Abstract
Brother of regulator of imprinted sites (BORIS) is a DNA-binding protein that is normally expressed in the testes. However, aberrant expression of BORIS is observed in various carcinomas, indicating a malignant role for this protein. Furthermore, abolishment or reduction of suppressor of cytokine signaling 3 (SOCS3) expression directed by promoter methylation is considered significant in hepatocellular carcinoma (HCC) carcinogenesis. This study aims to investigate BORIS and SOCS3 expression in HCC specimens and assess the prognostic significance of these proteins.
BORIS and SOCS3 expression was examined using immunohistochemistry in HCC tissues, along with corresponding paracarcinomatous, cirrhosis, hepatitis, and normal liver tissues. The expression levels of these 2 proteins in HCC were evaluated for their association with clinicopathological parameters. Survival analysis was performed using Kaplan–Meier curves, the log-rank test, and multivariate Cox regression analysis.
BORIS expression was significantly higher in HCC tissues than in normal liver tissues. In contrast, SOCS3 expression was dramatically lower in HCC tissues. BORIS expression was associated with tumor size, differentiation grade, satellite lesions, and recurrence while SOCS3 expression correlated with differentiation grade, vascular invasion, and recurrence. A significant negative correlation between BORIS and SOCS3 was observed. Patients with high BORIS expression and/or low SOCS3 expression had poorer postoperative survival. Patients with both these characteristics had the poorest prognostic outcome.
BORIS and SOCS3 are promising as valuable indicators for predicting HCC prognosis.
Keywords: BORIS, hepatocellular carcinoma, immunohistochemistry, prognosis, SOCS3
1. Introduction
Hepatocellular carcinoma (HCC) is the 5th most common malignancy and the 3rd leading cause of cancer-related mortality worldwide.[1] Most cases of HCC are diagnosed at an advanced stage.[2] Despite great improvements in surgical treatments and early diagnostic technologies, the long-term survival of HCC after surgical resection still remains unsatisfactory because of a high rate of postoperative recurrence.[3,4] Therefore, it is extremely important to identify sensitive and reliable biomarkers for the diagnosis, prognosis, and target therapy of HCC.
Brother of the regulator of imprinted sites (BORIS) was first reported as a DNA-binding protein. It exhibits high homology with its paralog CCCTC-binding factor (CTCF) through the sharing of an 11 zinc-finger domain, and is thus termed CTCFL (CTCF-like).[5] BORIS protein is not expressed in normal somatic tissues and is only found in male testicular tissue.[6] CTCF, acting as a DNA-binding protein, silences genes through DNA methylation. Lobanenkov et al[7] found that CTCF negatively regulates the c-myc gene, and CTCF was consequently identified as a tumor suppressor. However, in some cancer cell lines, conditional expression of BORIS induces replacement of CTCF by BORIS, leading to DNA demethylation and gene activation.[8,9] In addition, recent studies indicate that aberrantly high expression of BORIS was detected in a variety of human malignances, including ovarian,[10] prostate,[11] esophageal,[12] and hepatocellular cancers.[13] High expression of BORIS in ovarian cancers also conferred a poor prognosis.[10] All of this evidence implies a malignant phenotype for BORIS. Although our previous study found increased expression of BORIS in HCC,[13] the prognostic value of BORIS for HCC patients after curative resection has not been clarified.
The suppressors of cytokine signaling (SOCS) family proteins function as cytokine signaling inhibitors of the JAK/STAT signaling pathway. At present, 8 SOCS proteins have been identified. These proteins have similar structures but variant mechanisms for inhibiting the JAK/STAT pathway cytokines.[14,15] Suppressor of cytokine signaling 3 (SOCS3) is a major member of the SOCS family. As a part of a classical feedback loop, SOCS3 negatively regulates STAT3 by inhibiting its phosphorylation. Furthermore, SOCS3 induces inhibition of JAK by binding to cytokine receptors that contain JAK proximal sites.[16,17] Frequent hypermethylation of the SOCS3 gene has been observed in diverse malignancies, including head and neck cancer,[18] lung cancer,[19] prostate cancer,[20] and HCC.[21] Moreover, Niwa et al[22] revealed that the methylation-directed silencing of the SOCS3 gene may promote cell growth and migration in HCC. Deletion of the SOCS3 gene in hepatocytes has been proved to promote hepatitis-induced hepatocarcinogenesis.[23] Yang et al[24] reported that a high level of SOCS3 in HCC is associated with a poor survival rate. In contrast, Zhang et al[21] found that loss of SOCS3 predicted a poor prognosis for HCC patients with hepatitis B virus (HBV) infection. Similar results were observed in cholangiocarcinoma.[25] Because these results are in conflict, it is imperative to clarify the role of SOCS3 in the prognostic outcome of HCC.
The present study aims to investigate the status of BORIS and SOCS3 expression in a cohort of various liver lesions, including HCC, paracarcinomatous nontumor, liver cirrhosis, hepatitis, and normal liver tissues, using immunohistochemical (IHC) staining and to determine their correlations with clinicopathological parameters in HCC tissues. Survival analyses were also performed to assess the prognostic relevance of these 2 biomarkers. Our results indicate that BORIS and SOCS3 may serve as novel prognostic biomarkers for HCC patients.
