Abstract
Although coronary artery disease (CAD) is common in patients with heart failure (HF), little is known about the prognostic significance of coronary lesion complexity in patients with prior HF undergoing percutaneous coronary intervention (PCI). The aim of this study was to investigate whether the coronary Synergy between Percutaneous Coronary Intervention with TAXus and Cardiac Surgery (SYNTAX) score could improve risk stratification in HF patients with CAD. Two hundred patients (mean age 73 ± 11 years, left ventricular ejection fraction 49 ± 15 %) with prior HF who underwent PCI were divided into two groups stratified by SYNTAX score (median value 12) and tracked prospectively for 1 year. The study endpoint was the composite of major adverse cardiovascular events (MACE), including all-cause death, myocardial infarction, stroke, and hospitalization for worsening HF. Adverse events were observed in 39 patients (19.5 %). Patients with high SYNTAX scores (n = 100) showed worse prognoses than those with low scores (n = 100) (26.0 vs. 13.0 %, respectively, P = 0.021). In multivariate Cox-regression analysis, SYNTAX score ≥12 was significantly associated with MACE (hazard ratio: 1.99, 95 % confidence interval: 1.02–3.97; P = 0.045). In patients with prior HF and CAD, high SYNTAX scores predicted a high incidence of MACE. These results suggest that the SYNTAX score might be a useful parameter for improving risk stratification in these patients.
Keywords: Coronary artery disease, Heart failure, SYNTAX score, Prognosis
Introduction
Heart failure (HF) is a serious healthcare problem in today’s aging society. Despite significant advances in the treatment of chronic HF, the disease tends to follow a progressive course with high mortality and morbidity rates [1–3]. Patients with HF are at significant risk for recurrent cardiovascular events such as death, myocardial infarction (MI), stroke, and hospitalization for worsening HF. Therefore, the secondary prevention of cardiovascular events is invaluable for improving the prognostic outlook of HF patients. A novel risk stratification system would provide critical information that could result in more aggressive therapy and lead to improved patient survival. Coronary artery disease (CAD) has contributed to the increased prevalence of HF and is associated with cardiovascular events in patients with HF [4–7]. The Synergy between Percutaneous Coronary Intervention with TAXus and Cardiac Surgery (SYNTAX) score, a measure of coronary lesion complexity, has been proposed for use in the risk stratification of patients with untreated left main trunk or 3-vessel CAD [8, 9]. However, the prognostic significance of the SYNTAX score for risk stratification in HF patients is poorly understood. We hypothesized that the SYNTAX score would predict adverse cardiovascular events in patients with HF. The aim of this study was to investigate whether the coronary SYNTAX score could improve risk stratification in HF patients with CAD.
Materials and methods
Study population
This cohort study retrospectively reviewed data available from the SHINANO registry (Shinshu prospective multi-center study of elderly patients with CAD undergoing percutaneous coronary intervention (PCI)) obtained between August 2012 and July 2013. A detailed summary of the methods and design of this registry has been published previously [10]. Briefly, the SHINANO registry is a prospective, multi-center observational registry of patients with any CAD diagnosis, including stable angina, ST-segment elevation MI, non-ST-segment elevation MI, and unstable angina, undergoing PCI at 16 collaborating hospitals located in the Nagano prefecture, Japan. As it is an all-comer registry, there are no exclusion criteria. The institutional review board approved the protocol, which was registered at the University Hospital Medical Information Network (UMIN000010070), and informed consent was obtained from each patient before enrollment. This study was performed in accordance with the Declaration of Helsinki.
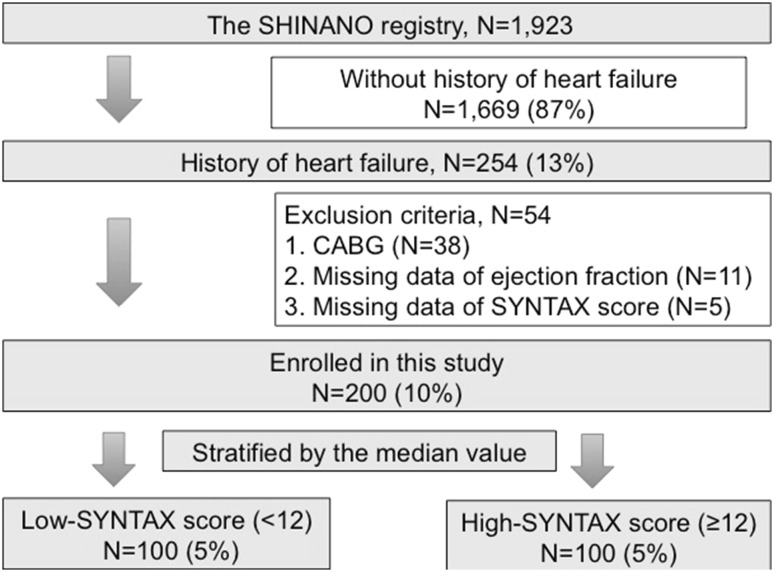
Among the 1923 patients registered in the SHINANO registry, we screened 254 patients with a history of HF. After excluding patients with a history of coronary artery bypass grafting (CABG), as well as those with missing data concerning left ventricular ejection fraction (LVEF) or no SYNTAX score, we enrolled 200 patients with prior HF into the final study. All patients were prospectively followed for 12 months from the date of the PCI procedure. The study endpoint was the composite of major adverse cardiovascular events (MACE), including all-cause death, MI, stroke, and hospitalization for worsening HF using a time-to-first-event analysis.
