Abstract
Background:
Studies examining the efficiency of drug-coated balloon (DCB) + bare metal stent (BMS) compared with stents alone for de novo lesions have reported inconsistent results. The present comprehensive meta-analysis of randomized controlled trials (RCTs) assessed and compared the clinical efficacy and safety of DCB + BMS with those of stents alone for de novo coronary artery disease.
Methods:
We formally searched electronic databases before September 2016 to identify potential studies. All RCTs were eligible for inclusion if they compared DCB + BMS with a control treatment (drug-eluting stent [DES] alone or BMS alone) in patients with de novo coronary artery disease.
Results:
Eleven RCTs with a total of 2196 patients met the inclusion criteria were included in our meta-analysis. Subgroup analysis indicated DCB plus BMS was associated with poorer outcomes when compared with DES alone in primary endpoint {(in-segment late lumen loss [LLL]: mean difference [MD], 0.19; 95% confidence interval [CI], 0.06–0.32; P = 0.0042) and (major adverse cardiovascular events [MACEs]: risk ratio [RR], 1.88; 95% CI, 1.44–2.45; P < 0.0001)}. However, DCB + BMS had nonsignificantly lower LLL than BMS alone (in-segment LLL: MD, −0.14; 95% CI, −0.33–0.04; P = 0.24), and was more advantageous in reducing MACE incidence, with borderline significance (MACEs: RR, 0.67; 95% CI, 0.45–0.99; P = 0.05).
Conclusions:
In summary, the present results do not favor the DCB + BMS strategy as an alternative therapeutic method to DES implantation for de novo coronary artery lesions in percutaneous coronary intervention (PCI). Additional well-designed large RCTs with long-follow-up periods are required to clarify the inconsistent results.
Keywords: bare metal stent, de novo coronary artery disease, drug-coated balloon, drug-eluting stent
1. Introduction
Recent evidences support using paclitaxel drug-coated balloon (DCB) catheters as a therapeutic method for de novo coronary lesions,[1,2] in-stent restenosis (ISR),[2,3] small coronary vessels,[4,5] and coronary bifurcation lesions.[6,7] DCB was designed to achieve comparable efficacy in neointimal proliferation through local drug delivery without requiring foreign body implantation or prolonged dual antiplatelet therapy (DAPT). The advantages of DCB include homogeneous and high concentration's drug delivery to the entire vessel wall, absence of stent layer, and absence of the polymer that could lead to chronic inflammation. DCB is a promising device to overcome some limitations of DES in percutaneous coronary intervention (PCI), such as ISR,[8] late and very late stent thrombosis,[9] and risk of bleeding caused by prolonged DAPT.[10] Although DCB has shown remarkable angiographic and clinical effects in coronary artery interventional therapy, it has some limitations in the treatment of de novo coronary lesions. Elastic recoil and flow-limiting dissections may be the main reasons for therapy failure.[11] As the lack of mechanical scaffolding provided by stent struts, the use of DCB may not be ideal for complex coronary lesions. Therefore, a strategy combining DCB and bare metal stent (BMS) is a potential solution to overcome these limitations. The more rapid endothelialization and shorter DAPT duration of BMS than DES should be beneficial in certain scenarios. However, studies examining the efficiency of DCB + BMS compared with stents alone for de novo lesions have yielded inconsistent results,[11,12] and whether this strategy provides additional benefits remains unclear. Hence, we conducted a comprehensive meta-analysis of randomized controlled trials (RCTs) to assess and compare the clinical efficacy and safety of DCB + BMS with those of stents alone for de novo coronary lesions.
2. Methods
2.1. Search strategy
We comprehensively searched related papers in electronic databases (PubMed, Web of Science, and the Cochrane Central Register of Controlled Trials) before September 2016 to identify potential RCTs. The keywords were “paclitaxel-coated balloon,” “paclitaxel-eluting balloon,” “drug-eluting balloon,” and “drug-coated balloon.” Moreover, we evaluated relevant publications, including review articles and editorials.
Ethical approval was not required due to that this is a systematic review and meta-analysis. All included studies were approved by the notified ethics committees and institutional review boards. And this study was performed in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.
2.2. Study selection and data extraction
Studies met the following inclusion criteria were included in the meta-analysis: RCTs of de novo coronary artery lesions intervention, DCB + BMS as a treatment arm, and eligible angiographic and clinical outcome data obtained during follow-up. The exclusion criteria were incomplete data and cases number less than 50. No restrictions were applied regarding the language of publication. Data abstraction was performed independently by 2 investigators (Lu and Zhu), and discrepancies were resolved by consensus. The following features of each eligible study were extracted using a standardized form: study and patient characteristics, intervention procedures, and angiographic and clinical outcomes.
2.3. Quality assessment
The Cochrane Collaboration tool[13] was used to methodologically assess the risk of bias to evaluate the quality of included trials. The following methodological domains were considered: random sequence generation, allocation concealment, blinding, drop-out rates (incomplete outcome data), addressing incomplete outcome data, selective reporting, and other potential sources of bias. After assessment, the included study were labeled as “low risk (L),” “high risk (H),” or “unclear risk (U).”
