Abstract
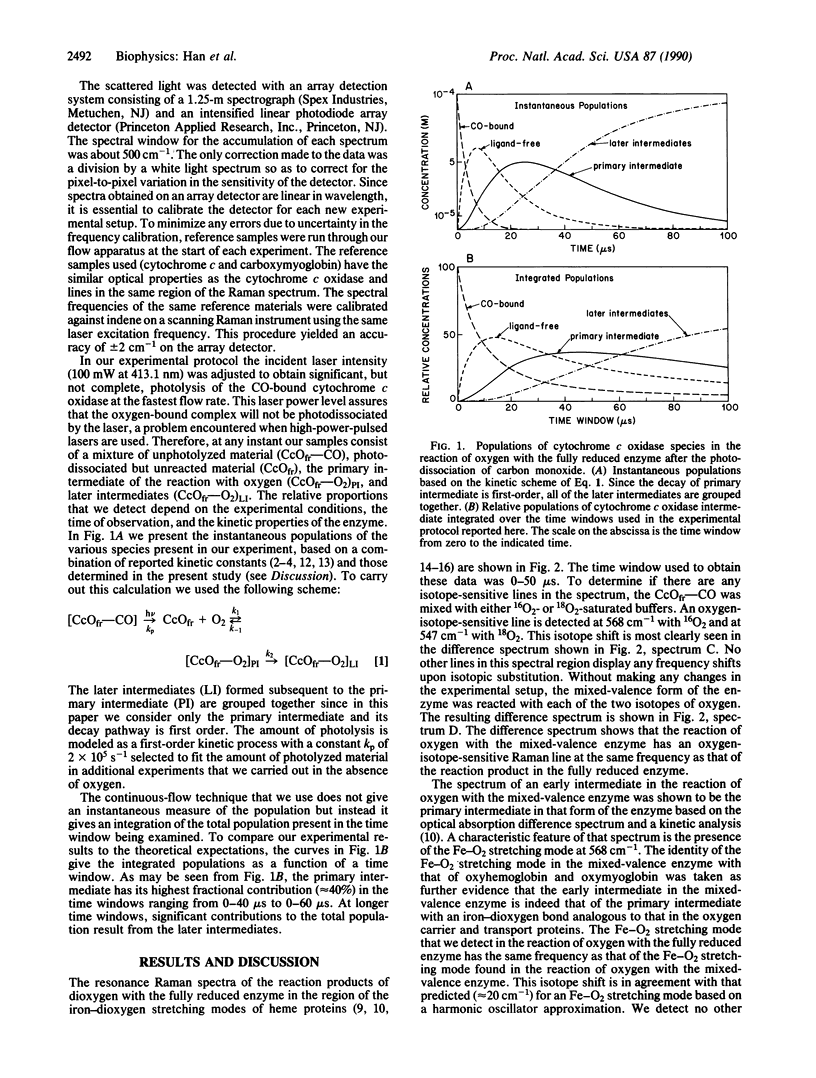
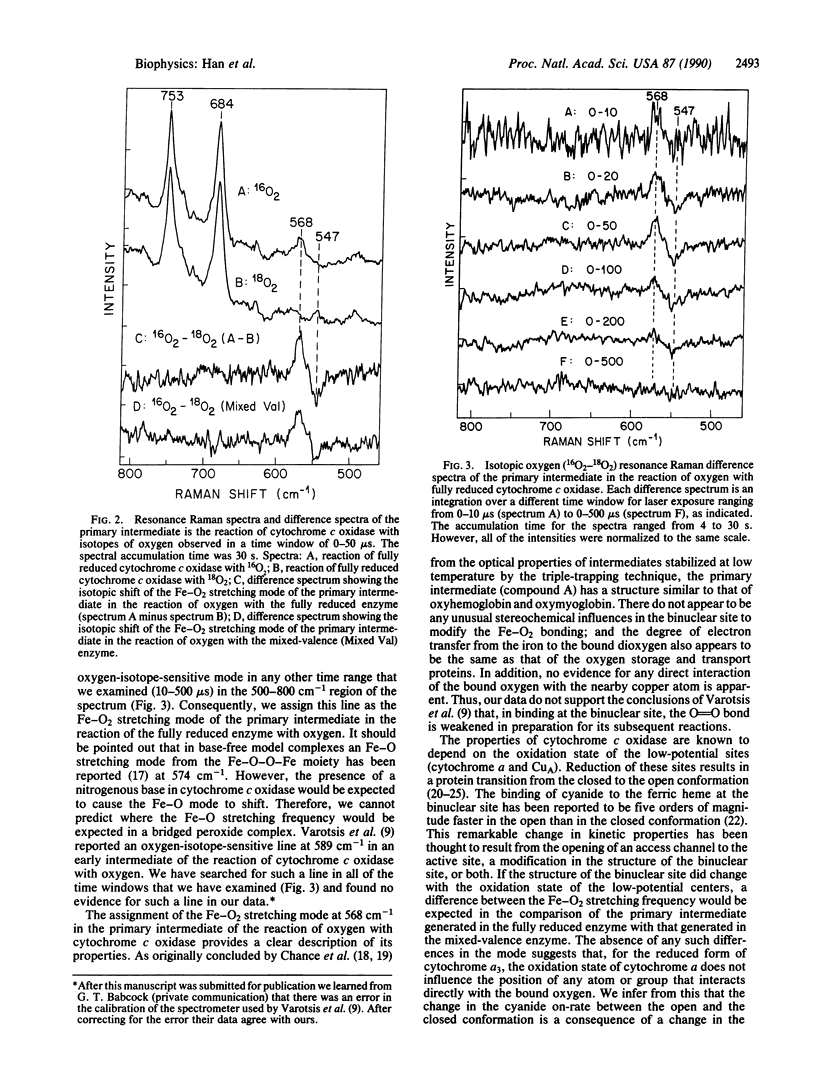
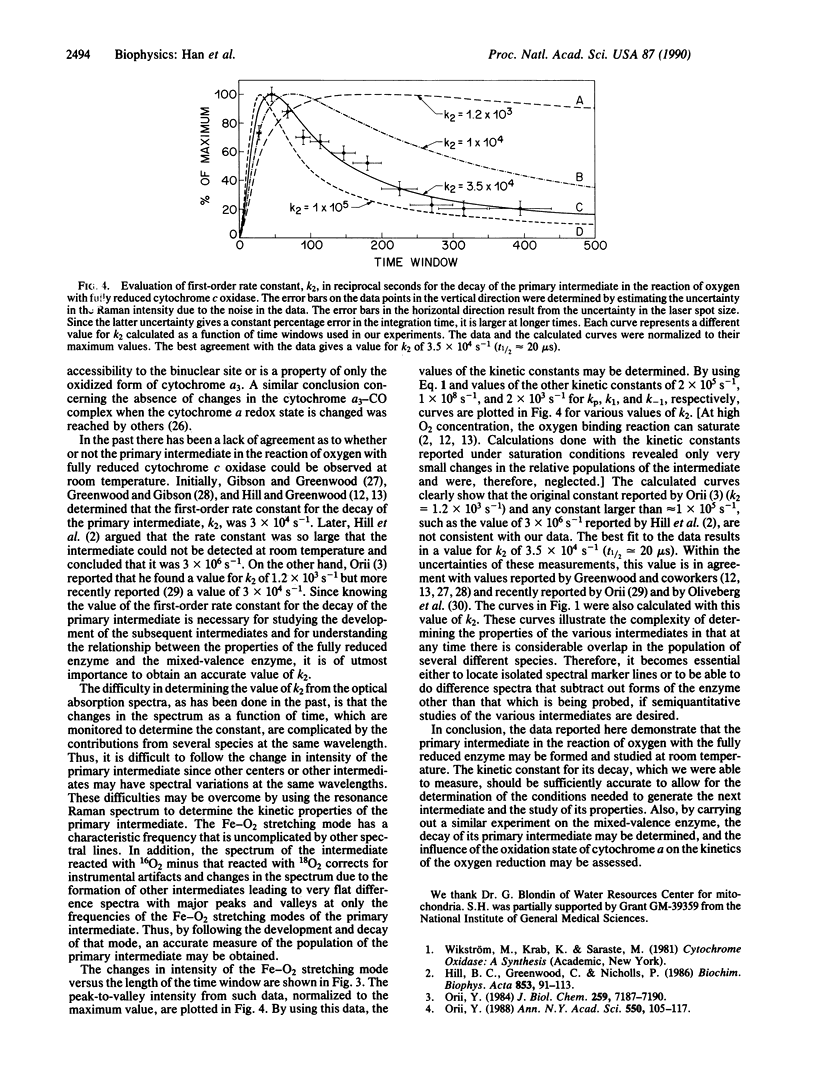
The primary intermediate in the reaction of oxygen with cytochrome c oxidase was generated by photodissociating carbon monoxide in a continuous flow rapid mixing apparatus. The presence of the primary intermediate was confirmed by a comparison of the iron-dioxygen stretching frequency with that obtained in the reaction of oxygen with the mixed-valence enzyme. For both of these preparations, the Fe-O2 stretching mode is detected at 568 cm-1, the same frequency as that found in oxyhemoglobin and oxymyoglobin. These data illustrate that the primary intermediate may be generated and detected at room temperature in the fully reduced enzyme and that the oxidation state of cytochrome a does not affect the structure of the iron-dioxygen complex. By following the changes in the intensity of the Fe-O2 stretching mode in the resonance Raman spectrum as a function of time, the first-order rate constant for the decay of the primary intermediate was found to be 3.5 x 10(4) s-1 (t1/2 = 20 microseconds).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babcock G. T., Jean J. M., Johnston L. N., Woodruff W. H., Palmer G. Flow-flash, time-resolved resonance Raman spectroscopy of the oxidation of reduced and of mixed valence cytochrome oxidase by dioxygen. J Inorg Biochem. 1985 Mar-Apr;23(3-4):243–251. doi: 10.1016/0162-0134(85)85031-5. [DOI] [PubMed] [Google Scholar]