2. Methods
2.1. Patients and tissue samples
HCC and corresponding paracarcinomatous nontumor tissues were obtained from 85 patients who underwent curative hepatic resection at West China Hospital (Chengdu, China) between August 2009 and July 2011. Patients who died within 1 month after surgery or died from unrelated conditions were not included. The diagnosis of HCC was confirmed by senior pathologists. All paracarcinomatous nontumor samples were obtained from tissue at least 3 cm away from the tumor edge. None of the HCC patients had other accompanying malignancies or received radiotherapy or chemotherapy treatment before surgery. The patient cohort consisted of 67 males and 18 females, with a median age of 50.4 years (range, 29–79 years). Pathological parameters such as tumor size, differentiation grade, tumor encapsulation, satellite lesion, and vascular invasion (including vascular invasion and/or cancer thrombus in the portal vein or hepatic vein) were obtained from pathological reports and medical records, along with preoperative serum alpha-fetoprotein levels and HBV infection status (Table 2). All pathology data for HCC were evaluated based on the 7th edition of the American Joint Committee on Cancer. Follow-up was terminated in March, 2016. The median follow-up period was 30 months (range, 3–72 months). The overall survival (OS) and disease-free survival (DFS) were calculated from the date of surgery to the date of death/latest follow-up and recurrence, respectively.
Table 2.
Correlation between clinicalpathological characteristics and expression of CD90 and OCT4 in HCC patients.
In addition, 25 cirrhosis samples and 10 hepatitis samples were collected from individuals without malignant tumors who underwent liver transplantation, hepatic resection, or puncture biopsy. Another 12 normal liver tissues were obtained from adult patients who underwent hepatectomy for benign conditions such as hemangioma. All excised samples were made into formalin-fixed and paraffin-embedded blocks and stored in the pathology department.
2.2. Immunohistochemical (IHC) staining
IHC was performed as previously described.[26] Formalin-fixed and paraffin-embedded specimens were cut into serial sections with a thickness of 4 μm and heated at 55 °C for 30 minutes. Slides were then deparaffinized in xylene and subsequently rehydrated in a series of graded ethanol solutions. Afterwards, the slides were incubated with 3% hydrogen peroxide solution at room temperature for 10 minutes to eliminate endogenous peroxidase activity. Next, the slides were subjected to antigen retrieval in sodium citrate buffer (10 mM, pH 6.0) for 30 minutes at 98 °C and then cooled at room temperature. After incubation with 5% normal goat serum for 30 minutes at 37 °C to block nonspecific binding, the slides were incubated with anti-BORIS rabbit polyclonal antibody (ab126766, Abcam, Cambridge, USA, at 1:200 dilution) or anti-SOCS3 rabbit polyclonal antibody (ab16040, at 1:150 dilution) overnight at 4 °C. After washing with PBS solution 3 times, the slides were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody for 40 minutes at 37 °C. Slides were stained with 3,3′-diaminobenzidine tetrahydrochloride (DAB) and observed under the microscope, followed by counterstaining with hematoxylin. Negative controls were treated the in same way, replacing the primary antibody with rabbit IgG.
2.3. Assessment of IHC staining
Two pathologists, who were blinded to the clinical data follow-up information, examined the slides independently. A semiquantitative immunoreactivity score (IRS) was applied to evaluate expression levels. The chromogenic reaction intensity was classified as 1 of 4 grades using a scale of 0–3 (0, negative; 1, weak; 2, moderate; and 3, strong). The percentage of positive-staining cells in 5 random, nonoverlapping fields of each slide at a magnification of 400 × was also classified as 1 of 5 grades as follows: 0 (<5%), 1 (5%–25%), 2 (26%–50%), 3 (51%–75%), and 4 (>75%). The IRS (0–12) was then calculated by multiplying the score values.[27] The final IRS scores from the 2 pathologists were compared. When divergence occurred in assigning a score, the slide was reappraised until a consensus was reached. For categorical analyses, sections were graded as high expression (total score >4) and low expression (total score ≤4).[28]
2.4. Ethics
This study was approved by the Human Research Ethics committee of the West China hospital, Sichuan University (Chengdu, China). All participating patients voluntarily offered written informed consent. All clinical investigations were conducted in accordance with the principles expressed in the Declaration of Helsinki.
2.5. Statistics
Statistical analyses were performed using SPSS version 19.0 software (Chicago, IL). The Mann–Whitney U test was employed to compare expression levels of BORIS and SOCS3 between different liver lesions. The correlation between the expression of 2 biomarkers and clinicopathological parameters was evaluated using the Chi-square test. The Spearman correlation test was used to evaluate linear correlations between the 2 biomarkers with GraphPad Prism v5.0. Survival analyses were conducted using the Kaplan–Meier method with GraphPad Prism v5.0. Comparisons of survival distributions were performed using the log-rank test. Multivariate survival analyses were accomplished by a Cox-proportional hazards model with forward stepwise selection. P < 0.05 was considered statistically significant, with all P-values based on 2-sided statistical analysis.
3. Results
3.1. Expression levels of BORIS in different types of liver disease
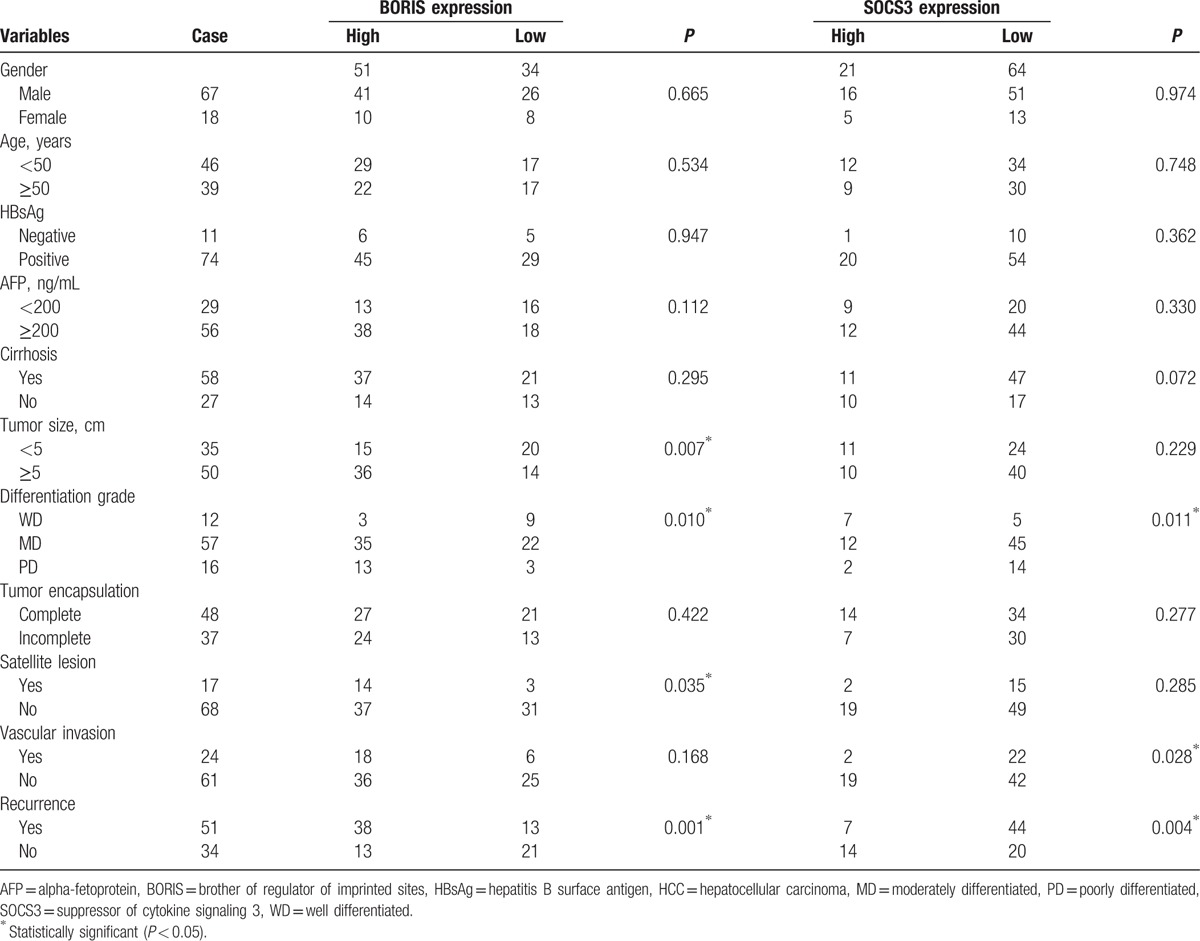
IHC analysis showed that BORIS staining was present in the cytoplasm and nuclei of tumor cells. For paracarcinomatous nontumor, cirrhosis, and hepatitis tissues, weak BORIS expression was mainly localized in the cytoplasm of hepatocytes. BORIS was nearly negative in normal livers (Fig. 1A). BORIS staining scores were significantly higher in HCC tissues than in the corresponding paracarcinomatous nontumor (P < 0.0001), cirrhosis (P < 0.0001), hepatitis (P = 0.0026), and normal liver tissues (P < 0.0001). Notably, the levels of BORIS expression in paracarcinomatous nontumor tissues were higher than those of normal liver (P = 0.0014). No statistically significant differences were observed in other comparisons (Fig. 1B).
Figure 1.
BORIS expression in different liver diseases. (A) Immunohistochemical staining in (a) HCC, (b) paracancerous nontumor, (c) cirrhosis, (d) hepatitis, and (e) normal liver (magnification 400×). (B) Comparison of staining score for BORIS expression between different liver diseases. –, no statistical significance; ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001; and ∗∗∗∗P < 0.0001. BORIS = brother of regulator of imprinted sites, HCC = hepatocellular carcinoma.
3.2. Expression levels of SOCS3 in different liver diseases
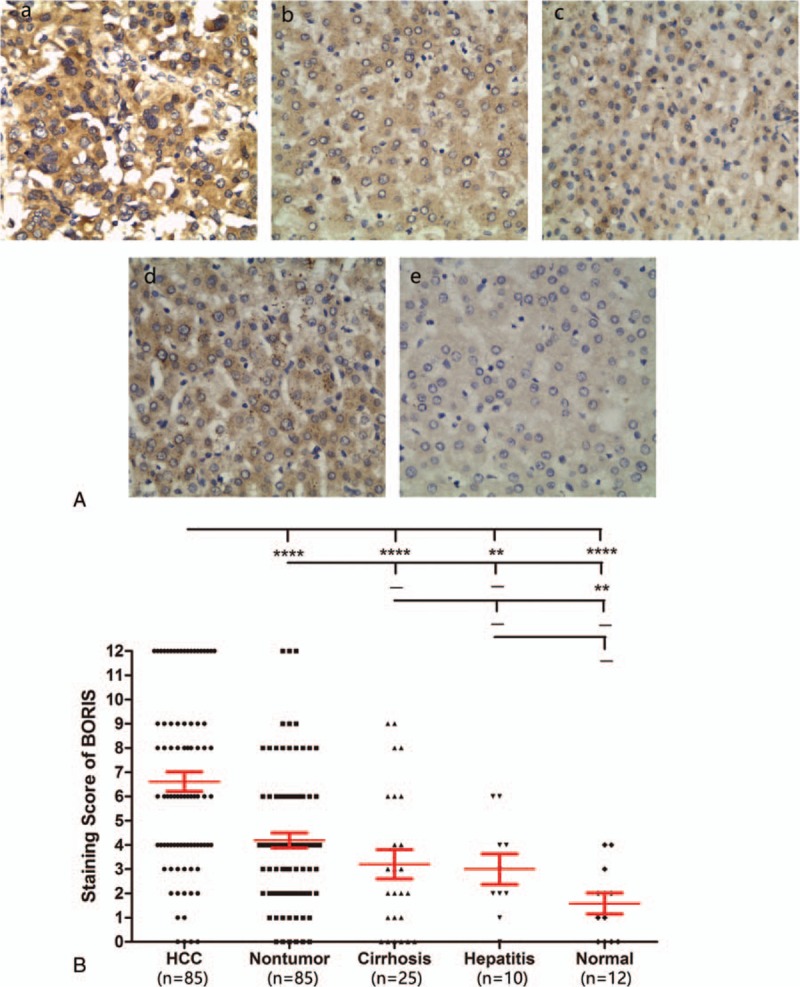
In normal liver, moderate SOCS3 expression was found in the cytoplasm of the majority of hepatocytes, vascular endothelial cells, and leukocytes. In cirrhosis and paracarcinomatous nontumor specimens, SOCS3 was barely observed in fractional hepatocytes and a few mesenchymal cells. However, SOCS3 was almost indiscernible in cancer cells. Interestingly, SOCS3 expression levels were dramatically elevated in hepatitis tissues, with some staining of hepatocyte nuclei (Fig. 2A). Quantitative comparisons showed that the expression levels of SOCS3 in HCC were significantly lower than in paracarcinomatous nontumor (P = 0.0031), hepatitis (P = 0.0002), and normal liver tissues (P = 0.0034). Notably, although no statistically significant differences were detected, hepatitis tissues exhibited a trend of higher expression of SOCS3 compared to normal liver (Fig. 2B).
