Abstract
Rationale:
Paroxysmal nocturnal hemoglobinuria (PNH) is a nonmalignant acquired hematopoietic stem cell disease, which can be revealed by hemolytic anemia, thromboembolism, or bonemarrow failure. Thrombosis can occur at any site, but coronary thrombosis is extremely rare. Controlled trials have demonstrated that eculizimab, an inhibitor of the terminal complement cascade, was able to reduce both hemolysis and thrombosis, but its efficacy in cases of PNH with coronary thrombosis is unknown.
Patient concerns and diagnoses:
We report herein the unusual case of a 73-year-old patient presenting with recurrent coronary syndromes without associated stenosis, fever, marked inflammatory syndrome, and anemia, leading to a delayed diagnosis of PNH.
Intervention and outcomes:
Eculizumab allowed the resolution of fever and inflammation, and prevented further thromboembolism.
Lessons:
This case emphasizes the importance of performing aflow cytometry test for PNH in front of unusual or unexplained recurrent thromboses. Thromboses, as observed in our case, may be associated with fever and marked inflammation. This case also provides useful information on eculizumab ability to prevent further thromboembolism in PNH patients with a medical history of arterial thrombosis.
Keywords: coronary thrombosis, eculizumab, PNH
1. Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare nonmalignant acquired clonal hematopoietic stem cell disorder that manifests with hemolytic anemia, bone marrow (BM) failure, and thrombosis. In nearly all the cases of PNH, an acquired somatic mutation of the X-linked PIG-A (phosphatidylinositol glycan class A) gene can be found, which is responsible for the expansion of hematopoietic stem cells lacking functional glycosylphosphatidylinositol (GPI) anchor. This results in the deficiency of complement inhibitory proteins, leading to complement-mediated hemolysis, and also activation of monocytes, granulocytes, and platelets with formation of prothrombotic microparticles. Moreover, hemolysis with liberation of high levels of free hemoglobin leads to scavenging of nitric oxide, which contributes to platelet activation and aggregation.[1,2]
In PNH, thrombosis can occur at any sites, the most common of which being hepatic vein (Budd–Chiari syndrome), mesenteric and portal veins, cerebral veins, and dermal veins. The reasons underlying the occurrence of thrombosis in these particular locations remain largely unknown.[3–5] Arterial thrombosis is far less frequent and such less common presentation may delay the diagnosis. Thromboembolism is the most common cause of mortality in PNH, and the 4-year survival of patients presenting with thrombosis at diagnosis was only 40% before the era of eculizumab.[6] Eculizumab is a humanized monoclonal antibody that blocks terminal complement pathway by binding to C5. This drug has dramatically changed the natural history of PNH.[7–12] Current evidence indicates that compared with placebo, eculizumab increases transfusion independency and health-related quality of life. Open-label studies and case reports suggest that eculizumab also reduces the risk of further thrombotic events.[7,12]
We report herein the case of a patient presenting with recurrent febrile arterial coronary thromboses, despite both antiplatelet treatment and curative anticoagulation. Fever and marked inflammatory syndrome delayed both diagnosis and initiation of eculizimab, and this delay was life-threatening for the patient. Finally, he was successfully treated with eculizumab and did no longer experience thrombotic events during the period of follow-up.