Study definitions
MI was diagnosed according to the American College of Cardiology/American Heart Association (ACC/AHA) guidelines [11]. Stroke was defined as an ischemic cerebrovascular event that persisted for ≥24 h and was diagnosed by a neurologist [12]. Prior HF was based on a previous diagnosis of HF according to the Framingham criteria [13] or a history of hospitalization for worsening HF. Body mass index was defined as weight in kilograms divided by the square of height in meters. Patients with systolic blood pressure >140 mmHg and/or diastolic pressure >90 mmHg and those taking anti-hypertensive agents were considered to have hypertension. Dyslipidemia was defined as a serum total cholesterol concentration ≥220 mg/dL, low-density lipoprotein cholesterol ≥140 mg/dL, or the need for treatment with lipid-lowering agents. Diabetes mellitus was defined as hemoglobin (Hb) A1c ≥6.5 %, random plasma glucose ≥220 mg/dL, or a clinical history of oral hypoglycemic agent and/or insulin use. Patients were considered smokers if they were current smokers. CAD was defined as >50 % stenosis in a coronary vessel on angiography, history of CABG or PCI, or history of MI. Multi-vessel disease was defined as the presence of a ≥75 % lesion in ≥2 major coronary arteries. The SYNTAX score was calculated as previously described [9]. Complete revascularization was considered to have occurred when all stenotic main vessels and all side branches greater than 2 mm in diameter were revascularized [14]. Estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet and Renal Disease equation with coefficients modified for Japanese patients [15]. Chronic kidney disease was defined as an eGFR <60 ml/min/1.73 m2. LVEF was calculated using the Teichholz method in patients without regional wall motion abnormality or the biplane Simpson’s method in those with regional wall motion abnormality [16, 17]. All PCI procedures and selection of medical treatments after PCI were at the discretion of the treating physician.
Statistical analysis
Continuous variables were summarized as mean ± standard deviation if normally distributed or as median and interquartile range otherwise. Normality was evaluated using the Shapiro–Wilk W test. Comparisons of baseline categorical data between the two groups were analyzed using two-sided Chi-squared tests, whereas differences between continuous variables were analyzed using an unpaired t test or the Mann–Whitney U test. The optimal cutoff value for MACE prediction was chosen as the value that maximized sensitivity and specificity on the receiver operating characteristics (ROC) curve. Kaplan–Meier curves were constructed from the date of the PCI procedure to the MACE and were compared using the log-rank test. Cox proportional hazards regression analysis was performed to identify the MACE predictors, using variables that included clinical characteristics and risk factors. Multivariate analysis was performed using all variables with a P value <0.1 in the univariate analysis. A P value <0.05 was considered to indicate statistical significance. All analyses were performed using SPSS version 22.0 (SPSS, Chicago, IL, USA).
Results
Patient characteristics
All of the patients initially enrolled in the study completed the follow-up. Using the median value of the SYNTAX score (12), patients were divided into a high-SYNTAX score group (n = 100) and a low-SYNTAX group (n = 100) (Fig. 1). During the 12-month follow-up period, adverse events were observed in 39 patients (19.5 %) and included deaths (n = 25), MIs (n = 5), strokes (n = 5), and hospitalizations for HF (n = 18) (Table 1). The baseline clinical characteristics are listed in Table 2. Patients with high SYNTAX scores did not differ from those with low scores with respect to age, sex, the prevalence of hypertension, dyslipidemia, diabetes mellitus, atrial fibrillation, chronic kidney disease, prior stroke, hemodialysis, or peripheral artery disease. Patients with high SYNTAX scores had significantly more severe HF symptoms, as estimated by New York Heart Association (NYHA) functional class, compared with those with low SYNTAX scores. As expected, the prevalence of multi-vessel disease was significantly higher in patients with high SYNTAX scores compared with those with low scores. Complete revascularization was significantly rarer in patients with high SYNTAX scores. The use of angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, and statins was similar between the two groups. Hb, low-density lipoprotein cholesterol, eGFR, and HbA1c levels were similar between the two groups, whereas levels of brain natriuretic peptide tended to be higher in patients with high SYNTAX scores than in those with low scores. LVEF was significantly lower in patients with high SYNTAX scores than in those with low scores.