2.4. Endpoints and statistical analysis
The primary endpoints were in-segment late lumen loss (LLL) and major adverse cardiac events (MACEs). The secondary endpoints were in-segment binary restenosis (BR), in-segment minimum lumen diameter (MLD), and target lesion revascularization (TLR), myocardial infarction (MI), and death. MACEs were defined as a composite of death, MI, and TLR. The most similar endpoint was used if data for mentioned endpoint were unavailable. We conducted the meta-analysis by using the Cochrane Program Review Manager (v.5.0; Oxford, England) and STATA software (version 12.0; StatCorp, College Station, TX). According to the inverse variance fixed-effect model, categorical variables were calculated as the pooled risk ratio (RR) and 95% confidence intervals (CIs). Continuous variables were presented as estimated mean difference (MD) with a 95% CI. The I2 index was used to assess heterogeneity among studies. If I2 > 50% (substantial and important heterogeneity), a random effect model was used for quantitative data synthesis, whereas a fixed model was adopted. Begger Funnel plots and Egger tests were used to assess publication bias, with P < 0.05 as the threshold for statistical significance.[14,15]
3. Results
3.1. Characteristics of included studies
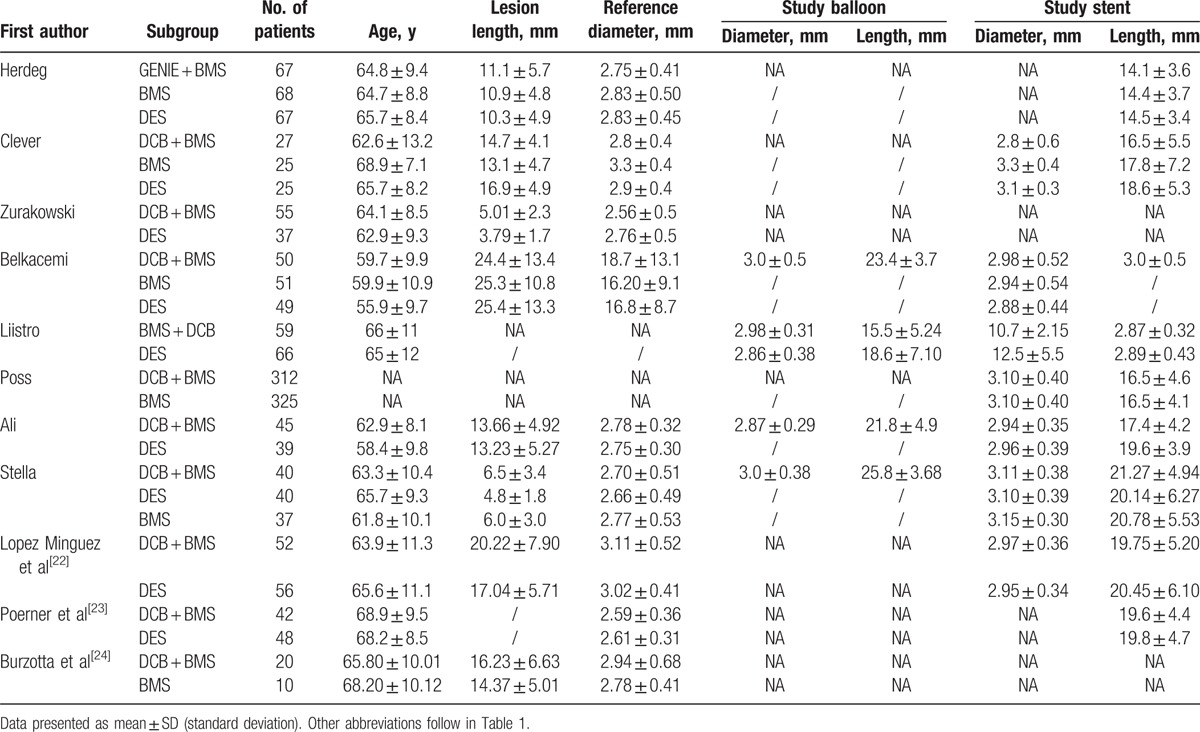
We initially screened a total of 7668 potential studies through a number of searches. After eliminating duplicates, 505 articles were examined. Of these, 11 RCTs[11,12,16–24] with a total of 2196 patients met the inclusion criteria were included in our meta-analysis. Figure 1 presents a flowchart of the overall search strategy. Among these 11 studies, 7 were multicenter studies and 4 were single-center studies. Four studies were 3-arm trials comparing the subgroups DCB + BMS, BMS alone, and DES alone; therefore, these studies were considered as 2 separate trials. We finally selected 9 studies comparing DCB + BMS with DES alone and 6 comparing DCB + BMS with BMS alone. The clinical and angiographic primary endpoints were provided in all trials, with follow-up durations of 6 to 24 months. Furthermore, DCB + BMS was used in 714 patients, whereas control treatments, namely BMS alone and DES alone, were used in 190 and 715 patients, respectively. The key demographic and angiographic characteristics of included the studies are summarized in Tables 1 and 2, respectively.
Figure 1.
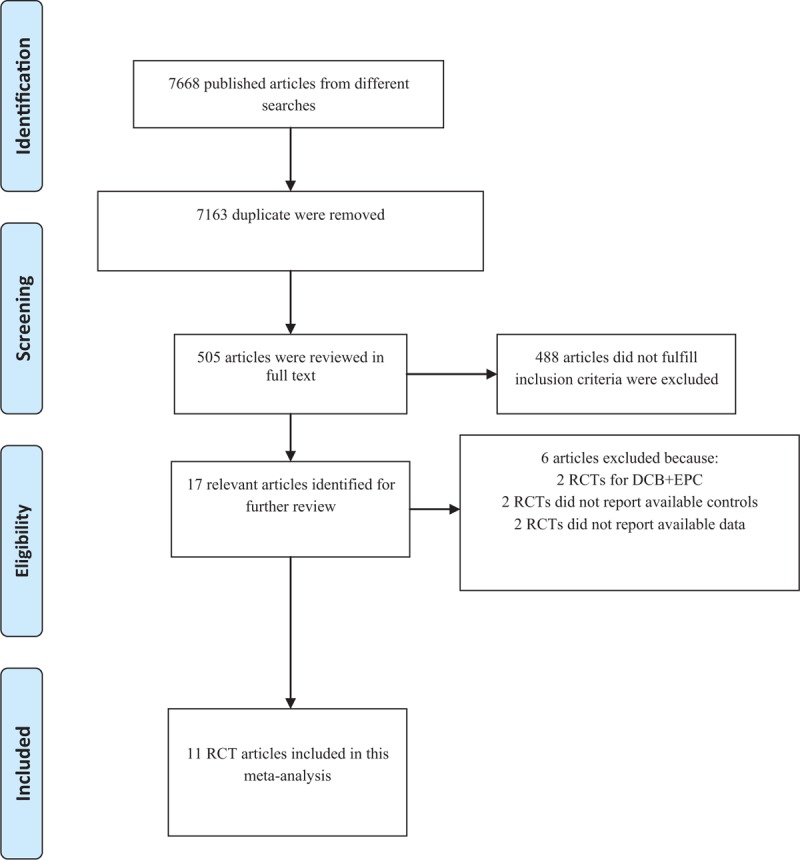
Flow diagram for identification processes.
Table 1.
General characteristics of studies included in this meta-analysis.
