Abstract
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of early myeloid progenitors and precursors at different stages of differentiation into granulocytes, macrophages, and dendritic cells. Blockade of their differentiation into mature myeloid cells in cancer results in an expansion of this population. High-grade gliomas are the most common malignant tumours of the central nervous system (CNS), with a poor prognosis despite intensive radiation and chemotherapy. Histopathological and flow cytometry analyses of human and rodent experimental gliomas revealed the extensive heterogeneity of immune cells infiltrating gliomas and their microenvironment. Immune cell infiltrates consist of: resident (microglia) and peripheral macrophages, granulocytes, myeloid-derived suppressor cells, and T lymphocytes. Intratumoural density of glioma-associated MDSCs correlates positively with the histological grade of gliomas and patient’s survival. MDSCs have the ability to attract T regulatory lymphocytes to the tumour, but block the activation of tumour-reactive CD4+ T helper cells and cytotoxic CD8+ T cells. Immunomodulatory mechanisms employed by malignant gliomas pose an appalling challenge to brain tumour immunotherapy. In this mini-review we describe phenotypic and functional characteristics of MDSCs in humans and rodents, and their occurrence and potential roles in glioma progression. While understanding the complexity of immune cell interactions in the glioma microenvironment is far from being accomplished, there is significant progress that may lead to the development of immunotherapy for gliomas.
Keywords: tumour microenvironment, myeloid-derived suppressor cells, antitumor responses, immunosuppression
Phenotypical and molecular characteristics of myeloid-derived suppressor cells
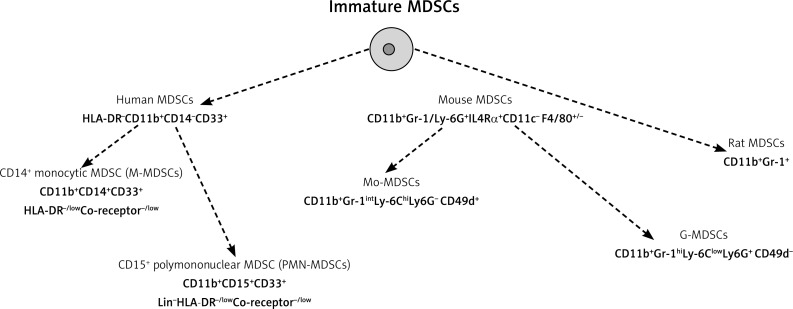
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of early myeloid progenitors and precursors at different stages of differentiation into granulocytes, macrophages, and dendritic cells. In healthy individuals, immature myeloid cells generated in bone marrow quickly differentiate into mature granulocytes, macrophages, or dendritic cells. Under pathological conditions such as cancer, infectious diseases, sepsis, trauma, bone marrow transplantation, or autoimmune disorders, a blockade in their differentiation into mature myeloid cells results in an expansion of this population [1]. Human MDSCs are identified as HLA-DR−CD11b+CD14−CD33+ cells that express the common myeloid marker CD33, and lack the expression of markers of mature myeloid and lymphoid cells and the MHC-class-II molecule HLA-DR [2, 3] (Fig. 1). Moreover, this heterogeneous population is composed of cells defined as CD14+ monocytic MDSC (CD11b+CD33+C14+) and CD15+ granulocytic MDSC (CD11b+CD33+CD15+CD14–) [4]. Human MDSCs commonly express Siglec-3/CD33 and lack lineage markers and HLA-DR, but heterogeneous expression of CD14 and CD15 suggests the presence of multiple subsets. Several other surface molecules have been used to identify additional subsets of MDSCs in cancer, including CD80, also known as B7.1 [5], CD115 (the macrophage colony-stimulating factor receptor), and CD124 (the IL-4 receptor α-chain) [6, 7]. Although these proteins are expressed by MDSCs, they do not define a specific MDSC population with distinct suppressive functions. Human MDSCs do not express a marker homologous to mouse Gr-1.
Fig. 1.
The currently accepted phenotypic definitions of MDSCs
In humans, identification of G- and M-MDSC subsets is difficult, they have been described by the combination of several myeloid markers that, in some instances, overlap partially or completely. Additionally, a more immature subset of human MDSCs characterised by the absence of staining for the lineage cocktail (CD3–, CD14–, CD15–, CD19–, CD56–), HLA-DR−, and expression of the common myeloid markers CD33 and CD11b has been identified as an “early stage” e-MDSC, defined. Using a multi-colour staining protocol and taking into account all the reported myeloid subsets endowed with a suppressive activity, a recent study discriminated six human MDSC phenotypes: MDSC1 (CD14+IL-4Rα+), MDSC2 (CD15+IL-4Rα+), MDSC3 (Lineage−HLA-DR−CD33+), MDSC4 (CD14+HLA-DRlow/−), MDSC5 (CD11b+CD14−CD15+), and MDSC6 (CD15+FSClowSSChigh) (Table 1) [8–10]. The detection of different human MDSC phenotypes highlighted the lack of standardisation in this area and led to the establishment of a human MDSC proficiency panel according to the guidance of the Association of Cancer Immunotherapy (CIMT) Immunoguiding Program in order to promote the standardisation of MDSC immunophenotyping across different groups. The currently accepted phenotypic definitions of human MDSCs are CD11b+CD14+CD33+HLA-DR−/lowCo-receptor–/low for macrophage M-MDSCs, and CD11b+CD15+CD33+Lin–HLA-DR−/low for polymononuclear PMN-MDSCs, present in the mononuclear fraction [11]. According to the recommendations the current nomenclature of polymononuclear PMN-MDSCs, present in the mononuclear fraction, replaced former granulocytic G-MDSCs. Growing evidence shows that the phenotype and mechanisms of action of MDSCs are tumour-dependent; therefore, it is important to determine the presence of all MDSC subsets in each cancer patient [12].