2. Case report
A 73-year-old male patient with a medical history of multiple thromboses (2 episodes of deep vein thrombosis in 1995 and 2011, and 2 episodes of pulmonary embolism in 1995 and 2004) presented in 2013 two consecutive acute coronary syndromes within a 1-month interval, while on continuous anticoagulation with vitamin K antagonists (fluindione). The patient was first admitted to cardiology department for acute thoracic pain in May 2013. Electrocardiogram evidenced inferior Q waves with ST elevation. Echography revealed antero-inferior akinesia. Serum troponins were not quantified because the patient was immediately transferred for coronarography. This coronary catheterization revealed an intraluminal thrombus in the right coronary artery. Balloon angioplasty was performed, but no stent was inserted because of unexplained fever. Antiplatelet therapy with both acetylsalicylic acid and clopidogrel was added to fluidione. A month later, the patient presented again with typical acute chest pain. Electrocardiogram always evidenced inferior Q waves with no other abnormalities. Echography found the known antero-inferior akinesia. Ultrasensitive troponins were elevated (560 ng/L, n < 14). A new coronary catheterization was performed and again evidenced a thrombosis in the right coronary artery and a new asymptomatic incomplete intraluminal obstruction of the circumflex artery (see Fig. 1, which illustrates the 2 successive coronary catheterizations). Again, angioplasty was performed without any vascular stenting because of unexplained fever since 2 months. Because of this persistent fever and marked inflammatory syndrome, additional investigations were performed. Clinically, the patient also presented with fatigue and signs of anemia. Hemoglobin level was 90 g/L, reticulocytes were 52 × 109/L, white blood cells 6.6 × 109/L with normal differential, and platelet count was 52 × 109/L. C-reactive protein (CRP) level was 147 mg/L. All tests for infectious, systemic inflammatory diseases, and vasculitis remained negative. Serum haptoglobin was decreased (<0.10 g/L) and lactate dehydrogenase (LDH) level was markedly elevated. Direct antiglobulin test was negative. A computerized tomography (CT) scan was performed and showed a thrombosis of the hepatic vein. Given the association of multiple thromboses, despite anticoagulation and signs of hemolysis, a flow cytometry test was performed and disclosed a big-sized PNH clone with 80% of neutrophils lacking expression of CD66b, CD16, and CD24 antigens, and more than 80% of monocytes lacking CD24 antigen. BM smears were normocellular and there were only mild signs of dysmegacaryocytopoiesis without excess blasts (2%). Karyotype was normal. The diagnosis of classical PNH was retained. Because of the presence of fever and inflammatory syndrome, and given the increased risk of severe infections after terminal complement blockade, the decision to start eculizumab was postponed. Rapidly, the patient presented with acute abdomen, hypotension, and functional renal insufficiency. He was admitted to the intensive care unit. A CT scan was not helpful in making a diagnosis, and a surgical exploration of the abdomen was performed. As no major abnormality was found, acute abdomen was hypothesized to be linked to multiple thromboses of small abdominal vessels, and treatment with eculizumab was finally started. Required prophylaxis, including vaccination against meningococcal and pneumococcal infection and penicillin V therapy, was performed. In addition, both anticoagulation and antiplatelet treatments were maintained. Shortly after the initiation of eculizumab, the patient recovered. Fever, abdominal pain, and inflammatory syndrome resolved. The main biological data observed during eculizumab therapy are reported in Table 1. During the entire follow-up period, the patient did not experience any further episode of thrombosis. LDH level returned to normal, but the patient still required repeated transfusions of red blood cell packs.
