Abstract
IgG4-related sclerosing cholangitis (IgG4-SC) is a rare biliary manifestation in which many other organs might be affected. The purpose of our study was to investigate the different clinical characteristics and initial steroid response between IgG4-SC patients with and without other organs affected.
A series of patients with IgG4-SC in the period from January 2006 to December 2015 at our hospital were included. The pancreas and major salivary glands were screened, and the initial corticosteroid therapy was given. Clinical information was collected and analyzed including demographics, clinical presentation, IgG4 serology, imaging features, and treatment outcomes.
The study identified 72 IgG4-SC patients, including 60 males and 12 females. The mean age was 59.8 years old. Among these IgG4-SC patients, 10 patients had only bile duct involved, 42 patients had 2 organs involved and 20 patients had multiple organs involved. In patients with multiple organs involved, more complaints were given (mean 2.9 kinds), higher serum IgG4 levels were found (23458 ± 19402.7 mg/L), and more stricture lesions of biliary tract were shown. All 72 patients exhibited a disease response within 4 to 6 weeks of starting steroids. The remission rate in the multiple lesions group was lower (60%), and the recurrence rate is higher (83.3%). The relapse-free survival was 20.0 months in the single lesion group, which is longer than that in the multiple lesions group (3.1 months, P < 0.05).
The IgG4-SC patients with multiple organs affected had more complaints, higher serum IgG4 levels, and poor response to initial steroids.
Keywords: IgG4-related disease, IgG4-related sclerosing cholangitis, manifestation, treatment
1. Introduction
Immunoglobulin G4-related sclerosing cholangitis (IgG4-SC) is characterized by lymphoplasmacytic tissue infiltration with a predominance of IgG4-positive plasma cells, increased IgG4 production, leading to bile duct wall thickening and good response to steroid therapy. It was regarded as the most frequent extrapancreatic manifestation of type 1 autoimmune pancreatitis, present in over 70 percent of such patients.[1] Now IgG4-SC belongs to the spectrum of immunoglobulin G4-related disease (IgG4-RD), which encompasses a large number of medical conditions that share similar histopathological features.[2]
As an independent type of IgG4-RD, many IgG4-SC cases could affect multiple organs. Up to 60 to 90 percent of IgG4-RD patients have multiple organs affected.[3,4] The most often affected organs are pancreas and biliary duct.[5] Likewise, many IgG4-SC patients have other organs involved, such as pancreas and major salivary glands (submandibular gland, parotid).[6] The clinical and pathological characteristics of isolate IgG4-SC had been described[7]; however, rare investigations investigated the differences of clinical characteristics of IgG4-SC between with and without other organ affected. Does the IgG4-SC patient without other organ involved has a mild manifestation, good response to steroid therapy? It is still unclear. Considering the susceptibility of pancreas and salivary glands and the screening facility, we focused on its differential characteristics between IgG4-SC patients with or without these glands affected.
2. Materials and methods
A series of patients with IgG4-SC in the period from January 2006 to December 2015 at our hospital and who did not receive any therapy before were included. Our hospital is one of the largest units treating patients with IgG4-RD. The diagnosis were based on recognized international criteria, including the HISORt criteria and the Japan Pancreas Society criteria.[8,9] Each patient accepted magnetic resonance cholangiopancreatography (MRCP) and endoscopic retrograde cholangiopancreatography (ERCP) for biliary and pancreatic ducts, ultrasound and thin-layer computed tomography for pancreas and major salivary glands. For mass or diffusely enlarged glands, biopsy was performed. Pathological evaluation was performed at each site by a dedicated pathologist.
The features of pancreas, submandibular gland, and parotid affected included a focal mass or a diffusely enlarged gland (focal or diffuse duct stricture), a lymphoplasmacytic infiltrated within the tissues and a subsequent prompt response to steroid therapy. Aimed to investigate the differential characteristics of IgG4-SC with or without these glands affected, these patients were divided into 3 groups: single lesion group (without extra-biliary involved), double lesions group (1 extra-biliary organ involved), and multiple lesions group.
