Abstract
The ethnopharmacology, chemistry and pharmacology of four Malian medicinal plants, Biophytum umbraculum, Burkea africana, Lannea velutina and Terminalia macroptera are reviewed. These plants are used by traditional healers against numerous ailments: malaria, gastrointestinal diseases, wounds, sexually transmitted diseases, insect bites and snake bites, etc. The scientific evidence for these uses is, however, limited. From the chemical and pharmacological evidence presented here, it seems possible that the use in traditional medicine of these plants may have a rational basis, although more clinical studies are needed.
Keywords: Malian medicinal plants, Biophytum umbraculum, Burkea africana, Lannea velutina, Terminalia macroptera
1. Introduction
Africa has a very varied flora, and the study of African medicinal plants has engaged many scientists for a long time. The oldest written documents on this are found in the Papyrus Ebers, written ca. 3500 years ago [1,2]. One early study by the Norwegian medical doctor Henrik Greve Blessing, carried out in 1901–1904, but only existing as a handwritten manuscript, was recently discovered and has now been published [3].
Studies on African medicinal plants have in nearly all cases been limited to geographically limited areas—this is necessary, due to the very wide floral variation throughout this huge continent. Some examples of the large number of books dealing with African medicinal plants are the classical work by Watt and Breyer-Brandwijk [4] and Burkill’s multivolume treatise [5]. A few more recent examples include the books by Iwu [1], Kuete [6], Neuwinger [7] and van Wyk [8]—this list is very far from complete! Journals such as African Journal of Traditional, Complementary and Alternative Medicines, Journal of Ethnopharmacology, Journal of Ethnobiology and Ethnomedicine, as well as many others, are also rich sources of knowledge of African medicinal plants and their properties and use.
For many years, scientists in Mali and Norway have collaborated in investigating Malian medicinal plants, and this has resulted in more than sixty publications in peer-reviewed international journals, numerous contributions at national and international conferences, nine Ph.D. degrees and more than 100 M.Sc. degrees awarded. A description of this project is available on the web [9].
In this brief survey, I intend to review the ethnopharmacology, chemistry and pharmacology of some Malian medicinal plants. The plants discussed have been studied in my research group in the Section of Pharmacognosy, School of Pharmacy, University of Oslo. Our research has in the main been directed towards isolation and identification of secondary metabolites of plant origin and their properties as antioxidants, radical scavengers and inhibitors of enzymes involved in peroxidative processes.
Four of the less known species are chosen for this treatise, viz. Biophytum umbraculum, Burkea africana, Lannea velutina and Terminalia macroptera (Table 1). References to previous work were found through the SciFinder database, which covers Medline and Chemical Abstracts. Titles in other languages than English, French or German have been used in translation.
Table 1.
Data for herbarium voucher samples of plants discussed in this review.
| Plant | Deposited in | Registry Number | Article Reference |
|---|---|---|---|
| Biophytum umbraculum | DMT | 2653 | [10] |
| Burkea africana | DMT | no number | [11] |
| (registered under plant name) | |||
| Lannea velutina | DMT | 1014 | [12] |
| Terminalia macroptera | DMT | 2468 | [13,14] |
DMT: Department of Traditional Medicine, Bamako, Mali.
A recent review [15] covers medicinal plants from Mali. That review is, however, differently angled, and the plants covered in detail in the present article are only briefly mentioned.
2. Biophytum umbraculum
Biophytum umbraculum Welw. (Oxalidaceae) is a small herb, up to 15 cm in height. It is widespread in the tropical parts of Africa and Asia [16]. Several synonyms exist for this plant, including B. petersianum Klotzsch (the name most commonly found in the scientific literature) [17].
2.1. Ethnopharmacology
Few systematic surveys on the traditional use of this plant have been done. Diallo et al. [18] reported that leaves are used in wound healing, using the dried and powdered plant. Another survey [19] in Mali found that while the plant was used against pain, insect bites and snake bites, treatment of cerebral malaria was by far the most common indication.
2.2. Chemistry
The polysaccharides of this plant are fairly well investigated. Pectic polysaccharides with several biological activities have been isolated and identified [20,21,22]. These polysaccharides contain rhamnogalacturonan, xylogalacturonan, and arabinogalactan regions. Very little was known about the low molecular weight constituents prior to our studies. Saponins were known to be present in the plant [23,24], but their structures still remain unknown.
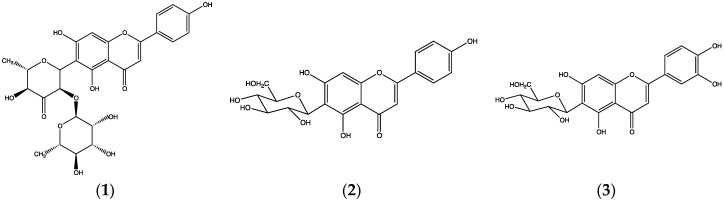
From a methanol extract of above ground parts of B. umbraculum, we isolated the C-glycosylflavones cassiaoccidentalin A (1), isovitexin (2) and isoorientin (3) (Figure 1) [10]. Isovitexin and isoorientin are not rare substances, but this appears to the first report on their occurrence in B. umbraculum. They have, however, been reported from another Biophytum species, B. sensitivum [25].
Figure 1.
Cassiaoccidentalin A (1); isovitexin (2) and isoorientin (3) from B. umbraculum.
Cassiaoccidentalin A was first reported from Cassia occidentalis (Leguminosae) [26] along with substances (2) and (3). After our report on this, cassiaoccidentalin A was found in Serjania marginata (Sapindaceae) leaves [27]. To our knowledge, no other sources for this compound are known.
2.3. Biological Activity
Crude extracts of the plant show complement fixing activity [18], hypotensive effects [28], calcium antagonism [29], and increased corticosteroid secretion [30]. Decreased methane production was observed in cattle fed B. petersianum [24].
The polysaccharides referred to in Section 2.2 have been investigated for immunological effects. Complement fixing, macrophage activation, dendritic cell activation and immunomodulating activity against Peyer’s patch cells has been reported [20,21,22]. The pectic polysaccharides from this plant exhibited protective ability against Streptococcus pneumoniae in a mouse model [31]. Cassiaoccidentalin A was investigated for suppression of the HIV promoter [32], but was found to be without effect. The plant where it was first found, C. occidentalis, is a known antimalarial plant and is used in Mali as one of the components of the “Improved Traditional Medicine” Malarial [33], which has been subjected to clinical studies. In view of the traditional use in Mali against cerebral malaria and the related chemistry between B. umbraculum and C. occidentalis, we investigated the malaria-related and antiinflammatory properties of B. umbraculum extracts and pure substances [34]. The ethyl acetate extract showed antiplasmodial, anti-complement and antiinflammatory activity and inhibited lipopolysaccharide stimulation in macrophages, but this appears to be due to other constituents than the isolated flavonoids. These findings may be relevant for the ethnopharmacological use of the plant against cerebral malaria. No clinical studies have been done, however.
3. Burkea africana
The tree Burkea africana Hook. (Leguminosae), usually less than 10 m in height, grows over large parts of tropical and subtropical Africa [35]. No synonyms are given for this plant [17].
