Abstract
Introduction
We conducted a randomized, double-blinded, placebo-controlled study, to evaluate the effect of dehydroepiandrosterone (DHEA), on diminished ovarian reserve (DOR).
Materials and Methods
Twenty patients with DOR received DHEA (oral 25 mg three times a day). Post-supplementation 12 weeks, D2/3 age-specific follicle-stimulating hormone (FSH), anti-mullerian hormone (AMH) levels, and antral follicle count (AFC), were repeated to evaluate response. Spontaneous pregnancy rates and regularization of menstrual cycles were also studied as secondary outcome.
Results
Predominant risk factors were age >35 years (28 %) and poor responders to ovarian stimulation (23 %). There was significant improvement of AMH levels (1.15 ± 1.49 vs. 1.53 ± 1.62) found before and after supplementation in the DHEA group. When the AMH values between DHEA and placebo group were compared, pre- and post-supplementation, no significant difference was found. There was decrease in FSH levels and increase in AFC value post-supplementation in both DHEA and placebo groups which was not statically significant. DHEA supplementation benefited clinically, as evidenced by the improvement in the menstrual abnormality spontaneous conception in two cases each.
Conclusions
A significant improvement in AMH levels pre- and post-supplementation of DHEA was noted. The same was not seen for FSH and AFC values.
Keywords: Diminished ovarian reserve, Dehydroepiandrosterone, FSH, Anti-mullerian hormone, Antral follicle count
Introduction
Poor ovarian response reflecting diminished ovarian reserve (DOR) still remains among the foremost challenging problems in artificial reproductive medicine. It is estimated that despite of all protocol, 5–18 % of all IVF cycles are ended because of poor ovarian response [1–3]. The treatment of diminished ovarian reserve is debatable. The possible options for fulfilling parenthood for such couples are limited, like in vitro fertilization with donor oocyte or adoption. Since this may not be socially acceptable or sometimes economically feasible, alternate options need to be explored. It has been proposed based on various studies that oral administration of Dehydroepiandrosterone (DHEA), a food supplement found naturally in wild yam and soy products, may improve ovarian response and pregnancy rates in reduced ovarian reserve [4, 5]. DHEA ovarian impact needs to be reassessed, as so far only few and small double-blinded randomized case control studies has been done in this regard, with limited statistical significance and those too in the setting of in vitro fertilization [1–3].