The initial corticosteroid therapy, which was prednisone 40 mg/day orally for 4 to 6 weeks then taper by 5 mg/week for total of 13 weeks of treatment with regular monitoring of biochemistry and repeated imaging were given to all included patients.[10] There was no steroid-sparing agents (azathioprine, methotremate, cyclophosphamide) for these patients, and no use of maintenance therapy in the initial therapy in the study. Four treatment outcomes were defined according to the previous description.[11] (1) Disease response was defined as symptomatic, biochemical and radiologic improvement after the commencement of treatment. (2) Disease remission referred to the maintenance of the improvements after cessation of treatment. (3) Disease relapse was defined as recurrences of disease activity after achievement of remission and cessation of treatment. (4) Failed weaning was defined as an inability to wean steroids completely because of a flare of disease activity. We excluded the probable or possible patients because of their incomplete profiles. Clinical information was collected and analyzed including demographics, clinical presentations, IgG4 serology levels, imaging features and treatment outcomes.
The statistic difference was performed by using ANOVA and chi-square (SPSS 19, SPSS, Inc, Chicago). Nonparametric quantitative variables were compared using the Mann–Whitney U test. Kaplan–Meier curves were used to assess differences in relapse-free survival rates between groups. Differences associated with a P value less than 0.05 were considered statistically significant. All procedural protocols were approved and supervised by the Ethics Committee of our hospital, and informed consent was signed by each patient.
3. Results
The study identified 72 IgG4-SC patients, including 60 males and 12 females (the ratio is 5:1). The mean age at admission is 59.8 years old (28–83 years old). The initial presentation included obstructive jaundice in 59 of 72 patients (81.9%), whereas 9 (12.5%) with abdominal pain alone. In 4 patients an incidental pancreatic mass was noted. 56 patients (77.8%) had undergone surgery eventually, the other had puncture biopsy. All the diagnosis had the pathological confirmations.
Among all the IgG4-SC patients, 10 patients had only bile duct involved, and the other 62 patients had pancreas involved. In total, 36 patients had a focal pancreatic mass at presentation, and 26 patients had diffuse pancreatic enlargement. Pancreatic ductal disease was seen on MRCP and/or in 39 of 62 (62.9%) patients. Focal pancreatic duct strictures were found in 22 of 62 (35.5%) patients, whereas the diffuse structuring was seen in 17 of 62 (27.4%) patients.
Besides the bile duct and pancreas, 12 patients had submandibular gland involved, 9 patients had parotid involved, and 1 patient had both (Table 1). However, 10 patients had submandibular gland mass and 8 patients had parotid gland mass; the other patients only had diffusely enlarged glands. They all had biopsies and the pathological results confirmed the involvement of these glands.
Table 1.
Comparison of clinical features between single and multiple lesions in immunoglobulin G4-related sclerosing cholangitis patients.