- Chance B., Saronio C., Leigh J. S., Jr Functional intermediates in reaction of cytochrome oxidase with oxygen. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1635–1640. doi: 10.1073/pnas.72.4.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Saronio C., Leigh J. S., Jr Functional intermediates in the reaction of membrane-bound cytochrome oxidase with oxygen. J Biol Chem. 1975 Dec 25;250(24):9226–9237. [PubMed] [Google Scholar]
- Copeland R. A., Smith P. A., Chan S. I. Cytochrome c oxidase exhibits a rapid conformational change upon reduction of CuA: a tryptophan fluorescence study. Biochemistry. 1987 Nov 17;26(23):7311–7316. doi: 10.1021/bi00397a017. [DOI] [PubMed] [Google Scholar]
- Einarsdóttir O., Choc M. G., Weldon S., Caughey W. S. The site and mechanism of dioxygen reduction in bovine heart cytochrome c oxidase. J Biol Chem. 1988 Sep 25;263(27):13641–13654. [PubMed] [Google Scholar]
- GIBSON Q. H., GREENWOOD C. Reactions of cytochrome oxidase with oxygen and carbon monoxide. Biochem J. 1963 Mar;86:541–554. doi: 10.1042/bj0860541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood C., Gibson Q. H. The reaction of reduced cytochrome C oxidase with oxygen. J Biol Chem. 1967 Apr 25;242(8):1782–1787. [PubMed] [Google Scholar]
- Hill B. C., Greenwood C., Nicholls P. Intermediate steps in the reaction of cytochrome oxidase with molecular oxygen. Biochim Biophys Acta. 1986;853(2):91–113. doi: 10.1016/0304-4173(86)90006-6. [DOI] [PubMed] [Google Scholar]
- Hill B. C., Greenwood C. Spectroscopic evidence for the participation of compound A (Fea32+-O2) in the reaction of mixed-valence cytochrome c oxidase with oxygen at room temperature. Biochem J. 1983 Dec 1;215(3):659–667. doi: 10.1042/bj2150659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill B. C., Greenwood