We planned a randomized, double-blinded, placebo-controlled study, to evaluate the effect of DHEA on diminished ovarian reserve (DOR) and its impact on objective parameters such as serum anti-mullerian hormone (AMH), age-specific follicle-stimulating hormone (FSH) and Antral follicle count (AFC).
Materials and Methods
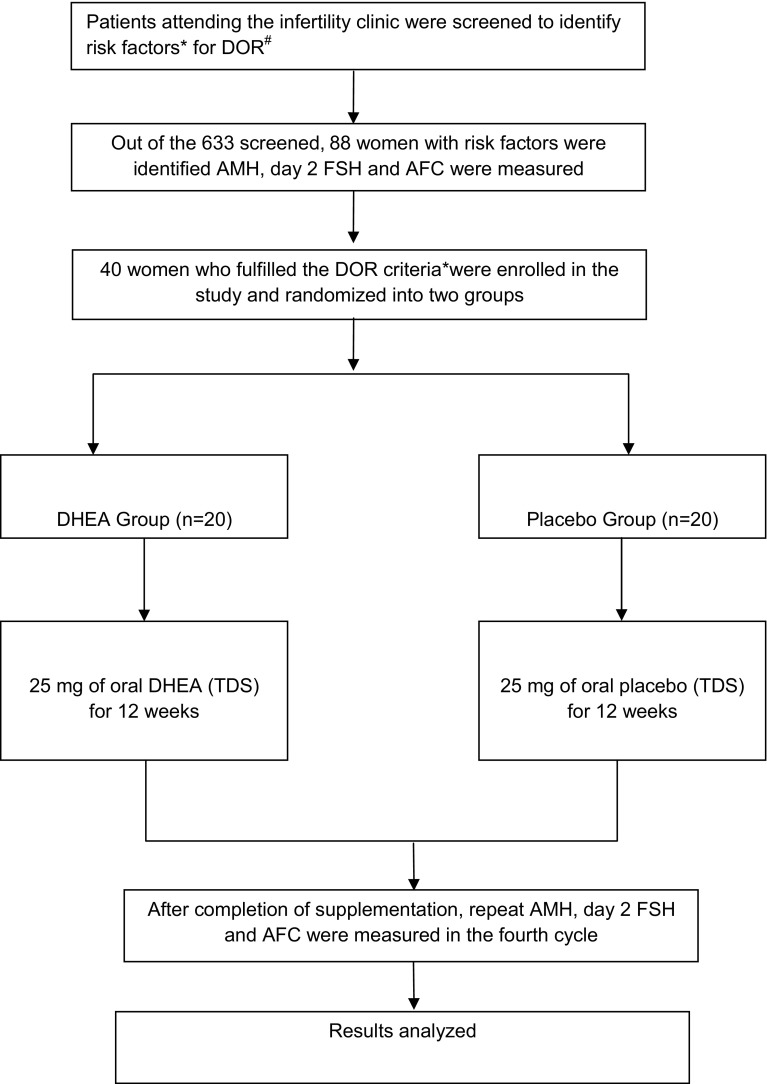
The study (2012–2014) was approved by the hospital ethics committee, and an informed consent from all the participants obtained. Our centre does not have IVF and assisted reproduction facilities. The inclusion criteria for enrolled in the study were patients (age group 18–45 years) of infertility (primary or secondary), with high-risk factors for DOR and rest of the infertility workup was normal (tubal and male factor), who screened positive for any of the tests of diminished ovarian reserve (Fig. 1) [6, 7]. Women with polycystic ovarian disorder, premature ovarian failure (FSH levels >30 mIU/ml), hormone-dependent disorders (e.g., breast cancer), diagnosed liver disease, diabetes or psychiatric illness were excluded.
Fig. 1.
Consort statement and methodology. *High-risk factors in infertile patients, who were subjected to tests of ovarian reserve, were age >35 years, previous ovarian surgery, family history of premature ovarian failure, poor responders to standard ovulation induction protocol, with clomiphene or gonadotrophins, suspected unexplained infertility. #DOR: Any one criteria for diagnosis of diminished ovarian reserve (DOR) in cases of infertility were [4, 6–12]. (1) Age-specific FSH levels using the ELFA technique (enzyme-linked fluorescent assay) (>7 mIU/ml for age <33 years; >7.9 mIU/ml for age 33–37 years; >8.4 mIU/ml for age >38 years). (2) AMH levels done using the AMH Gen II enzyme-linked immunosorbent assay <1.05 ng/ml. (3) Sonographic antral follicle count AFC ≤ 4. The diameter of the follicle in the range of 2–10 mm was counted in both ovaries and added to give total antral follicle count
Intervention/Procedure
Computerized random number table for 40 packets was generated (Fig. 1). The patients were allocated precoded packets in a consecutive manner, as they were enrolled in the study. Oral DHEA supplementation was given at the dosage of 25 mg three times a day for duration of 12 weeks to one group and placebo to the other in a double-blind manner. Patients were followed up weekly for the first 2 weeks for tolerance and side effects, every fortnightly thereafter to ensure compliance. After 12 weeks, serum D2/3 FSH, serum AMH levels, and AFC were repeated in their fourth cycle. Spontaneous pregnancy rates, if any, during the course of supplementation were also recorded. Regularization of menstrual cycles in patients with previous menstrual problems was also studied as a secondary outcome.