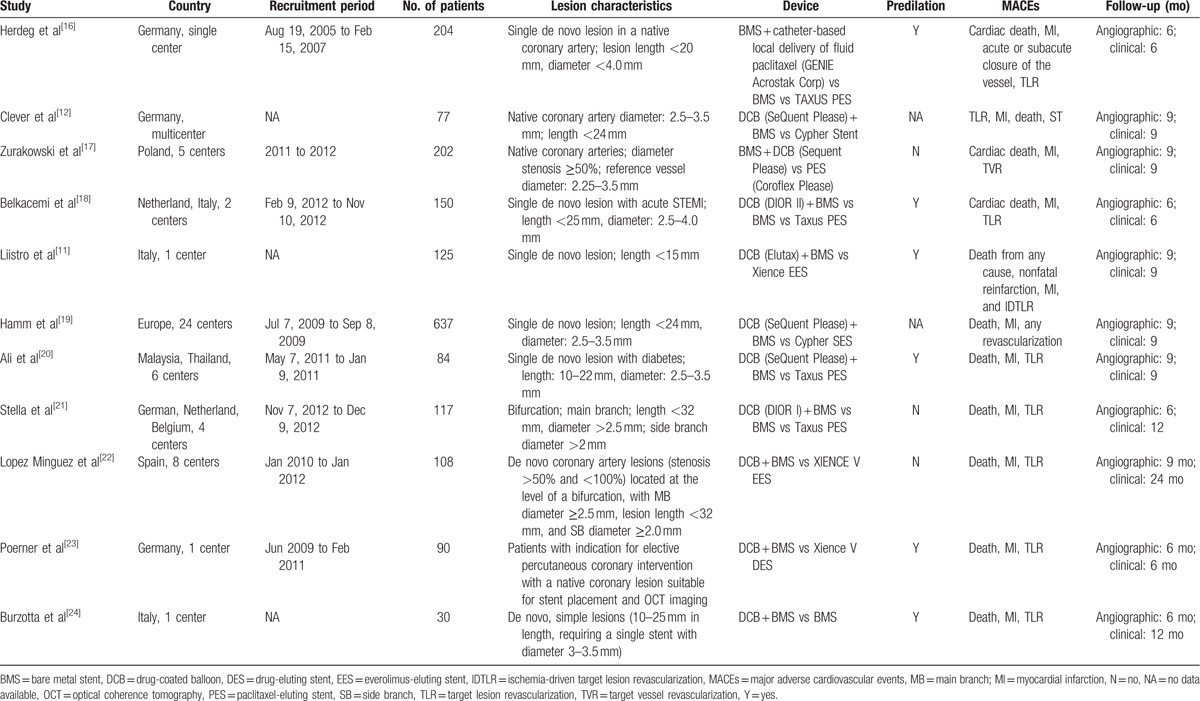
Table 2.
Lesions and devices characteristics of included studies.
3.2. Primary endpoint
LLL: This was reported in 9 of the 11 studies within follow-up periods of 6 to 9 months. The random effect model was used to quantitative analysis. Nine studies were included in the DCB + BMS versus DES subgroup analysis, whereas 5 studies were included in the DCB + BMS versus BMS subgroup analysis. Compared with the DES alone subgroup, the DCB + BMS subgroup exhibited a significant increase in LLL (MD, 0.19; 95% CI, 0.06–0.32; P = 0.0042). However, the DCB + BMS subgroup showed nonsignificantly lower LLL than did the BMS alone subgroup (MD, −0.14; 95% CI, −0.33–0.04; P = 0.24; Fig. 2 A).
Figure 2.
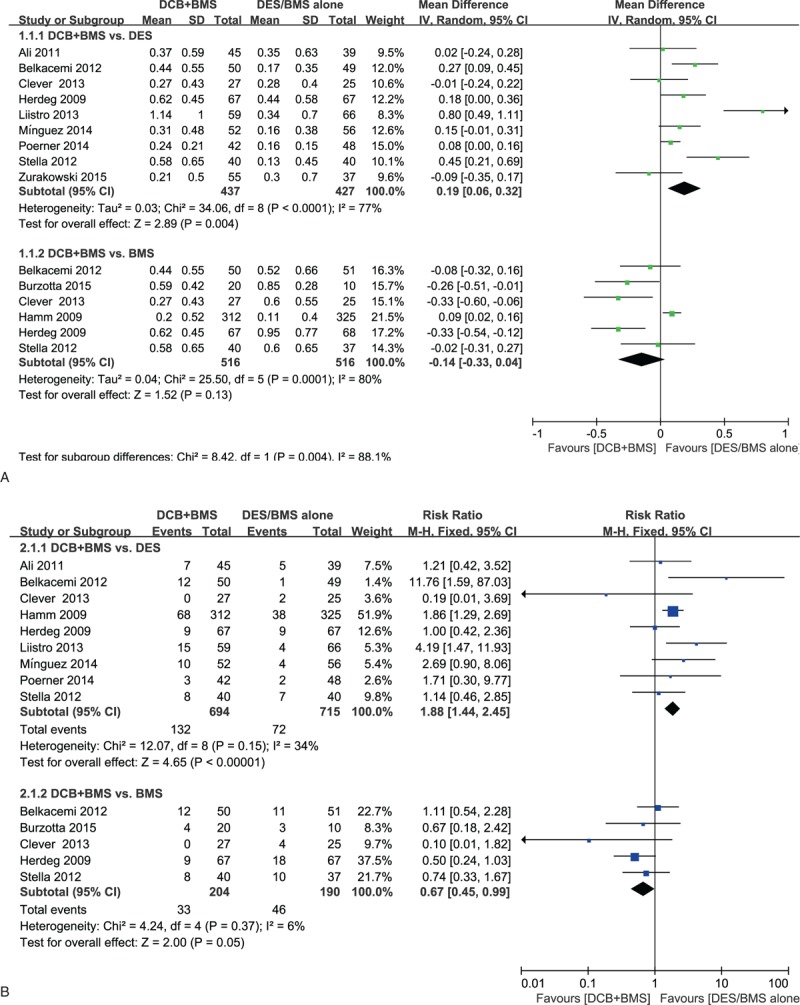
Effectiveness of “DCB + BMS strategy” versus “DES alone” or “BMS alone” for treating de novo lesions. (A) Primary angiographic endpoint: in-segment late lumen loss. (B) Primary clinical endpoint: major adverse cardiovascular events (MACEs).