Fig. 1.
Study design. CABG coronary artery bypass graft
Table 1.
Clinical outcomes
| Overall (n = 200) | Low-SYNTAX group (<12) (n = 100) | High-SYNTAX group (≥12) (n = 100) | P value | |
|---|---|---|---|---|
| MACE, n (%) | 39 (19.5) | 13 (13.0) | 26 (26.0) | 0.021 |
| All-cause death, n (%) | 25 (12.5) | 4 (4.0) | 21 (21.0) | <0.001 |
| Cardiac death, n (%) | 13 (6.5) | 1 (1.0) | 12 (12.0) | 0.001 |
| Myocardial infarction, n (%) | 5 (2.5) | 3 (3.0) | 2 (2.0) | 0.58 |
| Stroke, n (%) | 5 (2.5) | 2 (2.0) | 3 (3.0) | 0.60 |
| Hospitalization for heart failure, n (%) | 18 (9.0) | 7 (7.0) | 11 (11.0) | 0.24 |
Values are number (%)
MACE major adverse cardiac events (including all-cause death, myocardial infarction, stroke, and hospitalization for heart failure)
Table 2.
Baseline characteristics of patients according to SYNTAX score
| Overall (n = 200) | Low-SYNTAX group (<12) (n = 100) | High-SYNTAX group (≥12) (n = 100) | P value | |
|---|---|---|---|---|
| Age | 73 ± 11 | 73 ± 11 | 74 ± 11 | 0.58 |
| Female sex, n (%) | 47 (23.4) | 18 (18.0) | 29 (29.0) | 0.067 |
| Body mass index (kg/m2) | 22.6 ± 4.1 | 23.1 ± 3.9 | 22.2 ± 4.3 | 0.14 |
| Ischemic etiology (%) | 66.7 | 58.3 | 75.6 | 0.001 |
| NYHA functional class ≥III, n (%) | 60 (30.0) | 23 (23.0) | 37 (37.0) | 0.031 |
| Comorbidities | ||||
| Hypertension, n (%) | 157 (78.1) | 78 (78.0) | 79 (79.0) | 0.86 |
| Dyslipidemia, n (%) | 127 (63.2) | 62 (62.0) | 65 (65.0) | 0.66 |
| Diabetes mellitus, n (%) | 76 (37.8) | 40 (40.0) | 36 (36.0) | 0.56 |
| Current smoker, n (%) | 25 (12.4) | 12 (12.0) | 13 (13.0) | 0.87 |
| Atrial fibrillation, n (%) | 56 (28.0) | 34 (34.0) | 22 (22.0) | 0.059 |
| Chronic kidney disease, n (%) | 132 (66.0) | 72 (72.0) | 60 (60.0) | 0.10 |
| Prior stroke, n (%) | 27 (13.5) | 12 (12.0) | 15 (15.0) | 0.68 |
| Hemodialysis, n (%) | 22 (11.0) | 15 (15.0) | 7 (7.0) | 0.11 |
| Peripheral artery disease, n (%) | 37 (18.5) | 17 (17.0) | 20 (20.0) | 0.72 |
| Angiographic data | ||||
| Target coronary lesion | ||||
| Right coronary artery, n (%) | 72 (36.0) | 41 (41.0) | 31 (31.0) | 0.19 |
| Left anterior descending artery, n (%) | 92 (46.0) | 40 (40.0) | 52 (52.0) | 0.12 |
| Left circumflex artery, n (%) | 31 (15.5) | 18 (18.0) | 13 (13.0) | 0.44 |
| Left main trunk, n (%) | 5 (2.5) | 1 (1.0) | 4 (4.0) | 0.37 |
| De novo lesion, n (%) | 170 (67.5) | 83 (83.0) | 87 (87.0) | 0.55 |