Figure 1.
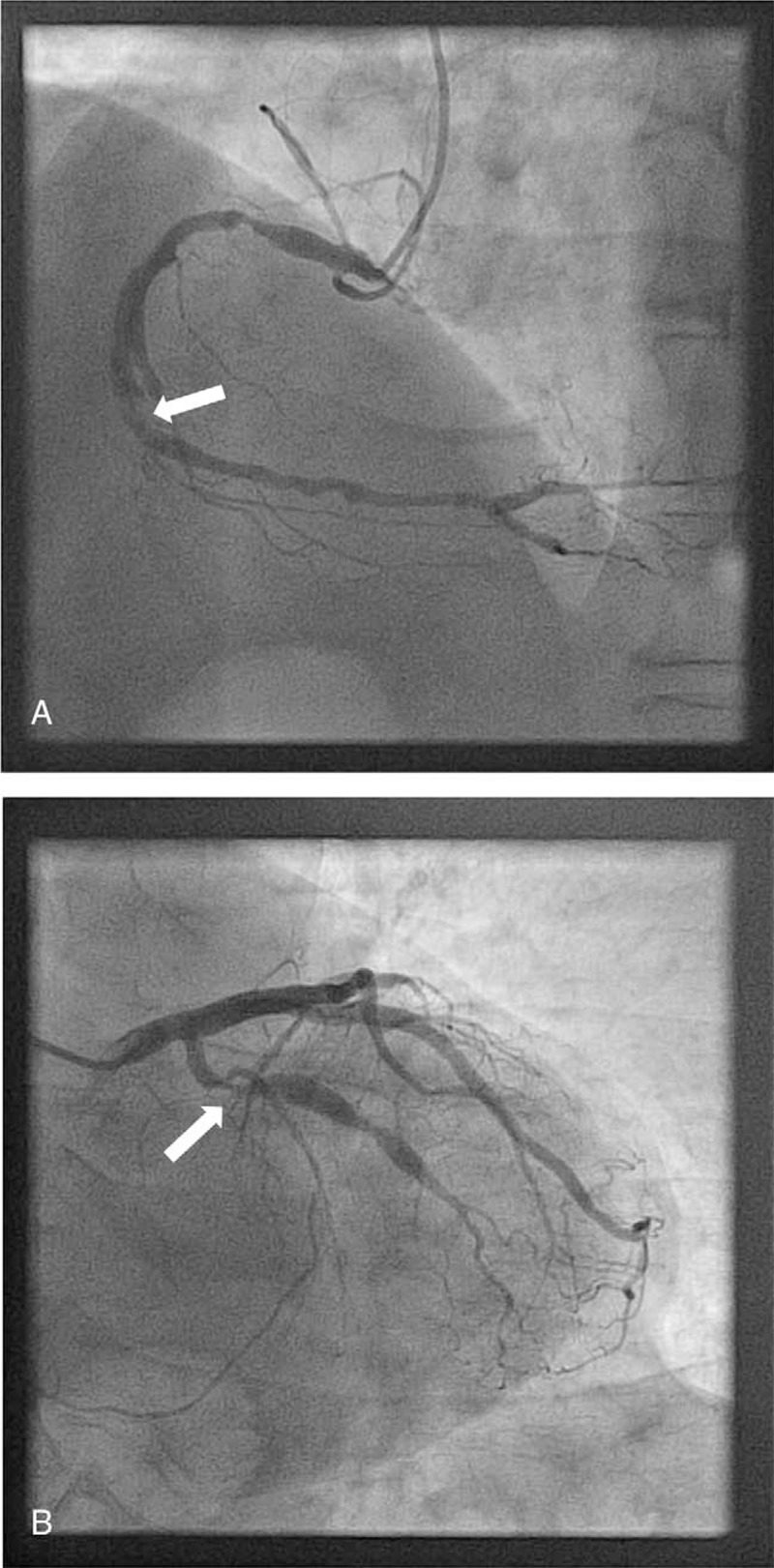
Coronary catheterization images. A, The patient was admitted in cardiology department for acute thoracic pain in May 2013. Electrocardiogram evidenced inferior Q waves with ST elevation. Echography revealed antero-inferior akinesia. Coronary catheterization showed a thrombosis in the right coronary artery (arrow). B, A month later, the patient presented again with typical acute chest pain despite dual antiplatelet therapy and vitamin K antagonist. Electrocardiogram always evidenced inferior Q waves with no other abnormalities. Echography found the known antero-inferior akinesia. Ultrasensitive troponins were elevated (560 ng/L, n < 14). A new coronary catheterization was performed and evidenced a thrombosis in the right coronary artery and a new asymptomatic incomplete intraluminal obstruction of the circumflex artery (arrow).
Table 1.
Evolution of biological tests on eculizumab therapy.
Eight months later, in March 2014, a severe thrombocytopenia occurred. There were no signs of hemolysis. A new BM examination showed a normocellular BM with dysplasia of the 3 myeloid lineages and 11% blasts. This led to the diagnosis of refractory anemia with excess blast-2 (myelodysplastic syndrome with an excess of blasts in the BM comprised within the range of 11%–19%) associated with PNH.BM karyotype was still normal. Vitamin K antagonists were stopped because of thrombocytopenia, but antiplatelet treatment was maintained. Both platelet and red blood cell transfusions were performed, only leading to very transient improvement. Because of his age and poor physical condition, the patient was not eligible for BM transplantation. Hypomethylating treatment with 5-azacytidine (AZA) was added to eculizumab. After 2 cycles of AZA, the cytopenias remained unchanged. The patient was admitted in July 2014 to the intensive care unit because of septic shock due to the infection of his implantable chamber by Aeromonas veronii, rapidly leading to multiorgan failure and death, despite broad-spectrum antibiotics and vasopressor medication.
2.1. Patient consent
Patient's wife and children have given their informed consent for the case report to be published.
3. Discussion
This case reports an unusual presentation of PNH, as PNH was revealed by 2 acute coronary syndromes and elevated makers of inflammation mimicking vasculitis, infectious or connective tissue diseases, and delaying both diagnosis and treatment.
One of the particularities of this case is the presence of fever and marked inflammatory syndrome at diagnosis of PNH. Inflammation is not a classical feature of PNH, but may be observed in case of extensive thrombosis independently of the underlying etiology. It can also be driven by activation of the complement pathway as observed in PNH. Because of both inflammation and the increased risk of severe infections related to terminal complement blockade, diagnosis and treatment were delayed. During this time, the state of the patient worsened, leading to his transfer to an intensive care unit and to useless abdominal surgery. Recovery of the patient along with regression of both fever and inflammatory biological signs on eculizumab confirmed that extensive thromboses and complement activation were probably responsible for this critical state. Consequently, marked inflammation does not exclude the diagnosis of PNH and does not have to delay treatment with eculizumab if acute infection is not evidenced.