C. The reaction of fully reduced cytochrome c oxidase with oxygen studied by flow-flash spectrophotometry at room temperature. Evidence for new pathways of electron transfer. Biochem J. 1984 Mar 15;218(3):913–921. doi: 10.1042/bj2180913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P., Wilson M. T., Aasa R., Malmström B. G. Cyanide inhibition of cytochrome c oxidase. A rapid-freeze e.p.r. investigation. Biochem J. 1984 Dec 15;224(3):829–837. doi: 10.1042/bj2240829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. G., Bickar D., Wilson M. T., Brunori M., Colosimo A., Sarti P. A re-examination of the reactions of cyanide with cytochrome c oxidase. Biochem J. 1984 May 15;220(1):57–66. doi: 10.1042/bj2200057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai K., Kitagawa T., Morimoto H. Quaternary structures and low frequency molecular vibrations of haems of deoxy and oxyhaemoglobin studied by resonance raman scattering. J Mol Biol. 1980 Jan 25;136(3):271–289. doi: 10.1016/0022-2836(80)90374-5. [DOI] [PubMed] [Google Scholar]
- Ogura T., Yoshikawa S., Kitagawa T. Raman/absorption simultaneous measurements for cytochrome oxidase compound A at room temperature with a novel flow apparatus. Biochemistry. 1989 Oct 3;28(20):8022–8027. doi: 10.1021/bi00446a008. [DOI] [PubMed] [Google Scholar]
- Ogura T., Yoshikawa S., Kitagawa T. Resonance Raman spectra for catalytic intermediates of cytochrome c oxidase detected with a mixed flow transient apparatus. Biochim Biophys Acta. 1985 Nov 29;832(2):220–223. doi: 10.1016/0167-4838(85)90335-8. [DOI] [PubMed] [Google Scholar]