| Only POBA | 41 (20.5) | 21 (21.0) | 20 (20.0) | 0.86 |
| Type of implanted stent | ||||
| Drug-eluting stent, n (%) | 135 (67.5) | 69 (69.0) | 66 (66.0) | 0.76 |
| Bare metal stent, n (%) | 24 (12.0) | 10 (10.0) | 14 (14.0) | 0.52 |
| Calcification lesion, n (%) | 73 (36.5) | 32 (32.0) | 41 (41.0) | 0.24 |
| Bifurcation lesion, n (%) | 58 (29.0) | 20 (20.0) | 38 (38.0) | 0.008 |
| Ostial lesion, n (%) | 15 (7.5) | 5 (5.0) | 10 (10.0) | 0.28 |
| Multi-vessel, n (%) | 83 (41.3) | 23 (23.0) | 60 (60.0) | <0.001 |
| SYNTAX score | 13.7 ± 9.5 | 6.4 ± 2.4 | 21.1 ± 8.1 | <0.001 |
| Complete revascularization, n (%) | 123 (61.5) | 72 (72.0) | 51 (51.0) | 0.001 |
| Acute coronary syndrome, n (%) | 61 (30.3) | 27 (27.0) | 34 (34.0) | 0.28 |
| STEMI on admission, n (%) | 38 (19.0) | 10 (10.0) | 28 (28.0) | 0.002 |
| Killip class IV on admission, n (%) | 11 (5.5) | 1 (1.0) | 10 (10.0) | 0.010 |
| Medications at discharge | ||||
| Aspirin, n (%) | 187 (93.5) | 96 (96.0) | 91 (91.0) | 0.21 |
| Thienopyridines, n (%) | 167 (83.5) | 89 (89.0) | 78 (78.0) | 0.049 |
| Warfarin, n (%) | 56 (28.0) | 33 (33.0) | 23 (23.0) | 0.16 |
| ACE-inhibitor/ARB, n (%) | 157 (78.1) | 81 (81.0) | 76 (76.0) | 0.79 |
| Beta-blocker, n (%) | 118 (58.7) | 57 (57.0) | 61 (61.0) | 0.44 |
| Statin, n (%) | 134 (66.7) | 61 (61.0) | 73 (73.0) | 0.053 |
| Insulin user, n (%) | 18 (9.0) | 12 (12.0) | 6 (6.0) | 0.14 |
| Laboratory data | ||||
| Hemoglobin (g/dL) | 12.7 ± 3.0 | 12.7 ± 2.0 | 12.8 ± 3.9 | 0.84 |
| LDL-C (mg/dL) | 99.3 ± 33.8 | 96.0 ± 28.4 | 102.8 ± 38.6 | 0.17 |
| eGFR (mL/min/1.73 m2 surface area) | 50.3 ± 23.9 | 49.2 ± 22.5 | 51.3 ± 25.4 | 0.53 |
| Hemoglobin A1c (%) | 6.1 ± 1.0 | 6.1 ± 0.9 | 6.1 ± 1.0 | 0.86 |
| BNP (pg/mL) | 304 [133, 733] | 241 [123, 456] | 427 [137, 1021] | 0.067 |
| LV ejection fraction (%) | 49.4 ± 14.7 | 52.6 ± 15.7 | 46.2 ± 13.0 | 0.002 |
Values are number (%), mean ± standard deviation, or median [25th, 75th percentiles]
ACE angiotensin-converting enzyme, ARB angiotensin receptor blocker, BNP B-type natriuretic peptide, eGFR estimated glomerular filtration rate, LDL low-density lipoprotein cholesterol, LV left ventricular, MACE major adverse cardiac events (including all-cause death, myocardial infarction, stroke, and hospitalization for heart failure), POBA plain old balloon angioplasty
Predictors of MACE
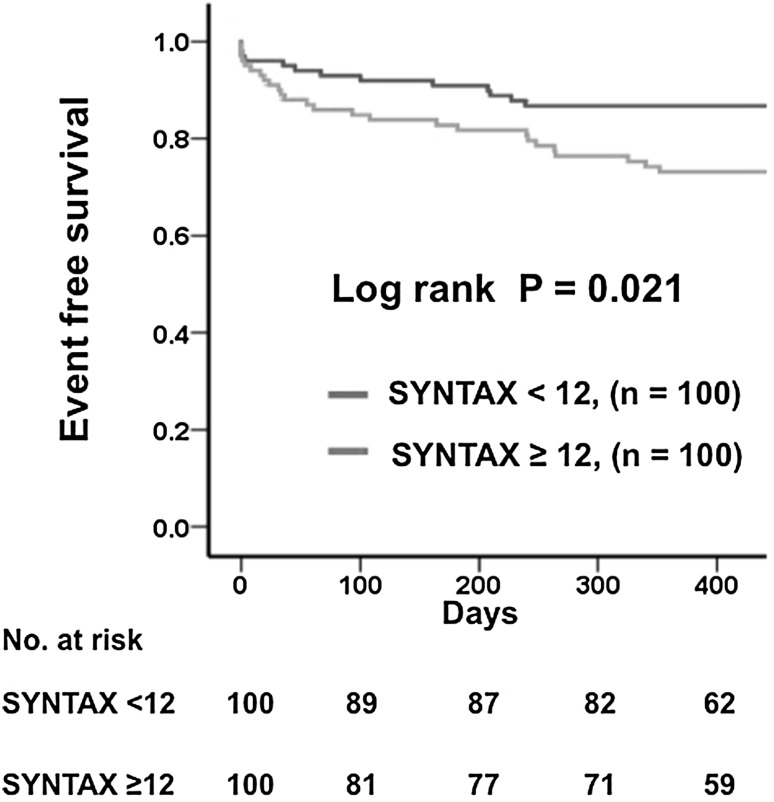
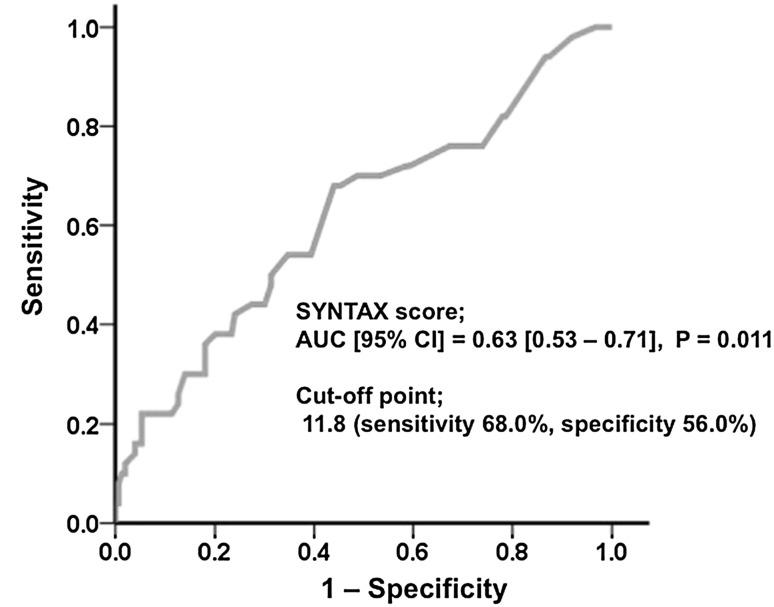