MACEs: These were observed in 10 of the 11 studies within a follow-up period of 6 to 24 months. The fixed effect model was used. Subgroup analysis indicated that compared with DES alone, DCB + BMS significantly increased MACEs (RR, 1.88; 95% CI, 1.44–2.45; P < 0.0001). The subgroup analysis showed that the DCB + BMS strategy was advantageous over the BMS treatment in reducing MACEs incidence, with borderline significant (RR, 0.67; 95% CI, 0.45–0.99; P = 0.05; Fig. 2B).
3.3. Secondary endpoint
In-segment BR rate. Seven and 3 studies with follow-up periods of 6 to 9 months were included in the DCB + BMS versus DES alone and DCB + BMS versus BMS alone subgroup analyses, respectively. We adopted the random effect model for analysis. Subgroup analysis showed the DCB + BMS strategy was inferior to DES alone strategy in reducing BR incidence (RR, 2.15; 95% CI, 1.07–4.31, P = 0.03). The DCB + BMS versus BMS subgroup analysis showed that DCB + BMS was beneficial, but the difference between both strategies was nonsignificant (RR, 0.74; 95% CI, 0.34–1.60, P = 0.44, respectively; Fig. 3 A).
Figure 3.
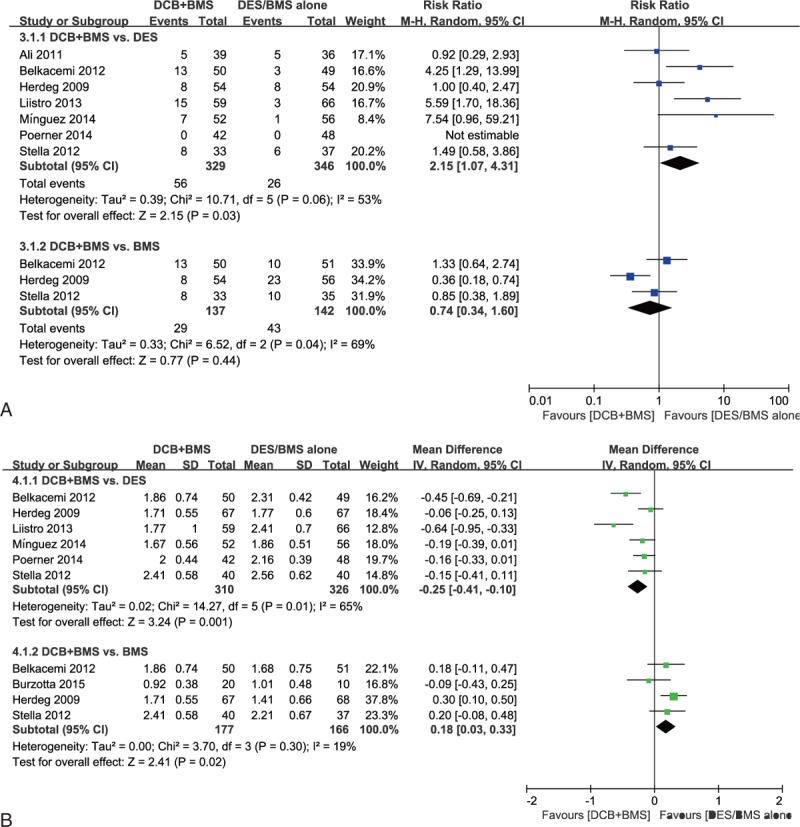
Effectiveness of “DCB + BMS strategy” versus “DES alone” or “BMS alone” for treating de novo lesions. Secondary angiographic endpoints: (A) in-segment binary restenosis rate; and (B) in-segment minimum lumen diameter.
In-segment MLD. Six and 3 studies with follow-up periods of 6 to 9 months were included in the DCB + BMS versus DES alone and DCB + BMS versus BMS alone subgroup analyses, respectively. Compared with DES alone, DCB + BMS had a significant lower MLD (MD, −0.25; 95% CI, −0.41 to −0.10; P = 0.001). A significant effect favoring DCB + BMS was detected in the DCB + BMS versus BMS alone subgroup analysis (MD, 0.18; 95% CI, 0.03–0.33; P = 0.02; Fig. 3B).
TLR, MI, and Death. All 3 endpoints were reported in 9 of the 11 studies within follow-up periods of 6 to 24 months. Because of the low degree of heterogeneity, we used the fixed effect model for the quantitative analysis. TLR: The analysis indicated a significantly higher risk of TLR in the DCB + BMS subgroup than in the DES alone subgroup (RR, 1.94; 95% CI, 1.27–2.98; P = 0.002), and the incidence rate of TLR did not differ significantly between the DCB + BMS subgroup and BMS alone subgroup (RR, 0.71; 95% CI, 0.47–1.09; P = 0.012; Fig. 4 A). MI: The analysis showed no significant difference in MI incidence between the DCB + BMS and DES alone subgroups (RR, 0.88; 95% CI, 0.32–2.42; P = 0.81). Similarly, the incidence rate of MI was comparable following DCB + BMS and BMS alone implantation (RR, 0.51; 95% CI, 0.16–1.67; P = 0.27; Fig. 4B). Death: The analysis revealed that death did not differ significantly in the DCB + BMS and DES subgroups (RR, 5.91; 95% CI, 0.72–48.39; P = 0.10); similar results were observed in the DCB + BMS versus BMS subgroup analysis (RR, 0.20; 95% CI, 0.02–1.70; P = 0.14).
Figure 4.
