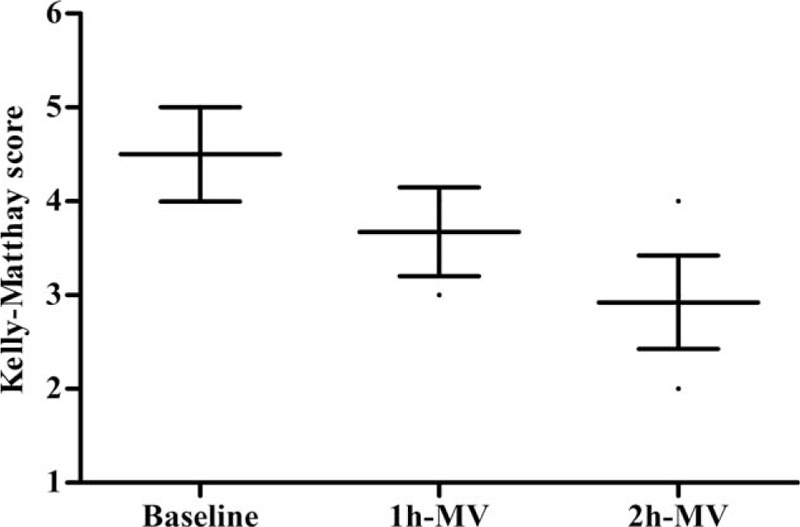
Kelly–Matthay scale (KMS) score at baseline, and 1 and 2 hours in the noninvasive positive-pressure ventilation (NPPV) group. Values are expressed as the mean ± SD. Value at 2 hours versus that at baseline, P < 0.01. MV = mechanical ventilation, SD = standard deviation.
3.3. Adverse reactions and complications
Subjects receiving CMV had a higher complication rate than those receiving NPPV due to a greater occurrence of nosocomial infections and use of more invasive devices.
3.4. Outcome measures
Hospital mortality was lower in the NPPV group than in the CMV group, but 1-year mortality rate, de novo initiations of long-term oxygen therapy, and HMV rate were similar between the 2 groups (Table 2).
Table 2.
Prognostic indicators in NPPV and CMV groups.
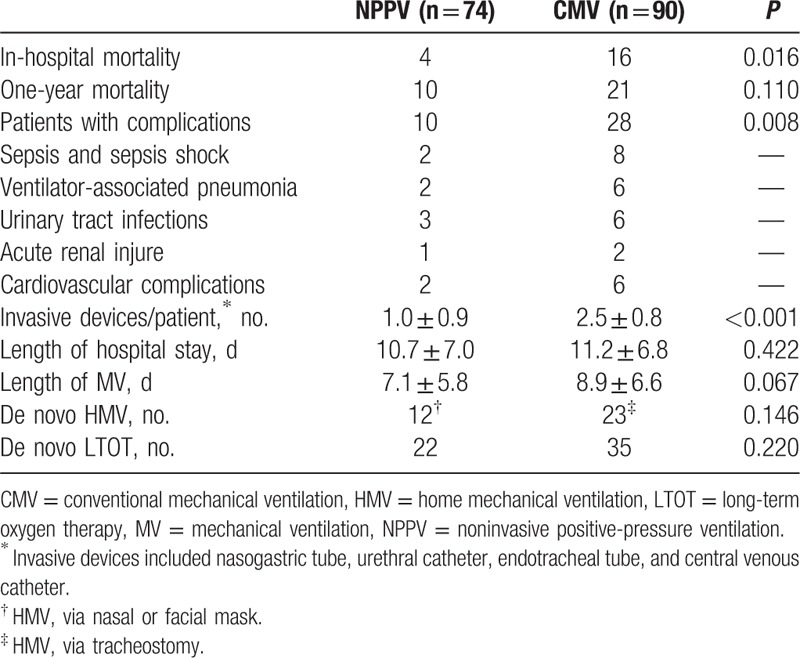
NPPV failed in 12 of 74 (16%) participants after a total of 13.5 ± 5.9 days of ventilation due to worsening of ABGs (n = 5), retention of secretions (n = 3), mask intolerance (n = 2), or worsening level of consciousness (n = 2). Nasogastric tubes were used in 33 participants for gastric distension that proved to be fully reversible. Mild facial skin erythema occurred in 22 participants. Four participants were not intubated after NPPV failure because ETI was refused, and these participants died from septic shock (n = 2) and cardiac arrest (n = 2). The causes of in-hospital death in the CMV group were septic shock (n = 8), cardiovascular complications (n = 6), and acute kidney injury (n = 2). Three subjects unweaned from CMV received tracheostomy and HMV treatment.