Figure 2.
SOCS3 expression in different liver diseases. (A) Immunohistochemical staining in (a) HCC, (b) paracancerous nontumor, (c) cirrhosis, (d) hepatitis, and (e) normal liver (magnification 400×). (B) Comparison of staining score of SOCS3 expression among different types of liver diseases. –, no statistical significance; ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001; and ∗∗∗∗P < 0.0001. HCC = hepatocellular carcinoma, SOCS3 = suppressor of cytokine signaling 3.
3.3. Correlation of BORIS and SOCS3 expression with clinicopathological parameters in HCC
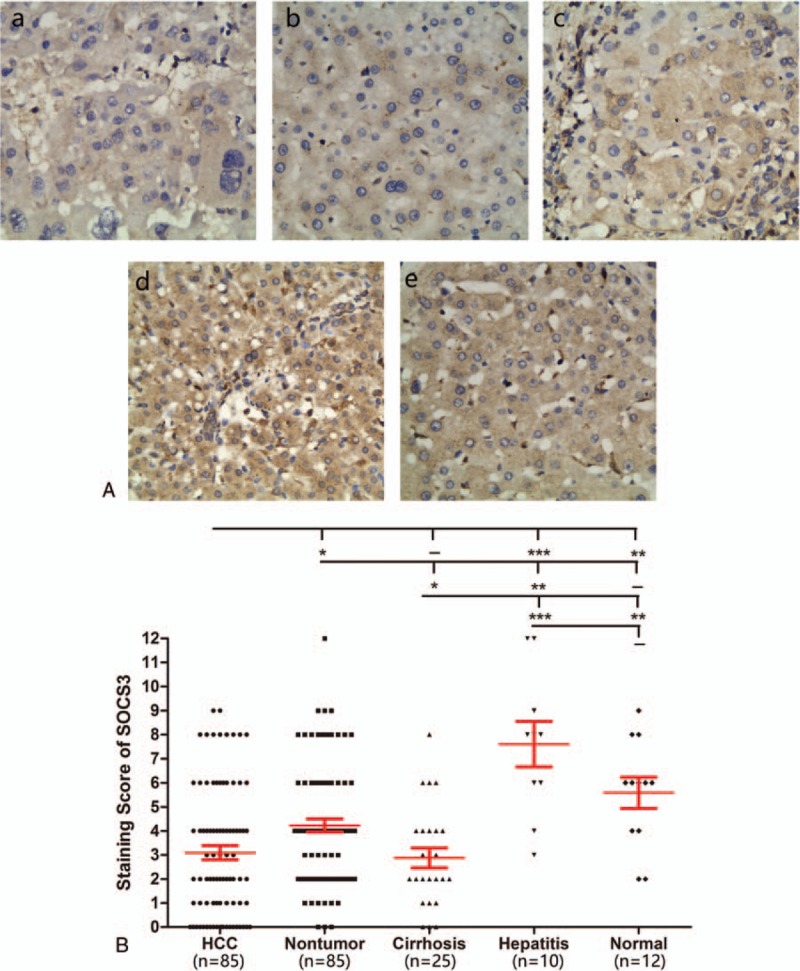
Next, the expression levels of BORIS and SOCS3 were categorized into high and low expression groups (Table 1). We then analyzed the correlation between the expression of these 2 categories in HCC and clinicopathological parameters. As shown in Table 2, we found that the expression of BORIS significantly correlated with tumor size (P = 0.007), differentiation grade (P = 0.010), satellite lesion (P = 0.035), and recurrence (P = 0.001). The expression of SOCS3 was associated with differentiation grade (P = 0.011), vascular invasion (P = 0.028), and recurrence (P = 0.004). No significant correlation was found with gender, age, tumor size, HBV infection, serum alpha-fetoprotein levels, cirrhosis of the nontumor parenchyma, or tumor encapsulation.
Table 1.
The expression of BORIS and SOCS3 in different types of liver lesions.
In addition, we found a significant negative correlation between the expression levels of BORIS and SOCS3 using the Spearman correlation (r = −0.361; P = 0.0007) (Fig. 3).
Figure 3.