Table 1.
Phenotyping of human MDSC subsets with the use of multi-colour staining protocol
| Human MDSCs | Phenotype (multi-colour staining protocol) |
|---|---|
| MDSC1 | CD14+IL-4Rα+ |
| MDSC2 | CD15+IL-4Rα+ |
| MDSC3 | Lineage–HLA-DR–CD33+ |
| MDSC4 | CD14+HLA-DRlow/– |
| MDSC5 | Cd11b+CD14−CD15+ |
| MDSC6 | CD15–FSClowSSChigh |
In mice, MDSCs are defined as CD11b+Gr-1/Ly-6G+ IL4Rα+CD11c−F4/80+/− cells [13], but the relative expression levels of Ly-6G and Ly-6C also identified two specific subsets: granulocytic (G-MDSCs) CD11b+Gr-1hiLy-6ClowLy-6G+CD49d− and monocytic (Mo-MDSCs) CD11b+Gr-1intLy-6ChiLy-6G−CD49d+ MDSCs [14–16]. Gr-1 antigen is a cell surface protein that belongs to the Ly-6 family of proteins. In rats MDSCs could be identified as CD11b+Gr-1+ cells (Fig. 2).
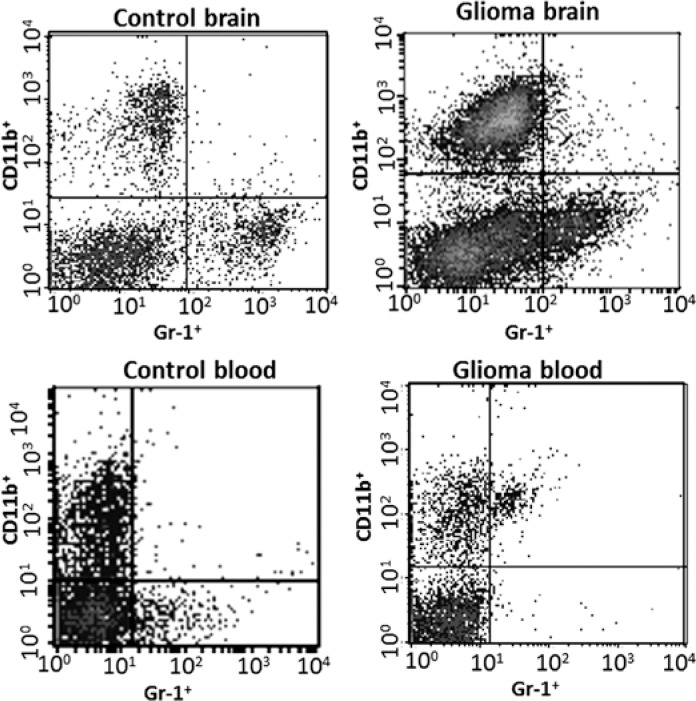
Fig. 2.
An example of flow cytometry studies of double positive cells – CD11b+Gr-1+ from experimental C6 gliomas
MDSCs are of great interest because they have the capacity to suppress the cytotoxic activities of natural killer (NK) and NKT cells, and the adaptive immune response mediated by CD4+ and CD8+ T cells, and they induce apoptosis of T-cell subsets [17]. Multiple mechanisms responsible for MDSC-mediated T-cell suppression have been described. The two MDSC subsets inhibit immune responses through different mechanisms: PNM-MDSCs suppress antigen (Ag)-specific CD8+ T cells mainly by producing reactive oxygen species (ROS), while M-MDSCs function primarily by expressing nitric oxide synthase 2 (NOS2) and arginase 1 (ARG1), and by generating reactive nitrogen species [18]. ARG1 and NOS2 metabolise L-arginine and block translation of the T cell CD3 zeta chain, inhibit T-cell proliferation, and promote T-cell apoptosis. Moreover, MDSCs secrete immunosuppressive cytokines and induce regulatory T-cell progression. The proportions of PMN-MDSCs and M-MDSCs are variable in different tumour models. While the majority of MDSCs in peripheral lymphoid organs are PMN-MDSCs, the ratio between PMN-MDSCs and M-MDSCs is much lower at tumour sites. It has been proposed that chemokines produced by tumour cells could induce a preferential migration of M-MDSCs to the tumour site. Alternatively, special features of the tumour microenvironment such as hypoxia, low pH, and metabolic products might not support PMN-MDSC survival [18].