Statistical Analysis
The study groups were matched, regarding age, BMI, primary and secondary infertility, and the two groups were comparable. Nonparametric tests were applied to obtain between the group comparison (Mann–Whitney test) and within the group comparison (Wilcoxon signed-rank test). Repeated measure ANOVA was applied to obtain the between and within the group comparisons for FSH levels.
Results
Forty women fulfilled the inclusion criteria and fit into the diagnosis of DOR, making the prevalence rate of 6.3 % (40 out of 633) at our centre (Table 1). The predominant risk factors in our study population (n = 40) were age >35 years (28 %) and poor responders to ovarian stimulation (23 %). The percentage prevalence of POA women <38 years with DOR was 85 % (n = 34). The mean age (35.8 vs. 30.4 years) and duration of infertility (10.5 vs. 6.6 years) were more in cases of secondary infertility when compared to cases of primary infertility in DHEA and placebo group (Table 2).
Table 1.
Diagnostic criteria fulfilled for enrollment into study
| Diagnostic criteria | DOR n = 40 (percentage) | DHEA group (n = 20) | Placebo group (n = 20) |
|---|---|---|---|
| Age-specific FSH alone | 4 (10) | 2 | 2 |
| AMH alone cutoff <1.05 ng/ml) | 6 (15) | 4 | 2 |
| AFC alone cutoff ≤4 | 6 (15) | 3 | 3 |
| Age-specific FSH + AMH | 6 (15) | 5 | 1 |
| Age-specific FSH + AFC | 6 (15) | 1 | 5 |
| AMH + AFC | 2 (5) | 0 | 2 |
| Age-specific FSH + AMH + AFC | 10 (25) | 5 | 5 |
Table 2.
Clinical characteristic profile in study group (n = 40)
| DHEA | Placebo | |
|---|---|---|
| Age (mean) in years | 33.10 ± 4.29 | 32.30 ± 4.07 |
| BMI (mean) kg/cm2 | 25.01 ± 3.31 | 25.04 ± 3.77 |
| No. of cases with menstrual abnormalities (n = 14) | 6 | 8 |
| No of cases with amenorrhea (n = 8) | 4 | 4 |
| No of cases with oligomenorrhea (n = 6) | 2 | 4 |
| No of cases with failed ovulation induction (n = 9) | 5 | 4 |
| Past h/o treated chronic infection (n = 10) | 4 | 6 |
After the completion of supplementation, the values of FSH, AFC, and AMH from pre- and post-supplementation were compared (Table 3). It was seen that there is a decrease in the mean value of FSH in both groups, but it was not statistically significant (p = 0.899). When the post-supplementation values between DHEA and placebo group were compared, it was also not statistically significant (p = 0.235). There was significant improvement of AMH levels (1.15 ± 1.49 vs. 1.53 ± 1.62) found before and after supplementation in the DHEA group (p = 0.048), whereas in the placebo group, although the mean value improved, but it was not found to be statistically significant (p = 0.776). When the AMH values between DHEA and placebo group were compared, post-supplementation (p = 0.725), no significant difference was found. Although the mean value of AFC increased pre- and post-supplementation, on within the group comparison, it was not statistically significant (p = 0.092). When the post-supplementation values between DHEA and placebo group were compared, it was also not statistically significant (p = 0.097). Thus there was slight decrease in FSH levels, increase in AMH levels, and increase in AFC value post-supplementation in both DHEA and placebo groups. On statistical analysis, only the increase in AMH value was found to be statistically significant.
Table 3.