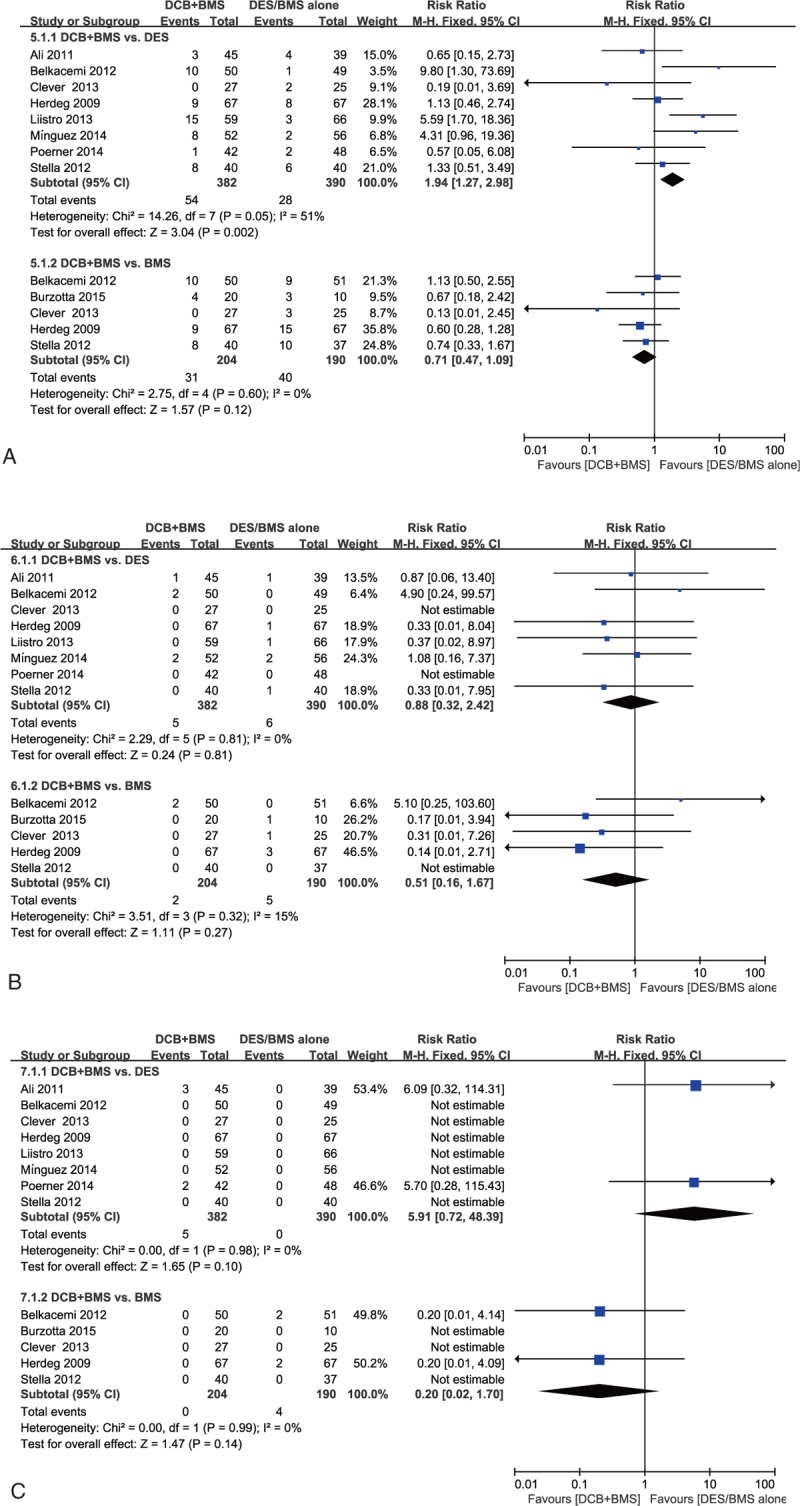
Effectiveness of “DCB + BMS strategy” versus “DES alone” or “BMS alone” for treating de novo lesions. Secondary clinical endpoints: (A) target lesion revascularization, (B) MI, and (C) death.
3.4. Sensitivity analysis
According to the results of heterogeneity analysis, we conducted sensitivity analysis between the DCB + BMS and control groups (DCB + BMS vs DES and DCB + BMS vs BMS subgroups) at all observed endpoints. We sequentially eliminated one study at a time and observed that no study strongly influenced the overall results.
3.5. Publication bias
Egger test showed no evidence of significant publication bias in this meta-analysis (P > 0.05). In addition, the funnel plot was symmetrical, suggesting no publication bias (Fig. 5).
Figure 5.
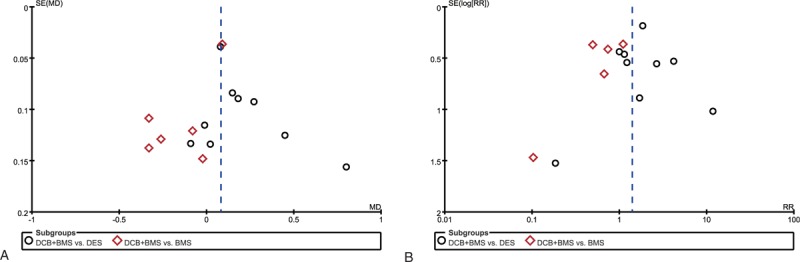
Funnel plot for publication bias. (A) Primary angiographic endpoint: in-segment late lumen loss. (B) Primary clinical endpoint: major adverse cardiovascular events (MACEs).
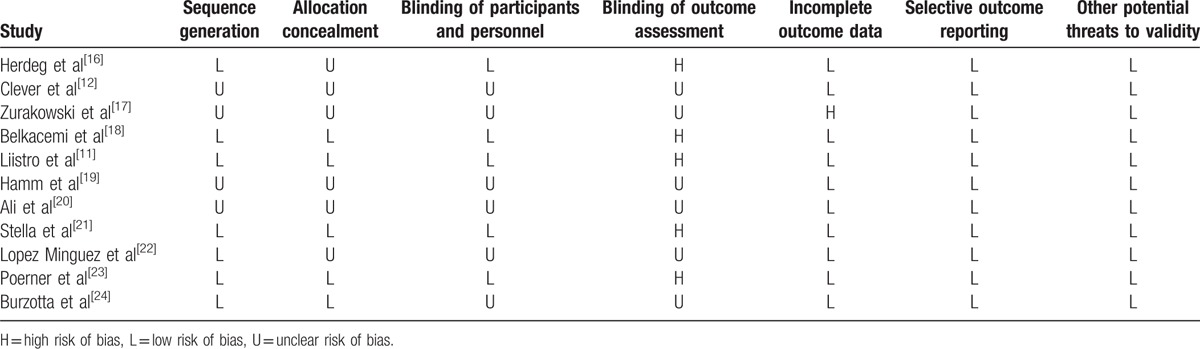
3.6. Risk of bias assessment
The assessment of the risk of bias is presented in Table 3. Seven and 5 of the included studies showed a low risk of bias in random sequence generation and allocation concealment, respectively. Five studies showed a low risk of bias in the blinding of participants, and 5 had a high risk of bias in the blinding of the outcome assessment. All studies have a low risk of bias regarding incomplete outcome data and selective outcome reporting.
