Abstract
Several previously published studies revealed a hazardous role of pretreatment lactate dehydrogenase (LDH) in survival of advanced or metastatic pancreatic cancer (PC) patients. Nevertheless, in early stage PC patients who are eligible for curative resection, the prognostic role of postresection LDH has never been discussed. In this study, we aimed to explore the prognostic significance of varying postresection LDH among early stage PC patients. In total, 80 PC patients who received curative resection were retrospectively selected from a population-based electronic inpatients database which originated from Shanghai, China. A dynamic survival analysis method, counting process approach in combination with the multiple failure-time Cox model, was applied to evaluate the association between postresection LDH and OS. The multiple failure-time Cox model found that age, resection modality, and postresection LDH were significantly associated with OS: an elevated LDH (defined as > 250 U/L) was related to 2.93 (95% CI: 1.26–6.79) folds of death hazard. Further analysis disclosed an identifiable dose–response association between LDH and OS: compared with LDH≤155 U/L, the HRs for 155 U/L < LDH < 196 U/L, and LDH≥196 U/L were 2.07 (95% CI: 0.88–4.88) and 3.15 (95% CI: 1.30–7.59), respectively. Our study results suggest that postresection LDH is a prominent prognostic factor in this group of early stage PC patients. Maintaining normally ranged LDH after resection might bring about survival benefit in early stage PC patients.
Keywords: early stage pancreatic cancer, lactate dehydrogenase, overall survival
1. Introduction
Pancreatic cancer (PC) remains one of the most lethal malignant tumors, for nearly 95% patients will die within 5 years after diagnosis.[1] The lack of specific symptoms in the early stage of disease mainly contributes to the dismal survival of PC. It has been estimated that, among all newly diagnosed patients, only 15% to 20% will be eligible for curative resection. More depressing is that, even in resected PC patients, the overall 5-year survival rate only ranges from 18% to 24%.[2,3] Thus, in early stage PC patients, other nontreatment factors of possible prognostic significance should be intensively searched and investigated.
Compared with normal cells, the most distinctive feature in metabolism of cancer cells is the enhanced glycolysis capacity even in the presence of sufficient oxygen. This phenomenon is well known as the Warburg effect. Recently, along with the uncovering of laboratory evidences which connect aerobic glycolysis to cancer initiation and proliferation,[4,5] lactate dehydrogenase (LDH), a central enzyme involved in the final step of the Warburg effect in converting pyruvate to lactate, is attracting growing study interest.
The prognostic value of serum LDH has been widely discussed in many types of cancer. For example, an elevated pretreatment serum LDH has been found associated with deteriorated survival of small-cell lung cancer, nasopharyngeal cancer, colon cancer, and aggressive lymphoid cancer.[6–11] In PC, although several studies also reported a significant inverse association between pretreatment LDH and survival in advanced or metastatic patients,[12–14] in early stage patients who are eligible for curative resection, the prognostic role of postresection LDH has never been discussed.
Similar to other blood indicators, within a given period, usually LDH constantly varies from one single test to another; in this case, when discussing the association between LDH and cancer survival, this variation should not be ignored. Nevertheless, nearly all currently available studies chose to analyze the prognostic role of LDH measured at certain transient moments by using the common Cox proportional hazards model; dynamic survival analysis methods were seldom seen.
In this study, we aimed to discuss the relationship between varying postresection LDH and the overall survival (OS) of early stage PC patients. To effectively adjust for LDH variation, we restructured the original survival data into counting process style and adopted multiple failure-time Cox model subsequently.
2. Methods
2.1. Patients
After Institutional Research Ethics Board of Fudan University approved, we retrospectively selected PC patients from a mega population-based electronic database. This database was established at the end of 2011 and has been accumulating on daily basis ever since. Relevant information of patients who were admitted in selected county-level and above hospitals within the Shanghai Metropolitan area, China, was mandatorily collected and reported. In this study, we chose early stage PC patients based on the following criteria: (1) histopathologically confirmed exocrine cancer of pancreas; (2) resection with curative intention was performed (patients who only received palliative resection were excluded); (3) the date of diagnosis was between January 1, 2012, and December 31, 2013; (4) had at least 1 serum LDH test result after resection; (5) other vital information for analysis, such as age, gender, date of operation, modality of resection, and adjuvant chemotherapy, was complete. In the end, 80 patients were eligible for inclusion.
2.2. Outcome
The outcome of interest was OS. The survival period was defined as time interval between the date of curative resection and the date of death, which was ascertained through external matching with death registration system on January 31, 2015.
2.3. Adjuvant chemotherapy
Adjuvant chemotherapy was defined as the administration of gemcitabine alone or in combination with the following agents after curative resection: nab-Paclitaxel, 5-fluorouracil, Irinotecan, and Oxaliplatin.
2.4. Counting process approach for time-varying covariates
Based on commonly used Cox proportional hazards model, a simple extension called “counting process approach” was initially forwarded by Anderson and Gill[15] to cope with recurrent event or time-varying covariates. The logic behind this method is simple: suppose a study subject has intermittent measurements of a time-varying covariate during the whole survival period, we can then split the original observation into a group of “subobservations” at the time points this covariate varied. Therefore, in the transformed database, for every subobservation, this covariate will be treated as constant, and multiple failure-time Cox model, which extra adjusts for inter-correlations among subobservations stemmed from the same subject, by using “sandwich” estimator for instance,[16] can be applied.
2.5. Statistical analysis
At first, we transformed the original survival data into counting process style based on LDH fluctuations, then, we applied multiple failure-time Cox model to evaluate the association between postresection LDH and OS. A LDH level no higher than 250 units/liter (U/L) is widely used to define normally raged LDH as suggested by some previous publications. [12,13,17] Thus, in the current study, LDH was categorized as either “normal” or “elevated” by using the cut-off of 250 U/L. The influence of other available potential confounders, such as age, gender, resection modality, adjuvant chemotherapy, was simultaneously controlled for. In order to simplify the problem, we assumed that the effect of LDH and the baseline hazard were constant during the whole survival period. We used the univariate method to screen candidate covariates, and variables with P values less than 0.10 were included in the multivariate model.
We further divided LDH into subgroups by using percentiles to explore dose–response association between LDH and OS. All statistical analyses were executed by SAS (version 9.2, SAS Institute Inc., Cary, NC), and the significance level was defined as 2-tailed probability less than 0.05.
3. Results
3.1. Characteristics of patients
The general characteristics of 80 early stage PC patients were briefly summarized in Table 1. The mean age at diagnosis for all patients was 60.93 years. Males and females were almost equivalent in count. Nearly a half of patients (48.75%) received gemcitabine-based adjuvant chemotherapy after curative resection. As to resection modality, 46 (57.50%) patients went through Whipple pancreaticoduodenectomy. In total, 45 patients had experienced death before January 31, 2015, accounted for 56.25%. The shortest and longest survival lengths were 53 days and 744 days, respectively, and the median of survival length was 489 days. Monthly means of LDH were calculated to show the longitudinal variation of postresection LDH in Fig. 1: along with the extension of survival length, monthly means of LDH randomly fluctuated, no prominent pattern was discerned.
Table 1.
Characteristics of 80 resected PC patients.
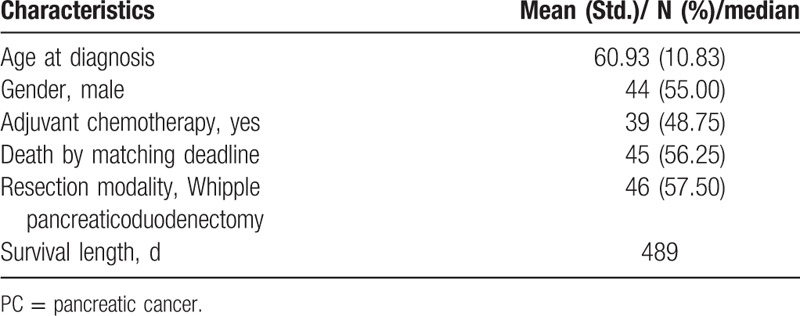
Figure 1.
Postresection LDH fluctuation among PC patients. LDH = lactate dehydrogenase, PC = pancreatic cancer.