Normal bone marrow contains 20–30% of cells with this phenotype, but these cells constitute only a small proportion (2–4%) of spleen cells and are absent from the lymph nodes in mice. In healthy individuals, immature myeloid cells with the described above phenotype comprise ∼0.5% of peripheral blood mononuclear cells [3]. Granulocyte-macrophage colony stimulating factor (GM-CSF), granulocyte colony stimulating factor (G-CSF), and macrophage colony stimulating factor (M-CSF) are haematopoietic growth factors responsible for the recruitment, proliferation, and maturation of myeloid cells [19]. One of the main concepts of MSDC generation, the emergency myelopoiesis states that an expansion signal 1, mediated mainly by STAT3 (induced by, e.g., GM-CSF, G-CSF, and IL-6), mobilises the immature myeloid cells from the bone marrow. An activation signal 2, mediated mainly by the transcription factor NFκB (induced by pro-inflammatory stimuli, e.g. TLR signalling and cytokines), follows [20]. The concept of block in differentiation states that immature myeloid cells are arrested in their immature phase by inflammatory mediators such as S100A8, S100A9, VEGF, IL-10, and COX-2/prostaglandin PGE2 [21, 22]. The classical definition of MDSCs as immature myeloid cells blocked from terminal differentiation has been challenged by recent studies suggesting that M-MDSCs and PMN-MDSCs may represent monocytes and granulocytes that have acquired immunosuppressive properties [10]. Both emergency myelopoiesis and block in differentiation are linked to an abnormal and persistent activation of STAT3. It is believed that activation of STAT3 in myeloid progenitor cells leads to the induction of S100A8 and S100A9 expression, which subsequently acts in an autocrine manner to arrest the cells in their immature phase [23].
All MDSCs originate from common myeloid progenitors, and their development is probably governed by the same growth factors that control normal myelopoiesis, e.g. GM-CSF, G-CSF, and M-CSF. MDSC percentages increase under pathological conditions, as a result of signals released by tumour cells or chronic inflammation [24]. Emerging evidence suggests that M-MDSCs are generated by reprogramming of monocytes into M-MDSCs, whereas PMN-MDSCs might be generated from neutrophils through the activation of immature or mature granulocytes and represent different stages of activation [10].
The frequency of MDSCs positively correlates with clinical stage and tumour burden in patients with breast [25], gastric [26], and colorectal cancers [27]. Increased levels of CD14+HLA-DR–/low MDSCs were correlated with extrathoracic metastasis and poor response to chemotherapy in non-small cell lung cancer patients [28].
The analysis of ten different experimental tumour models revealed that the expansion of the PMN-MDSC population was much greater than that of the monocytic subset, and the two subpopulations used different mechanisms to suppress T-cell function. The ability to differentiate into mature DCs and macrophages in vitro has been shown to be restricted to monocytic M-MDSCs [14]. Several studies have shown that bone marrow precursor cells treated with G-CSF or GM-CSF acquire a surface phenotype similar to MDSCs found in blood of cancer patients [29–31].
Accumulation and functions of myeloid cells in gliomas
Gliomas are tumours of the central nervous system (CNS), originating from transformed neural stem or progenitor glial cells [32]. The World Health Organisation (WHO), based on histopathological characteristics, divided gliomas into groups: low-grade gliomas (grades I and II) are benign, well-differentiated, slow-growing tumours; whereas high-grade gliomas (grades III and IV) are poorly differentiated or anaplastic, rapidly proliferating, and strongly infiltrate brain parenchyma. The most frequent and malignant primary brain tumour is glioblastoma (GBM, grade IV). Histological classification is currently assisted by molecular genetic features that provide diagnostic, prognostic, and predictive values [33, 34], facilitating patient stratification, prognosis, and treatment response [35]. GBM is one of the most aggressive and difficult to treat human malignancies, due to the frequent dysfunctions of tumour suppressors or oncogenes and highly diffusive growth, which prevents tumour resection and contributes to rapid tumour recurrence [36]. GBMs show alterations in EGFR, PDGFRA, PTEN, TP53, NF1, and CDKN2A/B, TERT promoter mutations, and rarely IDH1/2 mutations (5%, mainly secondary GBMs that develop from lower grade tumours) [37, 38]. The golden standard of GBM treatment is surgery combined with chemo- and radiotherapy; however, it remains only palliative and the median survival time of adult patients with GBM is 14–15 months after diagnosis [39].
Glioma cells secrete numerous chemokines, cytokines, and growth factors that promote infiltration of various cells, including a vast majority of immune cells such as microglia, peripheral macrophages, MDSCs, leukocytes, mostly CD4+ T cells, and Treg [40–43]. These non-neoplastic cells create a specific niche within a tumour microenvironment, which plays an important role in glioma growth, metastasis, and response to treatment. Locally produced cytokines and chemokines and their crosstalk with components of the extracellular matrix re-educate infiltrating immune cells to acquire distinct functional properties, directing the immune system towards immunosuppressive responses. Like many other non-CNS malignant cancers, GBMs developed multiple strategies to inhibit host antitumour responses [44]. Several recent transcriptomic studies of GBM tissues have identified “immune and myeloid/macrophage” gene expression signatures associated with GBM pathology, overall survival (OS), or response to treatment [45–47]. These data strongly support a link between a type of the immune response, accumulation of specific immune cell subsets, and glioma progression.