3.1. Ethnopharmacology
In traditional medicine, the powdered stem bark is used in Mali on wounds in the mouth [18]. Other sources [4,35,36,37,38] cite a number of uses in different African countries.
3.2. Chemistry
Like many African plants, B. africana has only been subjected to a limited number of scientific studies. It has been reported [39] that the activity of the bark, which was stated as being used in folk medicine against gastrointestinal symptoms and headache, was due to its content of tyramine. The finding of tyramine was later corroborated [40], and the occurrence of harman-type alkaloids was reported, as well [41,42]. 5-Hydroxypipecolic acid, an unusual amino acid, was reported from the seeds of this plant [43].
Work in Ferreira’s group in the late 1980s [44,45] led to the identification of several new oligomeric flavonoids (proanthocyanidins) based on the flavan-3-ol fisetinidol (5-deoxycatechin) in the heartwood of B. africana. The leaf lipids [46] and the stem bark polysaccharides [18] have been investigated, as well.
In view of the widespread medicinal use and the limited knowledge of the constituents of this plant, we decided to investigate the chemistry and the antioxidative properties of the stem bark, which appears to be the part of the plant that is most commonly used for medicinal purposes in Mali. The putative wound-healing and antiinflammatory activities ascribed to preparations of B. africana stem bark might well be correlated to antioxidative effects.
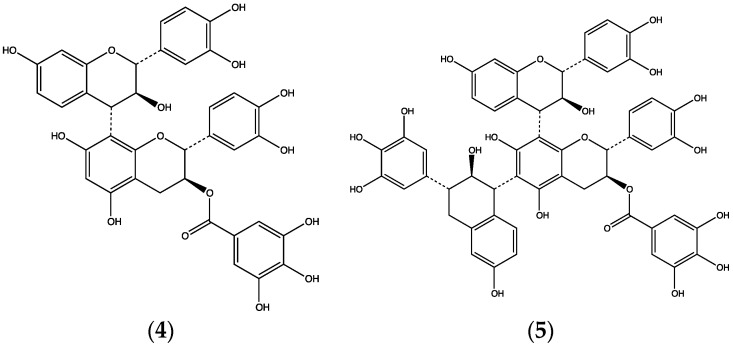
Using stem bark collected in Blendio, about 320 km south-east of the capital Bamako, we made an ethanolic extract which was subjected to liquid-liquid partition and repeated chromatography. This led to two major constituents (Figure 2), one of which was identified as fisetinidol-(4β→8)-catechin-3-O-gallate (4), previously known only from the heartwood of this tree [45]. The other substance was given the structure bis-fisetinidol-(4→6, 4→8)-catechin-3-O-gallate (5). Compound (5) was a new natural product [11].
Figure 2.
Structures of compounds (4) and (5) from Burkea africana stem bark.
3.3. Biological Activity
A pharmacological screening of Malian medicinal plants [47] revealed that a methanolic bark extract of B. africana had considerable radical scavenging activity as well as molluscicidal properties, and that both dichloromethane and methanol extracts of the bark were fungicidal. The methanolic bark extract has later been shown to attenuate oxidative stress in cells [48]. Fungicidal effects have been reported for heartwood extracts, as well [49], and moderate activity of water and methanol extracts of the bark against Candida albicans was reported [50]. An ethanol extract of the stem bark was shown to be antidiarrhoeal in mice [51]. Moderate inhibition of hyaluronidase and phospholipase A2 as well as antiproteolytic activity of methanol and water extracts of B. africana was reported [52]. An acetone leaf extract was tested for some antioxidant and antiinflammatory activities, finding inhibition of 15-lipoxygenase, acetylcholinesterase and nitric oxide production, as well as radical scavenging activity in different assay systems [53].
We found [11,54] that the crude aqueous ethanolic extract (80% ethanol) had very high radical scavenging activity, good 15-lipoxygenase inhibitory activity, and was also active as an inhibitor of iron-induced peroxidation of phospholipids. Of the subfractions from liquid-liquid partition, the ethyl acetate part was the most active, indicating that the active constituents were semipolar.
Substances 4 and 5 were about equiactive as radical scavengers and 15-lipoxygenase inhibitors, as might be expected from their polyphenolic structures, but differed in their inhibitory activity against phospholipid peroxidation.
We concluded that the hydroethanolic extract of Burkea africana stem bark showed strong activity against several peroxidative processes, probably due to its high content of polyphenolic substances, most of which appear to be proanthocyanidin-type tannins [11,54]. Wound-healing properties of tannins are well described in the literature (e.g., [55,56]), so our findings may be related to the ethnopharmacological use of the plant. Although animal experiments have been reported, it appears that no clinical studies have been carried out.
4. Lannea velutina
Lannea velutina A. Rich. (Anacardiaceae), syn. Calesiam velutinum (A. Rich.) Kuntze, Odina velutina (A. Rich.) Oliv. [17] is a shrub or tree growing from Senegal to Ghana.
4.1. Ethnopharmacology
Several sources report on the medicinal use of this plant, but without details [57,58]. More specifically, decoctions are said to be used against diarrhoea, rachitis, muscle pains, gastric pains, and as a tonic. The bark is applied to wounds and leprosy [59]. In Mali, the stem bark is used in wound healing [18], and the leaves are sold in markets for making a decoction used as an antidote to poisoning [60]. The fruits are edible [61]. According to one source, the smoke from the twigs is used in witchcraft [62]. In our field work, we found that in Dioila and Kolokani, leaf decoctions, stem bark decoctions and root decoctions of L. velutina are used against a multitude of diseases, including skin diseases, fever, gastrointestinal diseases, and in wound treatment [12,63].
4.2. Chemistry
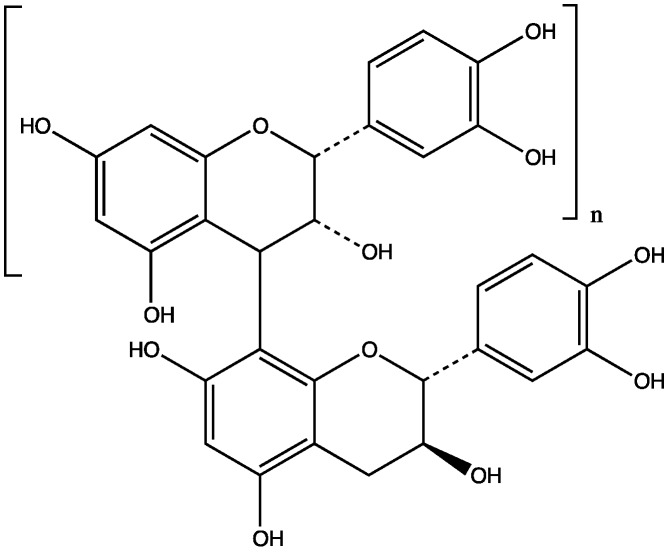
Prior to our studies, this plant appeared to be nearly uninvestigated. Unidentified tannins are reported to occur in all parts of the plant, mainly in the bark [64]. We found that the root bark of this plant was a rich source of procyanidins [63]. These had a linear 4→8 structure and commonly had catechin as starter unit and epicatechin as extender unit. Monomeric (catechin), dimeric, trimeric, tetrameric, hexameric, heptameric, nonameric, decameric and dodecameric procyanidin fractions were isolated (Figure 3). Most of the procyanidins were decameric or higher.