3.2. Multiple failure-time Cox model fitting results
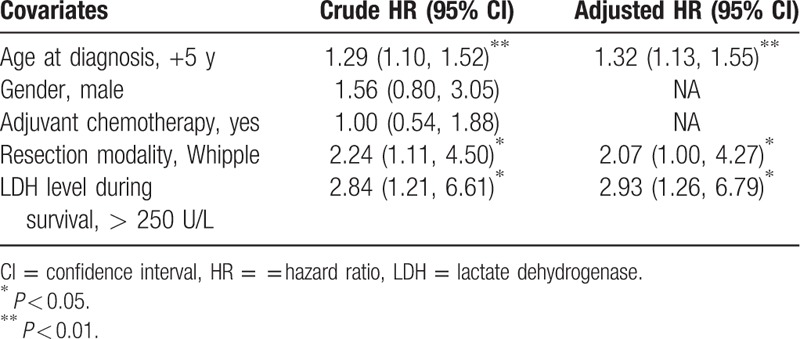
After transforming the original survival data into “counting process” style, totally we obtained 455 subobservations from 80 patients. Based on univariate results, other than the LDH level, 2 covariates from the original 4 available potential confounders were included into a multivariate model: age at diagnosis and resection modality. Interactions between age and LDH, resection modality, and LDH were all insignificant (χ2 = 0.55, P = 0.46; χ2 = 1.29, P = 0.26). After adjusting for inter-correlations by using the sandwich estimator, we found that age, resection modality, and LDH level were all prominently associated with OS in resected PC patients: every 5-year increase in age corresponded to 32% extra hazard of death; PC patients who received Whipple resection exhibited 2.07 (95% CI: 1.00, 4.27) times of death hazard; compared with patients of normally ranged LDH, the HR for patients with an elevated postresection LDH was 2.93 (95% CI: 1.26–6.79) (Table 2).
Table 2.
Multiple failure-time Cox model fitting results.
3.3. Dose–response association between LDH and OS
LDH test results were divided into 3 subgroups by using the 33rd (155 U/L) and the 66th (196 U/L) percentiles. After adjusting for age and resection modality, the multiple failure-time Cox model disclosed a discernible dose–response association: along with the increase of LDH value, the hazard of death was also increasing: compared with LDH≤155 U/L, the HRs for 155 U/L < LDH < 196 U/L and LDH≥196 U/L were 2.07 (95% CI: 0.88–4.88) and 3.15 (95% CI: 1.30–7.59), respectively (Fig. 2).
Figure 2.
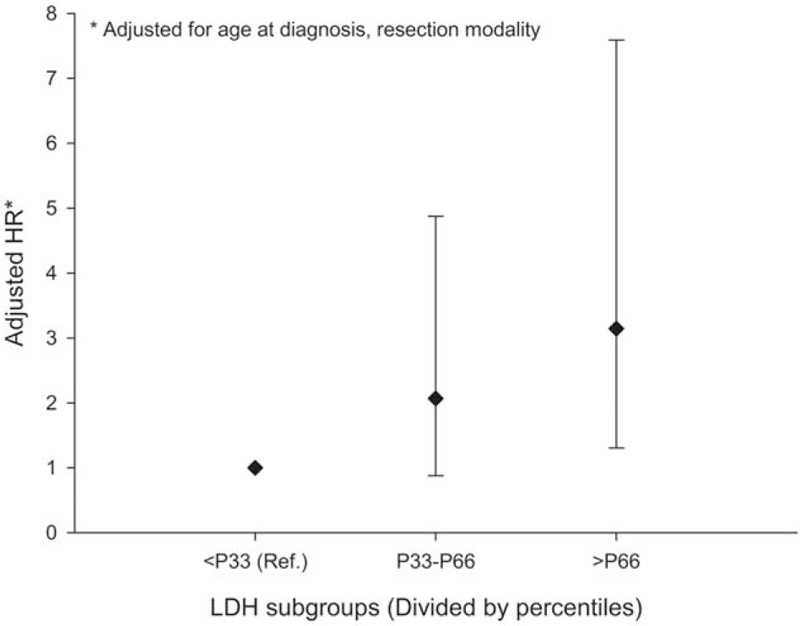
Dose–response association between LDH and OS of PC. LDH = lactate dehydrogenase, OS = overall survival, PC = pancreatic cancer.
4. Discussion
In this retrospective study, through the application of dynamic survival analysis method, we evaluated the influence of varying LDH measured after curative resection on OS in 80 early stage PC patients. Based on analytical results, we found that an elevated LDH was in general significantly associated with compromised OS of PC.
In their newly published study, Wan et al[18] reported that high pretreatment serum LDH level was correlated with a shortened disease-free survival in nasopharyngeal carcinoma. Although currently, similar evidences in other malignant tumors are absent, we can still suspect that in resected PC patients, the overconcentration of serum LDH may also result in expedited relapse of the cancer. Besides, it has been suggested that there exists a positive feedback loop between hypoxia inducible factors (HIFs) and LDH in hypoxia microenvironment[19]; thus, in relapsed PC patients, an elevated serum LDH will stimulate the production of HIFs, and the overexpression of HIFs is closely associated with metastasis of solid tumors.[20] Moreover, LDH has been found directly promoted the growth of PC cells in vitro.[21] All these enumerated evidences may collectively contribute to the deteriorated OS in PC patients with elevated postresection LDH.
The major novelty of our study is the application of dynamic survival analysis method in estimating the association between varying postresection LDH and OS of PC. Although counting process adjustment in tandem with multiple failure-time Cox model is not novel in dealing with time-dependent covariates, their application in cancer survival literatures was seldom seen.
Nevertheless, our study does have several limitations. At first, we lack the data of some clinical characteristics of PC, such as tumor stage, size, location, and lymph node implication. It is highly likely that these unadjusted factors can bring confounding to our study results. Second, selection bias cannot be precluded, as we only analyzed PC patients whose vital information was complete. Besides, the sample size of patients was comparatively small, which impeded more thorough analysis of data, and to some extent, it may influence the accuracy of estimation. Finally, compared with OS, the association between postresection LDH and disease-free survival of PC undoubtedly bears a much more prominent clinical significance. However, because of data limitation, we can only expect to discuss this issue in future studies.
Despite aforementioned limitations, our study is the very first to analyze the prognostic significance of varying postresection LDH in early stage PC patients, and the credibility of results can be substantially consolidated by properly applied dynamic survival analysis method. Through the application of multiple failure-time Cox model, we successfully identified a prominent inverse association between LDH and OS. Our findings probably suggest that, for early stage PC patients, maintaining normally ranged LDH after resection may result in survival benefit. In 2 previously published studies, Le et al[22] and Xie et al[23] revealed that the inhibition of LDH activity showed a notable antiproliferation effect in animal xenograft models of human lymphoma, lung cancer, and PC. More importantly, various effective LDH inhibitors with minimum side-effects are already available now.[24–26]
To conclude, in the current study, we found that postresection LDH was significantly associated with OS in a group of early stage PC patients. Our study results hint a promising prospect of LDH maintenance in survival of early stage PC patients.
Footnotes
Abbreviations: HR = hazard ratio, LDH = lactate dehydrogenase, OS = overall survival, PC = pancreatic cancer.
YX and ZX contributed equally to this study.
Availability of data and materials: The datasets analyzed during the current study are not publicly available due to confidentiality agreement with the cooperative institutions, but are available by direct contact with the corresponding author under reasonable request.
Funding: This study was supported by National Natural Science Foundation of China (No. 81273187), National Science and Technology Major Project of the People's Republic of China (2012ZX09303–013–014).
The authors have no conflicts of interest to disclose.