Table 3.
Assessment of risk of bias in the included studies using Cochrane criteria.
4. Discussion
Our present meta-analysis included the largest number of RCTs to date showed that although the DCB + BMS strategy performed more favorably than did the BMS alone strategy, it was not superior to DES alone strategy in the treatment of de novo coronary lesions.
DES implantation is the first choice of treatment in PCI. Its dramatic ability to inhibit neointimal hyperplasia through sustained elution of cytostatic drugs turns into a significantly reduced repeat revascularization rate in clinical trials.[25,26] Nevertheless, cases of treatment failure, mainly because of ISR and stent thrombosis (ST),[27,28] have attracted more attention considering the sizeable number of patients with DES implantation. Various factors are required to satisfactorily resolve, such as slow drug release, polymer-induced inflammation, endothelial dysfunction, and coronary vasoconstriction disturbance.[29,30] Therefore, paclitaxel DCB may be an emerging therapeutic alternative that has the advantages of operative simplicity and homogeneous antiproliferative agent release along the entire device.[20] To avoid the disadvantages of DES, researchers have tried to combine DCB and BMS to achieve benefits by DCB provided local release antiproliferative agents and BMS prevented acute postangioplasty recoil.
Determining an optimal treatment for de novo lesions remains challenging. Although BELLO[4] study showed that, compared with PES in small vessels (reference diameter 2.8 mm), DCB yielded significantly lower in-stent (in-balloon) late loss and similar rates of restenosis and revascularization. However, there have been few well-designed “head to head” studies comparing the DCB and DES strategies for lesions with lumen diameters of more than 2.5 mm. All studies included in the present meta-analysis had applied the DCB + BMS therapeutic strategy for de novo coronary lesions (lumen diameter >2.5 mm). Nevertheless, the pooled results of our research showed that the clinical efficacy and safety of the DCB + BMS strategy were not equivalent to those of the DES alone strategy for de novo coronary lesions. Regarding the MACEs rate, replacing DES implantation with DCB + BMS was not beneficial in simple de novo coronary lesion intervention.
This finding may be explained by various factors. First, the lack of sufficient uncoated balloon predilation in some included study[17,21] may have contributed to the result. Predilation before DCB use could improve drug uptake by the vessel wall because of the creation of microdissections, thus facilitating drug transport through the intima and media, particularly for calcified lesions.[18] The Valentines II[1] trial adopted regular balloon predilatation of the target lesion followed DIOR II DCB reported low in-segment LLL and TLR rates. Meanwhile, 1 RCT, which adopted regular balloon predilatation, compared the efficacy of BMS and DCB combination versus BMS alone in patients with non-ST elevation acute coronary syndrome also reported significantly lower LLL but the absence of a favorable effect on patient clinical outcomes.[31] Second, we speculated “geographical miss” caused by unfavorable geometric proportions as a potential influencing factor because the reference point for stent or balloon placement was missing. One clinical trial reported that patients treated with DCB predilatation with an additional BMS implantation had a very high proportion of geographical miss, which was identified as an independent significant predictor of restenosis.[32] If stent deployment precedes DCB dilatation, the contact surface between the balloon and vessel wall is reduced by approximately 15% owing to the surface of the stent struts.[33]
Another possible reason is intimal hyperplasia. The OCTOPUS trial, which used optical coherence tomography, reported that DCB + BMS was associated with more pronounced neointimal proliferation than DES.[23,34] The IVUS study used intravascular ultrasound also showed more pronounced neointimal hyperplasia in the DCB + BMS group, leading to more revascularization than that in the DES group.[35] The reason for this finding is not yet satisfactorily explained. Possible influencing factors are the interaction of the mounted stent with drug release from DCB, stent and balloon lengths, drug concentrations, and stent system.
Our meta-analysis included 2 strategies for DCB application: pre- and post-BMS implantation. Theoretically, DCB used before BMS implantation could increase the risk of geographical mismatch, because the stent may be implanted partly outside the DCB-treated segment. By contrast, DCB used after BMS implantation might affect the drug delivery to the vessel because of interposition of the stent struts.[24] An optical coherence tomography (OCT) study investigated the effects of the sequence of DCB and BMS (i.e., DCB first and BMS first) and stated that the BMS-first sequence translated into more favorable apposition than did the DCB-first sequence, as evidenced by the significantly low proportion of incomplete stent apposition (ISA) struts and nonsignificantly low ISA areas and volumes in the former.[36] However, the INDICOR trial[33] and another OCT study[36] used DCB from different manufacturers suggested that, the sequence of DCB application does not affect LLL, MACEs, and in-stent neointimal hyperplasia. Similar clinical and angiographic results were reported by the IN-PACT CORO trial.[24]
Finally, a possible explanation for these findings is that the currently used DCB, particularly first-generation DCBs, failed to warrant sufficient bioavailability of paclitaxel at the lesion site. Bondesson et al[37] reported the differential treatment outcomes of various DCBs, and this variation may be even larger than that caused by DES because drug delivery to the vessel wall is crucial during balloon inflation. Regarding LLL, the pharmacokinetics of paclitaxel with first-generation DCBs may have been insufficient to provide comparable benefits. A recent experimental study[38] showed much higher drug concentrations into the vessel wall by using the DIOR-II DCB than DIOR-I, combined with a shorter inflation time. Hence, using a second-generation DCB with a BMS, higher tissue drug delivery dose, might lead to better angiographic and clinical outcomes for de novo lesions.
The present meta-analysis has several potential limitations. First, the sample sizes were small in all except one of the studies.[19] Second, because the studies had a relatively short follow-up durations, definitive conclusions will necessitate clinical follow-up for several additional years. Finally, most included studies were conducted in Western countries, hence, data from non-Western countries were inadequate to precisely assess the clinical efficacy and safety of the DCB + BMS strategy for de novo lesions. Thus, further large, multicenter, well-designed randomized trials recruiting patients from more countries are required to provide additional insights.