Thrombosis appears as a common complication of PNH, occurring in 40% of the patients during the course of the disease. The pathogenesis of such event is still unclear, and seems to involve both platelet and endothelial activation due to hemolysis, nitric oxide deficiency, and activation of the complement pathway. Arterial thromboembolism is far less frequent than venous thromboembolism in the setting of PNH (15% of the thromboses), and less than 20 cases of coronary thrombosis associated with PNH have been reported until now.[5,13–16] In a meta-analysis on 363 PNH cases with thrombosis, 12 episodes of myocardial infarction were described. Despite a very low frequency, thromboses of the coronary arteries were found to be a significant predictor of thrombosis-related mortality (Risk Ratio 20.53, 95% confidence interval [CI] 4.42–95.28).[5] This emphasizes the importance of performing rapidly flow cytometry analysis for PNH in patients with unexpected or recurrent thromboses even in arterial vessels.
As observed in this case and in previous reports, recurrent thromboses may occur despite curative anticoagulation and antiplatelet therapy in PNH.[10,17,18] In our patient, eculizumab was effective to prevent further venous or arterial thrombotic events. Eculizumab treatment has demonstrated its efficacy in reducing hemolysis and the need for red cell transfusions in a controlled trial.[8] It has been shown to also reduce the risk of further thrombosis in open-label studies[10] and in case reports, but not in a controlled trial.[19] Eculizumab blocks terminal complement pathway, which appears to be 1 of the key factors of thrombosis in PNH. Consequently, eculizumab is recommended in PNH patients not only in the presence of symptomatic hemolysis but also in cases with thrombosis, even if there are no prospective data concerning this complication.[3,12] In addition, an important question to be addressed is whether anticoagulation can be discontinued after either venous or arterial thromboses in the context of PNH treated with eculizumab. Successful discontinuation of anticoagulation in patients with previous thrombosis receiving eculizumab has been reported.[20] Nevertheless, continued anticoagulation is still recommended for PNH patients with a prior thrombosis receiving eculizumab, unless there are clear contra-indications to anticoagulation.[3] In our case, both vitamin K antagonists and antiplatelet treatment were initially maintained, but anticoagulation was stopped when the patient developed a myelodysplastic syndrome with severe thrombocytopenia. However, antiplatelet treatment was pursued, and no major hemorrhagic event occurred.
A variable degree of BM failure is present in all patients with PNH. In some patients, evidence of BM dysfunction might be subtle (inappropriately low reticulocyte count), whereas some other will present with marked cytopenias at diagnosis. In our patient, BM cellularity was initially normal, morphology was near normal, and there were no cytogenetic abnormalities. The PNH clone was over 50% by flow cytometry. The diagnosis of classical PNH was retained and there was no argument for “PNH in the setting of another specified BM disorder (eg, PNH/aplastic anemia or PNH/refractory anemia-MDS)”, as defined by the classification developed by the International PNH Interest Group.[2] Clonal evolution such as the development of a myelodysplastic syndrome or an acute myeloid leukemia is classical in PNH. In our case, the short delay between PNH diagnosis and the onset of myelodysplastic syndrome raises the question of a putative role of complement blockade in the arising of clonal evolution. In a recent retrospective treatment versus no treatment study, no significant difference regarding clonal evolution between patients receiving or not receiving eculizumab was found, even if a longer follow-up is needed.[12]
4. Conclusions
This case reports an uncommon presentation of PNH, with recurrent coronary syndrome and systemic inflammation. It emphasizes the importance of looking for hemolysis markers and performing rapidly a flow cytometry test for PNH in front of an unusual or unexplained thrombosis, even more if it occurs while on anticoagulation or antiplatelet treatment. This observation also provides information on the efficacy of eculizumab to prevent recurrences after arterial thrombosis.
Acknowledgment
The authors are grateful to Ms Wuibout for her corrections of the manuscript.
Footnotes
Abbreviations: AZA = 5-azacytidine, BM = bone marrow, CRP = C-reactive protein, CT = computerized tomography, GPI = glycosylphosphatidylinositol, LDH = lactate dehydrogenase, PIG-A = phosphatidylinositol glycan class A, PNH = paroxysmal nocturnal hemoglobinuria.
Conflicts of interest: Professor Peffault de Latour received research grant from Alexion and declared consultancy and international board membership for Alexion. The other authors stated they have no relevant conflicts of interest.