References
- [1].Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277–300. [DOI] [PubMed] [Google Scholar]
- [2].Wagner M, Redaelli C, Lietz M, et al. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg 2004;91:586–94. [DOI] [PubMed] [Google Scholar]
- [3].Yeo CJ, Abrams RA, Grochow LB, et al. Pancreaticoduodenectomy for pancreatic adenocarcinoma: postoperative adjuvant chemoradiation improves survival. A prospective, single-institution experience. Ann Surg 1997;225:621–33. discussion 633-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009;324:1029–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sun X, Sun Z, Zhu Z, et al. Clinicopathological significance and prognostic value of lactate dehydrogenase A expression in gastric cancer patients. PLoS One 2014;9:e91068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Souhami RL, Bradbury I, Geddes DM, et al. Prognostic significance of laboratory parameters measured at diagnosis in small cell carcinoma of the lung. Cancer Res 1985;45:2878–82. [PubMed] [Google Scholar]
- [7].Cohen MH, Makuch R, Johnston-Early A, et al. Laboratory parameters as an alternative to performance status in prognostic stratification of patients with small cell lung cancer. Cancer Treat Rep 1981;65:187–95. [PubMed] [Google Scholar]
- [8].Terpos E, Katodritou E, Roussou M, et al. High serum lactate dehydrogenase adds prognostic value to the international myeloma staging system even in the era of novel agents. Eur J Haematol 2010;85:114–9. [DOI] [PubMed] [Google Scholar]
- [9].Scartozzi M, Giampieri R, Maccaroni E, et al. Pre-treatment lactate dehydrogenase levels as predictor of efficacy of first-line bevacizumab-based therapy in metastatic colorectal cancer patients. Br J Cancer 2012;106:799–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jin Y, Ye X, Shao L, et al. Serum lactic dehydrogenase strongly predicts survival in metastatic nasopharyngeal carcinoma treated with palliative chemotherapy. Eur J Cancer 2013;49:1619–26. [DOI] [PubMed] [Google Scholar]
- [11].Ferraris AM, Giuntini P, Gaetani GF. Serum lactic dehydrogenase as a prognostic tool for non-Hodgkin lymphomas. Blood 1979;54:928–32. [PubMed] [Google Scholar]
- [12].Haas M, Heinemann V, Kullmann F, et al. Prognostic value of CA 19-9, CEA, CRP, LDH and bilirubin levels in locally advanced and metastatic pancreatic cancer: results from a multicenter, pooled analysis of patients receiving palliative chemotherapy. J Cancer Res Clin Oncol 2013;139:681–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tas F, Karabulut S, Ciftci R, et al. Serum levels of LDH, CEA, and CA19-9 have prognostic roles on survival in patients with metastatic pancreatic cancer receiving gemcitabine-based chemotherapy. Cancer Chemother Pharmacol 2014;73:1163–71. [DOI] [PubMed] [Google Scholar]
- [14].Stocken DD, Hassan AB, Altman DG, et al. Modelling prognostic factors in advanced pancreatic cancer. Br J Cancer 2008;99:883–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Anderson PK, Gill RD. Cox's regression model for counting processes: a large sample study. Ann Stat 1982;10:1100–20. [Google Scholar]
- [16].Lin DY, Wei LJ. The robust inference for the Cox proportional hazard model. J Am Stat Assoc 1989;84:1074–8. [Google Scholar]
- [17].Haas M, Laubender RP, Stieber P, et al. Prognostic relevance of CA 19-9, CEA, CRP, and LDH kinetics in patients treated with palliative second-line therapy for advanced pancreatic cancer. Tumor Biol 2010;31:351–7. [DOI] [PubMed] [Google Scholar]
- [18].Wan XB, Wei L, Li H, et al. High pretreatment serum lactate dehydrogenase level correlates with disease relapse and predicts an inferior outcome in locally advanced nasopharyngeal carcinoma. Eur J Cancer 2013;49:2356–64. [DOI] [PubMed] [Google Scholar]
- [19].Lu H, Forbes RA, Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J Biol Chem 2002;277:23111–5. [DOI] [PubMed] [Google Scholar]
- [20].Lu X, Kang Y. Hypoxia and hypoxia-inducible factors (HIFs): master regulators of metastasis. Clin Cancer Res 2010;16:5928–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rong Y, Wu W, Ni X, et al. Lactate dehydrogenase A is overexpressed in pancreatic cancer and promotes the growth of pancreatic cancer cells. Tumour Biol 2013;34:1523–30. [DOI] [PubMed] [Google Scholar]
- [22].Le A, Cooper CR, Gouw AM, et al. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U S A 2010;107:2037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Xie H, Hanai J, Ren JG, et al. Targeting lactate dehydrogenase-A inhibits tumorigenesis and tumor progression in mouse models of lung cancer and impacts tumor initiating cells. Cell Metab 2014;19:795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Maftouh M, Avan A, Sciarrillo R, et al. Synergistic interaction of novel lactate dehydrogenase inhibitors with gemcitabine against pancreatic cancer cells in hypoxia. Br J Cancer 2014;110:172–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Manerba M, Vettraino M, Fiume L, et al. Galloflavin (CAS 568-80-9): a novel inhibitor of lactate dehydrogenase. ChemMedChem 2012;7:311–7. [DOI] [PubMed] [Google Scholar]
- [26].Granchi C, Roy S, De Simone A, et al. N-Hydroxyindole-based inhibitors of lactate dehydrogenase against cancer cell proliferation. Eur J Med Chem 2011;46:5398–407. [DOI] [PubMed] [Google Scholar]