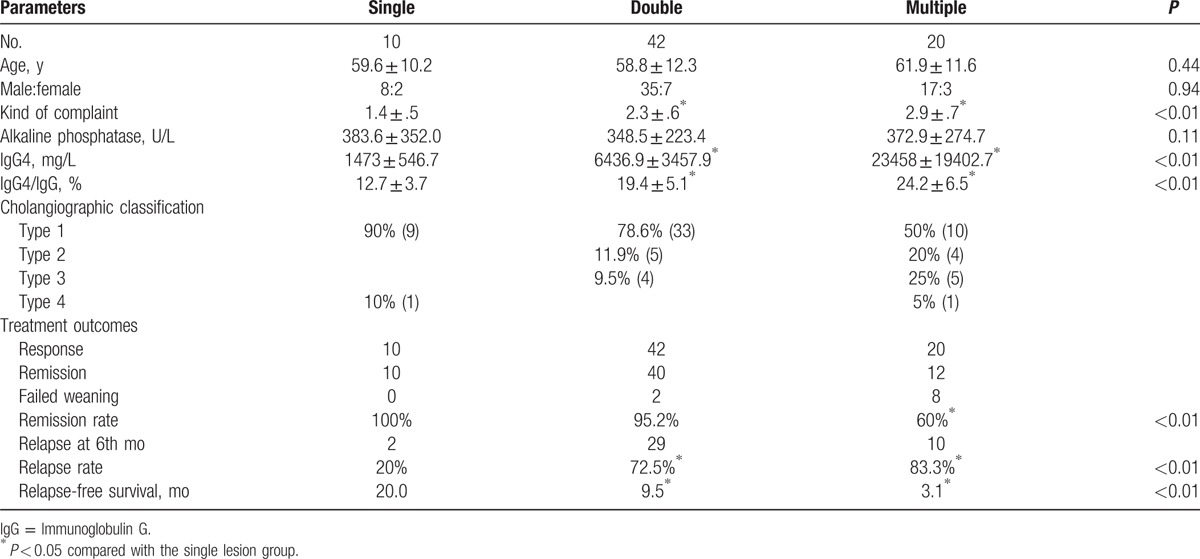
To compared the differential characteristics between IgG4-SC patients with or without other organs affected. The patients were divided into single lesion group, double lesions group, and multiple lesions group. As to the manifestation, the complaint was not more serious with more organs involved, but more complaints were given. The mean number of complaint was 2.9 kinds in the multiple lesions group, whereas the complaint was 1.4 kinds in the single lesion group (P < 0.01). Of the immunoglobulins (IgA, IgM, IgG), the IgG4 level is remarkably higher in 68 (94.4%) IgG4-SC patients. Besides, serum IgG4 levels were significantly higher in patients with multiple lesions (23458 ± 19402.7 mg/L) than in those with a single lesion (1473 ± 546.7 mg/L, P < .05). And the ratio of IgG4/IgG was higher in patients with multiple lesions. It was (24.2 ± 6.5) % in the multiple lesions group and (19.4 ± 5.1) % in the double lesions group, whereas (12.7 ± 3.7)% in the single lesion group (P < 0.05). There were no significant differences in the alkaline phosphatase level of 3 groups (P = 0.11). Imaging performances of IgG4-SC are characteristic stricture of bile duct tree. Distal bile duct involved is most common (97.2%). According to the different stricture part by imaging, type 1 patients account for 72.2% (52/72), type 2 for 12.5% (9/72), type 3 for 12.5% (9/72), and type 4 for 2.8% (2/72). The ratio of type 1 cholangiographic classification in the single lesion group was higher, whereas more type 2 and type 3 patients existed in the double lesion group or multiple lesions group (Table 1).
The patients all accept initially corticosteroid therapy. The median follow-up from the start of the initial steroid course was 12 months (range, 6–32 months). All 72 patients exhibited a disease response within 4 to 6 weeks of starting steroids as defined. Steroids were reduced and stopped after disease remission in 62 of 72 (86.1%) patients after a total treatment period. Ten patients (13.9%) failed weaning of their initial steroid course. The remission rate of the single lesion group was higher than that of the multiple lesions group.
Of the 62 patients who achieved remission, 41 (66.1%) relapsed at 6th month after cessation of treatment. Advanced studies indicate that the more extra-biliary organs involved or the more segments of bile duct involved the higher rate of recurrence. The recurrence rate in the multiple lesions group is 83.3%, which is higher than that in the single lesion group (20%, P < 0.05, Table 1). The median duration of relapse-free was 5.9 months (range, 0.3–25.2 months). The relapse-free survival was 20.0 months in the single lesion group, which is longer than that in the double lesions group (9.5 months) or in the multiple lesions group (3.1 months, P < 0.05, Fig. 1).
Figure 1.
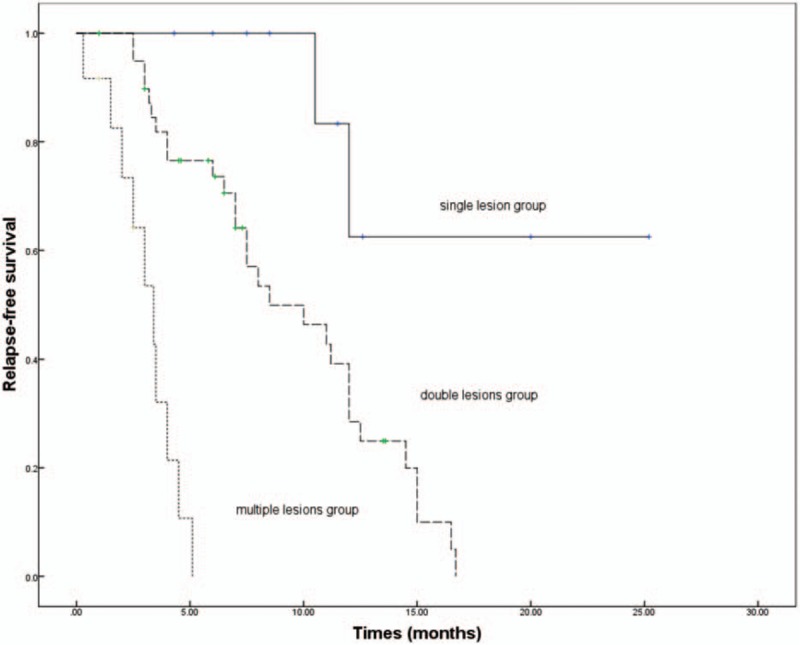
Kaplan–Meier curve of relapse-free survival in IgG4-SC patients. The relapse-free survival was 20.0 months in the single lesion group, which is longer than that in the double lesions group (9.5 mo) or in the multiple lesions group (3.1 mo, P < 0.05). IgG4-SC = IgG4-related sclerosing cholangitis.