Figure 3.
General structure of procyanidins from L. velutina (lower part: catechin, upper part: epicatechin, n = 0 (catechin monomer) to 11 (procyanidin dodecamer).
Mass spectroscopy revealed that O-methylation, O-galloylation and substitution of epiafzelechin (deoxyepicatechin) for epicatechin occurred, although to a minor extent.
4.3. Biological Activity
In a survey of Malian medicinal plants [47], extracts (dichloromethane, methanol, water) of the leaves, bark and roots of L. velutina were investigated for antifungal, larvicidal, molluscicidal, antioxidant and radical scavenging activities. Although activities varied between plant parts and extracts, positive results were obtained for antioxidant activity (bark and root methanol extracts), antifungal activity (leaf dichloromethane extract active against both Candida albicans and Cladosporium cucumerinum, the other extracts had more selective activity), larvicidal activity against the malaria mosquito Anopheles gambiae (dichloromethane bark and leaf extract, methanol leaf extract), and molluscicidal activity towards the schistomiasis-transmitting snail Biomphalaria pfeifferi. Antioxidant and antibacterial activity of an ethanol extract of L. velutina bark were reported [65], with the highest activity towards Bacillus subtilis, Staphylococcus aureus (Gram-positive), Pseudomonas aeruginosa and Salmonella typhimurium (Gram-negative).
We investigated the radical scavenger and 15-lipoxygenase inhibitory activity of different extracts of root bark and stem bark [12]. Lipophilic extracts (petrol ether, dichloromethane, chloroform) were inactive as scavengers of the DPPH radical, water extracts had moderate activity, while semipolar extracts (methanol, 80% aqueous ethanol) of both root bark and stem bark were highly active. Similar observations (high activity of semipolar extracts) were made for inhibition of the peroxidative enzyme 15-lipoxygenase, although lipophilic extracts were more active than aqueous extracts in this assay. In a continuation of these experiments [63], radical scavenging activity and 15-lipoxygenase inhibition were investigated for the purified proanthocyanidins. All of them were highly active, although there was a slight trend towards higher activity on a weight basis for the higher molecular weight substances in both assays, translating to a clear molecular weight—activity correlation on a molecular basis. Two of the smaller compounds, epicatechin and trimeric proanthocyanidin (mostly catechin-→epicatechin→epicatechin) were tested for ability to counteract pro-oxidant toxicity induced by glutamate in cerebellar neurons. The trimer gave a significant decrease in cell death. Although a decrease was observed for epicatechin, as well, this did not reach statistical significance.
As mentioned above (Section 3.3), the contents of proanthocyanidins may be of relevance to the ethnopharmacological use of the plants against wounds. Proanthocyanidins may also be involved in the use of the plant against gastric pains and diarrhea [66]. As for many ethnopharmacologically used plants, clinical data are lacking.
5. Terminalia macroptera
Terminalia macroptera Guill. & Perr. (Combretaceae) (syn. Terminalia chevalieri Diels, Myrobalanus macroptera (Guill. & Perr.) Kuntze) [17] is a tree up to 20 m in height. It is widespread in West Africa from Senegal to Cameroon, often on wet land [67].
5.1. Ethnopharmacology
Different parts of T. macroptera have been used for numerous ailments, including malaria [68,69,70], GI tract ailments (e.g., diarrhoea, gastritis, colitis, piles) [67,71,72], and infectious diseases including sexually transmitted diseases [73,74].
We carried out a systematic survey on the medicinal use of T. macroptera by traditional healers in three districts in Mali; Siby, Dioila and Dogonland [75]. Although there were regional differences, major areas of use were against pain and rheumatism (all areas), wounds (mainly in Siby), and hepatitis (mainly in Dogonland). Cough, diarrhoea and fever/malaria were also treated with T. macroptera preparations. Root bark, stem bark and leaves were used, but Loranthus parasitic plants on this tree were often employed in Dogonland.
5.2. Chemistry
The first published research on the constituents of T. macroptera appears to be by Prista et al. [76], reporting on the identification of chlorogenic acid and quercetin, two very common phenolic natural products. Other flavonoids isolated from T. macroptera flowers include the flavone C-glucosides orientin, isoorientin [77], vitexin, isovitexin [78,79] and the flavonol glycosides isorhamnetin 3-O-(6-O-α-l-rhamnosyl)-β-d-glucoside, quercetin 3-O-(6-O-β-d-glucosyl)-α-l-rhamnoside, quercetin 3-O-β-d-glucoside and quercetin 3-O-(6-O-α-l-rhamnosyl)-β-d-glucoside [80]. Unidentified proantho-cyanidins have been reported to occur in the leaf extract [81].
Other polyphenols have been found, as well. Conrad et al [82] reported a new phenolic glucoside, vanillic acid 4-O-β-d-(6´-O-galloyl) glucopyranoside from the bark, and Kone et al [79] found a series of benzoic and cinnamic acids in the stem and root bark extracts of the plant. Ellagitannins, some of them new natural products, have been reported [82,83,84]. Ellagic acid and methylellagic acids were reported from the heartwood in an early investigation [85].
The other major group of natural products reported from T. macroptera is the terpenoids, more specifically triterpenoids. Idemudia [85] reported the presence of terminolic acid; more recently, 23-galloylarjunolic acid and its glucosyl ester [86] and glucosides of 24-deoxysericoside and chebuloside II [78] have been identified.
Research on the polysaccharides of T. macroptera has recently been carried out in our department [87,88,89]. Pectins are important in this plant, and their biological activities have been discussed below (Biological activity, Section 5.3).
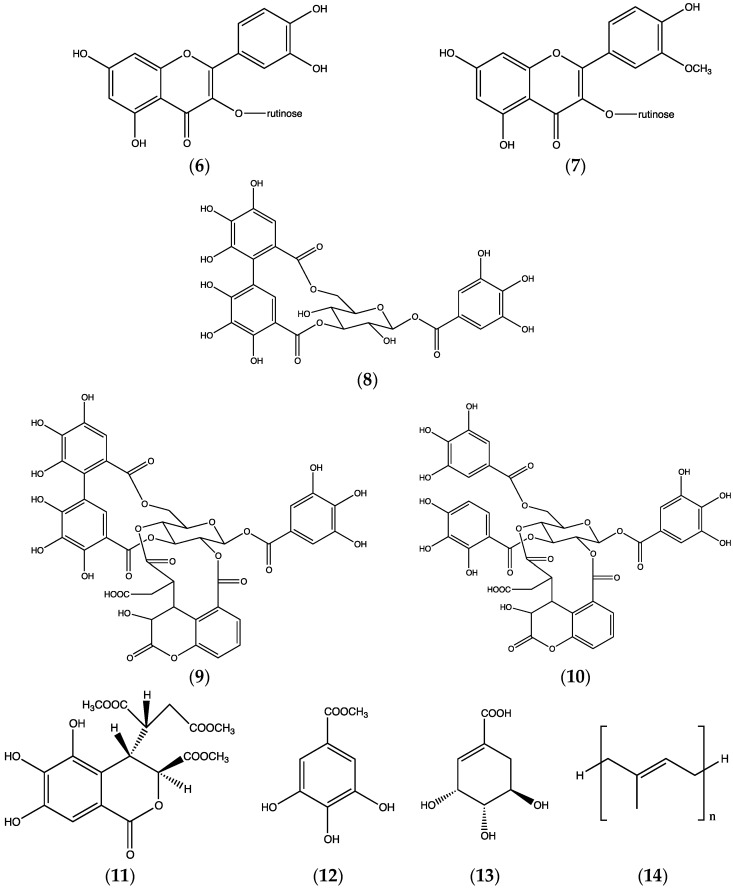
We investigated leaf constituents in T. macroptera, since this part of the plant was not well known. A series of compounds was isolated and identified [13] from the methanolic extract (Figure 4): The flavonoids rutin (6) and narcissin (7), the hydrolyzable tannins corilagin (8), chebulagic acid (9), chebulinic acid (10) and chebulic acid trimethyl ester (11), methyl gallate (12) and shikimic acid (13). In the dichloromethane extract, poly-cis-isoprene (14; calculated average chain length ca 25 units) was the main constituent. All of these compounds are new to T. macroptera, and chebulic acid trimethyl ester is a new compound. It may, however, be an artifact, formed from chebulic acid during methanol extraction.
Figure 4.
Constituents of Terminalia macroptera leaves: Flavonoids (6–7), ellagitannins (8–10), other constituents (11–14).
5.3. Biological Activity
Pharmacological studies of T. macroptera were started in the 1990s by Silva and co-workers. In a series of papers [74,90,91,92,93], extracts of roots and leaves were investigated for antimicrobial activity towards a series of pathogenic microorganisms, including Neisseria gonorrheae and Helicobacter pylori. Other groups have investigated antimicrobial effects, as well: Antibacterial and/or antifungal effects were reported [81,82,94,95,96]. Effects on the malaria protozoa Plasmodium falciparum [68,97] as well as on another pathogenic protozoa, Trypanosoma brucei [97] might be of special importance in view of the serious nature of the diseases caused by these organisms.
The pharmacology of the pectic polysaccharides from T. macroptera was recently investigated [87,88,89]. These polysaccharides were found to have immunomodulatory and complement fixing properties. Interestingly, such activities were present in preparations made in the same way as traditional healers do [88].
We found [13] that the methanol crude extract had high radical scavenger activity (6.2 ± 0.4 μg/mL) and showed moderate inhibition (52 ± 5 μg/mL) of xanthine oxidase, an enzyme involved in production of superoxide radical anion. Xanthine oxidase inhibition is of medicinal importance in the treatment of gout. While poly-cis-isoprene was inactive in all our assays, the other isolated compounds showed various activities. Corilagin and chebulagic acid were very good radical scavengers, with IC50 values less than half of the positive control quercetin. Due to lack of material, chebulinic acid could not be tested.
Rutin and chebulagic acid inhibited xanthine oxidase. They were, however, less active than quercetin (positive control). Shikimic acid was inactive [13]. Toxicity towards brine shrimp was low (LD50 > 100 μg/mL for all extracts, >200 μM for all pure compounds) [14]. This test is commonly used as an indication of general toxicity [98]. The crude methanol extract had good activity as a 15-lipoxygenase inhibitor (IC50 27.9 ± 1.5 μg/mL, comparable to the positive control quercetin) and an α-glucosidase inhibitor (IC50 0.47 ± 0.03 μg/mL, much more active than the positive control acarbose with an IC50 value of 130 ± 18 μg/mL). In 15-lipoxygenase inhibition, both chebulagic acid, corilagin and narcissin were considerably more active than the positive control quercetin, while chebulagic acid showed remarkable inhibitory activity towards α-glucosidase (IC50 < 0.1 μM). Corilagin was less active, but being present in much higher concentration in the extract, it may well be the most important component in this respect [14,15]. In sum, we have found that T. macroptera leaves constitute a rich source of bioactive compounds, with good activity both as an antioxidant and radical scavenger and as an inhibitor of α-glucosidase. This might be of importance for their use by traditional healers.
Biological activities in vitro (radical scavenging; enzyme inhibition (α-glucosidase, 15-lipoxygenase, xanthine oxidase); complement fixation) and in vivo (toxicity towards brine shrimp) of ethanol and water extracts of root bark, stem bark and leaves of T. macroptera were investigated in a separate set of experiments [99]. Ethanol extracts of root bark and stem bark showed the highest activity. Radical scavenging and enzyme inhibition was correlated to total phenolic content, while complement fixation was not. The extracts were non-toxic towards brine shrimp.
The ethnopharmacological use of the plant against GI tract ailments might be related to its content of gallotannins and ellagitannins. The polysaccharides of the plant have anti-inflammatory properties in vitro, but no clinical studies have been carried out.
6. Conclusions
The plants Biophytum umbraculum, Burkea africana, Lannea velutina and Terminalia macroptera are used in traditional medicine in Mali against diverse ailments. Extracts of these plants show a variety of biological effects in vitro and in vivo. These effects may be related to the medicinal use of these plants, and may therefore indicate that their use in traditional medicine could have a rational basis. Clinical studies are, however, needed to draw conclusions on this.
Acknowledgments
No funding was received for preparing and publishing this review. References to external funding of our work cited in this review are given in each paper. A special acknowledgement goes to the healers of Mali for generously sharing their knowledge with us.
Conflicts of Interest
The author declares no conflict of interest.
References
- 1.Iwu M.M. Handbook of African Medicinal Plants. 2nd ed. CRC Press; Boca Raton, FL, USA: 2014. [Google Scholar]
- 2.Petrovska B.B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012;6:1–5. doi: 10.4103/0973-7847.95849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paulsen B.S., Ekeli H., Johnson Q., Norum K.R. South African Traditional Medicinal Plants from Kwazulu-Natal. Fagbokforlaget; Oslo, Norway: 2012. [Google Scholar]
- 4.Watt J.M., Breyer-Brandwijk M.G. The Medicinal and Poisonous Plants of Southern and Eastern Africa: Being an Account of Their Medicinal and Other Uses, Chemical Composition, Pharmacological Effects and Toxicology in Man and Animal. Livingstone; Edinburgh, UK: 1962. [Google Scholar]
- 5.Burkill H.M. The Useful Plants of West Tropical Africa. Volumes 1–5 Royal Botanic Gardens; Kew, UK: 1985–2000. [Google Scholar]
- 6.Kuete V. Medicinal Plant Research in Africa: Pharmacology and Chemistry. Elsevier; Amsterdam, The Netherlands: 2013. [Google Scholar]
- 7.Neuwinger H.D. African Traditional Medicine. Medpharm; Stuttgart, Germany: 2000. [Google Scholar]
- 8.Van Wyk B.E., Van Oudtshoorn B., Gericke N. Medicinal Plants of South Africa. 2nd ed. Briza; Pretoria, South Africa: 2009. [Google Scholar]
- 9.Universitetet I Oslo The Malian Medicinal Plant Project. [(accessed on 14 November 2016)]. Available online: http://www.mn.uio.no/farmasi/english/research/projects/maliplants/
- 10.Pham A.T., Nguyen C., Malterud K.E., Diallo D., Wangensteen H. Bioactive flavone-C-glycosides of the African medicinal plant Biophytum umbraculum. Molecules. 2013;18:10312–10319. doi: 10.3390/molecules180910312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathisen E., Diallo D., Andersen Ø.M., Malterud K.E. Antioxidants from the bark of Burkea africana, an African medicinal plant. Phytother. Res. 2002;16:148–153. doi: 10.1002/ptr.936. [DOI] [PubMed] [Google Scholar]
- 12.Maiga A., Malterud K.E., Diallo D., Paulsen B.S. Antioxidant and 15-lipoxygenase inhibitory activities of the Malian medicinal plants Diospyros abyssinica (Hiern) F. White (Ebenaceae), Lannea velutina A. Rich. (Anacardiaceae) and Crossopteryx febrifuga (Afzel) Benth. (Rubiaceae) J. Ethnopharmacol. 2006;104:132–137. doi: 10.1016/j.jep.2005.08.063. [DOI] [PubMed] [Google Scholar]
- 13.Pham A.T., Malterud K.E., Paulsen B.S., Diallo D., Wangensteen H. DPPH radical scavenging and xanthine oxidase inhibitory activity of Terminalia macroptera leaves. Nat. Prod. Commun. 2011;6:1125–1128. [PubMed] [Google Scholar]
- 14.Pham A.T., Malterud K.E., Paulsen B.S., Diallo D., Wangensteen H. α-Glucosidase inhibition, 15-lipoxygenase inhibition, and brine shrimp toxicity of extracts and isolated compounds from Terminalia macroptera leaves. Pharm. Biol. 2014;52:1166–1169. doi: 10.3109/13880209.2014.880486. [DOI] [PubMed] [Google Scholar]
- 15.Wangensteen H., Diallo D., Paulsen B.S. Medicinal plants from Mali: Chemistry and biology. J. Ethnopharmacol. 2015;176:429–437. doi: 10.1016/j.jep.2015.11.030. [DOI] [PubMed] [Google Scholar]
- 16.Pham A.T. Ph.D. Thesis. University of Oslo; Oslo, Norway: 2014. [(accessed on 5 December 2016)]. Chemical, Biological and Ethnopharmacological Studies of Two Malian Medicinal Plants: Terminalia Macroptera and Biophytum Umbraculum. Available online: https://www.duo.uio.no/handle/10852/41479. [Google Scholar]
- 17.The Plant List. [(accessed on 13 November 2016)]. Available online: www.theplantlist.org.
- 18.Diallo D., Sogn C., Samaké F.B., Paulsen B.S., Michaelsen T.E., Keita A. Wound healing plants in Mali, the Bamako region. An ethnobotanical survey and complement fixation of water extracts from selected plants. Pharm. Biol. 2002;40:117–128. doi: 10.1076/phbi.40.2.117.5846. [DOI] [Google Scholar]
- 19.Grønhaug T.E., Glæserud S., Skogsrud M., Ballo N., Bah S., Diallo D., Paulsen B.S. Ethnopharmacological survey of six medicinal plants from Mali, West-Africa. J. Ethnobiol. Ethnomed. 2008;4:26. doi: 10.1186/1746-4269-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inngjerdingen K.T., Coulibaly A., Diallo D., Michaelsen T.E., Paulsen B.S. A complement fixing polysaccharide from Biophytum petersianum Klotzsch, a medicinal plant from Mali, West Africa. Biomacromolecules. 2006;7:48–53. doi: 10.1021/bm050330h. [DOI] [PubMed] [Google Scholar]
- 21.Inngjerdingen M., Inngjerdingen K.T., Patel T.R., Allen S., Chen X., Rolstad B., Morris G.A., Harding S.E., Michaelsen T.E., Diallo D., et al. Pectic polysaccharides from Biophytum petersianum Klotzsch, and their activation of macrophages and dendritic cells. Glycobiology. 2008;18:1074–1084. doi: 10.1093/glycob/cwn090. [DOI] [PubMed] [Google Scholar]
- 22.Grønhaug T.E., Kiyohara H., Sveaass A., Diallo D., Yamada H., Paulsen B.S. Beta-D-(1→4)-galactan-containing side chains in RG-I regions of pectic polysaccharides from Biophytum petersianum Klotzsch. contribute to expression of immunomodulating activity against intestinal Peyer’s patch cells and macrophages. Phytochemistry. 2011;72:2139–2147. doi: 10.1016/j.phytochem.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 23.Santoso B., Kilmaskossu A., Sambodo P. Effects of saponin from Biophytum petersianum Klotzsch on ruminal fermentation, microbial protein synthesis and nitrogen utilization in goats. Anim. Feed Sci. Technol. 2007;137:58–68. doi: 10.1016/j.anifeedsci.2006.10.005. [DOI] [Google Scholar]
- 24.Hariadi B.T., Santoso B. Evaluation of tropical plants containing tannin on in vitro methanogenesis and fermentation parameters using rumen fluid. J. Sci. Food Agric. 2010;90:456–461. doi: 10.1002/jsfa.3839. [DOI] [PubMed] [Google Scholar]
- 25.Bucar F., Jachak S.M., Kartnig T., Schubert-Zsilavecz M. Phenolic compounds from Biophytum sensitivum. Pharmazie. 1998;53:651–653. [Google Scholar]
- 26.Hatano T., Mizuta S., Ito H., Yoshida T. C-glycosidic flavonoids from Cassia occidentalis. Phytochemistry. 1999;52:1379–1383. doi: 10.1016/S0031-9422(99)00437-9. [DOI] [Google Scholar]
- 27.Heredia-Vieira S.C., Simonet A.M., Vilegas W., Macías F.A. Unusual C,O-fused glucosylapigenins from Serjania marginata leaves. J. Nat. Prod. 2015;78:77–84. doi: 10.1021/np500715x. [DOI] [PubMed] [Google Scholar]
- 28.Titrikou S., Aklikokou A.K., Gbeassor M. Effet de l’extrait de Biophytum petersianum (Oxalidaceae) Klotzsch sur le systeme cardiovasculaire de cobaye. Pharm. Med. Tradit. Afr. 1998;10:32–41. (In French) [Google Scholar]
- 29.Titrikou S., Eklu-Gadegbeku K., Mouzou A., Aklikokou K., Gbeassor M. Calcium antagonistic activity of Biophytum petersianum on vascular smooth muscles of Wistar rats. Iran. J. Pharmacol. Ther. 2007;6:185–189. [Google Scholar]
- 30.Kodjo K.M., Contesse V., Do Rego J.L., Aklikokou K., Titrikou S., Gbeassor M., Vaudry H. In vitro effects of crude extracts of Parkia biglobosa (Mimosaceae), Stereospermum kunthianum (Bignoniaceae) and Biophytum petersianum (Oxalidaceae) on corticosteroid secretion in rat. J. Steroid Biochem. Mol. Biol. 2006;100:202–208. doi: 10.1016/j.jsbmb.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Inngjerdingen K.T., Langerud B.K., Rasmussen H., Olsen T.K., Austarheim I., Grønhaug T.E., Aaberge I.S., Diallo D., Paulsen B.S., Michaelsen T.E. Pectic polysaccharides isolated from Malian medicinal plants protect against Streptococcus pneumoniae in a mouse pneumococcal pneumonia infection model. Scand. J. Immunol. 2013;77:372–388. doi: 10.1111/sji.12047. [DOI] [PubMed] [Google Scholar]
- 32.Uchiumi F., Hatano T., Ito H., Yoshida T., Tanuma S.I. Transcriptional suppression of the HIV promoter by natural compounds. Antivir. Res. 2003;58:89–98. doi: 10.1016/S0166-3542(02)00186-9. [DOI] [PubMed] [Google Scholar]
- 33.Willcox M., Sanogo R., Diakite C., Giani S., Paulsen B.S., Diallo D. Improved traditional medicines in Mali. J. Altern. Complement. Med. 2012;18:212–220. doi: 10.1089/acm.2011.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Austarheim I., Pham A.T., Nguyen C., Zou Y.F., Diallo D., Malterud K.E., Wangensteen H. Antiplasmodial, anti-complement and anti-inflammatory in vitro effects of Biophytum umbraculum Welw. traditionally used against cerebral malaria in Mali. J. Ethnopharmacol. 2016;190:159–164. doi: 10.1016/j.jep.2016.05.058. [DOI] [PubMed] [Google Scholar]
- 35.Nonyane F., Masupa T. Burkea africana Hook. [(accessed on 14 November 2016)]. Available online: http://www.plantzafrica.com/plantab/burkeaafricana.htm.
- 36.Delaveau P.A., Desvignes E., Adoux E., Tessier A.M. Baguettes frotte-dents d´ Afrique occidentale. Examen chimique et microbiologique. Ann. Pharm. Fr. 1979;37:185–190. (In French) [PubMed] [Google Scholar]
- 37.Pedersen M.E., Vestergaard H.T., Hansen S.L., Bah S., Diallo D., Jäger A.K. Pharmacological screening of Malian medicinal plants used against epilepsy and convulsions. J. Ethnopharmacol. 2009;121:472–475. doi: 10.1016/j.jep.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 38.Maroyi A. Burkea africana Hook. [(accessed on 16 February 2017)]. Available online: http://uses.plantnet-project.org/en/Burkea_africana_(PROTA)
- 39.Correira da Silva A., Paiva M.Q. Comparison of the pharmacodynamic activity of bark extracts from Burkea africana with one of its alkaloid constituents. An. Fac. Farm. Porto. 1971;31:107–118. [Google Scholar]
- 40.Ferreira M.A. Indole alkaloids from Burkea africana. An. Fac. Farm. Porto. 1972;32:37–41. [Google Scholar]
- 41.Ferreira M.A. Chemical study of Burkea africana. I. Identification of β-sitosterol and tetrahydroharman. Garcia de Orta. Ser. Farmacogn. 1973;2:7–21. [Google Scholar]
- 42.Ferreira M.A. Indolic alkaloids from Burkea africana. II. Characterization of harmane and dihydroharmane. Garcia de Orta Ser. Farmacogn. 1973;2:23–32. [Google Scholar]
- 43.Watson R., Fowden L. Amino acids of Caesalpinia tinctoria and some allied species. Phytochemistry. 1973;12:617–622. doi: 10.1016/S0031-9422(00)84454-4. [DOI] [Google Scholar]
- 44.Malan J.C.S., Young D.A., Steenkamp J.A., Ferreira D. Oligomeric flavonoids. Part 2. The first profisetinidins with dihydroflavonol constituent units. J. Chem. Soc. Perkin Trans. 1. 1988;9:2567–2572. doi: 10.1039/p19880002567. [DOI] [Google Scholar]
- 45.Bam M., Malan J.C.S., Young D.A., Brandt E.V., Ferreira D. Profisetinidin-type 4-arylflavan-3-ols and related δ-lactones. Phytochemistry. 1990;29:283–287. doi: 10.1016/0031-9422(90)89051-A. [DOI] [Google Scholar]
- 46.Davidson B.C. Seasonal changes in leaf lipid and fatty acid composition of nine plants consumed by two African herbivores. Lipids. 1998;33:109–113. doi: 10.1007/s11745-998-0186-x. [DOI] [PubMed] [Google Scholar]
- 47.Diallo D., Marston A., Terreaux C., Touré Y., Paulsen B.S., Hostettmann K. Screening of Malian medicinal plants for antifungal, larvicidal, molluscicidal, antioxidant and radical scavenging activities. Phytother. Res. 2001;15:401–406. doi: 10.1002/ptr.738. [DOI] [PubMed] [Google Scholar]
- 48.Cordier W., Gulumian M., Cromarty A.D., Steenkamp V. Attenuation of oxidative stress in U937 cells by polyphenolic-rich bark fractions of Burkea africana and Syzygium cordatum. BMC Complement. Altern. Med. 2013;13:116. doi: 10.1186/1472-6882-13-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neya B., Hakkou M., Pétrissans M., Gérardin P. On the durability of Burkea africana heartwood: Evidence of biocidal and hydrophobic properties responsible for durability. Ann. For. Sci. 2004;61:277–282. doi: 10.1051/forest:2004020. [DOI] [Google Scholar]
- 50.Steenkamp V., Fernandes A.C., Van Rensburg C.E.J. Screening of Venda medicinal plants for antifungal activity against Candida albicans. S. Afr. J. Bot. 2007;73:256–258. doi: 10.1016/j.sajb.2006.11.003. [DOI] [Google Scholar]
- 51.Tanko Y., Iliya B., Mohammed A., Mahdi M.A., Musa K.Y. Modulatory effect of ethanol stem bark extract of Burkea africana on castor oil induced diarrhoea on experimental animals. Arch. Appl. Sci. Res. 2011;3:122–130. [Google Scholar]
- 52.Molander M., Nielsen L., Søgaard S., Staerk D., Rønsted N., Diallo D., Chifundera K.Z., Van Staden J., Jäger A.K. Hyaluronidase, phospholipase A2 and protease inhibitory activity of plants used in traditional treatment of snakebite-induced tissue necrosis in Mali, DR Congo and South Africa. J. Ethnopharmacol. 2014;157:171–180. doi: 10.1016/j.jep.2014.09.027. [DOI] [PubMed] [Google Scholar]
- 53.Dzoyem J.P., Eloff J.N. Anti-inflammatory, anticholinesterase and antioxidant activity of leaf extract of twelve plants used traditionally to alleviate pain and inflammation in South Africa. J. Ethnopharmacol. 2015;160:194–201. doi: 10.1016/j.jep.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 54.Mathisen E. Master’s Thesis. University of Oslo; Oslo, Norway: 1999. Radical Scavengers and Antioxidants from Burkea Africana, an African Medicinal Plant. (In Norwegian) [Google Scholar]
- 55.Rane M.M., Mengi S.A. Comparative effect of oral administration and topical application of alcoholic extract of Terminalia arjuna bark on incision and excision wounds in rats. Fitoterapia. 2003;74:553–558. doi: 10.1016/S0367-326X(03)00118-7. [DOI] [PubMed] [Google Scholar]
- 56.Kisseih E., Lechtenberg M., Petereit F., Sendker J., Zacharski D., Brandt S., Agyare C., Hensel A. Phytochemical characterization and in vitro wound healing activity of leaf extracts from Combretum mucronatum Schum. & Thonn.: Oligomeric procyanidins as strong inductors of cellular differentiation. J. Ethnopharmacol. 2015;174:628–636. doi: 10.1016/j.jep.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 57.Taïta P. Use of woody plants by locals in Mare aux Hippopotames biosphere reserve in western Burkina Faso. Biodivers. Conserv. 2003;12:1205–1217. doi: 10.1023/A:1023045316329. [DOI] [Google Scholar]
- 58.Belem B., Nacoulma B.M.I., Gbangou R., Kambou S., Hansen H.H., Gausset Q., Lund S., Raebild A., Lompo D., Ouedraogo M., et al. Use of non wood forest products by local people bordering the “Parc national Kaboré Tambi”, Burkina Faso. J. Transdiscipl. Environ. Stud. 2007;6:1–21. [Google Scholar]