5. Conclusion
The present meta-analysis does not favor the DCB + BMS strategy as an alternative therapeutic method to DES implantation for de novo coronary artery lesions in PCI. Additional well-designed large RCTs with long follow-up periods are required to resolve this concern.
Footnotes
Abbreviations: BMS = bare metal stent, BR = in-segment binary restenosis, CI = confidence interval, DAPT = dual antiplatelet therapy, DCB = drug-coated balloon, ISR = in-stent restenosis, LLL = in-segment late lumen loss, MACEs = major adverse cardiovascular events, MD = mean difference, MI = myocardial infarction, MLD = in-segment minimum lumen diameter, PCI = percutaneous coronary intervention, PEB = paclitaxel-eluting balloon, RCTs = randomized controlled trials, RR = risk ratio, TLR = target lesion revascularization.
WL and YZ contributed equally to this work.
The authors have no conflicts of interest to disclose.
References
- [1].Waksman R, Serra A, Loh JP, et al. Drug-coated balloons for de novo coronary lesions: results from the Valentines II trial. EuroIntervention 2013;9:613–9. [DOI] [PubMed] [Google Scholar]
- [2].Mieres J, Fernandez-Pereira C, Risau G, et al. One-year outcome of patients with diabetes mellitus after percutaneous coronary intervention with three different revascularization strategies: results from the Diabetic Argentina Registry (DEAR). Cardiovasc Revasc Med 2012;13:265–71. [DOI] [PubMed] [Google Scholar]
- [3].Alfonso F, Perez-Vizcayno MJ, Cardenas A, et al. A randomized comparison of drug-eluting balloon versus everolimus-eluting stent in patients with bare-metal stent-in-stent restenosis: the RIBS V Clinical Trial (Restenosis Intra-stent of Bare Metal Stents: paclitaxel-eluting balloon vs. everolimus-eluting stent). J Am Coll Cardiol 2014;63:1378–86. [DOI] [PubMed] [Google Scholar]
- [4].Latib A, Colombo A, Castriota F, et al. A randomized multicenter study comparing a paclitaxel drug-eluting balloon with a paclitaxel-eluting stent in small coronary vessels: the BELLO (Balloon Elution and Late Loss Optimization) study. J Am Coll Cardiol 2012;60:2473–80. [DOI] [PubMed] [Google Scholar]
- [5].Unverdorben M, Kleber FX, Heuer H, et al. Treatment of small coronary arteries with a paclitaxel-coated balloon catheter in the PEPCAD I study: are lesions clinically stable from 12 to 36 months? EuroIntervention 2013;9:620–8. [DOI] [PubMed] [Google Scholar]
- [6].Worthley S, Hendriks R, Worthley M, et al. Paclitaxel-eluting balloon and everolimus-eluting stent for provisional stenting of coronary bifurcations: 12-month results of the multicenter BIOLUX-I study. Cardiovasc Revasc Med 2015;16:413–7. [DOI] [PubMed] [Google Scholar]
- [7].Kleber FX, Rittger H, Ludwig J, et al. Drug eluting balloons as stand alone procedure for coronary bifurcational lesions: results of the randomized multicenter PEPCAD-BIF trial. Clin Res Cardiol 2016;105:613–21. [DOI] [PubMed] [Google Scholar]
- [8].Kastrati A, Mehilli J, von Beckerath N, et al. Sirolimus-eluting stent or paclitaxel-eluting stent vs balloon angioplasty for prevention of recurrences in patients with coronary in-stent restenosis: a randomized controlled trial. JAMA 2005;293:165–71. [DOI] [PubMed] [Google Scholar]
- [9].Tada T, Byrne RA, Simunovic I, et al. Risk of stent thrombosis among bare-metal stents, first-generation drug-eluting stents, and second-generation drug-eluting stents: results from a registry of 18,334 patients. JACC Cardiovasc Interv 2013;6:1267–74. [DOI] [PubMed] [Google Scholar]
- [10].Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045–57. [DOI] [PubMed] [Google Scholar]
- [11].Liistro F, Porto I, Angioli P, et al. Elutax paclitaxel-eluting balloon followed by bare-metal stent compared with Xience V drug-eluting stent in the treatment of de novo coronary stenosis: a randomized trial. Am Heart J 2013;166:920–6. [DOI] [PubMed] [Google Scholar]
- [12].Clever YP, Cremers B, Speck U, et al. Influence of a paclitaxel coated balloon in combination with a bare metal stent on restenosis and endothelial function: comparison with a drug eluting stent and a bare metal stent. Catheter Cardiovasc Interv 2014;84:323–31. [DOI] [PubMed] [Google Scholar]
- [13].Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Higgins J, Thompson S, Deeks J, et al. Statistical heterogeneity in systematic reviews of clinical trials: a critical appraisal of guidelines and practice. J Health Serv Res Policy 2002;7:51–61. [DOI] [PubMed] [Google Scholar]
- [15].Bolanos Diaz R, Calderon Cahua M. [Introduction to traditional meta-analysis]. Rev Gastroenterol Peru 2014;34:45–51. [PubMed] [Google Scholar]
- [16].Herdeg C, Gohring-Frischholz K, Haase KK, et al. Catheter-based delivery of fluid paclitaxel for prevention of restenosis in native coronary artery lesions after stent implantation. Circ Cardiovasc Interv 2009;2:294–301. [DOI] [PubMed] [Google Scholar]
- [17].Zurakowski A, Buszman PP, Milewski KP, et al. Stenting and adjunctive delivery of paclitaxel via balloon coating versus durable polymeric matrix for de novo coronary lesions: clinical and angiographic results from the prospective randomized trial. J Interv Cardiol 2015;28:348–57. [DOI] [PubMed] [Google Scholar]
- [18].Belkacemi A, Agostoni P, Nathoe HM, et al. First results of the DEB-AMI (drug eluting balloon in acute ST-segment elevation myocardial infarction) trial: a multicenter randomized comparison of drug-eluting balloon plus bare-metal stent versus bare-metal stent versus drug-eluting stent in primary percutaneous coronary intervention with 6-month angiographic, intravascular, functional, and clinical outcomes. J Am Coll Cardiol 2012;59:2327–37. [DOI] [PubMed] [Google Scholar]