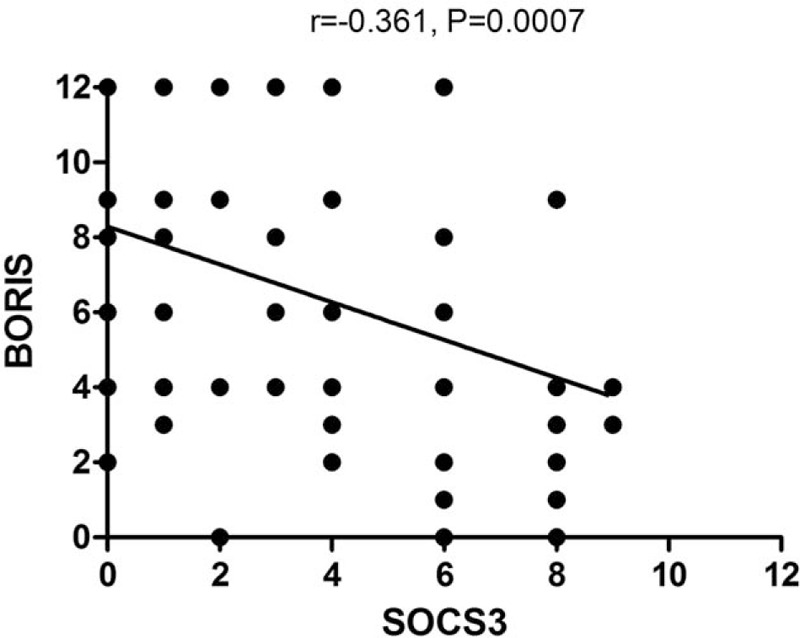
Correlation between BORIS and SOCS3 expression in HCC tissue. Spearman correlation analysis of BORIS and SOCS3 expression in HCC tissue. Each point represents a tissue sample, with n = 85 cases. The BORIS level was inversely correlated with SOCS3 expression. BORIS = brother of regulator of imprinted sites, HCC = hepatocellular carcinoma, SOCS3 = suppressor of cytokine signaling 3.
3.4. Independent prognostic factors of HCC
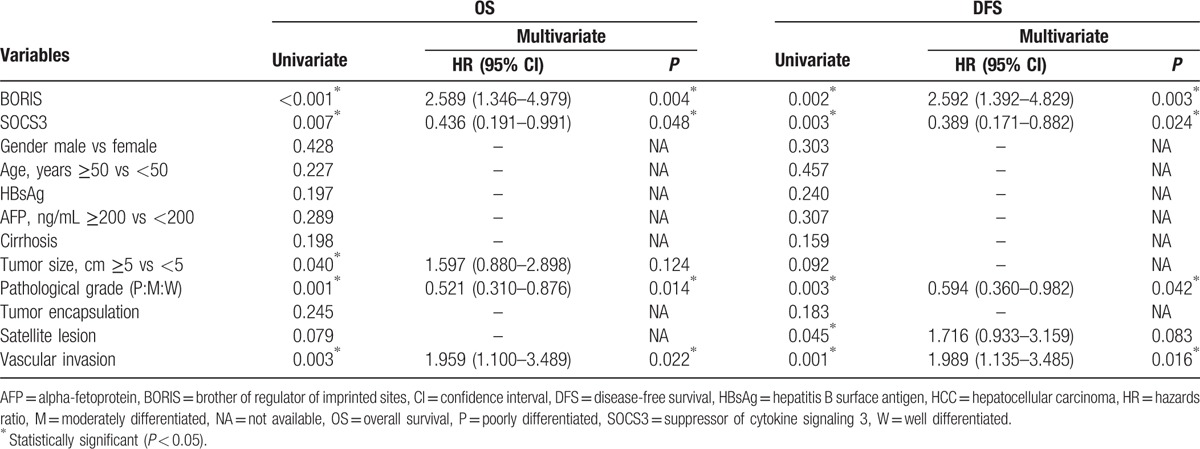
To determine potential risk factors linked to postoperative prognosis, we performed univariate and multivariate analysis to evaluate BORIS, SOCS3, and other clinicopathological parameters. As listed in Table 3, univariate analysis demonstrated that the significant prognostic factors for OS were BORIS expression (P < 0.001), SOCS3 expression (P = 0.007), tumor size (P = 0.040), differentiation grade (P = 0.001), and vascular invasion (P = 0.003). Similarly, the significant factors for DFS of HCC were BORIS expression (P = 0.002), SOCS3 expression (P = 0.003), differentiation grade (P = 0.003), satellite lesion (P = 0.045), and vascular invasion (P = 0.001). The 5 risk factors filtered by univariate analysis were further analyzed by multivariate analysis to determine the independent factors for prognosis. Ultimately, BORIS expression, SOCS3 expression, differentiation grade, and vascular invasion served as independent risk factors for both OS and DFS (all P < 0.05) (Table 3).
Table 3.
Prognostic factors for OS and DFS by univariate and multivariate analyses in HCC patients.
3.5. Association of BORIS and SOCS3 expression with survival in HCC patients
Kaplan–Meier survival analysis was applied to evaluate the relationship between BORIS/SOCS3 expression and OS and DFS in HCC patients (Fig. 4). Patients with high BORIS expression (median, 21 months) had a significantly shorter OS than did those with low expression (median, 41.5 months; P = 0.0003). Similarly, the DFS was significantly lower in patients with high BORIS expression (median, 12 months) than in those with low BORIS expression (median, 33 months; P = 0.0015). Conversely, patients with high SOCS3 expression had a longer OS than those with low (median, 41 vs 26.5 months, respectively; P = 0.0068) and consistent results for DFS (41 vs 14.5 months, respectively; P = 0.0035).
Figure 4.