There were no differences between the 2 groups in the overall duration of mechanical ventilation and the length of hospital stay (Table 2). However, Kaplan–Meier analysis showed that the percentage of subjects not weaned from mechanical ventilation within 30 days was significantly lower in the NPPV group than in the CMV group (log rank 9.635, P = 0.002; Fig. 8).
Figure 8.
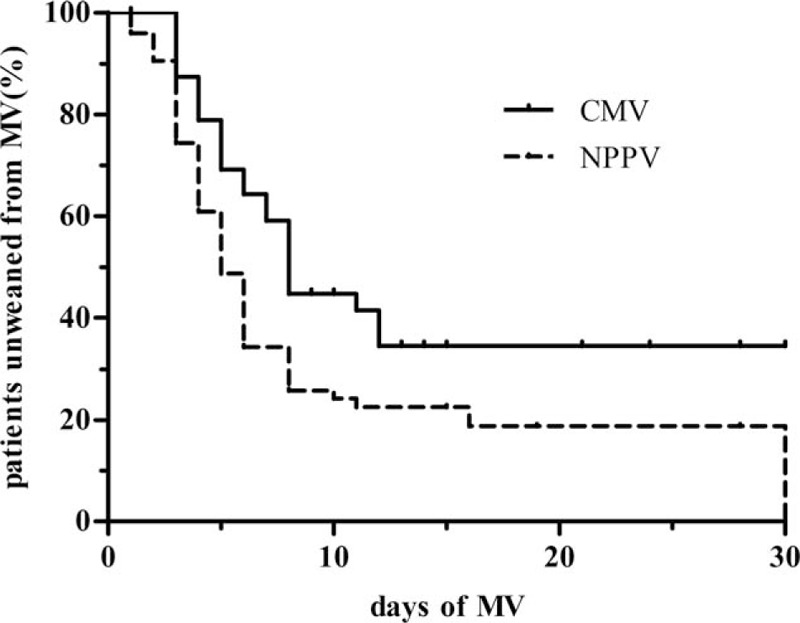
Kaplan–Meier curves showing that the percentage of participants unweaned from mechanical ventilation (MV) at 30 days was significantly lower in noninvasive positive-pressure ventilation (NPPV) group than in the conventional mechanical ventilation (CMV) group (log rank 9.635, P = 0.002). Participants who died or refused further treatment during MV were considered unweaned from the ventilator.
4. Discussion
The main finding of the present study was that noninvasive techniques to clear respiratory secretions during the first 2 hours of NPPV were safe and effective in patients with AECOPD and HE. Indeed, 2 hours of NPPV with clearance of secretions significantly improved KMS score and ABGs with no complications. Although the improvements in pH, PaO2/FiO2, and PaCO2, and the duration of hospitalization, were similar in the NPPV and CMV groups, the period required to wean from mechanical ventilation was significantly shorter in the NPPV group. Moreover, the NPPV group had a lower rate of nosocomial infection complications and lower hospital mortality than the CMV group. We conclude that NPPV in combination with a noninvasive strategy to clear respiratory secretions during the first 2 hours may be superior to CMV in the treatment of patients with AECOPD complicated by HE.