In the Kaplan–Meier analysis, patients with high SYNTAX scores (≥12) showed worse prognoses than those with low SYNTAX scores (<12) (26.0 vs. 13.0 %, respectively, P = 0.021) (Fig. 2). After multivariate Cox proportional hazards analysis, which included age, sex, and all variables with P < 0.1 in the univariate analysis, a high SYNTAX score predicted a poor prognosis (Table 3). Based on the SYNTAX scores in our study, the area under the ROC curve (AUC) was 0.63 (Fig. 3) and the optimal cutoff point for predicting adverse events was a SYNTAX score of 11.8 (sensitivity 68.0 %, specificity 56.0 %).
Fig. 2.
Kaplan–Meier curves for MACE according to SYNTAX score. MACE major adverse cardiac events (including all-cause death, myocardial infarction, stroke, and hospitalization for HF)
Table 3.
Cox Proportional Hazards Analyses of MACE
| Univariate | Multivariate* | |||
|---|---|---|---|---|
| HR (95 % CI) | P value | HR (95 % CI) | P value | |
| Age | 1.03 (1.02–1.10) | 0.087 | 1.02 (0.98–1.05) | 0.36 |
| Female sex | 1.55 (0.78–3.06) | 0.21 | 1.25 (0.62–2.52) | 0.53 |
| NYHA functional class ≥ III | 1.99 (1.06–3.75) | 0.033 | 1.87 (0.98–3.56) | 0.057 |
| Diabetes mellitus | 1.66 (0.89–3.11) | 0.11 | ||
| Atrial fibrillation | 1.56 (0.81–3.00) | 0.18 | ||
| Chronic kidney disease | 1.44 (0.63–3.10) | 0.35 | ||
| Hemodialysis | 0.19 (0.025–1.35) | 0.17 | ||
| Prior stroke | 0.77 (0.27–2.16) | 0.16 | ||
| Peripheral artery disease | 1.24 (0.57–2.70) | 0.59 | ||
| Multi-vessel disease | 1.14 (0.75–1.74) | 0.53 | ||
| SYNTAX score ≥12 | 2.14 (1.10–4.17) | 0.025 | 1.99 (1.02–3.90) | 0.045 |
| Aspirin | 0.81 (0.43–1.55) | 0.53 | ||
| Thienopyridines | 0.79 (0.42–1.48) | 0.46 | ||
| ACE-inhibitor/ARB | 1.16 (0.60–2.24) | 0.65 | ||
| Beta-blocker | 0.76 (0.50–1.45) | 0.41 | ||
| Insulin | 1.55 (0.60–4.00) | 0.36 | ||
| Hemoglobin | 0.97 (0.86–1.09) | 0.58 | ||
| eGFR | 0.99 (0.96–1.03) | 0.82 | ||
| BNP | 1.00 (1.00–1.01) | 0.75 | ||
| LV ejection fraction | 0.98 (0.96–1.00) | 0.17 | ||
ACE angiotensin-converting enzyme, ARB angiotensin receptor blocker, BNP B-type natriuretic peptide, CI confidence interval, eGFR estimated glomerular filtration rate, HR hazard ratio, LV left ventricular, MACE major adverse cardiac events (including all-cause death, myocardial infarction, stroke, and hospitalization for heart failure)
* Adjusted for age, sex, NYHA functional class ≥III, and SYNTAX score ≥12
Fig. 3.