4. Discussion
IgG4-RD is an increasingly recognized immune-mediated condition, and it could involve nearly every anatomic site. Patients often present with subacute development of a mass in the affected organ (e.g., an orbital pseudotumor, nodular lesions in the parotid gland) or diffuse enlargement of an organ (e.g., the pancreas).[12] IgG4-SC is a type of IgG4-RD with bad prognosis. It is associated with biliary obstruction and increased risk of malignancy, and also involves other organs. Some studies suggested that patients with multiorgan affected have different manifestations.[10] However, the different clinical characteristics and steroid response between IgG4-SC patients with or without multiorgan affected have not been defined.[6]
We screened bile duct, pancreas, submandibular gland, and parotid because of its susceptibility and feasibility, and divided all patients into single lesion group, double lesions group, and multiple lesions group. The ages of patients in these 3 groups were similar, with means from 58.8 to 61.9 years (ranges 28–83 years). The clinical symptoms of IgG4-SC patients were wide-ranging, manifesting in 1 or more organs synchronously or metachronously, although jaundice was the common presenting symptom. The complaint was not more serious with more organs involved, but more complaints were given. It is reasonable because when more organs were affected, a spectrum of mild localized symptoms to organ damage might occur.[13] The elevated serum level of IgG4 to some extent is the diagnostic hallmark of IgG4-RD, although it is neither necessary nor sufficient for the diagnosis.[14] In this study, 94.4% patients showed elevated serum IgG4 concentrations (≥135 mg/dL). IgG4>135 mg/dL in serum, as a cut-off value, demonstrated a sensitivity of 97% and a specificity of 79.6% in diagnosing IgG4-RD.[15] Measurements of the serum IgG4 concentration remain important for screening and evaluation of the disease. Significantly the higher IgG4 level was found in the multiple lesions group. The more organs the disease involved, the higher serum IgG4 level in our study. Imagings also showed different characteristic features in biliary tract. IgG4-related SC displays segmental and long strictures of bile duct tree.[6] In our cohort, the ratio of type 1 cholangiographic classification was 50% in triple or more lesions patients. That might indicate that the inflammatory IgG4-positive plasma cells infiltrated more lesions of bile duct tree with more extra-biliary organs involved.
Systemic glucocorticoids, which are well known to induce nonselective apoptosis of lymphocytes, are the first-line approach for the most patients with IgG4-RD. The majority of IgG4-RD patients respond to glucocorticoids, particularly in early stages of disease. In some subsets of organ disease (e.g., pancreatitis), glucocorticoids responsiveness is considered as a diagnostic criterion for the disorder.[16] Although the initial steroid response is excellent, relapses are common after early withdrawal of steroids for IgG4-SC patients. Therefore, re-treatment or improved therapy is required. Considering too many interference factors in the treatment of IgG4-SC, we only compared the initial response of glucocorticoids. The dosage and taper scenario were referred to the previous consensus and accordingly given to each patient. In our study, the IgG4-SC patients relapse rate was 66.1%. The high relapse rate is close to the results of the previous study.[17] In addition, our results reveal that the more extra-biliary organs involved or the more segments of bile duct involved, the higher rate of recurrence and the shorter relapse-free survival time. This indicates that the response of IgG4-SC with multiple organs affected is poorer than that with the single lesions group. The study of Hart had also shown that bile duct involvement were independent risk factors of IgG4-RD disease relapse.[17]
The high relapse rate raises the urgent need for classified management and improved therapy. Initial experience suggested that steroid-sparing agents and maintenance therapy can maintain long-term remission. Azathioprine, mycophenolate mofetil, 6-mercaptopurine, methotrexate, and tacrolimus have all been used as steroid-sparing agents.[16] Maintenance therapy refers to continuous low-dose glucocorticoids or any of the steroid-sparing agents following the achievement of remission. Further larger studies are needed to assess the efficacy. Our study indicates that if more complaints, higher serum IgG4 level and more stricture lesions of biliary tract were found, more organ lesions should be mentioned. Moreover, considering the high relapse rate and short relapse-free survival, when more lesions are certified, more aggressive treatment should be given.