- [19].Hamm CW, Cremers B, Moellmann H, et al. PEPCAD III: a randomised trial comparing a paclitaxel-coated balloon/stent system with a sirolimus eluting stent. Circulation 2009;120:2157. [Google Scholar]
- [20].Ali RM, Degenhardt R, Zambahari R, et al. Paclitaxel-eluting balloon angioplasty and cobalt-chromium stents versus conventional angioplasty and paclitaxel-eluting stents in the treatment of native coronary artery stenoses in patients with diabetes mellitus. EuroIntervention 2011;7suppl K:K83–92. [DOI] [PubMed] [Google Scholar]
- [21].Stella PR, Belkacemi A, Dubois C, et al. A multicenter randomized comparison of drug-eluting balloon plus bare-metal stent versus bare-metal stent versus drug-eluting stent in bifurcation lesions treated with a single-stenting technique: six-month angiographic and 12-month clinical results of the drug-eluting balloon in bifurcations trial. Catheter Cardiovasc Interv 2012;80:1138–46. [DOI] [PubMed] [Google Scholar]
- [22].Lopez Minguez JR, Nogales Asensio JM, Doncel Vecino LJ, et al. A prospective randomised study of the paclitaxel-coated balloon catheter in bifurcated coronary lesions (BABILON trial): 24-month clinical and angiographic results. EuroIntervention 2014;10:50–7. [DOI] [PubMed] [Google Scholar]
- [23].Poerner TC, Otto S, Gassdorf J, et al. Stent coverage and neointimal proliferation in bare metal stents postdilated with a Paclitaxel-eluting balloon versus everolimus-eluting stents: prospective randomized study using optical coherence tomography at 6-month follow-up. Circ Cardiovasc Interv 2014;7:760–7. [DOI] [PubMed] [Google Scholar]
- [24].Burzotta F, Brancati MF, Trani C, et al. Impact of drug-eluting balloon (pre- or post-) dilation on neointima formation in de novo lesions treated by bare-metal stent: the IN-PACT CORO trial. Heart Vessels 2016;31:677–86. [DOI] [PubMed] [Google Scholar]
- [25].Kirtane AJ, Gupta A, Iyengar S, et al. Safety and efficacy of drug-eluting and bare metal stents: comprehensive meta-analysis of randomized trials and observational studies. Circulation 2009;119:3198–206. [DOI] [PubMed] [Google Scholar]
- [26].Stefanini GG, Holmes DR., Jr Drug-eluting coronary-artery stents. N Engl J Med 2013;368:254–65. [DOI] [PubMed] [Google Scholar]
- [27].D’Ascenzo F, Bollati M, Clementi F, et al. Incidence and predictors of coronary stent thrombosis: evidence from an international collaborative meta-analysis including 30 studies, 221,066 patients, and 4276 thromboses. Int J Cardiol 2013;167:575–84. [DOI] [PubMed] [Google Scholar]
- [28].Alfonso F, Perez-Vizcayno MJ, Cardenas A, et al. A prospective randomized trial of drug-eluting balloons versus everolimus-eluting stents in patients with in-stent restenosis of drug-eluting stents: the RIBS IV randomized clinical trial. J Am Coll Cardiol 2015;66:23–33. [DOI] [PubMed] [Google Scholar]
- [29].Hamilos M, Sarma J, Ostojic M, et al. Interference of drug-eluting stents with endothelium-dependent coronary vasomotion: evidence for device-specific responses. Circ Cardiovasc Interv 2008;1:193–200. [DOI] [PubMed] [Google Scholar]
- [30].Byrne RA, Sarafoff N, Kastrati A, et al. Drug-eluting stents in percutaneous coronary intervention: a benefit-risk assessment. Drug Saf 2009;32:749–70. [DOI] [PubMed] [Google Scholar]
- [31].Besic KM, Strozzi M, Margetic E, et al. Drug-eluting balloons in patients with non-ST elevation acute coronary syndrome. J Cardiol 2015;65:203–7. [DOI] [PubMed] [Google Scholar]
- [32].Unverdorben M, Kleber FX, Heuer H, et al. Treatment of small coronary arteries with a paclitaxel-coated balloon catheter. Clin Res Cardiol 2010;99:165–74. [DOI] [PubMed] [Google Scholar]
- [33].Kaul U, Unverdorben M, Degenhardt R, et al. The paclitaxel-eluting PTCA-balloon in combination with a cobalt-chromium stent in two different sequences to treat de novo coronary artery lesions: an angiographic follow up study. Indian Heart J 2013;65:510–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Otto S, Gassdorf J, Nitsche K, et al. Time course of vascular response after an a priori strategy of bare metal stent implantation post-dilated with a paclitaxel-coated balloon: implementation of a three-dimensional analysis algorithm with optical coherence tomography. Cardiol J 2016;23:296–306. [DOI] [PubMed] [Google Scholar]
- [35].Fischer D, Scheller B, Schafer A, et al. Paclitaxcel-coated balloon plus bare metal stent vs. sirolimus-eluting stent in de novo lesions: an IVUS study. EuroIntervention 2012;8:450–5. [DOI] [PubMed] [Google Scholar]
- [36].Gutierrez-Chico JL, van Geuns RJ, Koch KT, et al. Paclitaxel-coated balloon in combination with bare metal stent for treatment of de novo coronary lesions: an optical coherence tomography first-in-human randomised trial, balloon first vs. stent first. EuroIntervention 2011;7:711–22. [DOI] [PubMed] [Google Scholar]
- [37].Bondesson P, Lagerqvist B, James SK, et al. Comparison of two drug-eluting balloons: a report from the SCAAR registry. EuroIntervention 2012;8:444–9. [DOI] [PubMed] [Google Scholar]
- [38].Posa A, Nyolczas N, Hemetsberger R, et al. Optimization of drug-eluting balloon use for safety and efficacy: evaluation of the 2nd generation paclitaxel-eluting DIOR-balloon in porcine coronary arteries. Catheter Cardiovasc Interv 2010;76:395–403. [DOI] [PubMed] [Google Scholar]