Receiver-operating characteristic (ROC) curve for predicting adverse events. The area under the ROC curve (AUC) for the SYNTAX score was 0.63, with an optimal ROC cutoff point of 11.8. CI confidence interval
Discussion
To the best of our knowledge, this is the first report to investigate the prognostic significance of the SYNTAX score for predicting cardiovascular events in patients with prior HF undergoing PCI. We demonstrated that a high SYNTAX score was an independent predictor of MACE in this patient population.
The SYNTAX score established itself as an important tool in the SYNTAX trial, which pioneered the Heart Team approach, in which the interventional cardiologist and cardiac surgeon determined the optimal revascularization modality for patients with untreated left main trunk or 3-vessel CAD [9]. SYNTAX is a very convenient scoring system for assessing the coronary lesion complexity in patients with CAD. Previous studies have demonstrated that a higher score is an independent marker of poor cardiovascular prognosis in patients with CAD [18, 19]. Importantly, risk stratification using the SYNTAX score has been validated in patients with CAD [20, 21]; however, there have been no studies using the SYNTAX score for risk stratification in HF patients undergoing PCI. In our study, we demonstrated that the SYNTAX score had predictive value for MACE in prior HF patients with CAD undergoing PCI. This result was consistent with previous reports on the prognostic value of the SYNTAX score in patients with acute MI [22].
We demonstrated that the AUC of the SYNTAX score for predicting adverse events was 0.63. This value was similar to that reported by a previous study which evaluated the value of the SYNTAX score for predicting 12-month clinical outcomes in acute MI (AUC: 0.65) [23]. Furthermore, the AUC of the SYNTAX score for predicting 12-month adverse events was 0.60 in the SHNINO registry study which included prior HF and non-HF patients. This AUC also approximates that of the SYNTAX score for predicting 5-year adverse events which was reported as 0.61 in the SYNTAX trial [18]. It remains unclear whether the SYNTAX score is a more useful parameter in patients with more severe cardiovascular diseases, such as HF and MI, than in those with lone CAD. A high SYNTAX score was also an independent predictor of MACE after multivariate analysis. Although the mechanism of the association between the SYNTAX score and MACE might be multifactorial, previous reports demonstrated that a higher SYNTAX score was associated with complex CAD and a higher prevalence of diabetes mellitus and peripheral artery disease, suggesting that the SYNTAX score may be related to advanced coronary and systemic atherosclerosis [24, 25].
Clinical implications
Our study demonstrated that the SYNTAX score is useful for assessing the prognosis of patients with a prior diagnosis of HF undergoing PCI. Given that prior HF patients have a high risk of recurrent cardiovascular events such as sudden death and hospitalization for worsening HF, we recommend that calculation of the SYNTAX score should be performed in prior HF patients with CAD to allow for precise risk stratification for MACE.
Study limitations
The major limitation of our study is that it was an observational study with a relatively small number of subjects and, therefore, the possibility of selection bias and unmeasured confounding factors might not have been completely excluded. Thus, our results should be interpreted cautiously until verified by large-scale multi-center studies. However, our study is the first to report on the utility of the SYNTAX score in prior HF patients with CAD undergoing PCI. Second, our definition of HF relied on an investigator diagnosis based on the Framingham criteria, rather than a requirement to fulfill all of the criteria recommended in the guidelines. Third, we analyzed the predictive value of SYNTAX score using an individual cutoff point and, therefore, further studies are needed to determine the optimal cutoff points in this patient population. Fourth, we did not assess biomarkers which are known predictors of cardiovascular events, despite the fact that certain novel biomarkers are associated with a higher SYNTAX score [26, 27]. Fifth, only prior HF patients diagnosed with CAD were included in this study and, therefore, our results may not apply to all HF patients. Despite these limitations, our findings provide new insight into the risk stratification for cardiovascular events in prior HF patients undergoing PCI. Furthermore, since our study was based on observational registry data from patients with CAD, we consider that our results represent a real-world unselected population of prior HF patients undergoing PCI.
Conclusions
In prior HF patients with CAD, high SYNTAX scores predicted a high incidence of MACE. The SYNTAX score might be a useful parameter for improving risk stratification in these patients.
Acknowledgments
The authors are grateful to the SHINANO Registry Investigators for providing data for this study and offer many thanks to all the participants including patients, caregivers, and staff.
Compliance with ethical standards
Conflict of interest
None.
References
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 2.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 3.Shimokawa H, Miura M, Nochioka K, Sakata Y. Heart failure as a general pandemic in Asia. Eur J Heart Fail. 2015;17:884–892. doi: 10.1002/ejhf.319. [DOI] [PubMed] [Google Scholar]
- 4.Authors/Task Force members. Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Jüni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Eur Heart J. 2014;35:2541–2619. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
- 5.WRITING COMMITTEE MEMBERS. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL, American College of Cardiology Foundation, American Heart Association Task Force on Practice Guidelines 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 6.Mentz RJ, Broderick S, Shaw LK, Fiuzat M, O’Connor CM. Heart failure with preserved ejection fraction: comparison of patients with and without angina pectoris (from the Duke Databank for Cardiovascular Disease) J Am Coll Cardiol. 2014;63:251–258. doi: 10.1016/j.jacc.2013.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hwang SJ, Melenovsky V, Borlaug BA. Implications of coronary artery disease in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63:2817–2827. doi: 10.1016/j.jacc.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 8.Mohr FW, Morice MC, Kappetein AP, Feldman TE, Ståhle E, Colombo A, Mack MJ, Holmes DR, Jr, Morel MA, Van Dyck N, Houle VM, Dawkins KD, Serruys PW. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet. 2013;381:629–638. doi: 10.1016/S0140-6736(13)60141-5. [DOI] [PubMed] [Google Scholar]
- 9.Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, Ståhle E, Feldman TE, van den Brand M, Bass EJ, Van Dyck N, Leadley K, Dawkins KD, Mohr FW, Investigators SYNTAX. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–972. doi: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 10.Miura T, Miyashita Y, Motoki H, Shimada K, Kobayashi M, Nakajima H, Kimura H, Akanuma H, Mawatari E, Sato T, Hotta S, Kamiyoshi Y, Maruyama T, Watanabe N, Eisawa T, Aso S, Uchikawa S, Hashizume N, Sekimura N, Morita T, Ebisawa S, Izawa A, Tomita T, Koyama J, Ikeda U. In-hospital clinical outcomes of elderly patients (≥ 80 years) undergoing percutaneous coronary intervention. Circ J. 2014;78:1097–1103. doi: 10.1253/circj.CJ-14-0129. [DOI] [PubMed] [Google Scholar]
- 11.Thygesen K, Alpert JS, White HD, Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Jaffe AS, Apple FS, Galvani M, Katus HA, Newby LK, Ravkilde J, Chaitman B, Clemmensen PM, Dellborg M, Hod H, Porela P, Underwood R, Bax JJ, Beller GA, Bonow R, Van der Wall EE, Bassand JP, Wijns W, Ferguson TB, Steg PG, Uretsky BF, Williams DO, Armstrong PW, Antman EM, Fox KA, Hamm CW, Ohman EM, Simoons ML, Poole-Wilson PA, Gurfinkel EP, Lopez-Sendon JL, Pais P, Mendis S, Zhu JR, Wallentin LC, Fernández-Avilés F, Fox KM, Parkhomenko AN, Priori SG, Tendera M, Voipio-Pulkki LM, Vahanian A, Camm AJ, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Morais J, Brener S, Harrington R, Morrow D, Lim M, Martinez-Rios MA, Steinhubl S, Levine GN, Gibler WB, Goff D, Tubaro M, Dudek D, Al-Attar N. Universal definition of myocardial infarction. Circulation. 2007;116:2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 12.Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, Elkind MS, George MG, Hamdan AD, Higashida RT, Hoh BL, Janis LS, Kase CS, Kleindorfer DO, Lee JM, Moseley ME, Peterson ED, Turan TN, Valderrama AL, Vinters HV, American Heart Association Stroke Council, Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Council on Nutrition, Physical Activity and Metabolism An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064–2089. doi: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 14.Høfsten DE, Kelbæk H, Helqvist S, Kløvgaard L, Holmvang L, Clemmensen P, Torp-Pedersen C, Tilsted HH, Bøtker HE, Jensen LO, Køber L, Engstrøm T, DANAMI 3 Investigators The Third DANish Study of Optimal Acute Treatment of Patients with ST-segment Elevation Myocardial Infarction: ischemic postconditioning or deferred stent implantation versus conventional primary angioplasty and complete revascularization versus treatment of culprit lesion only: Rationale and design of the DANAMI 3 trial program. Am Heart J. 2015;169:613–621. doi: 10.1016/j.ahj.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A, Collaborators developing the Japanese equation for estimated GFR Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 16.Teichholz LE, Kreulen T, Herman MV, Gorlin R. Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence of absence of asynergy. Am J Cardiol. 1976;37:7–11. doi: 10.1016/0002-9149(76)90491-4. [DOI] [PubMed] [Google Scholar]