Comparison of FSH, AMH levels, and AFC pre- and post-supplementation in DHEA and placebo group
| Pre-supplementation (mean ± SD) | Post-supplementation (mean ± SD) | p value | |
|---|---|---|---|
| FSH levelsa (mIU/ml) | |||
| DHEA | 10.68 ± 6.49 | 10.36 ± 5.84 | – |
| Placebo | 12.69 ± 8.01 | 11.53 ± 7.15 | |
| Log values | |||
| DHEA | 2.16 ± 0.74 | 2.16 ± 0.70 | 0.899b |
| Placebo | 2.43 ± 0.69 | 2.46 ± 0.98 | |
| Anti-mullerian hormone AMH (ng/ml) | |||
| DHEA | 1.15 ± 1.49 | 1.53 ± 1.62 | 0.048 |
| Placebo | 1.03 ± 0.81 | 1.07 ± 0.85 | 0.776 |
| Antral follicle count (AFC) | |||
| DHEA | 4.95 ± 1.95 | 5.25 ± 2.35 | 0.092b |
| Placebo | 3.75 ± 1.11 | 4.55 ± 2.38 | |
aThe data of FSH levels were not following the normal distribution curve, log transformation applied to normalize it
brepeated measure ANOVA
There were two cases of spontaneous conception which occurred after completion of DHEA supplementation. Both the patients had secondary infertility and had received DHEA. One patient was parous, and the other had repeated pregnancy loss. When statistical tests were applied, it was not found to be significant (p = 0.244). On follow-up, out of the two women, one had a missed abortion at 8 weeks of gestation and the other had viable conception.
Three women with menstrual abnormalities had normalization of the menstrual cycles at the end of 12 weeks, of which two were in DHEA group and one in placebo group. But, this also was not found to be statistically significant (p = 0.500).
All cases of DOR were reviewed for androgenic side effects as well such as acne, facial hair growth, and deepening of voice [8]. None of the women had these or any other side effects during the period of supplementation.
Discussion
Diminished ovarian reserve (DOR), one of the recently recognized causes of infertility, is a natural process by which follicular pool diminishes with time, as women ages. The prevalence of DOR is said to be around 10 % in the infertile population [1].
Conventional modalities of treatment in high-income countries would suggest IVF with donor oocyte as the next step in DOR. However, there is a need for cheaper alternatives to the expensive IVF treatment in low-income health care settings. Few small randomized prospective studies suggest that Dehydroepiandrosterone (DHEA) improves ovarian function, ovarian reserve, increases chances of live birth, by reducing miscarriage and aneuploidy [5, 6, 9, 10]. The rationale suggested is that oocytes, in their resting stage within the unrecruited primordial follicles, do not really age [4]. Once recruited, they enter into an age-dependent ovarian environment, where follicle maturation takes place. The quality of this environment deteriorates uniformly as women age and affects the segregation process of meiosis resulting in aneuploidies. DHEA probably acts by altering and rejuvenating this ovarian environment and hence preventing the aging of the follicles.
There have been many studies reporting the role of DHEA in DOR mainly in the setup of ART and IVF setting, where oocyte retrieval has been used principally as a predictor of improvement. Barad and Gleicher [4, 6] reported improvement in quality and quantity of oocytes, and embryos retrieved post-DHEA supplementation. Sonmezer evaluated DHEA in the intracytoplasmic sperm injection (ICSI) cycles. DHEA potentiated the effect of gonadotropins, in increasing the number and quality of oocytes, embryo quality, and increased pregnancy and decreased cancelation rate [2]. Contrarily, a recent metaanalysis based on 22 publications and 3 controlled studies indicated insufficient evidence for supporting the role of DHEA in IVF cycles [7]. We designed this randomized, double-blinded, placebo-controlled study to further evaluate the effects of DHEA in diminished ovarian reserve.