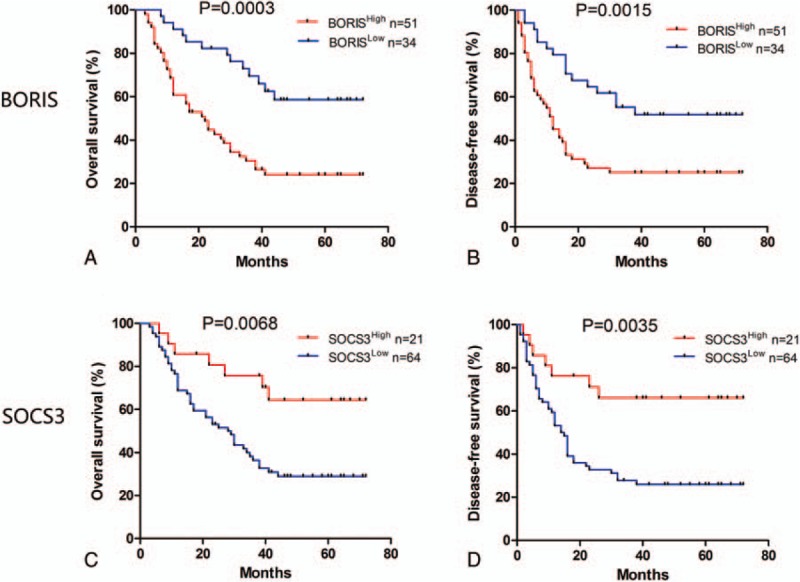
Kaplan–Meier survival curves for HCC patients after surgery according to BORIS and SOCS3 expression. (A, B) The OS and DFS were significantly longer in the BORISLow group than in the BORISHigh group. (C, D) The OS and DFS were significantly longer in the SOCS3High group than in the SOCS3Low group. BORIS = brother of regulator of imprinted sites, DFS = disease-free survival, HCC = hepatocellular carcinoma, OS = overall survival, SOCS3 = suppressor of cytokine signaling 3.
3.6. Low BORIS expression and high SOCS3 expression are associated with a favorable prognosis
To further analyze the association between BORIS and SOCS3 on the clinical outcomes of HCC, we divided patients into 4 groups as follows: BORISLow/SOCS3High, BORISLow/SOCS3Low, BORISHigh/SOCS3High, and BORISHigh/SOCS3Low (Fig. 5). The OS of the BORISLow/SOCS3High group (median = 62.5 months) was longer than that of the BORISLow/SOCS3Low group (median = 35 months), BORISHigh/SOCS3High group (median, 38 months), and BORISHigh /SOCS3Low group (median, 16.5 months). Similarly, the DFS was longest in the BORISLow/SOCS3High group (median, 62.5 months) and shortest in the BORISHigh/SOCS3Low group (median, 10.5 months).
Figure 5.
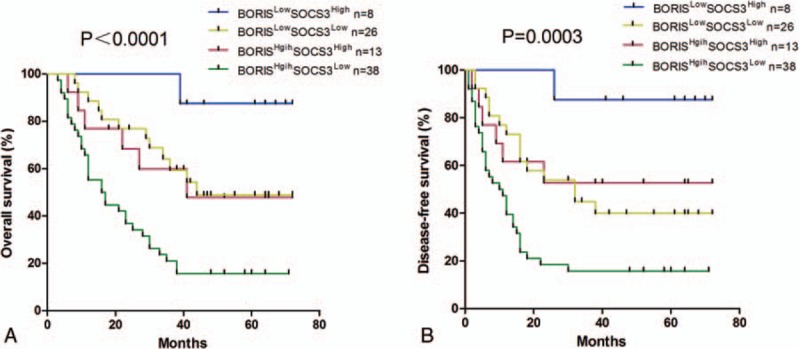
The combination of BORIS and SOCS3 was found to enhance accuracy of predicting prognosis of HCC patients. (A) OS curve. The BORISLow/SOCS3High group had the longest OS (median = 62.5 months) while the BORISHigh/SOCS3Low group suffered the shortest OS (median, 16.5 months). (B) DFS curve. The DFS was longest in the BORISLow/SOCS3High group (median, 62.5 months) and shortest in the BORISHigh/SOCS3Low group (median, 10.5 months). BORIS = brother of regulator of imprinted sites, DFS = disease-free survival, HCC = hepatocellular carcinoma, OS = overall survival, SOCS3 = suppressor of cytokine signaling 3.
4. Discussion
The BORIS gene is a member of the cancer-testis antigen family.[5] D’Arcy et al[29] found high levels of BORIS expression in the leukocytes of patients with breast cancer, indicating that BORIS can serve as a valuable marker. Furthermore, aberrant expression of BORIS in human cancer cells has been proposed to induce epigenetic deregulation. Expression of BORIS replaces CTCF in the genome and results in the proliferation of cancer cells.[6] In ovarian cancer, increased BORIS expression also correlates with advanced cancer stages and a decreased rate of survival.[10] These findings suggest that BORIS expression is greater in cancerous foci than in normal or benign lesions, which supports our findings. The typical transformation of normal liver to HCC is known to be a progressive, multistep process. For this reason, we also included hepatitis and cirrhosis tissues for examination in this study. Notably, the expression level of BORIS tended to increase gradually in normal liver, hepatitis, cirrhosis, paracarcinoma, and HCC tissues. This observation indicates that BORIS may play an important role in HCC tumorigenesis. A previous study suggested that BORIS may serve as a potential biomarker of cancer stem cells (CSC).[30] Alberti et al[31] found that BORIS is highly expressed in CSC-enriched populations. Moreover, BORIS plays an important role in the self-renewal of cancer cells and in the acquisition of epithelial mesenchymal transition capacity. Our results are in accordance with these findings, as high expression of BORIS is associated with larger tumors, poorer differentiation grade, and satellite lesion formation.