Clinical studies showed extensive infiltration of gliomas with myeloid cells; the majority of them are microglia and peripheral macrophages, collectively termed glioma-associated microglia/macrophages (GAMs). Immunohistochemical studies using various markers demonstrated the higher abundance of GAMs in high-grade gliomas than in low-grade gliomas [48–51]. Application of flow cytometry allowed for more detailed analyses of myeloid subpopulations. A flow cytometry phenotype for ramified microglia isolated from adult CNS was defined as CD45lowCD11b/c+ [52–54]. Intratumoural microglia/macrophage density increases during glioma progression and correlates with the grade of malignancy [55–57]. Flow cytometry-based analyses of myeloid cells in experimental gliomas showed accumulation of polarised GAMs within the tumour microenvironment. Using flow cytometry Badie and Schartner determined the proportion of microglia (CD11b/chigh CD45low), macrophages (CD11b/chighCD45high), and lymphocytes (CD11b/c–CD45high) in rat C6, 9L, and RG-2 glioma models, and demonstrated that microglia infiltration is dependent on the glioma cell line but does not correlate with the tumour size [54]. Microglia accounted for 13–34% of the tumour mass within the tumour periphery. Macrophages represented a smaller proportion of the infiltrating cells, accounting for 5–12% of cells, and this proportion was constant in different models [54]. In the transgenic RCAS-PDGFb tumour model in which RFP–/GFP+ microglia and RFP+/GFPlow macrophages/monocytes were isolated from tumours, flow cytometry studies demonstrated 14% microglia and 8.5% macrophages/monocytes in the RCAS-PDGFb gliomas, and a similar proportion of GAMs (16% microglia and 6.5% macrophages/monocytes) was observed in GL261 tumours [58].
Immunohistochemical evaluation of functional markers and gene expression profiling of human GBM infiltrating CD11b+ cells indicate immunosuppressive activation of GAMs and point to a lack of the classical “immune response” activation in GBMs [51, 58–60]. The studies using pharmacological or genetic ablation of CD11b+ cells in experimental glioma models demonstrated that tumours do not grow and invade the brain parenchyma if GAMs are ablated or functionally paralysed [59, 61–66]. In those studies the percentages of MDSCs and their functionality were not evaluated.
Myeloid-derived suppressor cells in gliomas
The numbers of MSDCs are frequently increased in blood, spleen, and tumour mass, and they correlate with cancer stage, metastasis, and chemotherapy response [18]. Information regarding their presence and roles in gliomas is scarce [4, 67]. Gielen et al. [4] reported increased percentages of both M-MDSCs and PMN-MDSCs in the blood of GBM patients when compared with healthy donors. The myeloid activation markers B7-1/CD80 and PD-L1 were not detected, and CD124, CD86, and CD40 were detected at similar levels on MDSCs in glioma patients and healthy donors. The MDSC population in tumour cell suspensions consisted almost exclusively of CD15+ PMN-MDSC cells. Immunohistochemistry confirmed infiltration of glioma tissues with CD15+/HLAII– cells [4]. Raychaudhuri et al. reported that patients with GBM have elevated levels of MDSCs compared with age-matched healthy donors and other cancer patients. The majority of the MDSCs in GBM patients were CD15+ CD14− PMN-MDSCs (82%), followed by lineage-negative e-MDSCs (15%) and M-MDSCs (3%) [67]. Another study of 52 GBM patients revealed a significantly higher frequency of CD14highCD15+ M-MDSCs and CD14lowCD15+ PMN-MDSCs in blood of GBM patients when compared with healthy controls. Correlation between the number of PMN-MDSCs and CD4+ effector memory T-cells (CD4+ Tem) within the tumours was detected. Tumour-derived CD4+ Tem expressed high levels of PD-1 and was functionally exhausted. The expression of PD-L1 was also significantly upregulated on tumour-derived MDSCs [68]. The presented findings provide evidence for the accumulation of different MDSC subsets in GBM patients and indicate that PMN-MDSCs in peripheral blood and at the tumour site may participate in GBM-induced T-cell suppression. Accumulation of MDSCs in peripheral blood in GBM patients may induce T-cell immunosuppression, and increased plasma levels of Arginase 1 and G-CSF may contribute to MDSC suppressor function and its expansion [4].
These results are corroborated by our studies, which show a large number of Arg1+ but not Iba1+ cells (GAMs) infiltrating experimental C6 gliomas in rats [66]. Flow cytometry studies of double-positive cells – CD11b+Gr-1+ from experimental C6 gliomas (Fig. 1B) show a negligible number of such cells in normal brain and an increase of up to 3–4% in the tumour-bearing hemispheres. In addition, an increased percentage of CD11b+Gr-1+ cells was detected in the peripheral blood (5.5–6.7%) of animals implanted with glioma cells (unpublished). It confirms the increase of MDSCs in experimental rat gliomas.
Recent studies indicate that GAMs (in particular CD163+ infiltrating macrophages) within the glioma microenvironment produce Ccl2, a chemokine recruiting both Ccr2+Ly-6C+ Mo-MDSCs and Ccr4+ Treg. In murine gliomas, tumour-derived Ccl20 and osteoprotegerin induced Ccl2 production by GAMs. Gliomas developing in Ccl2-deficient mice displayed reduced Tregs and monocytic MDSCs infiltration [69]. Macrophage migration inhibitory factor (MIF), which was produced at high levels by glioma stem-like cells (CSCs), increased the expression of the arginase-1 in MDSCs in a receptor Cxcr2-dependent manner. Reduction of MIF conferred a survival advantage to tumour-bearing animals and increased the cytotoxic T cell response towards the tumour [70]. M-MDSCs of GBM patients have increased levels of intracellular S100A8/9 and serum levels compared with M-MDSCs in healthy controls. Glioma patients also have increased S100A8/9 serum levels, which correlates with increased Arginase activity in serum [71].
Antitumour immune responses in glioma microenvironment
Accumulation of GAMs and MDSCs, and their functional polarisation into pro-invasive and immunosuppressive cells, have a profound effect on antitumour responses in gliomas. The glioma microenvironment is infiltrated with leukocytes, mostly CD4+ T helper (Th), CD8+ T cytotoxic (Tc), and CD4+CD25+FoxP3+ Treg [40–42, 55, 72, 73]. The presence of CD4+CD25+FoxP3+ Treg was correlated with tumour resistance to radiation and chemotherapy, poor prognosis, and higher grade [43, 44]. Treg cells are known as suppressors of the adaptive immune response inhibiting the proliferation of effector T cells. Treg suppressive mechanism depends on the cell-cell interaction and is implicated in a host tolerance in tumour growth [55]. The tumour-infiltrating CD8+ T cells were phenotypically CD8+CD25–, suggesting that these cells were not activated. CD4+ T cells were more numerous than CD8+ T cells within glioma tissues, and the percentages of CD4+ and CD8+ cells increased with tumour grade [74, 75].
Anti-tumour immune responses mediated by T cells are suppressed by TGF-β and IL-10 secreted by glioma cells [44]. Moreover, glioma cells lack B7.1/2 (CD80/86), costimulatory and immune regulatory molecules, but constitutively express B7-H1 mRNA and protein. Glioma-related B7-H1 was identified as a strong inhibitor of CD4+ as well as CD8+ T-cell activation [75]. GBM infiltrating T-cells had decreased INF-γ production and increased PD-1 gene expression [68]. As well as directly blocking effective activation of naive T cells, glioma cells promote accumulation of Treg and MDSCs that mediate inhibition of T cells and NK cells.
Immunotherapeutic approaches for GBM, such as immune checkpoint blockade and anti-tumour vaccines, are actively tested in preclinical models and the first combinations of immunotherapies with standard therapies are being translated to clinical trials. Phase I clinical trial studying the effects of anti-PD-1 and anti-CTLA-4 combination therapy for recurrent GBM (NCT02017717), and a number of studies of DC vaccines in recurrent and newly diagnosed GBM are in progress (NCT02010606, NCT02149225, NCT02049489, NCT01808820, NCT02078648), and an entire medical community awaits results. Further understanding the biology of MDSCs and conditions for their polarisation in the tumour microenvironment and peripheral blood may be crucial for the development of new therapeutic strategies.
Acknowledgements
Studies supported by the project DIMUNO: “Development of new cancer therapies based on selective antitumor immunomodulators” – co-financed by the National Centre for Research and Development in the framework of STRATEGMED-3 Prevention practices and treatment of civilisation diseases.
The authors declare no conflict of interest.
References
- 1.Kusmartsev S, Gabrilovich DI. Inhibition of myeloid cell differentiation in cancer: The role of reactive oxygen species. J Leukoc Biol. 2003;74:186–96. doi: 10.1189/jlb.0103010. [DOI] [PubMed] [Google Scholar]
- 2.Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–89. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 3.Nagaraj S, Gabrilovich DI. Myeloid-derived suppressor cells in human cancer. Cancer J. 2010;16:348–53. doi: 10.1097/PPO.0b013e3181eb3358. [DOI] [PubMed] [Google Scholar]
- 4.Gielen PR, Schulte BM, Kers-Rebel ED, Verrijp K, Petersen-Baltussen HM, ter Laan M, Wesseling P, Adema GJ. Increase in both CD14-positive and CD15-positive myeloid-derived suppressor cell subpopulations in the blood of patients with glioma but predominance of CD15-positive myeloid-derived suppressor cells in glioma tissue. J Neuropathol Exp Neurol. 2015;74:390–400. doi: 10.1097/NEN.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 5.Yang R, Cai Z, Zhang Y, Yutzy WH, 4th, Roby KF, Roden RB. CD80 in immune suppression by mouse ovarian carcinoma-associated Gr-1+CD11b+ myeloid cells. Cancer Res. 2006;66:6807–15. doi: 10.1158/0008-5472.CAN-05-3755. [DOI] [PubMed] [Google Scholar]
- 6.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–31. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 7.Gallina G, Dolcetti L, Serafini P, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–90. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dumitru CA, Moses K, Trellakis S, Lang S, Brandau S. Neutrophils and granulocytic myeloid-derived suppressor cells: immunophenotyping, cell biology and clinical relevance in human oncology. Cancer Immunol Immunother. 2012;61:1155–67. doi: 10.1007/s00262-012-1294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer. 2013;13:739–52. doi: 10.1038/nrc3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Millrud CR, Bergenfelz C, Leandersson K. On the origin of myeloid-derived suppressor cells. Oncotarget. 2016 Sep 27; doi: 10.18632/oncotarget.12278. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A, Ostrand-Rosenberg S, Rodriguez PC, Sica A, Umansky V, Vonderheide RH, Gabrilovich DI. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7:12150. doi: 10.1038/ncomms12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solito S, Marigo I, Pinton L, Damuzzo V, Mandruzzato S, Bronte V. Myeloid-derived suppressor cell heterogeneity in human cancers. Ann N Y Acad Sci. 2014;1319:47–65. doi: 10.1111/nyas.12469. [DOI] [PubMed] [Google Scholar]
- 13.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dolcetti L, Peranzoni E, Ugel S, et al. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur J Immunol. 2010;40:22–35. doi: 10.1002/eji.200939903. [DOI] [PubMed] [Google Scholar]
- 16.Haile LA, Gamrekelashvili J, Manns MP, Korangy F, Greten TF. CD49d is a new marker for distinct myeloid-derived suppressor cell subpopulations in mice. J Immunol. 2010;185:203–10. doi: 10.4049/jimmunol.0903573. [DOI] [PubMed] [Google Scholar]
- 17.Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment : expect the unexpected. J Clin Investig. 2015;125:3356–64. doi: 10.1172/JCI80005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Youn JI, Gabrilovich DI. The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol. 2010;40:2969–75. doi: 10.1002/eji.201040895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamilton JA, Achuthan A. Colony stimulating factors and myeloid cell biology in health and disease. Trends in Immunology. 2013;34:81–9. doi: 10.1016/j.it.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Condamine T, Mastio J, Gabrilovich DI. Transcriptional regulation of myeloid-derived suppressor cells. J Leukoc Biol. 2015;98:913–22. doi: 10.1189/jlb.4RI0515-204R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Obermajer N, Muthuswamy R, Lesnock J, Edwards RP, Kalinski P. Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood. 2011;118:5498–505. doi: 10.1182/blood-2011-07-365825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mao Y, Poschke I, Wennerberg E, et al. Melanoma-educated CD14+ cells acquire a myeloid-derived suppressor cell phenotype through COX-2-dependent mechanisms. Cancer Res. 2013;73:3877–87. doi: 10.1158/0008-5472.CAN-12-4115. [DOI] [PubMed] [Google Scholar]
- 23.Cheng P, Corzo CA, Luetteke N, et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205:2235–49. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The Nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016;37:208–20. doi: 10.1016/j.it.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bergenfelz C, Larsson AM, von Stedingk K, et al. Systemic monocytic-MDSCs are generated from monocytes and correlate with disease progression in breast cancer patients. PLoS One. 2015;10:e0127028. doi: 10.1371/journal.pone.0127028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, Chang EW, Wong SC, Ong SM, Chong DQ, Ling KL. Increased myeloid-derived suppressor cells in gastric cancer correlate with cancer stage and plasma S100A8/A9 proinflammatory proteins. J Immunol. 2013;190:794–804. doi: 10.4049/jimmunol.1202088. [DOI] [PubMed] [Google Scholar]
- 27.Sun HL, Zhou X, Xue YF, Wang K, Shen YF, Mao JJ, Guo HF, Miao ZN. Increased frequency and clinical significance of myeloid-derived suppressor cells in human colorectal carcinoma. World J Gastroenterol. 2012;18:3303–9. doi: 10.3748/wjg.v18.i25.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang A, Zhang B, Wang B, Zhang F, Fan KX, Guo YJ. Increased CD14(+)HLA-DR (-/low) myeloid-derived suppressor cells correlate with extrathoracic metastasis and poor response to chemotherapy in non-small cell lung cancer patients. Cancer Immunol Immunother. 2013;62:1439–51. doi: 10.1007/s00262-013-1450-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pak AS, Wright MA, Matthews JP, Collins SL, Petruzzelli GJ, Young MR. Mechanisms of immune suppression in patients with head and neck cancer: presence of CD34(+) cells which suppress immune functions within cancers that secrete granulocyte-macrophage colony-stimulating factor. Clin Cancer Res. 1995;1:95–103. [PubMed] [Google Scholar]
- 30.Abrams SI, Waight JD. Identification of a G-CSF-Granulocytic MDSC axis that promotes tumor progression. Oncoimmunology. 2012;1:550–551. doi: 10.4161/onci.19334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Annels NE, Shaw VE, Gabitass RF, et al. The effects of gemcitabine and capecitabine combination chemotherapy and of low-dose adjuvant GM-CSF on the levels of myeloid-derived suppressor cells in patients with advanced pancreatic cancer. Cancer Immunol Immunother. 2014;63:175–83. doi: 10.1007/s00262-013-1502-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ilkanizadeh S, Lau J, Huang M, et al. Glial progenitors as targets for transformation in glioma. Adv Cancer Res. 2014;121:1–65. doi: 10.1016/B978-0-12-800249-0.00001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weller M, Stupp R, Hegi ME, van den Bent M, Tonn JC, Sanson M, Wick W, Reifenberger G. Personalized care in neuro-oncology coming of age: Why we need MGMT and 1p/19q testing for malignant glioma patients in clinical practice. Neuro Oncol. 2012;14(Suppl 4):iv100–8. doi: 10.1093/neuonc/nos206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weller M, Pfister SM, Wick W, Hegi ME, Reifenberger G, Stupp R. Molecular neuro-oncology in clinical practice: A new horizon. Lancet Oncol. 2013;14:e370–9. doi: 10.1016/S1470-2045(13)70168-2. [DOI] [PubMed] [Google Scholar]
- 35.Ceccarelli M, Barthel FP, Malta TM, et al. Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell. 2016;164:550–63. doi: 10.1016/j.cell.2015.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarin H. Recent progress towards development of effective systemic chemotherapy for the treatment of malignant brain tumors. J Transl Med. 2009;7:77. doi: 10.1186/1479-5876-7-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tabatabai G, Stupp R, van den Bent MJ, Hegi ME, Tonn JC, Wick W, Weller M. Molecular diagnostics of gliomas: The clinical perspective. Acta Neuropathologica. 2010;120:585–92. doi: 10.1007/s00401-010-0750-6. [DOI] [PubMed] [Google Scholar]
- 38.Vigneswaran K, Neill S, Hadjipanayis CG. Beyond the World Health Organization grading of infiltrating gliomas: advances in the molecular genetics of glioma classification. Ann Transl Med. 2015;3:95. doi: 10.3978/j.issn.2305-5839.2015.03.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Meir EG, Hadjipanayis CG, Norden AD, Shu HK, Wen PY, Olson JJ. Exciting New Advances in Neuro-Oncology: The Avenue to a Cure for Malignant Glioma. CA. Cancer J Clin. 2010;60:166–93. doi: 10.3322/caac.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fecci PE, Mitchell DA, Whitesides JF. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66:3294–302. doi: 10.1158/0008-5472.CAN-05-3773. [DOI] [PubMed] [Google Scholar]
- 41.Lohr J, Ratliff T, Huppertz A. Effector T-cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-? Clin Cancer Res. 2011;17:4296–308. doi: 10.1158/1078-0432.CCR-10-2557. [DOI] [PubMed] [Google Scholar]
- 42.Alexiou GA, Vartholomatos G, Karamoutsios A, Batistatou A, Kyritsis AP, Voulgaris S. Circulating progenitor cells: A comparison of patients with glioblastoma or meningioma. Acta Neurol Belg. 2013;113:7–11. doi: 10.1007/s13760-012-0097-y. [DOI] [PubMed] [Google Scholar]
- 43.Wainwright DA, Dey M, Chang A, Lesniak MS. Targeting tregs in malignant brain cancer: Overcoming IDO. Front Immunol. 2013;15:116. doi: 10.3389/fimmu.2013.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perng P, Lim M. Immunosuppressive mechanisms of malignant gliomas: parallels at non-CNS Sites. Front Oncol. 2015;5:153. doi: 10.3389/fonc.2015.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Godard S, Getz G, Delorenzi M, et al. Classification of human astrocytic gliomas on the basis of gene expression: a correlated group of genes with angiogenic activity emerges as a strong predictor of subtypes. Cancer Res. 2003;63:6613–25. [PubMed] [Google Scholar]
- 46.Shirahata M, Iwao-Koizumi K, Saito S, Ueno N, Oda M, Hashimoto N, Takahashi JA, Kato K. Gene expression-based molecular diagnostic system for malignant gliomas is superior to histological diagnosis. Clin Cancer Res. 2007;13:7341–56. doi: 10.1158/1078-0432.CCR-06-2789. [DOI] [PubMed] [Google Scholar]
- 47.Vauléon E, Tony A, Hamlat A, et al. Immune genes are associated with human glioblastoma pathology and patient survival. BMC Med Genomics. 2012;5:41. doi: 10.1186/1755-8794-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Esiri M, Morris C. Immunocytochemical study of macrophages and microglial cells and extracellular matrix components in human CNS disease. 2. Non-neoplastic diseases. J Neurol Sci. 1991;101:59–7. doi: 10.1016/0022-510x(91)90018-3. [DOI] [PubMed] [Google Scholar]
- 49.Wierzba-Bobrowicz T, Kuchna I, Matyja E. Reaction of microglial cells in human astrocytomas (preliminary report) Folia Neuropathol. 1994;32:251–2. [PubMed] [Google Scholar]
- 50.Geranmayeh F, Scheithauer BW, Spitzer C, Meyer FB, Svensson-Engwall AC, Graeber MB. Microglia in gemistocytic astrocytomas. Neurosurgery. 2007;60:159–66. doi: 10.1227/01.NEU.0000249192.30786.67. [DOI] [PubMed] [Google Scholar]
- 51.Mieczkowski J, Kocyk M, Nauman P, et al. Down-regulation of IKKβ expression in glioma-infiltrating microglia/macrophages is associated with defective inflammatory/immune gene responses in glioblastoma. Oncotarget. 2015;6:33077–90. doi: 10.18632/oncotarget.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sedgwick JD, Schwender S, Imrich H, Dörries R, Butcher GW, ter Meulen V. Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc Natl Acad Sci U S A. 1991;88:7438–42. doi: 10.1073/pnas.88.16.7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sedgwick JD, Schwender S, Gregersen R, Dörries R, ter Meulen V. Resident macrophages (ramified microglia) of the adult brown Norway rat central nervous system are constitutively major histocompatibility complex class II positive. J Exp Med. 1993;177:1145–52. doi: 10.1084/jem.177.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Badie B, Schartner JM. Flow cytometric characterization of tumor-associated macrophages in experimental gliomas. Neurosurgery. 2000;46:957–62. doi: 10.1097/00006123-200004000-00035. [DOI] [PubMed] [Google Scholar]
- 55.Hussain SF, Yang D, Suki D, Aldape K, Grimm E, Heimberger AB. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro Oncol. 2006;8:261–79. doi: 10.1215/15228517-2006-008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Badie B, Schartner J. Role of microglia in glioma biology. Microsc Res Tech. 2001;54:106–13. doi: 10.1002/jemt.1125. [DOI] [PubMed] [Google Scholar]
- 57.Yi L, Xiao H, Xu M, et al. Glioma-initiating cells: A predominant role in microglia/macrophages tropism to glioma. J Neuroimmunol. 2011;232:75–82. doi: 10.1016/j.jneuroim.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 58.Szulzewsky F, Pelz A, Feng X, et al. Glioma-associated microglia/macrophages display an expression profile different from M1 and M2 polarization and highly express Gpnmb and Spp1. PLoS One. 2015;10:e0116644. doi: 10.1371/journal.pone.0116644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gabrusiewicz K, Rodriguez B, Wei J, et al. Glioblastoma-infiltrated innate immune cells resemble M0 macrophage phenotype. JCI insight. 2016;1:1–32. doi: 10.1172/jci.insight.85841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Szulzewsky F, Arora S, de Witte L, et al. Human glioblastoma-associated microglia/monocytes express a distinct RNA profile compared to human control and murine samples. Glia Glia. 2016;64:1416–36. doi: 10.1002/glia.23014. [DOI] [PubMed] [Google Scholar]
- 61.Sliwa M, Markovic D, Gabrusiewicz K, et al. The invasion promoting effect of microglia on glioblastoma cells is inhibited by cyclosporin A. Brain. 2007;130(Pt 2):476–89. doi: 10.1093/brain/awl263. [DOI] [PubMed] [Google Scholar]
- 62.Wesolowska A, Kwiatkowska A, Slomnicki L, et al. Microglia-derived TGF-β as an important regulator of glioblastoma invasion – an inhibition of TGF-β-dependent effects by shRNA against human TGF-β type II receptor. Oncogene. 2007;27:918–30. doi: 10.1038/sj.onc.1210683. [DOI] [PubMed] [Google Scholar]
- 63.Markovic DS, Vinnakota K, Chirasani S, Synowitz M, Raguet H, Stock K, Sliwa M, Lehmann S, Kälin R, van Rooijen N, Holmbeck K, Heppner FL, Kiwit J, Matyash V, Lehnardt S, Kaminska B, Glass R, Kettenmann H. Gliomas induce and exploit microglial MT1-MMP expression for tumor expansion. Proc Natl Acad Sci U S A. 2009;106:12530–5. doi: 10.1073/pnas.0804273106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gabrusiewicz K, Hossain MB, Cortes-Santiago N, Fan X, Kaminska B, Marini FC, Fueyo J, Gomez-Manzano C. Macrophage ablation reduces M2-like populations and jeopardizes tumor growth in a MAFIA-based glioma model. Neoplasia. 2015;17:374–84. doi: 10.1016/j.neo.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gabrusiewicz K, Ellert-Miklaszewska A, Lipko M, Sielska M, Frankowska M, Kaminska B. Characteristics of the alternative phenotype of microglia/macrophages and its modulation in experimental gliomas. PLoS One. 2011;6:e23902. doi: 10.1371/journal.pone.0023902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ellert-Miklaszewska A, Wisniewski P, Kijewska M, et al. Tumour-processed osteopontin and lactadherin drive the protumorigenic reprogramming of microglia and glioma progression. Oncogene. 2016:4. doi: 10.1038/onc.2016.55. [DOI] [PubMed] [Google Scholar]
- 67.Raychaudhuri B, Rayman P, Ireland J, et al. Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro Oncol. 2011;13:591–9. doi: 10.1093/neuonc/nor042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dubinski D, Wölfer J, Hasselblatt M, Schneider-Hohendorf T, Bogdahn U, Stummer W, Wiendl H, Grauer OM. CD4+ T effector memory cell dysfunction is associated with the accumulation of granulocytic myeloid-derived suppressor cells in glioblastoma patients. Neuro-Oncology. 2016;18:807–18. doi: 10.1093/neuonc/nov280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chang AL, Miska J, Wainwright DA, et al. CCL2 Produced by the glioma microenvironment is essential for the recruitment of regulatory T cells and myeloid-derived suppressor cells. Cancer Res. 2016;76:5671–82. doi: 10.1158/0008-5472.CAN-16-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Otvos B, Silver DJ, Mulkearns-Hubert EE, et al. Cancer stem cell-secreted macrophage migration inhibitory factor stimulates myeloid derived suppressor cell function and facilitates glioblastoma immune evasion. Stem Cells. 2016;34:2026–39. doi: 10.1002/stem.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gielen PR, Schulte BM, Kers-Rebel ED, Verrijp K, Bossman SA, Ter Laan M, Wesseling P, Adema GJ. Elevated levels of polymorphonuclear myeloid-derived suppressor cells in patients with glioblastoma highly express S100A8/9 and arginase and suppress T cell function. Neuro Oncol. 2016;18:1253–64. doi: 10.1093/neuonc/now034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wainwright DA, Sengupta S, Han Y, Lesniak MS. Thymus-derived rather than tumor-induced regulatory T cells predominate in brain tumors. Neuro Oncol. 2011;13:1308–23. doi: 10.1093/neuonc/nor134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Heimberger AB, Abou-Ghazal M, Reina-Ortiz C, Yang DS, Sun W, Qiao W, Hiraoka N, Fuller GN. Incidence and prognostic impact of FoxP3+ regulatory T cells in human gliomas. Clin. Cancer Res. 2008;14:5166–72. doi: 10.1158/1078-0432.CCR-08-0320. [DOI] [PubMed] [Google Scholar]
- 74.Kmiecik J, Poli A, Brons NH, Waha A, Eide GE, Enger PØ, Zimmer J, Chekenya M. Elevated CD3+ and CD8+ tumor-infiltrating immune cells correlate with prolonged survival in glioblastoma patients despite integrated immunosuppressive mechanisms in the tumor microenvironment and at the systemic level. J Neuroimmunol. 2013;264:71–83. doi: 10.1016/j.jneuroim.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 75.Wintterle S, Schreiner B, Mitsdoerffer M, Schneider D, Chen L, Meyermann R, Weller M, Wiendl H. Expression of the B7-related molecule B7-H1 by glioma cells: a potential mechanism of immune paralysis. Cancer Res. 2003;63:7462–7. [PubMed] [Google Scholar]