Much of psychiatric neuroscience has focused on studying neurons, with their relative health or dysfunction considered the key to mental health or disease. There are good reasons for this focus: neurons are the primary communicators of the central nervous system. Neurons form the most basic structure of synapses, exchanging information predominantly via chemical transmission. These synapses are the primary targets in psychiatry, with medications either interfering with synaptic communication (e.g., antipsychotics that antagonize dopaminergic transmission at D2 receptors) or augmenting it (e.g., benzodiazepines that allosterically enhance the function of gamma-aminobutyric acid receptors, or selective serotonin reuptake inhibitors, which prolong the actions of serotonin by preventing its reuptake and clearance). While the existence of other cells in the brain has long been known, their importance to neuronal functioning and their potential roles in psychiatric disorders is only now being fully appreciated.
Glia are the nonneuronal cells of the brain; they include astrocytes, oligodendrocytes, and microglia. Glia were classically considered the support cells of the brain, long regarded as inert structural “glue,” as their name suggests. Modern neuroscience, however, has begun to appreciate a more active, and at times critical role, for all of these cell types in health and disease. Here we focus on historical and current perspectives on the function and significance of microglia, particularly as they relate to mental health and disease.
Historical Perspectives on Microglial Function: Pathology Sensors
Microglia’s original claim to fame was their status as the immune cells of the brain. While neurons, astrocytes, and oligodendrocytes are all of neuroectodermal origin, microglia are largely thought to originate in the mesodermal lineage and migrate to the brain early in prenatal development (1), where they ultimately make up about 10% of all brain cells. While the gross structure of the brain is relatively fixed, a tantalizing recent finding suggested that microglia may be able to migrate as monocytes from the periphery later in life under the right conditions (2). Microglia were initially considered inert in the absence of a pathological neurological event. They sat in what is called a ramified form, with their multiple, branched processes extended, waiting for an “SOS” signal from neurons that were in trouble. In the event of ischemia, infection, or neurodegeneration, microglia would spring into action, changing from ramified to an ameboid form capable of engulfing cellular debris from dying neurons and of releasing a potent mix of cytokines and other inflammatory molecules. This process, called reactive microgliosis, is a prominent feature of neurodegenerative disorders, such as Alzheimer’s and Parkinson’s diseases (1).
Modern Perspectives on Microglial Function: Neuronal Homeostats
While this idea of microglia as “rescue” cells or “clean-up crews” carved out an important role for them, the question of what exactly resting microglia were doing continued to linger. Imaging microglia in vivo changed the field dramatically by showing that “resting” microglia were incredibly active, extending and retracting their processes in a constant surveillance of the cortical environment (3). Processes lingered at synapses, seeming to “check in.” If signs of danger were simulated (e.g., adding lipopolysaccharide to produce an inflammatory response), processes would linger even longer. Data from these initial studies suggested that microglia are the busiest cells in the central nervous system, collectively surveying the entire brain parenchyma—all 1014 synapses—every few hours (3).
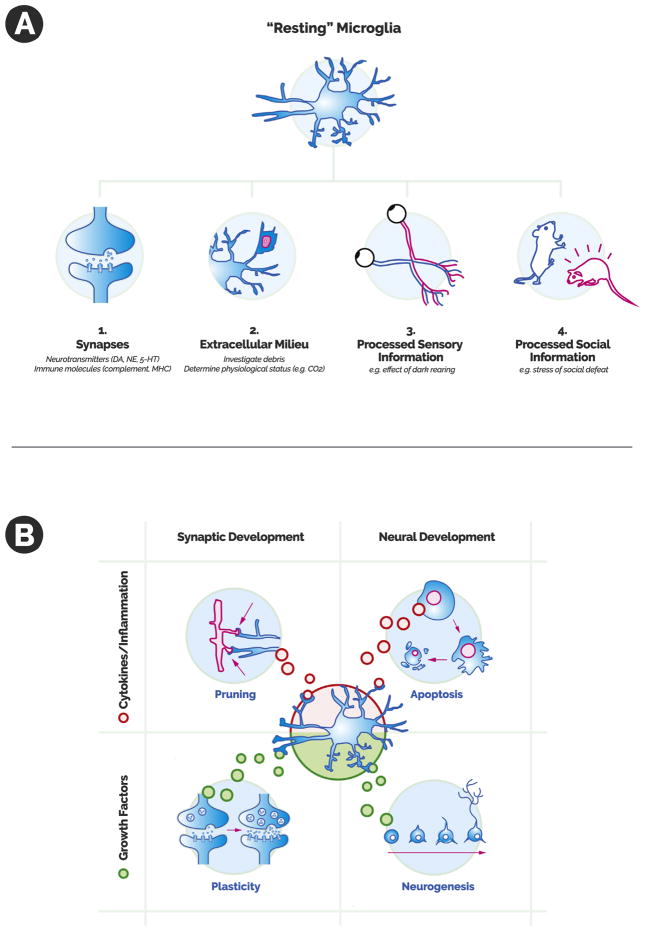
Microglia are now hypothesized to perform a homeostatic function in the nervous system, sensing the condition of the extracellular milieu, listening to the needs of synapses, and often participating in synaptic interactions based on the synthesis of this information. Microglia are able to “listen” to a large variety of synaptic signals (Figure 1A), expressing many receptors for traditional neurotransmitters (e.g., dopamine, serotonin, and norepinephrine), peptides (e.g., bradykinin and purines), and immune molecules (e.g., major histocompatibility complex and complement receptors) (1). They also express receptors that allow them to sample the extracellular milieu of the brain. For example, in this issue of Biological Psychiatry, research by Vollmer et al. suggests that microglia detect the acidotic conditions triggered by carbon dioxide exposure through the acid-sensing receptor T-cell death–associated gene-8 (4). Microglia are also sensitive to neuronal activity that is driven by sensory stimuli transmitted from the external world. For example, dark rearing of animals alters microglial activity in the visual cortex (1). Finally, microglia appear sensitive to more complex life experiences—activated, for example, by the stress of repeated social defeat in animals (2). Therefore, microglia integrate a range of incoming information regarding the status of the synapse, the extracellular environment, and perhaps more integrated neuronal information regarding the world at large.
Figure 1.
(A) There are several ways in which microglia survey the neuronal environment. Microglia are able to sense signals that include 1) synaptic crosstalk (via traditional neurotransmitters and immune signaling); 2) the physiologic status of the extracellular milieu; 3) neuronal interpretations of sensory information; and 4) neuronal interpretations of more complex social or emotional experience. (B) Possible microglial points of influence on synapses and neurons as a whole in a normal developmental context. When microglia release inflammatory molecules and cytokines, they may participate in synaptic pruning and developmental apoptosis. These same microglia also have the capacity to release growth factors, which likely promote synaptic plasticity and neurogenesis. 5-HT, 5-hydroxytryptamine (serotonin); DA, dopamine; MHC, major histocompatibility complex; NE, norepinephrine.
The question then becomes how microglia are able to act on this information—and it turns out that they have a considerable range of tools at their disposal (Figure 1B). With their classic immune function, they are able to release inflammatory molecules and cytokines that can encourage cell death and are also able to phagocytose synapses or entire dendritic trees. While these sound like destructive forces—and at times they certainly can be—the ability to promote apoptosis or to engulf synapses can serve critical normal developmental functions. Synapses are overproduced and then subsequently pruned back during childhood and adolescence, leading to the refinement of mature neural circuits. Microglia are likely critical mediators of developmental pruning with an important role for classical complement and chemokine signaling, as suggested by knockout studies showing that impaired microglial function (e.g., those lacking the fractalkine receptor) is associated with poorly pruned synapses and electrophysiological immaturity in those synapses that remain (5).
In addition to their capacity to promote inflammation and destroy, however, microglia also possess a considerable arsenal of neurotrophic factors, such as brain-derived neurotrophic factor (6), which can also be used at the synapse. In the right circumstances, microglia can use their signaling capacities to regulate the strength of synaptic connections between neurons, playing a role in synaptic plasticity and potentiating the relational strength between two neurons (6), with implications that range from learning and memory to drug addiction.
Like their roles in synapse development (i.e., supporting pruning vs. strengthening), microglia appear to play a critical part in determining the life or death of newborn neurons. Microglia’s ability to promote cell death likely serves an important homeostatic function in early neuronal proliferation and differentiation and also in adult neurogenesis (e.g., in the hippocampus). Although microglia were classically viewed as the harbingers of neuronal death (7), their access to growth factors endow microglia with the capacity to potently protect and support developing neurons (8). Microglia may therefore play a privileged role in synthesizing information about the environment and using this information to decide which neurons are appropriate to live or die in developing circuits.
In summary, microglia appear to synthesize information from the extracellular environment with the status or needs of synapses. They then have a considerable arsenal of signaling molecules to directly interact with neurons. The processes by which they “listen” and “talk back” to synapses and the environment include both traditional modes of communication (e.g., classical neurotransmitters like dopamine and growth factors) and also the arguably more exotic language of immune signaling in the nervous system (e.g., the classical complement system). The modern view of microglia, therefore, is one in which they play more than a role of mere support cell or sensor of pathology. They likely perform active, crucial homeostatic functions, acting as an integrator of the synaptic, extracellular, and sensory milieu.
Therapeutic Implications and Future Directions of Microglial Research
This potential important role in neuronal health then raises the question of the degree to which microglia could contribute to psychiatric disease. For example, the ability of microglia to detect carbon dioxide may represent a novel mechanism for translating hypercapnia (often associated with panic disorder) to “fear-associated behavioral and physiological responses” as described by Vollmer et al. (4). Genetic studies have implicated immune etiologies in disorders such as schizophrenia (9), making microglia a ripe target for exploration as the principal immune cells of the brain. One could imagine that misdirected or overzealous pruning or cell destruction could wreak havoc in neuronal circuits. In addition, positron emission tomography imaging studies have recently suggested that increased microglial activity is correlated with the intensity of psychotic symptoms in patients who are at high risk for psychosis who have not yet converted to schizophrenia (10). Microglia have been implicated in the etiologies of numerous psychiatric disorders, and the question remains as to whether microglia are simply attempting to rectify the bizarre behavior of diseased neurons or whether microglia may in fact have their own intrinsic pathologies. Either way, microglia now appear to be key players in the modern view of psychiatric neuroscience. The extent of their roles in health and mental illness are an area of active investigation, and it is hoped that better understanding their structure and function will enable researchers to create novel therapeutic interventions for psychiatric illness.
Acknowledgments
This work was supported by National Institutes of Health Grant Nos. R25 MH10107602S1 and R25 MH086466 07S1 (to DAR). DAR is the co-chair of the National Neuroscience Curriculum Initiative (NNCI), and this work was produced in collaboration with the NNCI.
We thank Drs. Mike Travis and Melissa Arbuckle for their contributions as NNCI editors and Amanda Wang for her role in developing the figure.
Footnotes
Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Schafer DP, Lehrman EK, Stevens B. The “quad-partite” synapse: microglia-synapse interactions in the developing and mature CNS. Glia. 2013;61:24–36. doi: 10.1002/glia.22389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wohleb ES, Powell ND, Godbout JP, Sheridan JF. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci. 2013;33:13820–13833. doi: 10.1523/JNEUROSCI.1671-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 4.Vollmer LL, Ghosal S, McGuire JL, Ahlbrand RL, Li KY, Santin JM, et al. Microglial acid sensing regulates carbon dioxide-evoked fear. Biol Psychiatry. 2016;80:541–551. doi: 10.1016/j.biopsych.2016.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 6.Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, 3rd, Lafaille JJ, et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marin-Teva JL, Dusart I, Colin C, Gervais A, van Rooijen N, Mallat M. Microglia promote the death of developing Purkinje cells. Neuron. 2004;41:535–547. doi: 10.1016/s0896-6273(04)00069-8. [DOI] [PubMed] [Google Scholar]
- 8.Walton NM, Sutter BM, Laywell ED, Levkoff LH, Kearns SM, Marshall GP, 2nd, et al. Microglia instruct subventricular zone neurogenesis. Glia. 2006;54:815–825. doi: 10.1002/glia.20419. [DOI] [PubMed] [Google Scholar]
- 9.Corvin A, Morris DW. Genome-wide association studies: findings at the major histocompatibility complex locus in psychosis. Biol Psychiatry. 2014;75:276–283. doi: 10.1016/j.biopsych.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 10.Bloomfield PS, Selvaraj S, Veronese M, Rizzo G, Bertoldo A, Owen DR, et al. Microglial activity in people at ultra high risk of psychosis and in schizophrenia: an [(11)C]PBR28 PET brain imaging study. Am J Psychiatry. 2016;173:44–52. doi: 10.1176/appi.ajp.2015.14101358. [DOI] [PMC free article] [PubMed] [Google Scholar]