- Oliveberg M., Brzezinski P., Malmström B. G. The effect of pH and temperature on the reaction of fully reduced and mixed-valence cytochrome c oxidase with dioxygen. Biochim Biophys Acta. 1989 Dec 7;977(3):322–328. doi: 10.1016/s0005-2728(89)80087-8. [DOI] [PubMed] [Google Scholar]
- Orii Y. Formation and decay of the primary oxygen compound of cytochrome oxidase at room temperature as observed by stopped flow, laser flash photolysis and rapid scanning. J Biol Chem. 1984 Jun 10;259(11):7187–7190. [PubMed] [Google Scholar]
- Orii Y. Intermediates in the reaction of reduced cytochrome oxidase with dioxygen. Ann N Y Acad Sci. 1988;550:105–117. doi: 10.1111/j.1749-6632.1988.tb35327.x. [DOI] [PubMed] [Google Scholar]
- Van Wart H. E., Zimmer J. Resonance Raman evidence for the activation of dioxygen in horseradish oxyperoxidase. J Biol Chem. 1985 Jul 15;260(14):8372–8377. [PubMed] [Google Scholar]
- Yoshikawa S., Choc M. G., O'Toole M. C., Caughey W. S. An infrared study of CO binding to heart cytochrome c oxidase and hemoglobin A. Implications re O2 reactions. J Biol Chem. 1977 Aug 10;252(15):5498–5508. [PubMed] [Google Scholar]
- van Buuren K. J., Nicholis P., van Gelder B. F. Biochemical and biophysical studies on cytochrome aa 3 . VI. Reaction of cyanide with oxidized and reduced enzyme. Biochim Biophys Acta. 1972 Feb 28;256(2):258–276. doi: 10.1016/0005-2728(72)90057-6. [DOI] [PubMed] [Google Scholar]
- van Buuren K. J., Zuurendonk P. F., van Gelder B. F., Muijsers A. O. Biochemical and biophysical studies on cytochrome aa 3 . V. Binding of cyanide to cytochrome aa 3 . Biochim Biophys Acta. 1972 Feb 28;256(2):243–257. doi: 10.1016/0005-2728(72)90056-4. [DOI] [PubMed] [Google Scholar]