5. Conclusion
In conclusion, the IgG4-SC patients with multiple organs affected had more complaints, higher serum IgG4 levels, and poor response to initial steroids.
Footnotes
Abbreviations: ERCP = endoscopic retrograde cholangiopancreatography, IgG4-RD = immunoglobulin G4-related disease, IgG4-SC = IgG4-related sclerosing cholangitis, MRCP = magnetic resonance cholangiopancreatography.
WL and WC contributed equally to this study.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Khosroshahi A, Stone JH. A clinical overview of IgG4-related systemic disease. Curr Opin Rheumatol 2011;23:57–66. [DOI] [PubMed] [Google Scholar]
- [2].Kamisawa T, Zen Y, Pillai S, et al. IgG4-related disease. Lancet 2015;385:1460–71. [DOI] [PubMed] [Google Scholar]
- [3].Okazaki K, Uchida K, Koyabu M, et al. Recent advances in the concept and diagnosis of autoimmune pancreatitis and IgG4-related disease. J Gastroenterol 2011;46:277–88. [DOI] [PubMed] [Google Scholar]
- [4].Sah RP, Chari ST, Pannala R, et al. Differences in clinical profile and relapse rate of type 1 versus type 2 autoimmune pancreatitis. Gastroenterology 2010;139:140–8. quiz e112-143. [DOI] [PubMed] [Google Scholar]
- [5].Koizumi S, Kamisawa T, Kuruma S, et al. Organ correlation in IgG4-related diseases. J Korean Med Sci 2015;30:743–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nakazawa T, Naitoh I, Hayashi K, et al. Diagnosis of IgG4-related sclerosing cholangitis. World J Gastroenterol 2013;19:7661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hamano H, Kawa S, Uehara T, et al. Immunoglobulin G4-related lymphoplasmacytic sclerosing cholangitis that mimics infiltrating hilar cholangiocarcinoma: part of a spectrum of autoimmune pancreatitis? Gastrointest Endosc 2005;62:152–7. [DOI] [PubMed] [Google Scholar]
- [8].Chari ST, Smyrk TC, Levy MJ, et al. Diagnosis of autoimmune pancreatitis: the Mayo Clinic experience. Clin Gastroenterol Hepatol 2006;4:1010–6. quiz 1934. [DOI] [PubMed] [Google Scholar]
- [9].Ohara H, Okazaki K, Tsubouchi H, et al. Clinical diagnostic criteria of IgG4-related sclerosing cholangitis 2012. J Hepatobiliary Pancreat Sci 2012;19:536–42. [DOI] [PubMed] [Google Scholar]
- [10].Khosroshahi A, Wallace ZS, Crowe JL, et al. International consensus guidance statement on the management and treatment of IgG4-related disease. Arthritis Rheumatol 2015;67:1688–99. [DOI] [PubMed] [Google Scholar]
- [11].Sandanayake NS, Church NI, Chapman MH, et al. Presentation and management of post-treatment relapse in autoimmune pancreatitis/immunoglobulin G4-associated cholangitis. Clin Gastroenterol Hepatol 2009;7:1089–96. [DOI] [PubMed] [Google Scholar]
- [12].Zen Y, Nakanuma Y. IgG4-related disease: a cross-sectional study of 114 cases. Am J Surg Pathol 2010;34:1812–9. [DOI] [PubMed] [Google Scholar]
- [13].Vasaitis L. IgG4-related disease: a relatively new concept for clinicians. Eur J Int Med 2016;27:1–9. [DOI] [PubMed] [Google Scholar]
- [14].Carruthers MN, Khosroshahi A, Augustin T, et al. The diagnostic utility of serum IgG4 concentrations in IgG4-related disease. Ann Rheum Dis 2015;74:14–8. [DOI] [PubMed] [Google Scholar]
- [15].Masaki Y, Kurose N, Yamamoto M, et al. Cutoff values of serum IgG4 and histopathological IgG4+ plasma cells for diagnosis of patients with IgG4-related disease. Int J Rheumatol 2012;2012:580814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Vasaitis L. IgG4-related disease: a relatively new concept for clinicians. Eur J Intern Med 2015;27:1–9. [DOI] [PubMed] [Google Scholar]
- [17].Hart PA, Kamisawa T, Brugge WR, et al. Long-term outcomes of autoimmune pancreatitis: a multicentre, international analysis. Gut 2013;62:1771–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