- 59.Jansen P.C.M. Lannea velutina A. Rich. PROTA. [(accessed on 16 February 2017)]. Available online: http://uses.plantnet-project.org/en/Lannea_velutina_(PROTA)
- 60.Maiga A., Diallo D., Fane S., Sanogo R., Paulsen B.S., Cisse B. A survey of toxic plants on the market in the district of Bamako, Mali: Traditional knowledge compared with a literature search of modern pharmacology and toxicology. J. Ethnopharmacol. 2005;96:183–193. doi: 10.1016/j.jep.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 61.Gueye M., Ayessou N.C., Koma S., Diop S., Akpo L.E., Samb P.I. Wild fruits traditionally gathered by the Malinke ethnic group in the edge of Niokolo Koba park (Senegal) Am. J. Plant Sci. 2014;5:1306–1317. doi: 10.4236/ajps.2014.59144. [DOI] [Google Scholar]
- 62.Pageard R. Plantes à brûler chez les Bambara. J. Soc. Afr. 1967;37:87–130. doi: 10.3406/jafr.1967.1419. [DOI] [Google Scholar]
- 63.Maiga A., Malterud K.E., Mathisen G.H., Paulsen R.E., Thomas-Oates J., Bergström E., Reubsaet L., Diallo D., Paulsen B.S. Cell protective antioxidants from the root bark of Lannea velutina A. Rich., a Malian medicinal plant. J. Med. Plants Res. 2007;1:66–79. [Google Scholar]
- 64.Sérémé A., Millogo Rasolodimby J., Guinko S., Nacro M. Concentration en tanins des organes de plantes tannifères du Burkina Faso. J. Soc. Ouest.-Afr. Chim. 2008;25:55–61. (In French) [Google Scholar]
- 65.Ouattara L., Koudou J., Zongo C., Barro N., Savadogo A., Bassole I.H.N., Ouattara A.S., Traore A.S. Antioxidant and antibacterial activities of three species of Lannea from Burkina Faso. J. Appl. Sci. 2011;11:157–162. doi: 10.3923/jas.2011.157.162. [DOI] [Google Scholar]
- 66.Cires M.J., Wong X., Carrasco-Pozo C., Gotteland M. The gastrointestinal tract as a key target organ for the health-promoting effects of dietary proanthocyanidins. Front. Nutr. 2017;3:57. doi: 10.3389/fnut.2016.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arbonnier M. Trees, Shrubs and Lianas of West African Dry Zones. Margraf; Weikersheim, Germany: 2004. [Google Scholar]
- 68.Sanon S., Ollivier E., Azas N., Mahiou V., Gasquet M., Ouattara C.T., Nebie I., Traore A.S., Esposito F., Balansard G., et al. Ethnobotanical survey and in vitro antiplasmodial activity of plants used in traditional medicine in Burkina Faso. J. Ethnopharmacol. 2003;86:143–147. doi: 10.1016/S0378-8741(02)00381-1. [DOI] [PubMed] [Google Scholar]
- 69.Benoit-Vical F., Soh P.N., Saléry M., Harguem L., Poupat C., Nongonierma R. Evaluation of Senegalese plants used in malaria treatment: Focus on Chrozophora senegalensis. J. Ethnopharmacol. 2008;116:43–48. doi: 10.1016/j.jep.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 70.Traore M.S., Baldé M.A., Diallo M.S.T., Baldé E.S., Diané S., Camara A., Diallo A., Balde A., Keïta A., Keita S.M., et al. Ethnobotanical survey on medicinal plants used by Guinean traditional healers in the treatment of malaria. J. Ethnopharmacol. 2013;150:1145–1153. doi: 10.1016/j.jep.2013.10.048. [DOI] [PubMed] [Google Scholar]
- 71.Etuk E.U., Uhwah M.O., Ajagbonna O.P., Onyeyili P.A. Ethnobotanical survey and preliminary evaluation of medicinal plants with antidiarrhoea properties in Sokoto state, Nigeria. J. Med. Plants Res. 2009;3:763–766. [Google Scholar]
- 72.Kayode J., Ige O.E., Adetogo T.A., Igbakin A.P. Conservation and biodiversity erosion in Ondo state, Nigeria: (3). Survey of plant barks used in native pharmaceutical extraction in Akoko region. Ethnobot. Leafl. 2009;13:665–667. [Google Scholar]
- 73.Kayode J., Jose R.A., Ige O.E. Conservation and biodiversity erosion in Ondo state, Nigeria: (4). Assessing botanicals used in the cure of sexually transmitted diseases in Owo region. Ethnobot. Leafl. 2009;13:734–738. [Google Scholar]
- 74.Silva O., Ferreira E., Pato M.V., Canica M., Gomes E.T. In vitro anti-Neisseria gonorrhoeae activity of Terminalia macroptera leaves. FEMS Microbiol. Lett. 2002;217:271–274. doi: 10.1111/j.1574-6968.2002.tb11487.x. [DOI] [PubMed] [Google Scholar]
- 75.Pham A.T., Dvergsnes C., Togola A., Wangensteen H., Diallo D., Paulsen B.S., Malterud K.E. Terminalia macroptera, its current medicinal use and future perspectives. J. Ethnopharmacol. 2011;137:1486–1491. doi: 10.1016/j.jep.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 76.Prista L.N., De Almeida e Silva L., Alves A.C. Phytochemical study of the barks and leaves of Terminalia macroptera. Garcia de Orta. 1962;10:501–509. [Google Scholar]
- 77.Nongonierma R., Proliac A., Raynaud J. Two mono-C-glycosyl flavonoids from the flowers of Terminalia macroptera Guill. et Perr. (Combretaceae) Pharmazie. 1987;42:871–872. [Google Scholar]
- 78.Nongonierma R., Proliac A., Raynaud J. Vitexin and isovitexin in the flowers of Terminalia macroptera Guill. et Perr. (Combretaceae) Pharmazie. 1988;43:293. [Google Scholar]
- 79.Kone D., Diop B., Diallo D., Djilani A., Dicko A. Identification, quantitative determination, and antioxidant properties of polyphenols of some Malian medicinal plant parts used in folk medicine. In: Uddin J., editor. Macro to Nano Spectroscopy. Intech; Rijeka, Croatia: 2012. pp. 131–142. [Google Scholar]
- 80.Nongonierma R., Proliac A., Raynaud J. O-glycosyl flavonoids of flowers from Terminalia macroptera Guill. et Perr. (Combretaceae) Pharm. Acta Helv. 1990;65:233–235. [Google Scholar]
- 81.Karou S.D., Tchacondo T., Tchibozo M.A.D., Anani K., Ouattara L., Simpore J., De Souza C. Screening Togolese medicinal plants for few pharmacological properties. Pharmacogn. Res. 2012;4:116–122. doi: 10.4103/0974-8490.94737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Conrad J., Vogler B., Klaiber I., Reeb S., Guse J.H., Roos G., Kraus W. Vanillic acid 4-O-β-d-(6′-O-galloyl) glucopyranoside and other constituents from the bark of Terminalia macroptera Guill. et Perr. Nat. Prod. Lett. 2001;15:35–42. doi: 10.1080/10575630108041255. [DOI] [PubMed] [Google Scholar]
- 83.Silva O., Gomes E.T., Wolfender J.L., Marston A., Hostettmann K. Application of high performance liquid chromatography coupled with ultraviolet spectroscopy and electrospray mass spectrometry to the characterization of ellagitannins from Terminalia macroptera roots. Pharm. Res. 2000;17:1396–1401. doi: 10.1023/A:1007598922712. [DOI] [PubMed] [Google Scholar]
- 84.Conrad J., Vogler B., Reeb S., Klaiber I., Papajewski S., Roos G., Vasquez E., Setzer M.C., Kraus W. Isoterchebulin and 4,6-O-isoterchebuloyl-d-glucose, novel hydrolyzable tannins from Terminalia macroptera. J. Nat. Prod. 2001;64:294–299. doi: 10.1021/np000506v. [DOI] [PubMed] [Google Scholar]
- 85.Idemudia O.G. Terpenoids of Nigerian Terminalia species. Phytochemistry. 1970;9:2401–2402. doi: 10.1016/S0031-9422(00)85748-9. [DOI] [Google Scholar]
- 86.Conrad J., Vogler B., Klaiber I., Roos G., Walter U., Kraus W. Two triterpene esters from Terminalia macroptera bark. Phytochemistry. 1998;48:647–650. doi: 10.1016/S0031-9422(98)00154-X. [DOI] [Google Scholar]
- 87.Zou Y.F., Zhang B.Z., Barsett H., Inngjerdingen K.T., Diallo D., Michaelsen T.E., Paulsen B.S. Complement fixing polysaccharides from Terminalia macroptera root bark, stem bark and leaves. Molecules. 2014;19:7440–7458. doi: 10.3390/molecules19067440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zou Y.F., Zhang B.Z., Inngjerdingen K.T., Barsett H., Diallo D., Michaelsen T.E., Paulsen B.S. Complement activity of polysaccharides from three different plant parts of Terminalia macroptera extracted as healers do. J. Ethnopharmacol. 2014;155:672–678. doi: 10.1016/j.jep.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 89.Zou Y.F., Barsett H., Ho G.T.T., Inngjerdingen K.T., Diallo D., Michaelsen T.E., Paulsen B.S. Immunomodulating pectins from root bark, stem bark and leaves of the Malian medicinal plant Terminalia macroptera: Structure activity relations. Carbohydr. Res. 2015;403:167–173. doi: 10.1016/j.carres.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 90.Silva O., Duarte A., Cabrita J., Pimentel M., Diniz A., Gomes E. Antimicrobial activity of Guinea-Bissau traditional remedies. J. Ethnopharmacol. 1996;50:55–59. doi: 10.1016/0378-8741(95)01323-7. [DOI] [PubMed] [Google Scholar]
- 91.Silva O., Duarte A., Pimentel M., Viegas S., Barroso H., Machado J., Pires I., Cabrita J., Gomes E. Antimicrobial activity of Terminalia macroptera root. J. Ethnopharmacol. 1997;57:203–207. doi: 10.1016/S0378-8741(97)00068-8. [DOI] [PubMed] [Google Scholar]
- 92.Silva O., Ferreira E., Vaz Pato M., Gomes E. Guinea-Bissau’s plants: In vitro susceptibility studies on Neisseria gonorrheae. Int. J. Pharmacogn. 1997;35:323–328. doi: 10.1080/09251619708951276. [DOI] [Google Scholar]
- 93.Silva O., Viegas S., De Mello-Sampayo C., Costa M.J.P., Serrano R., Cabrita J., Gomes E.T. Anti-Helicobacter pylori activity of Terminalia macroptera root. Fitoterapia. 2012;83:872–876. doi: 10.1016/j.fitote.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 94.Batawila K., Kokou K., Koumaglo K., Gbéassor M., De Foucault B., Bouchet P., Akpagana K. Antifungal activities of five Combretaceae used in Togolese traditional medicine. Fitoterapia. 2005;76:264–268. doi: 10.1016/j.fitote.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 95.Traoré M.S., Baldé M.A., Camara A., Baldé E.S., Diané S., Diallo M.S.T., Keita A., Cos P., Maes L., Pieters L., et al. The malaria co-infection challenge: An investigation into the antimicrobial activity of selected Guinean medicinal plants. J. Ethnopharmacol. 2015;174:576–581. doi: 10.1016/j.jep.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 96.Traore Y., Ouattara K., Ouattara A., Méité S., Bagré I., Konan K.F., Coulibaly A., Nathalie K.G. Evaluation of the antistaphylococcic activity of Terminalia macroptera Guill et Perr (Combretaceae) stem bark extracts. Am. J. Biosci. 2015;3:221–225. [Google Scholar]
- 97.Traore M.S., Diane S., Diallo M.S.T., Balde E.S., Balde M.A., Camara A., Diallo A., Keita A., Cos P., Maes L., et al. In vitro antiprotozoal and cytotoxic activity of ethnopharmacologically selected Guinean plants. Planta Med. 2014;80:1340–1344. doi: 10.1055/s-0034-1383047. [DOI] [PubMed] [Google Scholar]
- 98.McLaughlin J.L. Crown gall tumours on potato discs and brine shrimp lethality: Two simple bioassays for higher plant screening and fractionation. In: Hostettmann K., editor. Methods in Plant Biochemistry. Volume 6. Academic Press; London, UK: 1991. pp. 1–32. [Google Scholar]
- 99.Zou Y.F., Ho G.T.T., Malterud K.E., Le N.H.T., Inngjerdingen K.T., Barsett H., Diallo D., Michaelsen T.E., Paulsen B.S. Enzyme inhibition, antioxidant and immunomodulatory activities, and brine shrimp toxicity of extracts from the root bark, stem bark and leaves of Terminalia macroptera. J. Ethnopharmacol. 2014;155:1219–1226. doi: 10.1016/j.jep.2014.07.004. [DOI] [PubMed] [Google Scholar]