Abstract
Because of their high sensitivity, cost-efficiency, and great potential as point-of-care biodiagnostic devices, plasmonic biosensors based on localized surface plasmon resonance have gained immense attention. However, most plasmonic biosensors and conventional bioassays rely on natural antibodies, which are susceptible to elevated temperatures and nonaqueous media. Hence, an expensive and cumbersome “cold chain” system is necessary to preserve the labile antibodies by maintaining optimal cold temperatures during transport, storage, and handling. Herein, we introduce a facile approach to preserve the antibody activity on a biosensor surface even at elevated temperatures. We show that silk fibroin film could be used as a protective layer to preserve the activity of a model antibody (Rabbit IgG) and cardiac troponin antibody at both room temperature and 40 °C over several days. Furthermore, a simple aqueous rinsing process restores the biofunctionality of the biosensor. This energy-efficient and environmentally friendly method represents a novel approach to eliminate the cold chain and temperature-controlled packing of diagnostic reagents and materials, thereby extending the capability of antibody-based biosensors to different resource-limited circumstances such as developing countries, an ambulance, an intensive care unit emergency room, and battlefield.
Keywords: localized surface plasmon resonance, plasmonic biosensor, biopreservation, silk, gold nanorods
Graphical abstract
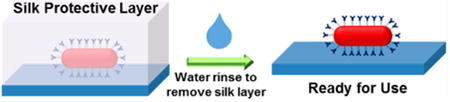
Introduction
Plasmonic biosensors based on refractive index sensitivity of localized surface plasmon resonance (LSPR) are considered to be highly promising for sensitive and cost-effective biodiagnostics in point-of-care and resource-limited settings.1 Similar to most of the conventional bioassays such as enzyme-linked immunosorbent assay (ELISA), immunoblotting, and immunoprecipitation assays, plasmonic biosensors mainly rely on natural antibodies as the recognition elements due to their high specificity and selectivity.2–5 Unfortunately, because of the poor stability of antibodies at elevated temperatures and in nonaqueous media (e.g., biosensor surface), natural antibodies are prone to lose their biofunctionality. Thus, it is imperative to maintain diagnostic reagents and biosensor chips within a specific refrigeration temperature range during transportation and storage.6 Hence, an extensive distribution network of refrigeration, the “cold chain”, is necessary to maintain an optimal temperature during transport, storage, and handling. Apart from being energy-intensive and expensive, a cold chain system is simply not feasible in prehospital or certain resource-limited settings such as developing countries, disaster struck regions, and battle field.7
Silk fibroin, a natural protein extracted from domesticated silkworm (Bombyx mori) cocoons, has been widely used for biomedical applications due to its biocompatibility, programmable biodegradation, and excellent mechanical properties.8,9 In a pioneering effort, Kaplan and co-workers have shown that silk fibroin can be used as a protective matrix to stabilize antibiotics, vaccines and enzymes.6,10 In a recent report, polydiacetylene/IgG/silk blended ink was used for colorimetric bacterial sensing.11 A dip-coated silk layer has been demonstrated to be effective in preserving macroscale objects such as fruits.12 In the aforementioned studies, the biological and biochemical reagents were preserved by mixing them with silk fibroin in solution. In these cases, additional processing is required to release and purify silk-encapsulated compounds, and without affecting their activity.13 However, this approach cannot be employed to preserve the recognition capabilities of antibody-based plasmonic biosensors, considering that the antibodies have already been immobilized on solid surfaces (i.e., plasmonic nanotransducer) before storage. Therefore, these considerations highlight the need for an alternate approach that preserves the antibody activity on a biosensor surface in situ by virtue of silk protective function.
In this paper, we report for the first time a facile approach to preserve the activity of antibody on biosensor surfaces against ambient and elevated temperatures using silk fibroin. Instead of mixing antibody with silk fibroin, an ultrathin silk film was employed to protect antibodies that have been immobilized on the surface. Plasmonic biosensor based on refractive index sensitivity of LSPR is used to monitor the fabrication steps including antibody conjugation, silk film formation and removal (before use), as well as analyte detection. We show that silk film increases the stability of two different antibodies (goat anti-rabbit IgG and anti-cTnI) conjugated to gold nanorods at room temperature. A simple water rinsing step can quickly remove the silk protective layer before exposing the sensor surface to the analyte solution. The increased temporal and thermal stability combined with the ease of formation and removal of the protective layer makes the encapsulated antibody-based biosensors ideal for use in point-of-care and resource-limited settings (Figure 1). Furthermore, we demonstrate that the silk stabilizes the antibody possibly through dehydration at the antibody–silk interface, forming hydrogen bonds and hydrophobic interactions, and limiting the mobility of antibody chain thus slowing down the degradation process. By eliminating the cold chain requirement in transportation and storage and preserving sensor functionality in an environmentally friendly and energy-efficient fashion, this facile approach opens up novel avenues for antibody-based biosensors that can be deployed in resource-limited settings such as ambulance, developing countries, battlefield and patient's home.
Figure 1.
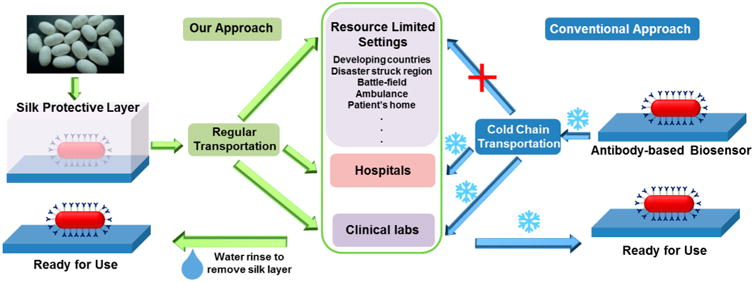
Schematic illustration depicting the concept of silk-encapsulated plasmonic biochips with enhanced thermal stability obviating the need for refrigerated transportation, handling, and storage of the plasmonic biochips.
Results and Discussions
Rabbit IgG and goat anti-Rabbit IgG (IgG and anti-IgG henceforth) were selected as model antibody and target bioanalyte, respectively, to establish the proof-of-concept. Because of their large refractive index sensitivity and excellent tunability of the LSPR wavelength, gold nanorods (AuNRs) have been employed as plasmonic nanotransducers. AuNRs were synthesized using a seed-mediated approach. From the TEM images, the length and diameter of AuNR (N > 100) were found to be 50.0 ± 2.4 nm and 18.4 ± 1.2 nm, respectively (Figure 2A).14 To conjugate AuNR with antibody, we initially derivatized the IgG molecules with a bifunctional polyethylene glycol (PEG) as described earlier.15 The AuNR are then functionalized with thiol-terminated PEG (SH-PEG) thus forming a link to immobilize the IgG on the AuNR surface via an Au–S bond. The flexible PEG chain increases the accessibility of IgG to target biomolecules and also forms a protein-resist layer around the AuNR surface to reduce nonspecific binding. The conjugation of SH-PEG-IgG on AuNR in solution resulted in a red shift of ∼8.3 nm in the longitudinal LSPR wavelength (Figure 2B). Subsequently, AuNR-IgG conjugates were adsorbed onto (3-mercaptopropyl)trimethoxysilane-modified glass substrates by incubating the glass substrates with AuNR-IgG conjugate solution followed by thorough rinsing with water to remove the weakly adsorbed nanorods. The biosensing ability of the plasmonic biosensor was demonstrated by using anti-IgG as a model bioanalyte, which is known to exhibit a strong and specific binding to IgG.16 A monotonic increase in the longitudinal LSPR shift was observed with an increasing concentration of the anti-IgG with a detection limit of 240 pg/mL (Figure 2C). The longitudinal LSPR wavelength red-shifted by ∼16.0 nm at the highest concentration (24 μg/mL) of anti-IgG tested here (Figure 2D). This concentration was employed to quantify the biorecognition ability of antibody (IgG) at elevated temperatures and extended incubation times in subsequent experiments.
Figure 2.
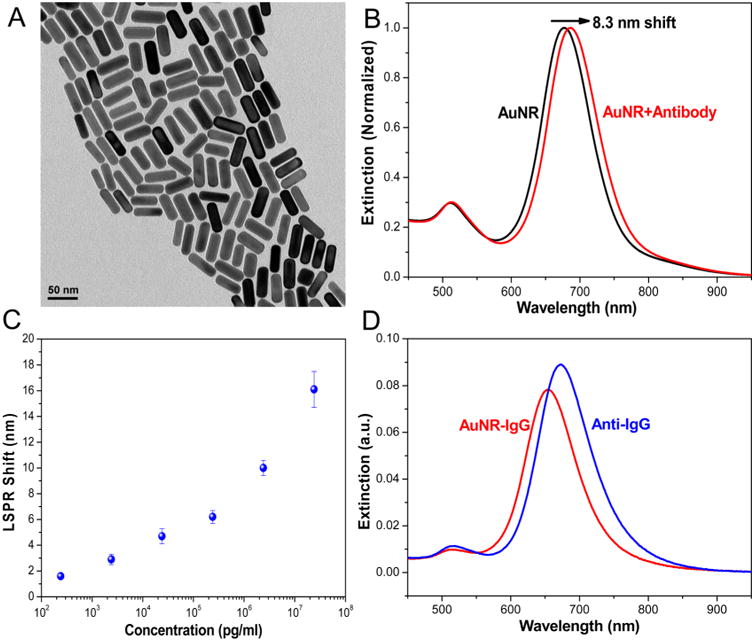
(A) TEM image of AuNRs used as plasmonic nanotransducers. The dimensions of the AuNRs were found to be 50 × 18 nm. (B) Extinction spectra showing the LSPR shift after conjugation of AuNR with IgG in solution. (C) LSPR shift of AuNR-IgG conjugates on glass substrates exposed to different concentrations of anti-IgG (mean ± standard deviation, N = 3) (D) Extinction spectra of AuNR-IgG conjugates on the glass substrate before (red) and after exposure to 24 μg/mL of anti-IgG (blue) showing a shift of ∼16 nm.
LSPR shift of AuNRs was used to monitor the deposition and removal of the silk film, as well as analyte (anti-IgG) binding after silk removal (Figure 3A, B). The LSPR wavelength exhibited a ∼ 70 nm red shift after deposition of the silk film. The silk film served as a protective layer for the antibody against elevated temperatures. After several days of storage at different temperatures, rinsing the substrate with nanopure water induced a ∼65 nm blue shift, suggesting that most of the silk film was removed. Subsequently, the rinsed substrate exhibited a red shift of ∼12.2 nm upon specific binding of anti-IgG (24 μg/mL) to IgG. Here, we used the percentage of retained sensitivity (%) to quantitatively evaluate the antibody preservation efficiency at different elevated temperatures after several days of storage. The retained sensitivity was calculated as the percentage of the red shift upon specific binding of anti-IgG (24 μg/mL) to IgG on a rinsed substrate after several days of storage at elevated temperatures compared to the red shift obtained from the same batch of freshly made substrate (which was considered as the reference sample tested instantly without silk coating process). For instance, as shown in Figure 3A, B, the red shift of ∼12.2 nm compared to the red shift of ∼15.8 nm obtained from the reference sample in the same batch corresponds to a retained sensitivity of ∼77%. In essence, after 3 days of storage at room temperature, ∼77% sensitivity of the biosensor was found to be preserved.
Figure 3.
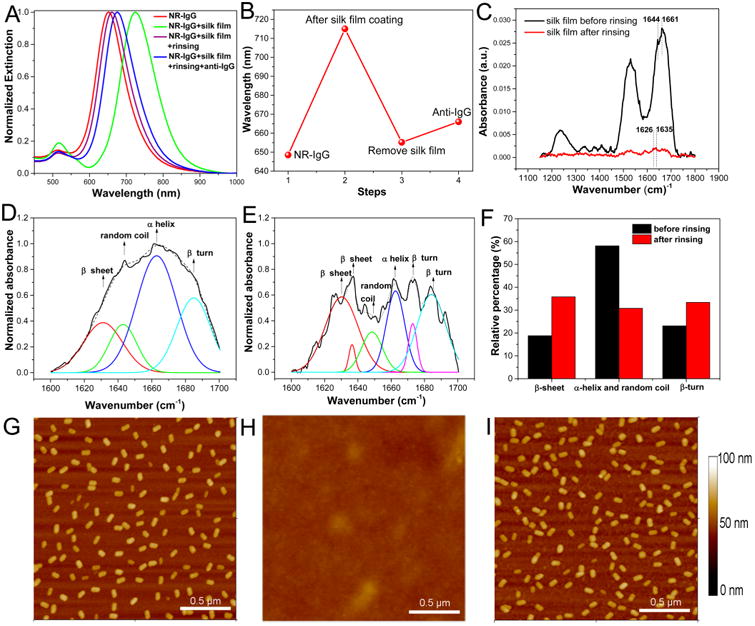
(A) Representative extinction spectra of AuNR-IgG conjugates on the glass substrate before (red) and after silk film coating (green), after rinsing silk film (purple) and after exposure to 24 μg/mL of anti-IgG (blue). (B) LSPR shift corresponding to each step in (A). (C) FTIR spectra of silk film before (black) and after rinsing (red) with water. (D) Peak deconvolution of amide I band of silk film before rinsing. (E) Peak deconvolution of amide I band of silk residue after rinsing. The black lines are the original FTIR spectra and the dotted lines are the fitted curves. (F) Histogram showing the relative content of different secondary structures of the silk before (black) and after rinsing (red). (G) AFM image showing uniformly adsorbed AuNR-IgG on glass substrate before silk film coating. (H) AFM image showing silk film deposited on AuNR-IgG conjugates adsorbed on glass substrate. (I) AFM image of the AuNR-IgG conjugates after rinsing the silk film with water evidencing the removal of most of the silk film.
Fourier transform infrared spectroscopy (FTIR) was employed to probe the conformation of the silk layer before and after rinsing (Figure 3C–E). Before rinsing, the infrared absorption peaks of random coil and α-helix in amide I band were observed at 1644 and 1661 cm−1, respectively, 17, 18 indicating that the coated silk film was predominantly a mixture of random coil and α-helix structures (Figure 3F). This was further confirmed by peak deconvolution of amide I band of the silk film, suggesting a structure with 58% α-helix and random coil, 19% β-sheet, and 23% β-turn. After rinsing, the peak intensity dropped significantly, indicating that most of the silk has been rinsed by water. Characteristic absorption bands of the residual silk were observed at 1626 and 1635 cm−1, indicating dominant β-sheet conformation.19 Peak deconvolution showed that the silk residue has a different structure compared to the film before rinsing with 36% β-sheet, 31% α-helix and random coil, and 33% β-turn. This secondary structure composition change is because most of the silk in α-helix and random coil conformation (which is water-soluble) is removed by water rinsing, whereas the small amount of insoluble β-sheet-rich silk at the substrate and AuNR surface remained bound on the surface. It is known that the spin-coating process induces a change in the secondary structure of silk (from silk I to silk II) at the interfacial regions (∼5 nm at substrate–film interface).20 It is possible that the residual silk observed here corresponds to the interfacial insoluble silk resulting from the spin coating process. Considering that silk I exhibits a transition to insoluble silk II state at elevated temperatures, it important to probe the possible conformational changes of the silk layer under incubation conditions tested here (40 °C). We have obtained FTIR spectra of deposited silk film on IgG conjugated AuNR at different time points over a 2 day incubation period at 40 °C (Figure S1). The FTIR spectra revealed no discernible changes in the shape and the relative intensity of various absorption bands indicating the absence of conformational changes in the silk film after incubation at 40 °C.
Atomic force microscopy (AFM) was performed to follow the silk coating and rinsing process. Before silk coating, AFM image revealed the uniform distribution of AuNR-IgG conjugates on the glass substrate (Figure 3G). After silk film deposition, the AuNRs were completely covered by the silk film as evidenced by the featureless silk film surface topography in the AFM image (Figure 3H). The complete coverage of AuNR bioconjugates with silk layer is further confirmed by AFM scratch test, which revealed the thickness of the silk film to be ∼120 nm (Figure S2). The thickness of the silk layer is nearly four times higher than the diameter of AuNR-IgG conjugates (∼22.8 nm), which ensures complete encapsulation of the bionanoconjugates. After rinsing the silk film with water, AFM images revealed the exposed AuNR-IgG conjugates (Figure 3I). It is worth noting that only a small amount of the silk residue was observed on the substrate after rinsing with nanopure water.
Before performing a detailed investigation of the efficacy of the silk layer in preserving the biorecognition of the antibody conjugated to AuNR under different storage conditions, it is important to understand the effect of the preservation process on the biosensor performance. An important aspect that needs to be addressed is the effect of silk deposition, rinsing and presence of residual silk on the specific recognition of antibody and the sensitivity of the plasmonic biosensor. To address this aspect, we deposited the silk film onto a freshly made AuNR-IgG adsorbed substrate and rinsed the silk film immediately. As noted above, a small amount of silk residue remained bound to the AuNR after rinsing. Following the exposure of the substrate to the analyte solution containing 24 μg/mL of anti-IgG, we noted a red shift of ∼16 nm, which is similar to the reference sample (Figure S3). The virtually complete preservation of the specific recognition capability of IgG-AuNR conjugates after subjecting the sensor to the preservation process (i.e., silk layer deposition and removal) indicates that the residual silk does not significantly block the binding sites of antibody and the steps involved in the preservation (spin-coating and rinsing) do not adversely affect the biosensor performance.
Considering the presence of residual silk on the AuNR after rinsing with water, it is also important to investigate the possible contribution of nonspecific adsorption of anti-IgG from the analyte solution on the silk residue. For this purpose, we employed bare AuNRs (without IgG conjugation) adsorbed on glass substrate, followed by the deposition of a silk film (Figure S4). Similar to AuNR-IgG bioconjugates, a small amount of silk remained on bare AuNRs after rinsing with water. However, the AuNRs exhibited no detectable shift upon exposure to 24 μg/mL of anti-IgG solution, implying that the red shift obtained on previous AuNR-IgG substrate stems from the specific binding between IgG and anti-IgG, rather than nonspecific interaction between residual silk (or AuNR) and anti-IgG. In addition, it should be noted that the rinsing step is critical to restore biofunctionality of the antibody-based biosensor. Otherwise, the thick film would cover the available binding sites of the antibody and eliminate the analyte detection capability. Considering the use of glycerol as antibody stabilizer in the buffer,21 we have investigated the effect of glycerol in silk solution. Glycerol resulted in the crystallization of silk film making it insoluble in water.22 Thus, most of the available binding sites of antibodies were covered by the insoluble silk film and could not capture anti-IgG in the analyte solution (Figure S5). These results further confirm the importance of near complete removal of the protective layer to restore the antibody based biosensor before using for biosensing.
Now we turn our attention to the efficacy of the silk layer in preserving the biorecognition ability of the antibody and sensitivity of plasmonic biosensor substrates. For this purpose, silk coated substrates were stored at room temperature and 40 °C for 1 week. Substrates were sampled at different time points to monitor the changes in the sensitivity of plasmonic biochip and the biorecognition ability of the antibody (Figure 4A). Substrates coated with silk exhibited only a ∼ 20% loss in sensitivity after storage at room temperature (25 °C) for 2 days as opposed to the nearly 90% loss in sensitivity for substrates without silk protective layer. After 1 week of storage at room temperature, substrates coated with silk retained ∼70% sensitivity. At 40 °C, silk-coated sensor substrates retained ∼70% sensitivity after 2 days and 42% sensitivity after 1 week. In contrast, substrates without a silk protective layer lost over 90% of sensitivity within the first day at the same temperature.
Figure 4.
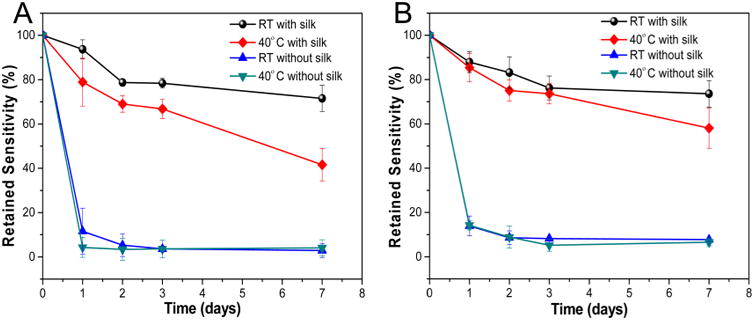
(A) Retained sensitivity of AuNR-IgG conjugates over 7 days at room temperature and 40 °C. (B) Retained sensitivity of AuNR-anti cTnI conjugates over 7 days at room temperature and 40 °C. Control experiments (without silk coating) show antibody on AuNR lost 90% of sensitivity in 2 days at room temperature and 40 °C (mean ± standard deviation of three measurements obtained from samples from three different batches).
Next, to assess the versatility of this approach and applicability of silk protection to clinically relevant biodiagnostics, we chose cardiac troponin I (cTnI) as the target biomarker. It is widely accepted that the concentration of troponin (cTnI) in blood serum serves as a highly sensitive and specific biomarker for the detection of myocardial damage and for risk stratification in such patients.23 The troponin concentration has been considered to be an important biomarker for the detection of myocardial damage, muscular fatigue, and hypoxia.24,25 Recent reports have demonstrated plasmonic biosensors based on natural antibodies as recognition elements.26 In our previous report, we have shown that troponin antibody based biosensor looses ∼60% sensitivity within 2 days even under storage at 4 °C.27 A similar time-lapse experiment was conducted to investigate the sensitivity of troponin antibody based plasmonic biosensor at different temperatures (Figure 4B). With the silk protective layer, troponin antibody-based biosensor also retained more than 70% sensitivity after 1 week storage at room temperature. At 40 °C, the biosensor exhibited 48% sensitivity, which is slightly higher compared to anti-IgG biosensor described above. In contrast, samples without silk film protection lost activity quickly at both temperatures. Apart from the generality of the silk protection approach, these results demonstrate the feasibility of enhancing the stability and preserving the sensitivity of a clinically relevant biosensor.
Furthermore, in order to demonstrate the generality of the approach (i.e., beyond the ability to protect the specific recognition ability of antibody in biosensing context), enzyme bound nanostructures were coated with silk film and incubated at 40 °C for 1 day. The well-studied horseradish peroxidase (HRP)—2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) enzyme–substrate system was used here. After rinsing the silk film, the biocatalytic activity of immobilized HRP was quantified by following ABTS oxidation in the presence of H2O2, which can be monitored by following the optical absorbance at 405 nm. With the silk film protection, the HRP retained ∼70% activity after 1 day storage at 40 °C, whereas control sample without silk film protection completely lost the enzymatic activity (Figure S6). This experiment further demonstrates the versatility of this approach to preserve different types of labile biomolecule recognition and catalytic activity in immobilized condition.
Finally, we turn our attention to the mechanistic aspects of the silk-based preservation of the bioactivity. So far, two contradictory mechanisms have been proposed. According to one of the hypotheses, proteins are more stable and retain their activity when surrounded by a hydration layer and form hydrogen bonds with these water molecules. The hydration layer of the proteins has a higher density and order than that of bulk water. The higher density and highly ordered water layer is slower to exchange and thus stabilizes the hydrophobic interactions of a folded protein through an increase in the solvent entropic penalty upon unfolding.28,29 In contrast, the other theory holds that the encapsulating matrix acts as a substitute to water.13,30 Hydrogen bonds between protein and water are replaced with hydrogen bonding formed between the stabilizing material and protein during the drying process. The hydrogen bonds between matrix and protein tend to drive the water out and restrict the mobility of the biomacromolecule, thus impeding denaturation pathways under unfavorable environments.
To elucidate the possible mechanism of silk preservation in this work, we designed a control experiment to determine whether or not the water molecules in the matrix play a favorable role in the preservation of antibody recognition capabilities. Hyaluronic acid (HA), a hydrophilic proteoglycan in the extracellular matrix of some tissues, has a high capacity for water-sorption and retention.31 Similar to silk, a HA film was deposited onto AuNR-IgG conjugates adsorbed substrates, leading to ∼70 nm red shift in the LSPR wavelength of AuNR. AFM scratch test further confirmed the complete coverage of the biochip surface with ∼80 nm thick film (Figure S7). After storing for 1 day at room temperature, most of the HA could also be removed by water rinsing (Figure 5A). However, upon exposure to the target analyte (anti-IgG at a concentration of 24 μg/mL), we observed only ∼1 nm red shift, suggesting over 90% loss in the sensitivity and biorecognition ability underneath the HA film within 1 day (Figure 5B). Considering the water-enriched environment created by HA film, this experiment suggests that the “hydration layer hypothesis” may not be tenable to rationalize the silk preservation mechanism here.
Figure 5.
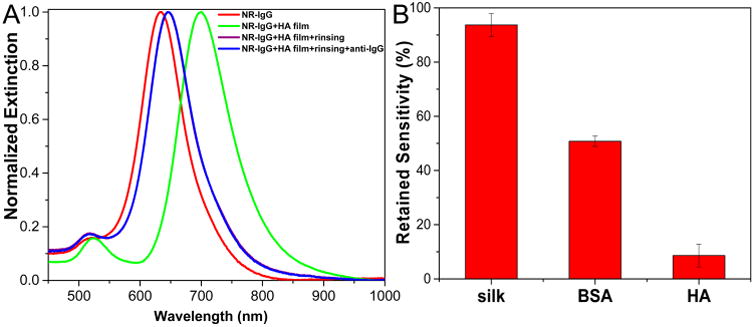
(A) Extinction spectra of AuNR-IgG conjugates on the glass substrate before (red) and after hyaluronic acid film coating (green), after hyaluronic acid film rinsing (purple) and after binding exposure to 24 μg/mL of anti-IgG (blue). (B) Histogram showing the comparison of retained sensitivity of IgG-AuNR conjugates underneath silk, BSA, and HA films after 1 day storage at room temperature (mean ± standard deviation of three measurements obtained from samples from three different batches).
The water-enriched environment might provide the antibody chain more free space to move and unfold, leading to the loss in activity. This result is consistent with a previous report, wherein excess moisture introduced to the silk film lead to a higher loss in the activity of a vaccine stabilized by silk.6 It should be noted that relative humidity of storage environment could be another factor that might affect the activity of antibody.6 In the present study, all the samples were stored in sealed containers after fabrication to minimize the influence of relative humidity variations in the standard laboratory environment.
The second theory involving “water replacement” by the matrix appears to be the possible preservation mechanism for plasmonic biosensor studies here. To further test this hypothesis, we used a globular protein, bovine serum albumin (BSA), as the protective layer. There was only ∼50% retained sensitivity after 1 day of storage at room temperature, ∼40% lower than silk protected IgG activity (Figure 5B and Figure S8). This observation can be rationalized by comparing the amino acid composition of silk fibroin with BSA. Silk fibroin has larger amount of alanine (30%) and glycine (43%),32 compared to BSA with only 6% alanine and 2% glycine.33 Since the side chains of alanine and glycine have a low hydration potential, the protein matrices containing high levels of alanine and glycine residues are prone to drive water out of the hydration layer and form hydrogen bonds between protein matrices (silk) and protected protein (antibody).34 In other words, silk is better suited to exclude water and form hydrogen bonding with the antibody when compared with BSA. A similar phenomenon was reported previously wherein silk had better performance than BSA in terms of virus stabilization.29 This experiment further supports the second hypothesis, in which hydrogen bonds limit the mobility of antibody and stabilized the antibody. At the same time, one should not exclude the possibility of hydrophobic interaction between silk and antibody considering that silk heavy chain comprises 12 repeating hydrophobic blocks connected by 11 relatively short hydrophilic spacers and large hydrophilic N- and C-termini ends.13 The stabilization of antibody may indeed be due to the combination of hydrogen bonding and hydrophobic interaction between silk and protein.
Conclusions
In conclusion, we have introduced a facile approach to preserve the antibody activity on the biosensor surface at elevated temperatures. We showed that silk film can be used as a protective layer to preserve the biorecognition capability of IgG and anti-troponin at both room temperature and 40 °C over several days. The possible preservation mechanism of silk is related to the formation of hydrogen bonds between silk matrix and antibody, and dehydration of the interface thus limiting the mobility of the antibody. This energy-efficient and environmentally friendly approach provides a novel approach to eliminate the cold chain system during antibody-based biosensor transportation and storage. Furthermore, a simple water rinsing step restores the biofunctionality of the biosensor, which can be used as a diagnostic device under different circumstances such as an ambulance, intensive care unit, emergency department, battlefield, and home use. The approach demonstrated here can be easily extended to numerous other biosensing platforms considering the simple water-based deposition and the removal of the protective layer.
Methods
Materials
Cetyltrimethylammonium bromide (CTAB), chloroauric acid, ascorbic acid, sodium borohydride, (3-mercaptopropyl)-triethoxy-silane (MPTES), horseradish peroxidase (HRP, type VI-A), hydrogen peroxide, and 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS, 10 mg/tablet) were purchased from Sigma-Aldrich. Silver nitrate was purchased from VWR international. 1-Ethyl-3-(3-(dimethylamino)propyl) carbodiimide (EDC) and N-hydroxy succinimide (NHS), Rabbit IgG, and Goat anti-Rabbit IgG (Mw = 150 kDa) were purchased from Thermo scientific. SH-PEG-COOH (Mw = 5000 g/mol) was purchased from Jenkem Technology. Recombinant human cardiac troponin-I was purchased from Life Diagnostics. Human cardiac troponin I antibody (H-41, Mw = 150 kDa) was obtained from Santa Cruz Biotechnology. All the chemicals have been used as received with no further purification.
Synthesis of Gold Nanorods (AuNRs)
Gold nanorods were synthesized using a seed-mediated approach. Seed solution was prepared by adding 0.6 mL of an ice-cold sodium borohydride solution (10 mM) into 10 mL of 0.1 M CTAB and 2.5 × 10−4 M chloroauric acid solution under vigorous stirring at room temperature. The color of the seed solution changed from yellow to brown. Growth solution was prepared by mixing 95 mL of CTAB (0.1 M), 0.5 mL of silver nitrate (10 mM), 5 mL of chloroauric acid (10 mM), and 0.55 mL of ascorbic acid (0.1 M) in the same order. The solution was homogenized by gentle stirring. To the resulting colorless solution, 0.12 mL of freshly prepared seed solution was added and set aside in dark for 14 h. Prior to use, the AuNR solution was centrifuged twice at 10k rpm for 8 min to remove excess CTAB and redispersed in nanopure water (18.2 MΩ cm).
AuNR-IgG and AuNR-Troponin Antibody Conjugate Preparation
To a solution of SH-PEG-COOH in water (37.5 μL, 20 μM), EDC, and NHS with the same molar ratio as SH-PEG-COOH were added followed by shaking for 1 h. The pH of the above reaction mixture was adjusted to 7.4 by adding 10 × concentrated phosphate buffered saline (PBS), followed by the addition of rabbit IgG (10 μL, 75 μM). The reaction mixture was incubated for 2 h, and then filtered to remove any byproduct during the reaction using centrifuge tube with 50 kDa filter. The final SH-PEG-IgG conjugates solution (0.75 μM) was obtained after washing with PBS buffer (pH 7.4) twice. AuNR-IgG conjugates solution was prepared by adding 50 μL of SH-PEG-IgG conjugates solution to 1 mL of twice-centrifuged AuNR solution followed by incubation for 1 h.
The preparation of AuNR-troponin antibody conjugates is similar to AuNR-IgG. A solution was prepared by adding 67 μL of SH-PEG-COOH in water (2 μM) with the same molar ratio of EDC and NHS, followed by shaking for 1 h. The pH of the above reaction was adjusted to 7.4, followed by the addition of 100 μL of troponin antibody (1.34 μM). The reaction mixture was incubated for additional 2 h and filtered to remove excess chemicals and byproducts by centrifugation using a centrifuge tube with 50 kDa filter. The AuNR-conjugate mixture was obtained by adding 6 μL of SH-PEG-antibody conjugate of concentration 1.34 μM to 1 mL of twice-centrifuged nanorods.
AuNR-HRP Conjugates Reparation and Enzyme Activity
HRP solution of 2.5 mg/mL in 100 mM potassium phosphate buffer (pH 5) was added to one time centrifuged AuNR solution and incubated at 4 °C overnight. The unbound HRP was removed by filtration at 3500 rpm using a 100 kDa filter. The HRP-AuNR conjugates were drop casted onto MPTES modified glass substrates and incubated for 3 h. Subsequently, the glass substrate was rinsed with water to remove weakly bound nanostructures. HRP-AuNR conjugates coated glass substrate were exposed to 300 μL of ABTS solution (9.1 mM in phosphate buffer, pH 5.0) followed by the addition of 100 μL of 0.3% H2O2. The absorbance at 405 nm is recorded as a function of time.
Adsorption of AuNR-IgG on Glass Surface
Glass substrates were cut into approximately 1×2 cm rectangular slides and cleaned in piranha solution (3:1 (v/v) mixture of H2SO4 and 30% H2O2) followed by extensive rinsing with nanopure water (Caution: Piranha solution is extremely dangerous and proper care needs to be executed in handling and disposal). AuNR-IgG conjugates were adsorbed onto glass substrates following the modification of the surface with MPTES by exposing the glass surface to 1% MPTES in ethanol for 1 h followed by ultrasonication in ethanol for 20 min and rinsing with water. Subsequently, the glass surface was exposed to AuNR-IgG conjugates solution for 3 h, followed by rinsing with water to remove the loosely bound nanorods. A similar approach was employed for adsorption of bare AuNR, AuNR-troponin antibody conjugates, and AuNR-HRP conjugates on glass surfaces.
Silk Film Deposition and Rinsing
Silk fibroin was reconstituted from Bombyx mori silkworm cocoon according to a reported protocol.35 The degumming time was performed for 30 min. The final concentration of silk fibroin was measured to be 4% (w/v). To fabricate silk films, 100 μL of silk fibroin solution was added onto glass adsorbed with AuNR-IgG conjugates and spun (model WS-400, Laurell Technologies Corporation) at 3000 rpm for 30 s. For rinsing, the silk-coated glass substrate was gently rinsed by nanopure water for 5 min.
Characterization
UV–vis extinction spectra were collected in air with a Shimadzu UV-1800 UV–vis spectrometer. Transmission electron microscopy (TEM) micrographs were recorded on a JEM-2100F (JEOL) field emission instrument. Samples were prepared by drying a drop of the solution on a carbon-coated grid, which had been previously made hydrophilic by glow discharge. Atomic force microscopy (AFM) images were obtained using Dimension 3000 (Digital instruments) AFM in light tapping mode. Fourier transform infrared spectroscopy (FTIR) measurements were performed using a Nicolette Nexus 470 spectrometer. The spectral data range was 400– 4000 cm−1 collected for an average of 1024 scans at a resolution of 1 cm−1. The amide I band from 1600 to 1700 cm−1 was deconvoluted using Gaussian curves in OriginPro 8.6 (Origin-Lab Corp.).
Supplementary Material
FTIR spectra of silk film at 40 °C; AFM scratch experiments measuring silk and HA film thickness; LSPR spectra of control experiments; HRP activity measurement (PDF)
Acknowledgments
We acknowledge support from Air Force Office of Scientific Research (FA9550-15-1-0228 and 12RX11COR), AFRL/711 HPW, and National Institutes of Health (R21DK100759 and R01 CA141521). The authors acknowledge the Nano Research Facility (NRF) at Washington University for providing access to electron microscopy facilities. We thank Ms. Beth Miller for technical help and Mr. Qisheng Jiang for TEM imaging.
Footnotes
Supporting Information: The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsami.6b07362.
Notes: The authors declare no competing financial interest.
References
- 1.Anker JN, Hall WP, Lyandres O, Shah NC, Zhao J, Van Duyne RP. Biosensing with Plasmonic Nanosensors. Nat Mater. 2008;7:442–453. doi: 10.1038/nmat2162. [DOI] [PubMed] [Google Scholar]
- 2.Johnson AJ, Martin DA, Karabatsos N, Roehrig JT. Detection of Anti-arboviral Immunoglobulin G by Using a Monoclonal Antibody-based Capture Enzyme-linked Immunosorbent Assay. J Clin Microbiol. 2000;38:1827–1831. doi: 10.1128/jcm.38.5.1827-1831.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Endo T, Kerman K, Nagatani N, Hiepa HM, Kim DK, Yonezawa Y, Nakano K, Tamiya E. Multiple Label-free Detection of Antigen-antibody Reaction Using Localized Surface Plasmon Resonance-based Core-shell Structured Nanoparticle Layer Nanochip. Anal Chem. 2006;78:6465–6475. doi: 10.1021/ac0608321. [DOI] [PubMed] [Google Scholar]
- 4.Ashley RL, Militoni J, Lee F, Nahmias A, Corey L. Comparison of Western Blot (Immunoblot) and Glycoprotein G-specific Immunodot Enzyme Assay for Detecting Antibodies to Herpes Simplex Virus Type-1 and Type-2 in Human Sera. J Clin Microbiol. 1988;26:662–667. doi: 10.1128/jcm.26.4.662-667.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scully R, Chen JJ, Plug A, Xiao YH, Weaver D, Feunteun J, Ashley T, Livingston DM. Association of BRCA1 with Rad51 in Mitotic and Meiotic Cells. Cell. 1997;88:265–275. doi: 10.1016/s0092-8674(00)81847-4. [DOI] [PubMed] [Google Scholar]
- 6.Zhang JE, Pritchard E, Hu X, Valentin T, Panilaitis B, Omenetto FG, Kaplan DL. Stabilization of Vaccines and Antibiotics in Silk and Eliminating The Cold Chain. Proc Natl Acad Sci U S A. 2012;109:11981–11986. doi: 10.1073/pnas.1206210109. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Wolfson LJ, Gasse F, Lee-Martin SP, Lydon P, Magan A, Tibouti A, Johns B, Hutubessya R, Salama P, Okwo-Bele JM. Estimating The Costs of Achieving The WHO-UNICEF Global Immunization Vision and Strategy, 2006-2015. Bull World Health Organ. 2008;86:27–39. doi: 10.2471/BLT.07.045096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tao H, Kaplan DL, Omenetto FG. Silk Materials - A Road to Sustainable High Technology. Adv Mater. 2012;24:2824–2837. doi: 10.1002/adma.201104477. [DOI] [PubMed] [Google Scholar]
- 9.Yucel T, Lovett ML, Kaplan DL. Silk-based Biomaterials for Sustained Drug Delivery. J Controlled Release. 2014;190:381–397. doi: 10.1016/j.jconrel.2014.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu Q, Wang XQ, Hu X, Cebe P, Omenetto F, Kaplan DL. Stabilization and Release of Enzymes from Silk Films. Macromol Biosci. 2010;10:359–368. doi: 10.1002/mabi.200900388. [DOI] [PubMed] [Google Scholar]
- 11.Tao H, Marelli B, Yang MM, An B, Onses MS, Rogers JA, Kaplan DL, Omenetto FG. Inkjet Printing of Regenerated Silk Fibroin: From Printable Forms to Printable Functions. Adv Mater. 2015;27:4273–4279. doi: 10.1002/adma.201501425. [DOI] [PubMed] [Google Scholar]
- 12.Marelli B, Brenckle MA, Kaplan DL, Omenetto FG. Silk Fibroin as Edible Coating for Perishable Food Preservation. Sci Rep. 2016;6:25263. doi: 10.1038/srep25263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li AB, Kluge JA, Guziewicz NA, Omenetto FG, Kaplan DL. Silk-based Stabilization of Biomacromolecules. J Controlled Release. 2015;219:416–430. doi: 10.1016/j.jconrel.2015.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orendorff CJ, Gearheart L, Jana NR, Murphy CJ. Aspect Ratio Dependence on Surface Enhanced Raman Scattering Using Silver and Gold Nanorod Substrates. Phys Chem Chem Phys. 2006;8:165–170. doi: 10.1039/b512573a. [DOI] [PubMed] [Google Scholar]
- 15.Tian LM, Morrissey JJ, Kattumenu R, Gandra N, Kharasch ED, Singamaneni S. Bioplasmonic Paper as a Platform for Detection of Kidney Cancer Biomarkers. Anal Chem. 2012;84:9928–9934. doi: 10.1021/ac302332g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian LM, Chen E, Gandra N, Abbas A, Singamaneni S. Gold Nanorods as Plasmonic Nanotransducers: Distance-Dependent Refractive Index Sensitivity. Langmuir. 2012;28:17435–17442. doi: 10.1021/la3034534. [DOI] [PubMed] [Google Scholar]
- 17.Lu Q, Hu X, Wang XQ, Kluge JA, Lu SZ, