Supplemental Digital Content is available in the text
Keywords: airway inflammation, continuous positive airway pressure, exhaled nitric oxide, obstructive sleep apnea
Abstract
Background:
Exhaled nitric oxide (eNO) has been proposed as a noninvasive measure of airway inflammation. However, its value in patients with obstructive sleep apnea (OSA) is still controversial. The authors aim to assess the difference in eNO levels between patients with OSA and controls by a meta-analysis.
Methods:
A systematic search was performed in the PubMed, EMBASE, the Cochrane Library, and MEDLINE databases to collect relevant studies published from 1996 to 2016. Eligible studies that reported eNO levels in patients with OSA were included. STATA (version 12.0) was used for data analysis.
Results:
Two hundred eighty-four studies were reviewed for inclusion, with 16 studies pooled for analysis (16 studies for fractional exhaled nitric oxide [FENO], 5 for alveolar nitric oxide [CANO], and 4 for the maximum airway wall flux of nitric oxide [J′awNO]). The FENO levels were significantly higher in patients with OSA compared with that in the control groups (6.32 ppb, 95% confidence interval [CI] 4.46–8.33, P < 0.001). Furthermore, FENO was significantly increased (4.00 ppb, 95% CI 1.74–6.27, P = 0.001) after overnight sleep in patients with OSA, but not in healthy controls. Additionally, long-term continuous positive airway pressure (CPAP) therapy reduced FENO levels (−5.82 ppb, 95% CI −9.6 to −2.01, P < 0.001). However, the CANO (−0.01 ppb, 95% CI −1.66 to 1.64, P = 0.989) and J’awNO levels (220.32 pl/s, 95% CI −49.31 to 489.94, P = 0.109) were not significantly different between the OSA groups and non-OSA groups.
Conclusion:
The results of the meta-analysis suggest that OSA is significantly associated with airway inflammation and elevated FENO levels can be modified by long-term CPAP therapy. J’awNO and CANO levels were not significantly different between the OSA groups and control groups.
1. Introduction
Obstructive sleep apnea (OSA) is a common sleep disorder characterized by repetitive episodes of upper airway obstruction during sleep leading to significant hypoxemia and frequent arousals. With a high prevalence, ranging from 9% to 38% in the overall population,[1] OSA is increasingly regarded as a public health concern because it is closely associated with cardiovascular disorders.[2] Because an altered upper airway structure and function is 1 etiology of OSA,[3,4] local pathophysiological processes such as upper airway inflammation could amplify these abnormalities and may further compromise upper airway patency during sleep. A large body of experimental evidence indicates that patients with OSA present with airway inflammation[5,6] mainly because of the mechanical trauma of recurrent snoring and oxidative stress. Furthermore, it has been reported that the repetitive hypoxia/reoxygenation phenomenon increases the levels of reactive oxygen species, which aggravate vascular endothelium injury in patients with OSA.[7] Therefore, an assessment of respiratory inflammation might be a predictor of OSA and its complications.[8]
Exhaled nitric oxide (eNO) levels have been suggested as a simple, noninvasive, and reproducible marker of respiratory inflammation.[9] Nitric oxide (NO) is a molecule synthesized by NO synthase (NOS). eNO derived from the central airway and alveolus can be separately assessed by obtaining measurements at different flow rates.[10] Fractional exhaled nitric oxide (FENO) reflects bronchial NO production and diffusion, the maximum airway wall flux of NO (J′awNO) represents NO from the airway tree, and alveolar NO (CANO) represents the steady-state alveolar NO concentration.[11] In the lungs, NO regulates pulmonary vascular tone and reduced NO levels may be associated with the development of pulmonary hypertension by contributing to pulmonary vascular smooth muscle proliferation and remodeling.[12] Hypoxia may impair NO release by reducing substrate availability or by inhibiting NOS. Increased levels of eNO are indicative of lung inflammation by over-expression of the inducible NOS, as observed in asthma,[13] while reduced eNO levels can be found in cardiovascular disorders such as pulmonary hypertension,[14] due to decreased NOS expression and activity caused by endothelial dysfunction. In patients with OSA, both principal pathological processes, hypoxia and endothelial dysfunction, coexist and may change eNO concentrations.
In the last 20 years, several studies measuring eNO levels in patients with OSA have been reported, but the results are controversial. Different studies have found increased eNO levels,[15–26] no significant difference,[27–30] or decreased eNO levels[31] in patients with OSA compared with healthy controls. Therefore, the aim of this meta-analysis was to evaluate the potential association between OSA and eNO levels. Our secondary purpose was to review the potential influence of OSA on the severity of airway inflammation to address the question of whether eNO can be a predictor of the diagnosis of OSA or a marker of treatment efficacy.
2. Materials and methods
2.1. Data sources and study selection
We searched for relevant articles in the following databases: PubMed, MEDLINE, EMBASE, and the Cochrane Library. Even though MEDLINE is a component of PubMed, they differ in update frequency and retrieval mechanism; we thus treated them as separate databases to avoid miss any eligible studies. The details of the search strategy are included in the supplementary material (Supplementary Digital Content 1). Briefly, we searched for the terms (“obstructive sleep apnea” or “apnea” or “hypopnea” or “OSA”) and (“FENO” or “exhaled nitric oxide” or “fractional exhaled nitric oxide” or “CANO” or “alveolar NO” or “J’awNO”) and in articles published from 1996 to 2016. Moreover, the references of the retrieved articles were checked, and the PubMed function “related articles” was used to identify additional papers. Two authors reviewed all abstracts independently to determine the eligibility criteria or establish the appropriateness of the research issue. Any disagreements were resolved by discussion with a third reviewer. All levels of evidence were considered, but because of a lack of higher levels of evidence, only controlled trials were included. Studies were included if they met the following criteria: All patients with OSA were newly diagnosed by polysomnography (PSG); patients with OSA and eligible controls had no history of asthma, rhinitis, or sinusitis, and none of them had an allergic disease or were receiving anti-inflammatory medications at the time of the study. All subjects were over 16 years old. Studies were excluded if they were case reports, editorials, reviews, or abstracts. Details of the exclusion criteria can be seen in Fig. 1.
Figure 1.
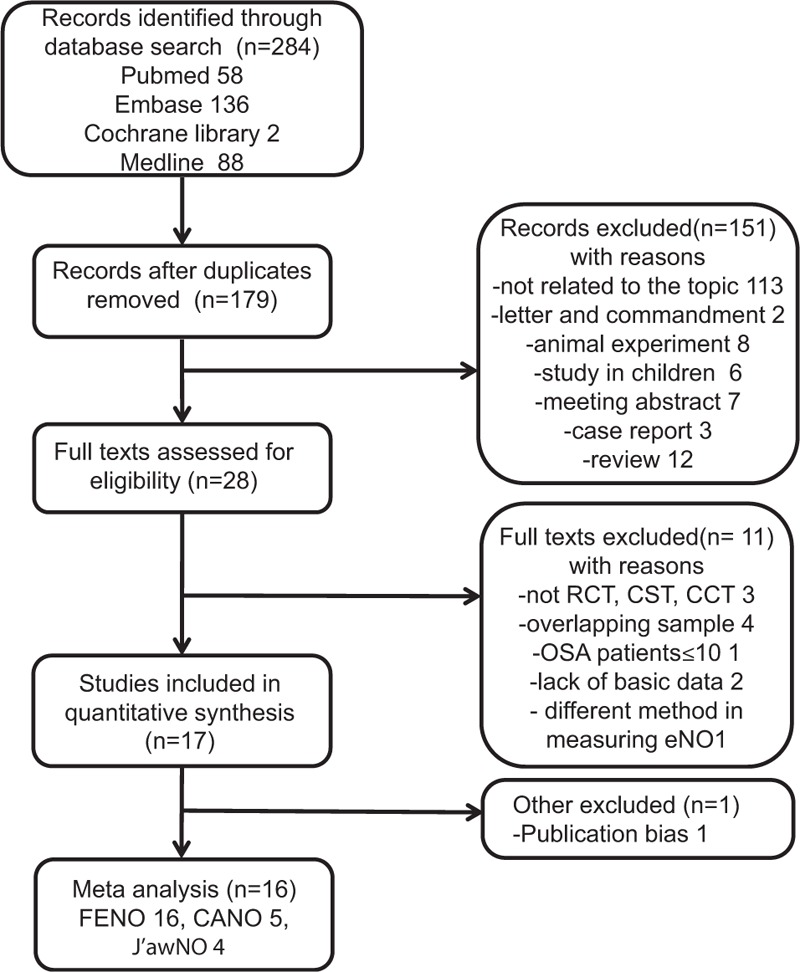
Flow diagram of the literature search.
2.2. Data extraction and analysis
The following information was extracted from the included studies: name of first author, year of publication, country, sample size, age, gender, diagnostic standard for OSA, type of study, and eNO measurement (method, parameters, and expiratory flow rates). When pertinent data were not included in a published article, the corresponding author was contacted to seek clarification. If the data could not be acquired, the article was excluded. The data were extracted by 2 authors independently. All data were expressed as mean ± standard deviation. For studies with data reported in median and range, or median and interquartile range, the mean and standard error were calculated utilizing the methods outlined by Hozo et al.[32]
For studies in which patients with OSA were compared with more than 1 group of control patients (e.g., obese and nonobese controls), only the one that was a better match for the patients with OSA in the study was included in the meta-analysis. For example, Petrosyan et al[33] compared FENO levels in obese patients with OSA with 2 control groups, obese controls and nonobese controls; we only extracted the data of the obese controls because the mean body mass index (BMI) in this group was not significantly different to that in the OSA group. Similarly, Foresi et al[31] compared patients with OSA with 2 control groups, but the hypertension group did not undergo PSG, so we included only the data of the healthy controls. For studies measuring eNO at different times, we used the parameters measured in the morning for our analysis. If eNO was measured at multiple flow rates, we extracted the data for an approximately 50-mL/s flow rate.
Heterogeneity was assessed by calculating the I2 statistics. An I2 of 25% to 49% was considered to represent a low level of heterogeneity, 50% to 74% a moderate level, and 75% to 100% a high level. Due to the high level of heterogeneity presented, a random-effects model was employed to combine the effect size. All statistical analysis was performed using STATA version 12.0 (Stata Statistical Software, College Station, TX). A meta-regression analysis was also performed to assess the influence of study-related factors on the outcomes and analysis of heterogeneity. Publication bias was checked by funnel plots, Begg test, and Egger test. A 2-sided P value ≤ 0.05 was considered statistically significant.
3. Results
3.1. Search results and study characteristics
The search identified 284 potential studies, 267 of which were excluded as described in Fig. 1. Seventeen studies were included in the analysis, but 1 study[34] had obvious publication bias and was excluded from the final meta-analysis. The remaining 16 studies differed in their study design: 5 were cross-sectional trials (CSTs) and 11 were case–control trials (Table 1). The number of patients included in the studies ranged from 13 to 97 in the OSA groups (total 688) and from 7 to 53 in the control groups (total 366). Among the studies, the mean age of participants ranged from 37 to 66 years in both the groups, and generally, more than half of them were male (Table 1). Among the included studies, 7 also analyzed the effects of continuous positive airway pressure (CPAP) treatment on airway inflammation: 2[24,31] evaluated the effects of short-term (2 nights) CPAP therapy while the other 5[21,22,26,30,33] followed CPAP treatment for more than 1 month. A total of 5 studies[21,24,25,29,31] including 359 subjects were pooled for CANO analysis, and 4 studies[21,24,25,29] with 296 subjects were pooled for J’awNO analysis.
Table 1.
Characteristics of included studies.
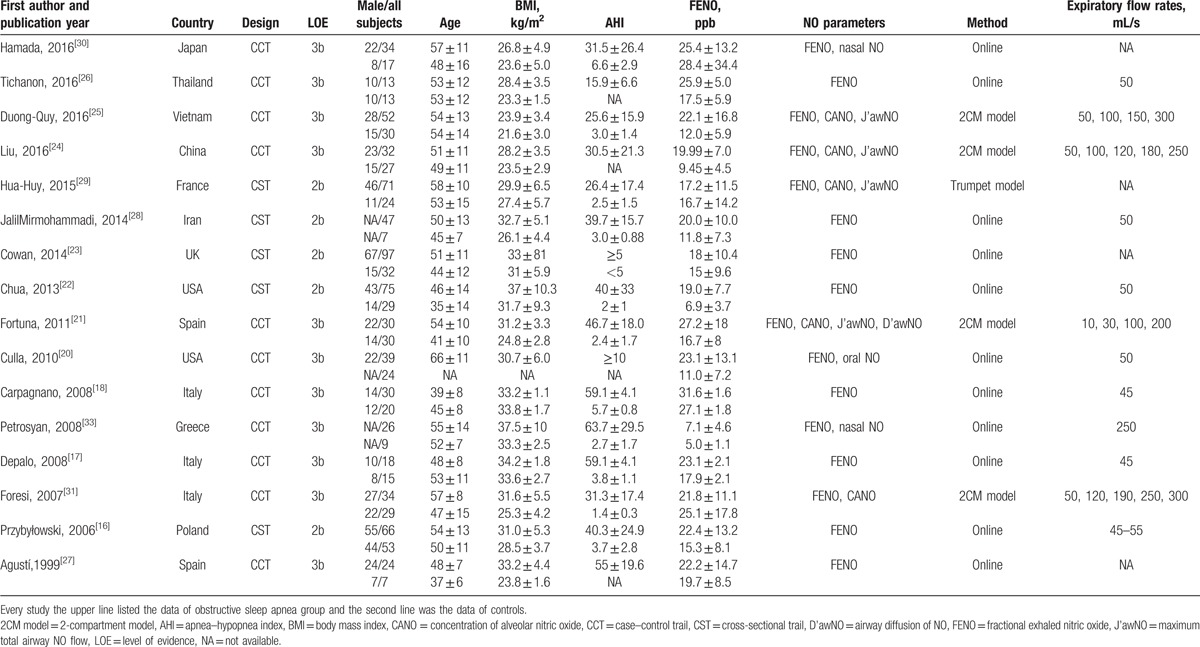
All the studies scored well in terms of adequate descriptions of selection criteria and reference tests, and the availability of clinical data. All included studies clearly described the data source, and patient inclusion and exclusion criteria, and presented measurements for the primary study end points.
3.2. FENO
Because there was heterogeneity in this endpoint (I2 = 82.8%), the random-effects model was used to combine the effect size. The pooled mean difference was 6.39, which indicates that FENO levels in the OSA group were 6.39 ppb (95% confidence interval [CI] 4.46–8.33, P < 0.001) higher than that in the control group (Fig. 2).
Figure 2.
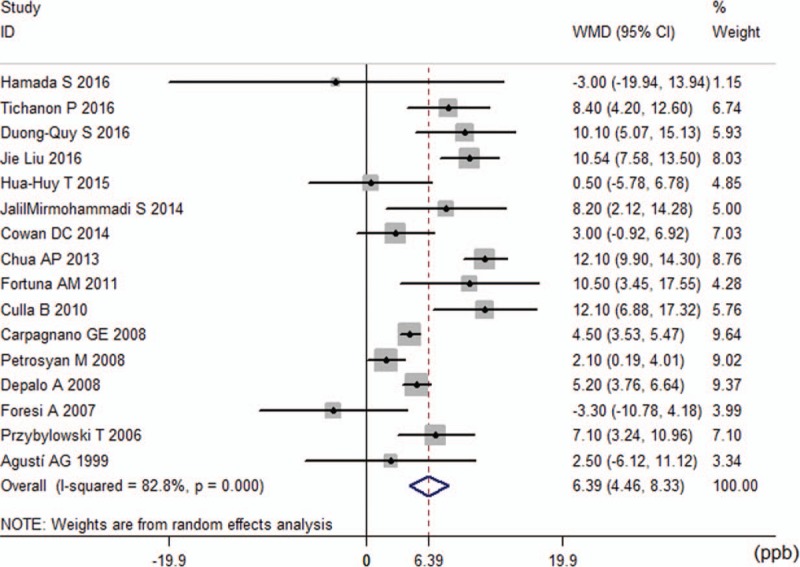
Comparison of fractional exhaled nitric oxide levels between obstructive sleep apnea groups and control groups in the 16 included studies.
Subgroup analyses were conducted based on BMI, age, apnea–hypopnea index (AHI), smoking status, and expiratory flow rate. In all subgroups, FENO levels were higher in the patients with OSA than they were in the controls, with a weighted mean difference (WMD) ranging from 3.49 ppb to 8.27 ppb (Supplemental Fig. 1A–C, Supplemental digital Content 2, which shows the results of the subgroup analyses). However, significant heterogeneities still existed in these subgroups (I2 41.7–92.0%).
In 5 studies,[16,22,25,28,29] exhaled NO was measured separately before and after overnight PSG to evaluate the variation of FENO levels in the morning and in the evening. For these studies, 2 analyses were performed. The first analysis was performed with the baseline parameters of morning and evening FENO levels in the patients with OSA and controls. The pooled WMD was 4.00 ppb (95% CI 1.74–6.27, P = 0.001) in the OSA groups compared with 0.29 ppb (95% CI −1.84 to 2.42, P = 0.791) in the control groups (Fig. 3). The second analysis included the changes after PSG (FENO levels in the morning minus FENO levels in the evening) in the OSA groups and controls, and the WMD was calculated to be 3.91 ppb (95% CI 0.55–7.28, P = 0.022), which indicated that in patients with OSA, the overnight increase of FENO levels was larger than that in non-OSA controls. There was medium heterogeneity in this endpoint (I2 = 65.3%) (Fig. 3).
Figure 3.
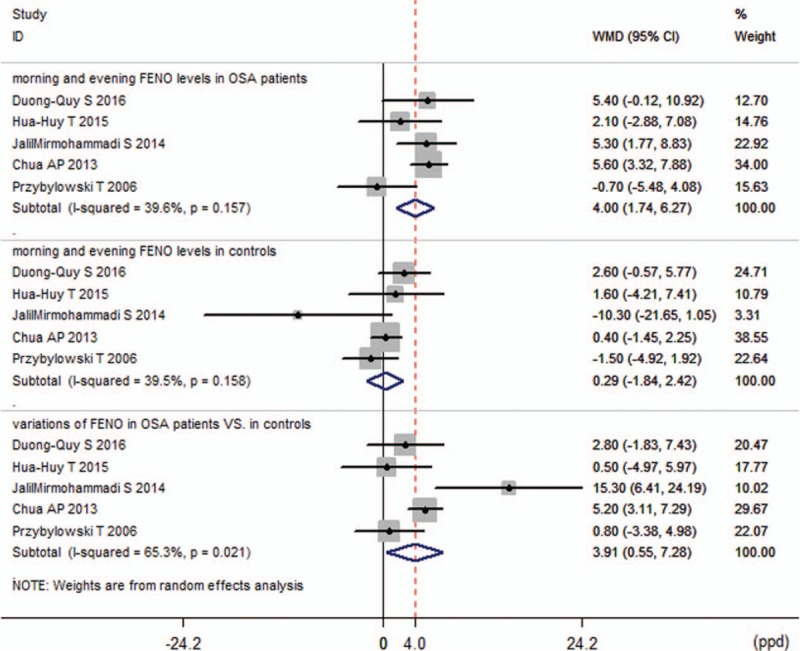
Variation of fractional exhaled nitric oxide (FENO) levels.
The random-effects analysis revealed that long-term CPAP therapy promoted a significant decrease in FENO levels (−5.82 ppb, 95% CI −9.64 to −2.01, P < 0.001; Fig. 4). However, short-term CPAP could not reduce FENO (−3.99 ppb, 95% CI −19.56 to 11.59, P = 0.616; Fig. 4). There was significant heterogeneity due to the limited number of trials, with an I2 of 81.6% and 94.2%, respectively.
Figure 4.
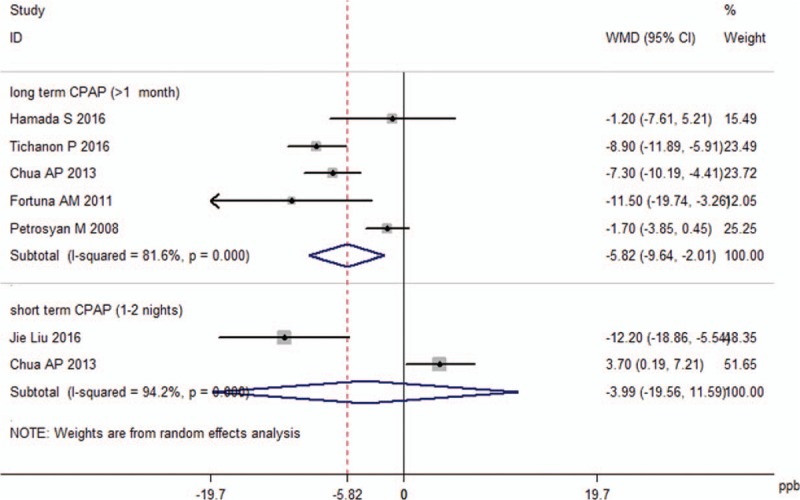
Effect of continuous positive airway pressure (CPAP) treatment on fractional exhaled nitric oxide levels.
3.3. CANO and J’awNO
For CANO, a random-effects analysis was applied since there was evidence of heterogeneity among the 5 studies (I2 = 96.1%, P < 0.001). The calculated WMD (−0.01 ppb, 95% CI −1.66 to 1.64, P = 0.989; Fig. 5) indicated that there was no statistical difference in the CANO levels between the OSA groups and control groups.
Figure 5.
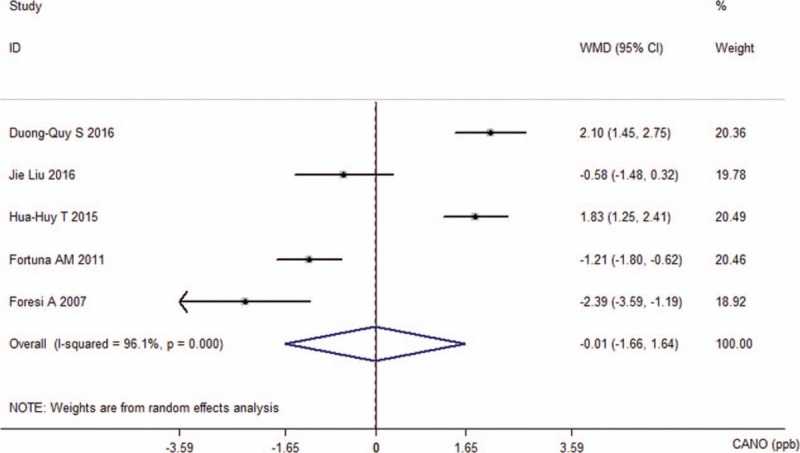
Comparison of concentration of alveolar nitric oxide between the obstructive sleep apnea groups and control groups in the 5 included studies.
For J’awNO, the pooled WMD was 220.32 pl/s (95% CI −49.31 to 489.94, P = 0.109). There was heterogeneity in this analysis (I2 = 74.2%, P = 0.009; Fig. 6).
Figure 6.
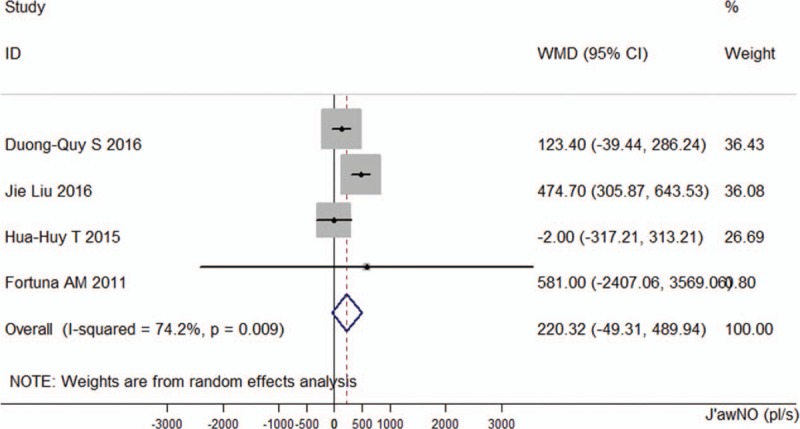
Comparison of the maximum airway wall flux of nitric oxide between the obstructive sleep apnea groups and control groups in the 4 included studies.
3.4. Publication bias
The funnel plot was almost perfectly symmetrical (Fig. 7) after excluding the study by Devouassoux et al,[34] suggesting that this study might have publication bias. The bias might have been caused by the inclusion of subjects with allergies. Subsequently, Begg tests (P = 0.822) and Egger tests (P = 0.463) were performed and found no evidence of publication bias in the remaining 16 studies. For the analysis of CANO levels and J’awNO levels, the Begg tests (P = 0.806 and P = 0.734, respectively) and Egger tests (P = 0.391 and P = 0.862, respectively) also found no evidence of bias.
Figure 7.
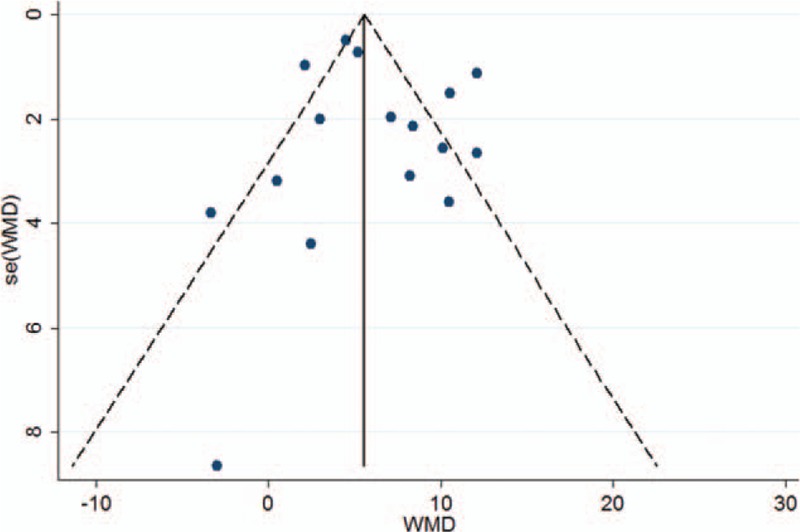
Funnel plot of fractional exhaled nitric oxide.
3.5. Sensitivity analysis
No single trial, when removed, significantly affected the overall estimate of the effects of the 3 eNO measures (Supplemental Fig. 2A–C, Supplemental digital Content 3, which shows the results of the sensitivity analyses). The pooled analysis using the random-effects model showed that FENO levels were increased significantly (WMD 6.39, 95% CI 4.46–8.33, P < 0.001). The fixed-effects model found a similar result (WMD 5.56, 95% CI 4.93–6.20, P < 0.001). After including the previously excluded study, the pooled analysis result was 7.25 (95% CI 3.49–11.00, P < 0.001) using the random-effects model.
3.6. Meta-regression analysis
Meta-regression analyses were performed to evaluate the effect of age, BMI, AHI, and minimum pulse oxygen saturation (SpO2) on the levels of FENO. The FENO levels were not significantly correlated with the age of the patients with OSA (P = 0.980) and BMI (P = 0.438), the age (P = 0.353) and BMI of normal participants (P = 0.362), or the AHI (P = 0.399) and minimum SpO2 (P = 0.964). The analyses of CANO levels and J’awNO levels described above were not reanalyzed by meta-regression because of the limited number of studies included.
4. Discussion
NO is a reactive molecule produced by nitric oxide synthase (NOS) in a reaction that converts l-arginine and oxygen to citrulline and NO. Three distinct forms of NOS have been identified: endothelial NOS (eNOS), neuronal NOS (nNOS), and inducible NOS (iNOS).[35] Among them, only iNOS can produce a large amount of NO for longer periods of time after activation by stimuli; it plays a role in pathophysiological processes such as inflammation. Exhaled NO levels are elevated in conditions associated with airway inflammation, for example, asthma, and are used as simple, noninvasive markers of airway inflammation.
To our knowledge, this is the first meta-analysis addressing the exhaled NO levels in patients with OSA. We found that there was an increase in FENO levels in subjects with OSA; however, CANO and J’awNO were not significantly different between the patients with OSA and the controls. Moreover, FENO levels were significantly increased on waking up in patients with OSA, but not in non-OSA groups, and long-term CPAP was able to modify FENO in OSA groups.
Our results showed that FENO was significantly increased in subjects with OSA, and that this might be linked to the over-expression of iNOS,[15,17,36] which was partially reversible after long-term CPAP treatment. In our pooled analysis, the level of FENO on waking up was significantly increased in subjects with OSA (Fig. 3) but not in healthy controls. Mechanical stress on the mucosa caused by intermittent airway closure and reopening as well as ischemia-reperfusion injury from intermittent nocturnal hypoxemia produces oxygen free radicals, which can lead to increased upper airway inflammation. The nasal, tonsillar, and oropharyngeal tissues are potential inflammatory sites in patients with OSA,[37–40] and plasma cell infiltration and interstitial edema have been documented in tissue biopsies.[40] Local upper-airway inflammation promotes oropharyngeal inspiratory muscle dysfunction and the formation of progressive local neurogenic lesions, thus amplifying the upper airway narrowing and collapsibility.[41] Additionally, tissue biopsies confirmed the significant effect of long-term CPAP treatment on reducing inflammation-related infiltration in upper airways.[38] Thus, FENO can be used as a simple, noninvasive marker of upper airway inflammation in patients with OSA, allowing a more accurate prediction of response to treatment.
Experimental studies have found that other exhaled markers of airway inflammation and oxidative stress such as interleukin 6, interleukin 10, and 8-isopentane were also raised in the breath condensate of adults with OSA,[42,43] and that there was a significantly higher percentage of neutrophils in the induced sputum of patients with OSA than in healthy volunteers.[6,18,34] These findings may suggest lower airway inflammation in patients with OSA. However, our results indicate that J’awNO is not a marker of lower airway inflammation in patients with OSA.
The CANO levels reflect the balance between the local production of NO from distal parts of the lung and diffusion across the alveolar capillary wall. Increased CANO has been found in patients with asthma,[44] alveolitis,[10] and chronic obstructive pulmonary disease,[45] mainly due to the increased NO production by inflammatory cells, epithelium, or endothelial cells. Although OSA is associated with systemic inflammation, increased oxidative stress, and endothelial dysfunction, we speculate that the extent of these processes was not enough to alter the concentration of CANO, as demonstrated by the lack of a significant difference in CANO levels between the OSA and control subjects. An alternative explanation might be the presence of endothelial dysfunction in patients with OSA; unfortunately, none of the included studies investigated this issue. The CANO levels in patients with OSA should be further investigated and characterized.
In the meta-regression, both the characteristics (age and BMI) and OSA severity (AHI and minimum SpO2) had no correlation with the increased FENO levels. In another experiment by Verhulst et al[46] involving the pediatric population, habitual snoring and age were the only variables associated with raised FENO levels in the morning and afternoon, leading them to conclude that snoring is more important than the actual obstructive respiratory events for increased upper airway inflammation. They proposed that snoring induced vibration of the soft tissues, which caused damage to these tissues contributing to the pathogenesis of OSA. In our analysis, only FENO was significantly different between the OSA and control subjects, implying that mechanical damage may play a more important role in the upper airway inflammation than that of hypoxemia. This hypothesis needs to be confirmed in further studies. Notably, even though FENO levels increased after sleep in patients with OSA, their FENO levels were still around the upper limit of the normal range (25 ppb). Therefore, FENO might not be an effective predictor of diagnosis of OSA, especially for distinguishing suspected OSA and the pure snorers.
The observed heterogeneity might be explained by the differences in patient inclusion criteria and the time of exhaled NO measurement. Further, only some of the studies included smokers. In the study by Fortuna et al,[21] currently smoking patients had lower FENO and CANO levels than ex-smokers and nonsmokers, possibly because cigarettes contain a large number of free radicals and pro-oxidant substances, which cause lower NO bioactivity, and the fact that smokers have a tetrahydrobiopterin deficit, which reduces the generation of NO.[47] However, in Liu et al's study,[24] FENO and CANO were not significantly different between the smokers and nonsmokers in both the OSA group and the healthy controls. Most studies measured the eNO from 6 am to 10 am and between 8 pm to 11 pm, a few studies did not report the measurement time,[17] and 1 study only evaluated the eNO from 11 am to 1 pm.[33] Dias et al confirmed that patients with OSA and healthy subjects showed a circadian pattern of FENO with a decrease of values across the day,[19] suggesting that the time of measurement may be a confounder in this analysis. Finally, some baseline data, such as age, BMI, and AHI, were significantly different between groups.
5. Limitations
Despite these meaningful findings, several limitations of this meta-analysis still need to be emphasized. Firstly, the available literature is largely low-level evidence; either case–control or CSTs of a relatively small size. Secondly, different techniques and instruments were used for the measurement of exhaled NO, which could be another source of study heterogeneity. Thirdly, there was also heterogeneity across the sample populations. We could not perform meta-regression for other confounding factors—snoring[25] or time of measurement[19]—since we did not have enough data on these variables. These factors have previously been found to be associated with eNO levels.
6. Conclusion
Our findings suggest that OSA was significantly associated with elevated FENO levels, especially after waking up, and long-term CPAP therapy can reduce FENO levels. Accordingly, FENO levels might be a noninvasive marker of upper airway inflammation in patients with OSA. However, J’awNO and CANO are not useful markers of airway inflammation for patients with OSA.
Supplementary Material
Acknowledgments
The authors would like to thank Xiaolu Gao for her help in the statistical analyses and developing the methods for performing the meta-analysis.
Footnotes
Abbreviations: AHI = apnea–hypopnea index, BMI = body mass index, CANO = concentration of alveolar nitric oxide, CI = confidence interval, CPAP = continuous positive airway pressure, CST = cross-sectional trail, eNO = exhaled nitric oxide, eNOS = endothelial nitric oxide synthase, FENO = fractional exhaled nitric oxide, iNOS = inducible nitric oxide synthase, J′awNO = the maximum airway wall flux of nitric oxide, nNOS = neuronal nitric oxide synthase, NO = nitric oxide, NOS = nitric oxide synthase, OSA = obstructive sleep apnea, PSG = polysomnography, SpO2 = pulse oxygen saturation, WMD = weighted mean difference.
Since it was a meta-analysis, it did not require ethical approval or patient consent.
Conceived and designed the experiments: YX, DZ, and JL; Performed the studies search and data integrity: DZ, YQ, and RH. Analyzed the data: JL, YX, RH, and XZ. Contributed reagents/materials/analysis tools: all authors. Wrote the paper: all authors.
This study was supported by the National Natural Science Foundation of China (81570085)
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Senaratna CV, Perret JL, Lodge CJ, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev 2016;[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [2].Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005;365:1046–53. [DOI] [PubMed] [Google Scholar]
- [3].Pham LV, Schwartz AR. The pathogenesis of obstructive sleep apnea. J Thorac Dis 2015;7:1358–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Owens RL, Eckert DJ, Yeh SY, et al. Upper airway function in the pathogenesis of obstructive sleep apnea: a review of the current literature. Curr Opin Pulm Med 2008;14:519–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Inancli HM, Enoz M. Obstructive sleep apnea syndrome and upper airway inflammation. Recent Pat Inflamm Allergy Drug Discov 2010;4:54–7. [DOI] [PubMed] [Google Scholar]
- [6].Salerno FG, Carpagnano E, Guido P, et al. Airway inflammation in patients affected by obstructive sleep apnea syndrome. Respir Med 2004;98:25–8. [DOI] [PubMed] [Google Scholar]
- [7].Lavie L. Obstructive sleep apnoea syndrome—an oxidative stress disorder. Sleep Med Rev 2003;7:35–51. [DOI] [PubMed] [Google Scholar]
- [8].Carpagnano GE, Lacedonia D, Foschino-Barbaro MP. Non-invasive study of airways inflammation in sleep apnea patients. Sleep Med Rev 2011;15:317–26. [DOI] [PubMed] [Google Scholar]
- [9].Barnes PJ, Kharitonov SA. Exhaled nitric oxide: a new lung function test. Thorax 1996;51:233–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lehtimäki L, Kankaanranta H, Saarelainen S, et al. Extended exhaled NO measurement differentiates between alveolar and bronchial inflammation. Am J Respir Crit Care Med 2001;163:1557–61. [DOI] [PubMed] [Google Scholar]
- [11].Condorelli P, Shin HW, Aledia AS, et al. A simple technique to characterize proximal and peripheral nitric oxide exchange using constant flow exhalations and an axial diffusion model. J Appl Physiol (1985) 2007;102:417–25. [DOI] [PubMed] [Google Scholar]
- [12].Cremona G, Wood AM, Hall LW, et al. Effect of inhibitors of nitric oxide release and action on vascular tone in isolated lungs of pig, sheep, dog and man. J Physiol 1994;481:185–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kharitonov SA, Yates D, Robbins RA, et al. Increased nitric oxide in exhaled air of asthmatic patients. Lancet 1994;343:133–5. [DOI] [PubMed] [Google Scholar]
- [14].Kharitonov SA, Cailes JB, Black CM, et al. Decreased nitric oxide in the exhaled air of patients with systemic sclerosis with pulmonary hypertension. Thorax 1997;52:1051–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Olopade CO, Christon JA, Zakkar M, et al. Exhaled pentane and nitric oxide levels in patients with obstructive sleep apnea. Chest 1997;111:1500–4. [DOI] [PubMed] [Google Scholar]
- [16].Przybyłowski T, Bielicki P, Kumor M, et al. Exhaled nitric oxide in patients with obstructive sleep apnea syndrome. Pneumonol Alergol Pol 2006;74:21–5. [PubMed] [Google Scholar]
- [17].Depalo A, Carpagnano GE, Spanevello A, et al. Exhaled NO and iNOS expression in sputum cells of healthy, obese and OSA subjects. J Intern Med 2008;263:70–8. [DOI] [PubMed] [Google Scholar]
- [18].Carpagnano GE, Spanevello A, Sabato R, et al. Exhaled pH, exhaled nitric oxide, and induced sputum cellularity in obese patients with obstructive sleep apnea syndrome. Transl Res 2008;151:45–50. [DOI] [PubMed] [Google Scholar]
- [19].Dias AR, Martinho C, Teixeira J, et al. Circadian pattern of exhaled nitric oxide in a group of obstructive sleep apnoea patients and healthy subjects. J Sleep Res 2010;19:164. [Google Scholar]
- [20].Culla B, Guida G, Brussino L, et al. Increased oral nitric oxide in obstructive sleep apnoea. Respir Med 2010;104:316–20. [DOI] [PubMed] [Google Scholar]
- [21].Fortuna AM, Miralda R, Calaf N, et al. Airway and alveolar nitric oxide measurements in obstructive sleep apnea syndrome. Respir Med 2011;105:630–6. [DOI] [PubMed] [Google Scholar]
- [22].Chua AP, Aboussouan LS, Minai OA, et al. Long-term continuous positive airway pressure therapy normalizes high exhaled nitric oxide levels in obstructive sleep apnea. J Clin Sleep Med 2013;9:529–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cowan DC, Allardice G, Macfarlane D, et al. Predicting sleep disordered breathing in outpatients with suspected OSA. BMJ Open 2014;4:e004519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Liu J, Li Z, Liu Z, et al. Exhaled nitric oxide from the central airway and alveoli in OSAHS patients: the potential correlations and clinical implications. Sleep Breath 2016;20:145–54. [DOI] [PubMed] [Google Scholar]
- [25].Duong-Quy S, Hua-Huy T, Tran-Mai-Thi HT, et al. Study of exhaled nitric oxide in subjects with suspected obstructive sleep apnea: a pilot study in Vietnam. Pulm Med 2016;2016:3050918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tichanon P, Wilaiwan K, Sopida S, et al. Effect of continuous positive airway pressure on airway inflammation and oxidative stress in patients with obstructive sleep apnea. Can Respir J 2016;2016:3107324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Agustí AG, Barbé F, Togores B. Exhaled nitric oxide in patients with sleep apnea. Sleep 1999;22:231–5. [PubMed] [Google Scholar]
- [28].JalilMirmohammadi S, Mehrparvar AH, Safaei S, et al. The association between exhaled nitric oxide and sleep apnea: the role of BMI. Respir Med 2014;108:1229–33. [DOI] [PubMed] [Google Scholar]
- [29].Hua-Huy T, Le-Dong NN, Duong-Quy S, et al. Increased alveolar nitric oxide concentration is related to nocturnal oxygen desaturation in obstructive sleep apnoea. Nitric Oxide 2015;45:27–34. [DOI] [PubMed] [Google Scholar]
- [30].Hamada S, Tatsumi S, Kobayashi Y, et al. Nasal nitric oxide improved by continuous positive airway pressure therapy for upper airway inflammation in obstructive sleep apnea. Sleep Breath 2016;[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [31].Foresi A, Leone C, Olivieri D, et al. Alveolar-derived exhaled nitric oxide is reduced in obstructive sleep apnea syndrome. Chest 2007;132:860–7. [DOI] [PubMed] [Google Scholar]
- [32].Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Petrosyan M, Perraki E, Simoes D, et al. Exhaled breath markers in patients with obstructive sleep apnoea. Sleep Breath 2008;12:207–15. [DOI] [PubMed] [Google Scholar]
- [34].Devouassoux G, Lévy P, Rossini E, et al. Sleep apnea is associated with bronchial inflammation and continuous positive airway pressure-induced airway hyperresponsiveness. J Allergy Clin Immunol 2007;119:597–603. [DOI] [PubMed] [Google Scholar]
- [35].Nathan C, Xie QW. Nitric oxide synthases: roles, tolls, and controls. Cell 1994;78:915–8. [DOI] [PubMed] [Google Scholar]
- [36].Bucca C, Cicolin A, Brussino L, et al. Tooth loss and obstructive sleep apnoea. Respir Res 2006;7:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Paulsen FP, Steven P, Tsokos M, et al. Upper airway epithelial structural changes in obstructive sleep-disordered breathing. Am J Respir Crit Care Med 2002;166:501–9. [DOI] [PubMed] [Google Scholar]
- [38].Gelardi M, Carbonara G, Maffezzoni E, et al. Regular CPAP utilization reduces nasal inflammation assessed by nasal cytology in obstructive sleep apnea syndrome. Sleep Med 2012;13:859–63. [DOI] [PubMed] [Google Scholar]
- [39].Kim J, Bhattacharjee R, Dayyat E, et al. Increased cellular proliferation and inflammatory cytokines in tonsils derived from children with obstructive sleep apnea. Pediatr Res 2009;66:423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Sekosan M, Zakkar M, Wenig BL, et al. Inflammation in the uvula mucosa of patients with obstructive sleep apnea. Laryngoscope 1996;106:1018–20. [DOI] [PubMed] [Google Scholar]
- [41].Friberg D, Ansved T, Borg K, et al. Histological indications of a progressive snorers disease in an upper airway muscle. Am J Respir Crit Care Med 1998;157:586–93. [DOI] [PubMed] [Google Scholar]
- [42].Carpagnano GE, Kharitonov SA, Resta O, et al. 8-Isoprostane, a marker of oxidative stress, is increased in exhaled breath condensate of patients with obstructive sleep apnea after night and is reduced by continuous positive airway pressure therapy. Chest 2003;124:1386–92. [DOI] [PubMed] [Google Scholar]
- [43].Li Y, Chongsuvivatwong V, Geater A, et al. Exhaled breath condensate cytokine level as a diagnostic tool for obstructive sleep apnea syndrome. Sleep Med 2009;10:95–103. [DOI] [PubMed] [Google Scholar]
- [44].Paraskakis E, Brindicci C, Fleming L, et al. Measurement of bronchial and alveolar nitric oxide production in normal children and children with asthma. Am J Respir Crit Care Med 2006;174:260–7. [DOI] [PubMed] [Google Scholar]
- [45].Brindicci C, Ito K, Resta O, et al. Exhaled nitric oxide from lung periphery is increased in COPD. Eur Respir J 2005;26:52–9. [DOI] [PubMed] [Google Scholar]
- [46].Verhulst SL, Aerts L, Jacobs S, et al. Sleep-disordered breathing, obesity, and airway inflammation in children and adolescents. Chest 2008;134:1169–75. [DOI] [PubMed] [Google Scholar]
- [47].Wever RM, van Dam T, van Rijn HJ, et al. Tetrahydrobiopterin regulates superoxide and nitric oxide generation by recombinant endothelial nitric oxide synthase. Biochem Biophys Res Commun 1997;237:340–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