Although the potential advantages of NPPV over CMV are well documented,[8–12] noninvasive ventilation has generally not been recommended for patients with altered consciousness syndrome due to poor patient compliance, the accumulation of secretions secondary to a depressed cough reflex, and the risk of aspiration pneumonia if the airways are not protected.[30] Indeed, early failure of NPPV is most often attributed to a weak cough reflex, excessive secretions, HE, intolerance, agitation, and patient–ventilator asynchrony.[31] For these reasons, only a small number of clinical studies have assessed the use of NPPV in patients with AECOPD and HE. Duenas-Pareja et al reported that 78% of patients treated with BiPAP–NPPV showed a reversal of coma and an improvement in pH within 48 hours, and that 69% survived.[9] Zhu et al found that NPPV was successful in 72% of their patients with AECOPD and HE, with a survival rate of 86%; the main cause of failure was excessive airway secretions.[32] Briones Claudett et al have also observed that BiPAP–NPPV has utility in patients with AECOPD and HE.[33] Very few studies have directly compared NPPV with CMV in patients with AECOPD and HE. Scala et al determined that NPPV (without specific interventions to clear respiratory secretions) was associated with a lower rate of complications (mainly due to fewer cases of nosocomial infection or sepsis) and a shorter ventilation time than CMV, although the 2 approaches did not differ in terms of ABGs, in-hospital mortality, 1-year mortality, and tracheostomy rates.[10] Moreover, a recent meta-analysis found that, compared with CMV, noninvasive ventilation significantly reduced the mortality rate, intubation rate, rate of ventilation-related complications, and duration of ventilation.[34] Although these data already support the use of NPPV in patients with HE due to AECOPD, the ability to clear respiratory secretions during NPPV in a noninvasive manner would likely make this an even more attractive option in this clinical setting.
As described earlier, a weak cough reflex leading to inefficient clearance of excessive airway secretions is a common cause of immediate NPPV failure,[35,36] and is considered a relative contraindication for NPPV, especially in patients with impaired consciousness and depressed cough.[37,38] NPPV does not allow direct access to the airways, which is a disadvantage in terms of the removal of secretions, although some investigations have indicated that specific “manual” or “mechanical” physiotherapeutic techniques may improve mucociliary clearance during NPPV and that NPPV can still be used in these circumstances.[13,14] Interestingly, a study by Scala et al reported the successful use of FBO to aspirate airway secretions during NPPV in patients with AECOPD and HE.[12] In their study, 2 hours of NPPV with FBO significantly improved ABGs, KMS score, and cough efficiency score without FBO-related complications, avoiding the need for intubation in 80% of patients. Furthermore, NPPV with FBO was associated with lower rates of overall and septic complications than CMV, and there were no differences between groups in ABG improvement, hospital mortality, length of hospital stay, and duration of ventilation. Nonetheless, FBO is an invasive technique, and the authors acknowledged that the combination of NPPV with FBO would be limited to units with expertise in these techniques. This highlights the need for a noninvasive method of clearing respiratory secretions during NPPV.
A novel aspect of the present study is that it used an OPA to facilitate the clearance of secretions during NPPV. The OPA helped in the establishment of a patent airway by preventing the tongue from covering the epiglottis, and facilitated manual ventilation using a face mask. In our study, the early use of an OPA permitted the effective clearance of mucus and induced a cough reflex. Therefore, this may represent a useful clearance technique in subjects with copious secretions and a depressed cough reflex.[19,39] We used a combination of techniques to maximize the clearance of secretions during NPPV. First, the patient was maintained in an appropriate body position to allow the removal of secretions and avoid aspiration. Studies using radioactive-labeled enteral feeding have shown that cumulative endotracheal counts were higher for participants in the completely supine position (0°C) than in those in the semirecumbent position (45°C), suggesting that aspiration might be decreased by semirecumbent positioning.[40] Second, we administered nebulized salbutamol and ambroxol. Patients with acute or acute-on-chronic respiratory failure who receive NPPV often require inhaled bronchodilators for relief of airway obstruction. Several investigations have employed in vitro models to determine the optimal techniques for aerosol delivery in subjects receiving NPPV, and have observed significant bronchodilator responses after albuterol administration with a jet nebulizer or pressurized metered-dose inhaler in stable subjects during NPPV with a mask.[41–44] Furthermore, aerosol delivery was highest when the nebulizer was close to the subject (between the leak port and the subject connection).[42,45] In our clinical experience, patients receiving inhaled therapy during NPPV show a greater improvement in oxygen saturation and breathlessness than those receiving nebulized drug alone.
There are several aspects of our strategy that need to be considered when our results are applied to clinical practice. First, unlike FBO, the use of an OPA to facilitate the clearance of secretions is a noninvasive, simple technique that would be straightforward to implement in other hospitals. Second, it was notable that our study showed a higher success rate than other recent reports.[10,12] This may have been because our participants were patients with severe AECOPD in an ICU, where they are likely to receive more intensive care and closer observation, and where the means to promptly intubate the patient if necessary are readily at hand. Third, it should be noted that 3 participants could not cooperate with NPPV because of agitation, and required restraining until they became more alert during NPPV. Fourth, only 2 cases of NPPV failure were induced by mask intolerance, a rate lower than that of a previous study.[10] This good level of compliance in patients with HE may have been because the masks were manufactured in China according to the facial features of Chinese people.