- 17.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/S0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 18.Girasis C, Garg S, Räber L, Sarno G, Morel MA, Garcia-Garcia HM, Lüscher TF, Serruys PW, Windecker S. SYNTAX score and Clinical SYNTAX score as predictors of very long-term clinical outcomes in patients undergoing percutaneous coronary interventions: a substudy of SIRolimus-eluting stent compared with pacliTAXel-eluting stent for coronary revascularization (SIRTAX) trial. Eur Heart J. 2011;32:3115–3127. doi: 10.1093/eurheartj/ehr369. [DOI] [PubMed] [Google Scholar]
- 19.Nam CW, Mangiacapra F, Entjes R, Chung IS, Sels JW, Tonino PA, De Bruyne B, Pijls NH, Fearon WF, FAME Study Investigators Functional SYNTAX score for risk assessment in multivessel coronary artery disease. J Am Coll Cardiol. 2011;58:1211–1218. doi: 10.1016/j.jacc.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 20.Vranckx P, Kalesan B, Stefanini GG, Farooq V, Onuma Y, Silber S, de Vries T, Jüni P, Serruys PW, Windecker S. Clinical outcome of patients with stable ischaemic heart disease as compared to those with acute coronary syndromes after percutaneous coronary intervention. EuroIntervention. 2015;11:171–179. doi: 10.4244/EIJV11I2A31. [DOI] [PubMed] [Google Scholar]
- 21.Kato S, Saito N, Kirigaya H, Gyotoku D, Iinuma N, Kusakawa Y, Iguchi K, Nakachi T, Fukui K, Futaki M, Iwasawa T, Taguri M, Kimura K, Umemura S. Incremental prognostic value of the SYNTAX score to late gadolinium-enhanced magnetic resonance images for patients with stable coronary artery disease. Heart Vessels. 2016;31:871–880. doi: 10.1007/s00380-015-0685-x. [DOI] [PubMed] [Google Scholar]
- 22.Garg S, Sarno G, Serruys PW, Rodriguez AE, Bolognese L, Anselmi M, De Cesare N, Colangelo S, Moreno R, Gambetti S, Monti M, Bristot L, Bressers M, Garcia-Garcia HM, Parrinello G, Campo G, Valgimigli M, STRATEGY and MULTISTRATEGY Investigators Prediction of 1-year clinical outcomes using the SYNTAX score in patients with acute ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention: a substudy of the STRATEGY (Single High-Dose Bolus Tirofiban and Sirolimus-Eluting Stent Versus Abciximab and Bare-Metal Stent in Acute Myocardial Infarction) and MULTISTRATEGY (Multicenter Evaluation of Single High-Dose Bolus Tirofiban Versus Abciximab With Sirolimus-Eluting Stent or Bare-Metal Stent in Acute Myocardial Infarction Study) trials. JACC Cardiovasc Interv. 2011;4:66–75. doi: 10.1016/j.jcin.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 23.Lee JH, Kim JH, Jang SY, Park SH, Bae MH, Yang DH, Park HS, Cho Y, Chae SC. A new tool for the risk stratification of patients undergoing primary percutaneous coronary intervention with ST-segment elevation myocardial infarction: bio-Clinical SYNTAX score. Int J Cardiol. 2015;187:193–195. doi: 10.1016/j.ijcard.2015.03.125. [DOI] [PubMed] [Google Scholar]
- 24.Head SJ, Davierwala PM, Serruys PW, Redwood SR, Colombo A, Mack MJ, Morice MC, Holmes DR, Jr, Feldman TE, Ståhle E, Underwood P, Dawkins KD, Kappetein AP, Mohr FW. Coronary artery bypass grafting vs. percutaneous coronary intervention for patients with three-vessel disease: final five-year follow-up of the SYNTAX trial. Eur Heart J. 2014;35:2821–2830. doi: 10.1093/eurheartj/ehu213. [DOI] [PubMed] [Google Scholar]
- 25.Sebastianski M, Narasimhan S, Graham MM, Toleva O, Shavadia J, Abualnaja S, Tsuyuki RT, McMurtry MS. Usefulness of the ankle-brachial index to predict high coronary SYNTAX scores, myocardium at risk, and incomplete coronary revascularization. Am J Cardiol. 2014;114:1745–1749. doi: 10.1016/j.amjcard.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Kato S, Saito N, Kirigaya H, Gyotoku D, Iinuma N, Kusakawa Y, Iguchi K, Nakachi T, Fukui K, Futaki M, Iwasawa T, Taguri M, Kimura K, Umemura S. Incremental prognostic value of the SYNTAX score to late gadolinium-enhanced magnetic resonance images for patients with stable coronary artery disease. Heart Vessels. 2016;31:871–880. doi: 10.1007/s00380-015-0685-x. [DOI] [PubMed] [Google Scholar]
- 27.Katagiri M, Takahashi M, Doi K, Myojo M, Kiyosue A, Ando J, Hirata Y, Komuro I. Serum neutrophil gelatinase-associated lipocalin concentration reflects severity of coronary artery disease in patients without heart failure and chronic kidney disease. Heart Vessels. 2016 doi: 10.1007/s00380-015-0776-8. [DOI] [PubMed] [Google Scholar]