References
- [1].Brodsky RA. Paroxysmal nocturnal hemoglobinuria. Blood 2014;124:2804–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Parker C, Omine M, Richards S, et al. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood 2005;106:3699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hill A, Kelly RJ, Hillmen P. Thrombosis in paroxysmal nocturnal hemoglobinuria. Blood 2013;121:4985–96. [quiz 5105]. [DOI] [PubMed] [Google Scholar]
- [4].Van Bijnen ST, Van Heerde WL, Muus P. Mechanisms and clinical implications of thrombosis in paroxysmal nocturnal hemoglobinuria. J Thromb Haemost 2012;10:1–0. [DOI] [PubMed] [Google Scholar]
- [5].Ziakas PD, Poulou LS, Rokas GI, et al. Thrombosis in paroxysmal nocturnal hemoglobinuria: sites, risks, outcome: an overview. J Thromb Haemost 2007;5:642–5. [DOI] [PubMed] [Google Scholar]
- [6].Socie G, Mary JY, de Gramont A, et al. Paroxysmal nocturnal haemoglobinuria: long-term follow-up and prognostic factors. French Soc Haematol Lancet (London, England) 1996;348:573–7. [DOI] [PubMed] [Google Scholar]
- [7].Hillmen P, Muus P, Roth A, et al. Long-term safety and efficacy of sustained eculizumab treatment in patients with paroxysmal nocturnal haemoglobinuria. Br J Haematol 2013;162:62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Brodsky RA, Young NS, Antonioli E, et al. Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood 2008;111:1840–7. [DOI] [PubMed] [Google Scholar]
- [9].Hillmen P, Young NS, Schubert J, et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med 2006;355:1233–43. [DOI] [PubMed] [Google Scholar]
- [10].Hillmen P, Muus P, Duhrsen U, et al. Effect of the complement inhibitor eculizumab on thromboembolism in patients with paroxysmal nocturnal hemoglobinuria. Blood 2007;110:4123–8. [DOI] [PubMed] [Google Scholar]
- [11].Weitz IC, Razavi P, Rochanda L, et al. Eculizumab therapy results in rapid and sustained decreases in markers of thrombin generation and inflammation in patients with PNH independent of its effects on hemolysis and microparticle formation. Thromb Res 2012;130:361–8. [DOI] [PubMed] [Google Scholar]
- [12].Loschi M, Porcher R, Barraco F, et al. Impact of eculizumab treatment on paroxysmal nocturnal hemoglobinuria: a treatment versus no-treatment study. Am J Hematol 2016;91:366–70. [DOI] [PubMed] [Google Scholar]
- [13].Taniguchi A, Ikezoe T, Takeuchi A, et al. Successful eculizumab treatment for multiple coronary thrombosis complicated in paroxysmal nocturnal hemoglobinuria. Rinsho Ketsueki 2014;55:965–9. [PubMed] [Google Scholar]
- [14].Melandri F, Gazzotti G, Fontana P, et al. Recurrent acute myocardial infarction in a patient with nocturnal paroxysmal hemoglobinuria. Ital Heart J Suppl 2001;2:792–4. [PubMed] [Google Scholar]
- [15].Klein KL, Hartmann RC. Acute coronary artery thrombosis in paroxysmal nocturnal hemoglobinuria. South Med J 1989;82:1169–71. [DOI] [PubMed] [Google Scholar]
- [16].Gerber B, Kyburz T, Reinhart WH, et al. Complement inhibition to treat myocardial infarction? BMJ Case Rep 2011;pii:bcr0120113701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].de Latour RP, Mary JY, Salanoubat C, et al. Paroxysmal nocturnal hemoglobinuria: natural history of disease subcategories. Blood 2008;112:3099–106. [DOI] [PubMed] [Google Scholar]
- [18].Moyo VM, Mukhina GL, Garrett ES, et al. Natural history of paroxysmal nocturnal haemoglobinuria using modern diagnostic assays. Br J Haematol 2004;126:133–8. [DOI] [PubMed] [Google Scholar]
- [19].Marti-Carvajal AJ, Anand V, Cardona AF, et al. Eculizumab for treating patients with paroxysmal nocturnal hemoglobinuria. Cochrane Database Syst Rev 2014;10: CD010340. [DOI] [PubMed] [Google Scholar]
- [20].Emadi A, Brodsky RA. Successful discontinuation of anticoagulation following eculizumab administration in paroxysmal nocturnal hemoglobinuria. Am J Hematol 2009;84:699–701. [DOI] [PubMed] [Google Scholar]