The data of IHC staining show that the expression level of SOCS3 is significantly lower in HCC than in normal liver or precancerous tissues. This result is consistent with the report that loss of SOCS3 induced by promoter methylation may contribute to the carcinogenesis and progression of malignances.[23] Interestingly, the level of SOCS3 was also dramatically lower in cirrhosis tissues. This observation might be explained by a previous report that decreased SOCS3 expression and the consequent activation of STAT3 are responsible for the hepatic fibrosis.[32] As reported, SOCS3 was activated and accumulated during inflammation.[33] It was observed that the hepatitis tissues had the highest level of SOCS3 expression, supporting the hypothesis that SOCS3 plays an important role in inflammatory damage repair. SOCS3 was found to be crucial for the suppression of tumor-associated angiogenesis, as it negatively regulates STAT3, which induces vascular endothelial growth factor (VEGF).[34] Similarly, we found that low expression of SOCS3 confers a higher risk of vascular invasion in HCC. Yang et al[24] reported that high expression of SOCS3 is associated with a poor OS in HCC patients. Strangely, this observation is contrary to the well-accepted concept that SOCS3 is a tumor suppressor. However, our data show that HCC patients with high SOCS3 expression had a favorable survival rate, which is consistent with the prognostic roles proposed for SOCS3 in lung cancer[19] and cholangiocarcinoma.[25]
Postoperative recurrence is a commonly known primary limitation to long-term survival of patients with HCC, and prediction of recurrence remains a great challenge.[35] Notably, our study found that BORIS and SOCS3 expression is significantly associated with HCC recurrence. Furthermore, high BORIS expression and low SOCS3 expression were also poor prognostic indicators in HCC patients who underwent surgical resection. The CSC hypothesis assumes that the recurrent tumors are derived from a transformed stem/early progenitor cancer cell with deregulated self-renewal and proliferation capacities.[36] Applying this hypothesis to our study, BORIS may act as a CSC marker that is involved in the recurrence of HCC. The use of a combination of markers is predicted to yield more information for predicting long-term survival of HCC patients.[37] Indeed, our results suggest that the combination of BORIS and SOCS3 provide better prognostic value than does either marker alone. Patients with both high BORIS expression and low SOCS3 expression had the poorest survival while those with low BORIS expression and high SOCS3 expression had the most favorable prognostic outcome.
Notably, we observed a negative correlation between BORIS and SOCS3 in HCC, which has not been reported before. CTCF is a kind of DNA-binding protein, which could bind to the promoter and thus regulates expression of a wide of human genes. As its paralog, BORIS was considered to have inverse function. Actually, it had been reported that expression of BORIS replaced CTCF in the genome and resulted in the proliferation of cancer cells.[6] Therefore, the aberrant activation of BORIS in HCC may shut down the expression of SOCS3 by epigenetic regulation, which could explain the negative correlation between these 2 biomarkers reasonably. However, clarification of this observation requires further investigation.
In conclusion, our study demonstrates that elevated BORIS expression and decreased SOCS3 expression are associated with tumor recurrence and a poor clinical outcome for patients with HCC. The predictive range of BORIS and SOCS3 expression combined was more sensitive than that of either BORIS or SOCS3 expression alone. Although the underlying mechanism is still unclear, a novel negative correlation between BORIS and SOCS3 was discovered. The reliable prognostic value of BORIS suggests its future use as a potential therapeutic target for HCC treatment.
Acknowledgments
The authors thank “The National Natural Science Foundation of China (no. 81172372)” for the support. The authors also thank Qun-Ying Li, Xiu-Qun Li, and Jian-Guo Qi for technical support.
Footnotes
Abbreviations: BORIS = brother of regulator of imprinted sites, CTCF = CCCTC-binding factor, DFS = disease-free survival, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, IHC = immunohistochemical, IRS = immunoreactivity score, OS = overall survival, SOCS3 = suppressor of cytokine signaling 3.
RZ and KC contributed equally to this work.
Funding/support: This study was supported by “The National Natural Science Foundation of China (no. 81172372).”
The authors have no conflicts of interest to disclose.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 2014;15:178–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cucchetti A, Trevisani F, Pecorelli A, et al. Estimation of lead-time bias and its impact on the outcome of surveillance for the early diagnosis of hepatocellular carcinoma. J Hepatol 2014;61:333–41. [DOI] [PubMed] [Google Scholar]
- [4].Altekruse SF, Henley SJ, Cucinelli JE, et al. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol 2014;109:542–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Klenova EM, Morse HC, 3rd, Ohlsson R, et al. The novel BORIS + CTCF gene family is uniquely involved in the epigenetics of normal biology and cancer. Semin Cancer Biol 2002;12:399–414. [DOI] [PubMed] [Google Scholar]
- [6].Loukinov DI, Pugacheva E, Vatolin S, et al. BORIS, a novel male germ-line-specific protein associated with epigenetic reprogramming events, shares the same 11-zinc-finger domain with CTCF, the insulator protein involved in reading imprinting marks in the soma. Proc Natl Acad Sci U S A 2002;99:6806–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lobanenkov VV, Nicolas RH, Adler VV, et al. A novel sequence-specific DNA binding protein which interacts with three regularly spaced direct repeats of the CCCTC-motif in the 5’-flanking sequence of the chicken c-myc gene. Oncogene 1990;5:1743–53. [PubMed] [Google Scholar]
- [8].Vatolin S, Abdullaev Z, Pack SD, et al. Conditional expression of the CTCF-paralogous transcriptional factor BORIS in normal cells results in demethylation and derepression of MAGE-A1 and reactivation of other cancer-testis genes. Cancer Res 2005;65:7751–62. [DOI] [PubMed] [Google Scholar]
- [9].Kang Y, Hong JA, Chen GA, et al. Dynamic transcriptional regulatory complexes including BORIS, CTCF and Sp1 modulate NY-ESO-1 expression in lung cancer cells. Oncogene 2007;26:4394–403. [DOI] [PubMed] [Google Scholar]
- [10].Woloszynska-Read A, Zhang W, Yu J, et al. Coordinated cancer germline antigen promoter and global DNA hypomethylation in ovarian cancer: association with the BORIS/CTCF expression ratio and advanced stage. Clin Cancer Res 2011;17:2170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cheema Z, Hari-Gupta Y, Kita GX, et al. Expression of the cancer-testis antigen BORIS correlates with prostate cancer. Prostate 2014;74:164–76. [DOI] [PubMed] [Google Scholar]
- [12].Okabayashi K, Fujita T, Miyazaki J, et al. Cancer-testis antigen BORIS is a novel prognostic marker for patients with esophageal cancer. Cancer Sci 2012;103:1617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chen K, Huang W, Huang B, et al. BORIS, brother of the regulator of imprinted sites, is aberrantly expressed in hepatocellular carcinoma. Genet Test Mol Biomarkers 2013;17:160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Newbern JM, Shoemaker SE, Snider WD. Taking off the SOCS: cytokine signaling spurs regeneration. Neuron 2009;64:591–2. [DOI] [PubMed] [Google Scholar]
- [15].Zhao RC, Zhou J, He JY, et al. Aberrant promoter methylation of SOCS-1 gene may contribute to the pathogenesis of hepatocellular carcinoma: a meta-analysis. J BUON 2016;21:142–51. [PubMed] [Google Scholar]
- [16].Aaronson DS, Horvath CM. A road map for those who don’t know JAK-STAT. Science 2002;296:1653–5. [DOI] [PubMed] [Google Scholar]
- [17].O'Shea JJ, Gadina M, Schreiber RD. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell 2002;109Suppl:S121–31. [DOI] [PubMed] [Google Scholar]
- [18].Weber A, Hengge UR, Bardenheuer W, et al. SOCS-3 is frequently methylated in head and neck squamous cell carcinoma and its precursor lesions and causes growth inhibition. Oncogene 2005;24:6699–708. [DOI] [PubMed] [Google Scholar]
- [19].He B, You L, Uematsu K, et al. SOCS-3 is frequently silenced by hypermethylation and suppresses cell growth in human lung cancer. Proc Natl Acad Sci U S A 2003;100:14133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pierconti F, Martini M, Pinto F, et al. Epigenetic silencing of SOCS3 identifies a subset of prostate cancer with an aggressive behavior. Prostate 2011;71:318–25. [DOI] [PubMed] [Google Scholar]
- [21].Zhang X, You Q, Zhang X, et al. SOCS3 methylation predicts a poor prognosis in HBV infection-related hepatocellular carcinoma. Int J Mol Sci 2015;16:22662–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Niwa Y, Kanda H, Shikauchi Y, et al. Methylation silencing of SOCS-3 promotes cell growth and migration by enhancing JAK/STAT and FAK signalings in human hepatocellular carcinoma. Oncogene 2005;24:6406–17. [DOI] [PubMed] [Google Scholar]
- [23].Ogata H, Kobayashi T, Chinen T, et al. Deletion of the SOCS3 gene in liver parenchymal cells promotes hepatitis-induced hepatocarcinogenesis. Gastroenterology 2006;131:179–93. [DOI] [PubMed] [Google Scholar]
- [24].Yang SF, Yeh YT, Wang SN, et al. SOCS-3 is associated with vascular invasion and overall survival in hepatocellular carcinoma. Pathology 2008;40:558–63. [DOI] [PubMed] [Google Scholar]
- [25].Wang Y, Wan M, Zhou Q, et al. The prognostic role of SOCS3 and A20 in human cholangiocarcinoma. PloS One 2015;10:e0141165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhao RC, Zhou J, Chen KF, et al. The prognostic value of combination of CD90 and OCT4 for hepatocellular carcinoma after curative resection. Neoplasma 2016;63:288–98. [DOI] [PubMed] [Google Scholar]
- [27].Ding BS, Cao Z, Lis R, et al. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature 2014;505:97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Weichert W, Roske A, Gekeler V, et al. Association of patterns of class I histone deacetylase expression with patient prognosis in gastric cancer: a retrospective analysis. Lancet Oncol 2008;9:139–48. [DOI] [PubMed] [Google Scholar]
- [29].D’Arcy V, Abdullaev ZK, Pore N, et al. The potential of BORIS detected in the leukocytes of breast cancer patients as an early marker of tumorigenesis. Clin Cancer Res 2006;12(20 Pt 1):5978–86. [DOI] [PubMed] [Google Scholar]
- [30].Alberti L, Renaud S, Losi L, et al. High expression of hTERT and stemness genes in BORIS/CTCFL positive cells isolated from embryonic cancer cells. PloS One 2014;9:e109921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Alberti L, Losi L, Leyvraz S, et al. Different effects of BORIS/CTCFL on stemness gene expression, sphere formation and cell survival in epithelial cancer stem cells. PloS One 2015;10:e0132977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ogata H, Chinen T, Yoshida T, et al. Loss of SOCS3 in the liver promotes fibrosis by enhancing STAT3-mediated TGF-beta1 production. Oncogene 2006;25:2520–30. [DOI] [PubMed] [Google Scholar]
- [33].Yin Y, Liu W, Dai Y. SOCS3 and its role in associated diseases. Hum Immunol 2015;76:775–80. [DOI] [PubMed] [Google Scholar]
- [34].Wang T, Niu G, Kortylewski M, et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med 2004;10:48–54. [DOI] [PubMed] [Google Scholar]
- [35].Toyoda H, Kumada T, Kaneoka Y, et al. Prognostic value of pretreatment levels of tumor markers for hepatocellular carcinoma on survival after curative treatment of patients with HCC. J Hepatol 2008;49:223–32. [DOI] [PubMed] [Google Scholar]
- [36].Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med 2006;355:1253–61. [DOI] [PubMed] [Google Scholar]
- [37].Li SX, Tang GS, Zhou DX, et al. Prognostic significance of cytoskeleton-associated membrane protein 4 and its palmitoyl acyltransferase DHHC2 in hepatocellular carcinoma. Cancer 2014;120:1520–31. [DOI] [PubMed] [Google Scholar]