The present study has several limitations. First, this was not a randomized controlled trial, which may have biased the results in favor of the therapy under investigation.[10] However, the cases (NPPV) and controls (CMV) were prospectively enrolled during the same period, using the same inclusion and exclusion criteria. Despite this limitation, it is accepted that well-designed observational studies can yield reliable results provided that the cases and controls are well balanced by careful matching, and the interference of confounding factors is minimized.[46] In this study, the cases were similar to the controls not only for the matching criteria but also for other historical/clinical/physiological features. Second, this was a single-center study, and hence it is not known whether our findings can be generalized. Third, the hospital setting of the CMV (RICU) and NPPV (ICU) groups differed, which potentially may have introduced bias into the study. Fourth, it would have been instructive to include an additional comparator group consisting of participants treated with NPPV alone (i.e., not in combination with OPA-facilitated clearance of secretions); this would have allowed the specific advantages of OPA use and mucus clearance to be determined.
In conclusion, the use of an OPA and suction aspiration, in combination with appropriate positioning of the patient and nebulized inhalation of salbutamol/ambroxol, was a feasible, simple, safe, and effective method for clearing respiratory secretions during the first 2 hours of NPPV in patients in ICU with AECOPD and HE. Moreover, compared with CMV, this innovative strategy reduced the risks of nosocomial infection, requirement for intubation, and hospital mortality, and showed superior results in terms of weaning from ventilation. Therefore, we propose that this novel NPPV strategy might be a successful alternative to CMV in selected patients with AECOPD in the ICU, where there is prompt access to ETI if needed. Large-scale, randomized controlled trials comparing our NPPV strategy to CMV in patients with AECOPD and HE are merited to confirm our results.
Acknowledgments
We would like to thank all the data collectors of Harrison International Peace Hospital. In addition, we thank Prof. Xiao-xue Ke from Southwest University for offering feedback and suggestions on this manuscript.
Footnotes
Abbreviations: ABG = arterial blood gas, AECOPD = acute exacerbation of chronic obstructive pulmonary disease, BiPAP = bilevel positive airway pressure, CMV = conventional mechanical ventilation, COPD = chronic obstructive pulmonary disease, ETI = endotracheal intubation, FBO = fiberoptic bronchoscopy, HE = hypercapnic encephalopathy, HMV = home mechanical ventilation, ICU = intensive care unit, KMS = Kelly–Matthay scale, NPPV = noninvasive positive-pressure ventilation, OPA = oropharyngeal airway, PEEP = positive end-expiratory pressure, PS = pressure support, RICU = respiratory ICU, RR = respiratory rate.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Fang X, Wang X, Bai C. COPD in China: the burden and importance of proper management. Chest 2011;139:920–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Murray CJ, Atkinson C, Bhalla K, et al. The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA 2013;310:591–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tobias JD. Conventional mechanical ventilation. Saudi J Anaesth 2010;4:86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Heyland DK, Cook DJ, Griffith L, et al. The attributable morbidity and mortality of ventilator-associated pneumonia in the critically ill patient. The Canadian Critical Trials Group. Am J Respir Crit Care Med 1999;159:1249–56. [DOI] [PubMed] [Google Scholar]
- [5].Esteban A, Anzueto A, Frutos F, et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA 2002;287:345–55. [DOI] [PubMed] [Google Scholar]
- [6].Stauffer JL, Olson DE, Petty TL. Complications and consequences of endotracheal intubation and tracheotomy. A prospective study of 150 critically ill adult patients. Am J Med 1981;70:65–76. [DOI] [PubMed] [Google Scholar]
- [7].Pierson DJ. History and epidemiology of noninvasive ventilation in the acute-care setting. Respir Care 2009;54:40–52. [PubMed] [Google Scholar]
- [8].Ambrosino N, Vagheggini G. Noninvasive positive pressure ventilation in the acute care setting: where are we? Eur Respir J 2008;31:874–86. [DOI] [PubMed] [Google Scholar]
- [9].Duenas-Pareja Y, Lopez-Martin S, Garcia-Garcia J, et al. Non-invasive ventilation in patients with severe hypercapnic encephalopathy in a conventional hospital ward. Arch Bronconeumol 2002;38:372–5. [DOI] [PubMed] [Google Scholar]
- [10].Scala R, Nava S, Conti G, et al. Noninvasive versus conventional ventilation to treat hypercapnic encephalopathy in chronic obstructive pulmonary disease. Intensive Care Med 2007;33:2101–8. [DOI] [PubMed] [Google Scholar]
- [11].Nava S, Hill N. Non-invasive ventilation in acute respiratory failure. Lancet 2009;374:250–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Scala R, Naldi M, Maccari U. Early fiberoptic bronchoscopy during non-invasive ventilation in patients with decompensated chronic obstructive pulmonary disease due to community-acquired-pneumonia. Crit Care 2010;14:R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bellone A, Spagnolatti L, Massobrio M, et al. Short-term effects of expiration under positive pressure in patients with acute exacerbation of chronic obstructive pulmonary disease and mild acidosis requiring non-invasive positive pressure ventilation. Intensive Care Med 2002;28:581–5. [DOI] [PubMed] [Google Scholar]
- [14].Vargas F, Bui HN, Boyer A, et al. Intrapulmonary percussive ventilation in acute exacerbations of COPD patients with mild respiratory acidosis: a randomized controlled trial [ISRCTN17802078]. Crit Care 2005;9:R382–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Antonaglia V, Lucangelo U, Zin WA, et al. Intrapulmonary percussive ventilation improves the outcome of patients with acute exacerbation of chronic obstructive pulmonary disease using a helmet. Crit Care Med 2006;34:2940–5. [DOI] [PubMed] [Google Scholar]
- [16].Scala R. Hypercapnic encephalopathy syndrome: a new frontier for non-invasive ventilation? Respir Med 2011;105:1109–17. [DOI] [PubMed] [Google Scholar]
- [17].Boidin MP. Airway patency in the unconscious patient. Br J Anaesth 1985;57:306–10. [DOI] [PubMed] [Google Scholar]
- [18].Roberts K, Allison KP, Porter KM. A review of emergency equipment carried and procedures performed by UK front line paramedics. Resuscitation 2003;58:153–8. [DOI] [PubMed] [Google Scholar]
- [19].Koga K, Sata T, Kaku M, et al. Comparison of no airway device, the Guedel-type airway and the cuffed oropharyngeal airway with mask ventilation during manual in-line stabilization. J Clin Anesth 2001;13:6–10. [DOI] [PubMed] [Google Scholar]
- [20].Pauwels RA, Buist AS, Calverley PM, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2014;163:10–8. [DOI] [PubMed] [Google Scholar]
- [21].Hall GJ, Gandevia B. Relationship of the loose cough sign to daily sputum volume. Observer variation in its detection. Br J Prev Soc Med 1971;25:109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kelly BJ, Matthay MA. Prevalence and severity of neurologic dysfunction in critically ill patients. Influence on need for continued mechanical ventilation. Chest 1993;104:1818–24. [DOI] [PubMed] [Google Scholar]
- [23].Adult Chronic Airway Disease Atomization Inhalation Treatment Expert Group. Expert recommendations of aerosol inhalation treatment for chronic airway disease in adults. Chin J Respir Crit Care Med. 2012;11:105–110. [Google Scholar]
- [24].Scala R, Naldi M, Archinucci I, et al. Noninvasive positive pressure ventilation in patients with acute exacerbations of COPD and varying levels of consciousness. Chest 2005;128:1657–66. [DOI] [PubMed] [Google Scholar]
- [25].Brochard L, Mancebo J, Wysocki M, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med 1995;333:817–22. [DOI] [PubMed] [Google Scholar]
- [26].Chen W, Qing-yuan Z. Guideline for mechanical ventilation in patients with acute exacerbation of chronic obstructive pulmonary disease (2007). Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2007;19:513–8. [PubMed] [Google Scholar]
- [27].Conti G, Antonelli M, Navalesi P, et al. Noninvasive vs. conventional mechanical ventilation in patients with chronic obstructive pulmonary disease after failure of medical treatment in the ward: a randomized trial. Intensive Care Med 2002;28:1701–7. [DOI] [PubMed] [Google Scholar]
- [28].Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013;39:165–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. [DOI] [PubMed] [Google Scholar]
- [30].Scala R. Non-invasive ventilation in acute respiratory failure with altered consciousness syndrome: a bargain or an hazard? Minerva Anestesiol 2013;79:1291–9. [PubMed] [Google Scholar]
- [31].Ozyilmaz E, Ugurlu AO, Nava S. Timing of noninvasive ventilation failure: causes, risk factors, and potential remedies. BMC Pulm Med 2014;14:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhu GF, Zhang W, Zong H, et al. Effectiveness and safety of noninvasive positive-pressure ventilation for severe hypercapnic encephalopathy due to acute exacerbation of chronic obstructive pulmonary disease: a prospective case–control study. Chin Med J (Engl) 2007;120:2204–9. [PubMed] [Google Scholar]
- [33].Briones Claudett KH, Briones Claudett M, Chung Sang Wong M, et al. Noninvasive mechanical ventilation with average volume assured pressure support (AVAPS) in patients with chronic obstructive pulmonary disease and hypercapnic encephalopathy. BMC Pulm Med 2013;13:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Liu Q, Chen R, Jia L, et al. Effect of noninvasive ventilation on hypercapnic encephalopathy syndrome: a meta-analysis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2016;28:57–62. [DOI] [PubMed] [Google Scholar]
- [35].Soo Hoo GW, Santiago S, Williams AJ. Nasal mechanical ventilation for hypercapnic respiratory failure in chronic obstructive pulmonary disease: determinants of success and failure. Crit Care Med 1994;22:1253–61. [DOI] [PubMed] [Google Scholar]
- [36].Carlucci A, Richard JC, Wysocki M, et al. Noninvasive versus conventional mechanical ventilation. An epidemiologic survey. Am J Respir Crit Care Med 2001;163:874–80. [DOI] [PubMed] [Google Scholar]
- [37].Organized jointly by the American Thoracic Society, the European Respiratory Society, the European Society of Intensive Care Medicine, the Société de Réanimation de Langue Française, approved by ATS Board of Directors, December 2000. International Consensus Conferences in Intensive Care Medicine: noninvasive positive pressure ventilation in acute Respiratory failure. Am J Respir Crit Care Med. 2001;163:283–291. [DOI] [PubMed] [Google Scholar]
- [38].British Thoracic Society Standards of Care Committee. Non-invasive ventilation in acute respiratory failure. Thorax. 2002;57:192–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Baskett TF. Arthur Guedel and the oropharyngeal airway. Resuscitation 2004;63:3–5. [DOI] [PubMed] [Google Scholar]
- [40].Orozco-Levi M, Torres A, Ferrer M, et al. Semirecumbent position protects from pulmonary aspiration but not completely from gastroesophageal reflux in mechanically ventilated patients. Am J Respir Crit Care Med 1995;152:1387–90. [DOI] [PubMed] [Google Scholar]
- [41].Parkes SN, Bersten AD. Aerosol kinetics and bronchodilator efficacy during continuous positive airway pressure delivered by face mask. Thorax 1997;52:171–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chatmongkolchart S, Schettino GP, Dillman C, et al. In vitro evaluation of aerosol bronchodilator delivery during noninvasive positive pressure ventilation: effect of ventilator settings and nebulizer position. Crit Care Med 2002;30:2515–9. [DOI] [PubMed] [Google Scholar]
- [43].Branconnier MP, Hess DR. Albuterol delivery during noninvasive ventilation. Respir Care 2005;50:1649–53. [PubMed] [Google Scholar]
- [44].Nava S, Karakurt S, Rampulla C, et al. Salbutamol delivery during non-invasive mechanical ventilation in patients with chronic obstructive pulmonary disease: a randomized, controlled study. Intensive Care Med 2001;27:1627–35. [DOI] [PubMed] [Google Scholar]
- [45].Dai B, Kang J, Sun LF, et al. Influence of exhalation valve and nebulizer position on albuterol delivery during noninvasive positive pressure ventilation. J Aerosol Med Pulm Drug Deliv 2014;27:125–32. [DOI] [PubMed] [Google Scholar]
- [46].Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med 2000;342:1887–92. [DOI] [PMC free article] [PubMed] [Google Scholar]