Decreased ovarian reserve diagnosed in younger women (<38 years) is defined as premature ovarian aging (POA) [8]. Thus a significant number of women in our study were POA (85 %) reflecting increased age of child bearing in career-driven women of the present generation. The incidence of poor ovarian response to stimulation (9–24 %) in infertile patients was also comparable in our study (23 %) [7]. In our study, there was significant increase in levels of AMH, pre- and post-supplementation in the DHEA group (p = 0.048), from 1.15 ± 1.49 to 1.53 ± 1.62 ng/ml, suggesting an improvement in ovarian reserve. Gleicher in his study observed an improvement from 0.22 ± 0.22 ng/ml in baseline to 0.35 ± 0.03 ng/ml after DHEA supplementation in 120 subjects, an increase of 60 % in mean value (p = 0.001) [8]. Significant improvement in AMH levels after DHEA supplementation was also noted by Yilmaz et al. [11]. We found no significant decrease in levels of FSH and no increase in AFC, pre- and post-supplementation in either DHEA or placebo group. Yilmazon the contrary, noticed a significant decrease in FSH levels (p = 0.001) and increase in AFC (p = 0.025) after DHEA supplementation in 41 patients [11]. The difference could probably be due to the limited subjects receiving DHEA in our study.
The occurrence of two spontaneous pregnancy in the DHEA group (p = 0.244), and none in placebo group, was an interesting observation. Similar incidence of spontaneous pregnancy while on DHEA supplementation was reported by other authors suggesting DHEA probable capacity in increasing fecundity [11, 12]. One of them had a missed abortion at 8 weeks of gestation, and the other resulted in full-term pregnancy. It is important to note that both these women were secondary infertility; this was comparable to the results obtained by Wiser et al. [5] where 14 of the clinical pregnancies observed were in secondary infertile women. Thus in DOR managed with DHEA, patients with previous pregnancy may have better prognosis than women of primary infertility.
The normalization of menstrual cycles post-DHEA supplementation noted in three women, although not statistically significant (p = 0.500), was also important as the normalization of the ovarian milieu, with associated improvement in the hormonal imbalance and anovulation, might have lead to regular cycles. Further research need to be done regarding this effect of DHEA.
Facilities for IVF and ART are not available at our centre. We were therefore unable to quantify oocyte retrieval and embryo quality. Data regarding subsequent ovulation induction or IVF cycles were not available due to limited scope and small time frame of our study. However, evaluation of a new emerging treatment modality for DOR using prospective double-blinded randomized placebo-controlled data collection and uniform monitoring of pre- and post-DHEA supplementation responses using three parameters (FSH, AMH and AFC) was the key strengths of this study. The increase in AMH values was significant supporting the beneficial role of DHEA in DOR. DHEA supplementation also benefited clinically, as evidenced by the improvement in menstrual abnormalities and spontaneous conceptions. DHEA can be one of the low-cost options in DOR in low-income countries like ours where facilities for IVF and ART are very limited and costly. Its true potential as a sole agent for DOR remains to be tested in larger trials.
Conclusions
A significant improvement in AMH levels pre- and post-supplementation of DHEA was noted supporting its beneficial role of DHEA in diminished ovarian reserve. The same was not seen for FSH and AFC values. DHEA supplementation benefited clinically, as evidenced by the improvement in the menstrual abnormality spontaneous conception in two cases each.
Dr. Rachna Agarwal
is a graduate and postgraduate from the prestigious All India Institute of Medical Sciences, New Delhi. She has over 40 publications, with 25 in indexed international journals. She has co-authored two books—Jaypee’s Video Atlas of Surgical Techniques in Gyneocology and Obstetrics and Step by Step Non-Descent Vaginal Hysterectomy. She was project facilitator of WHO Global Survey (2008)—Asia on Maternal and Perinatal health: mode of delivery and pregnancy outcomes and WHO RHL-EBM trial (2009). She has made several scientific presentations at international, national and regional conferences. She is also on the panel of reviewers for the international journals. She was past Joint Secretary of the Association of Obstetrics and Gynaecology of Delhi (2011) and Joint Secretary NARCHI Delhi branch (2014–2016). Her area of interest includes gynecology, oncology and high-risk pregnancy.
Compliance with Ethical Standards
Conflict of interest
None of the authors have any conflict of interest or financial conflicts. The authors have nothing to disclose.
Research Involving Human Participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants, and institutional ethical clearance was obtained for the study.
Footnotes
Dr. Rachna Agarwal is Professor at Department of Obstetrics and Gynaecology, Dr. R. Shruthi is Ex-Resident at Department of Obstetrics and Gynaecology, Dr. Gita Radhakrishnan is Director Professor and H.O.D at Department of Obstetrics and Gynaecology, Dr. Alpana Singh is Assistant Professor at Department of Obstetrics and Gynaecology
References
- 1.Ebner T, Sommergruber M, Moser M, et al. Basal level of anti Mullerian hormone is associated with oocytes quality in stimulated cycles. Hum Reprod. 2006;21:2022–2026. doi: 10.1093/humrep/del127. [DOI] [PubMed] [Google Scholar]
- 2.Sönmezer M, Özmen B, Cil AP, et al. Dehydroepiandrosterone supplementation improves ovarian response and cycle outcome in poor responders. Reprod Biomed Online. 2009;19:508–513. doi: 10.1016/j.rbmo.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Casson PR, Lindsay MS, Pisarska MD, et al. Dehydroepiandrosterone supplementation augments ovarian stimulation in poor responders: a case series. Hum Reprod. 2000;15:2129–2132. doi: 10.1093/humrep/15.10.2129. [DOI] [PubMed] [Google Scholar]
- 4.Gleicher N, Barad DH. Dehydroepiandrosterone (DHEA) supplementation in diminished ovarian reserve (DOR): review. Reprod Biol Endocrinol. 2011;9:67. doi: 10.1186/1477-7827-9-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiser A, Gonen O, Ghetler Y, et al. Addition of dehydroepiandrosterone (DHEA) for poor-responder patients before and during IVF treatment improves the pregnancy rate: a randomized prospective study. Hum Reprod. 2010;25:2496–2500. doi: 10.1093/humrep/deq220. [DOI] [PubMed] [Google Scholar]
- 6.Gleicher N, Ryan E, Weghofer A, et al. Dehydroepiandrosterone (DHEA) reduces miscarriage rates in women with diminished ovarian reserve: a multicenter study. Reprod Biol Endocrinol. 2009;7:108. doi: 10.1186/1477-7827-7-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narkwichean A, Maalouf W, Campbell BK, et al. Efficacy of dehydroepiandrosterone to improve ovarian response in women with diminished ovarian reserve: a meta-analysis. Reprod Biol Endocrinol. 2013;11:44. doi: 10.1186/1477-7827-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gleicher N, Weghofer A, Barad DH. Improvement in diminished ovarian reserve after dehydroepiandrosterone supplementation. Reprod Biomed Online. 2010;21:360–365. doi: 10.1016/j.rbmo.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Haydardedeoglu B. DHEA supplementation and ICSI outcomes: was this really randomized trial? Eur J Obstet Gynecol Reprod Biol. 2016 doi: 10.1016/j.ejogrb.2016.03.033. [DOI] [PubMed] [Google Scholar]
- 10.Gleicher N. Randomised controlled trials on dehydroepiandrosterone supplementation in female infertility still not conclusive. BJOG. 2016 doi: 10.1111/1471-0528.13945. [DOI] [PubMed] [Google Scholar]
- 11.Yilmaz N, Uygur D, Inal H, et al. Dehydroepiandrosterone supplementation improves predictive markers for diminished ovarian reserve: serum AMH, inhibin B and antral follicle count. Eur J Obstet Gynecol Reprod Biol. 2013;169:257–260. doi: 10.1016/j.ejogrb.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Barad D, Brill H, Gleicher N. Update on the use of Dehydroepiandrosterone supplementation among women with diminished ovarian function. J Assist Reprod Genet. 2007;24:629–634. doi: 10.1007/s10815-007-9178-x. [DOI] [PMC free article] [PubMed] [Google Scholar]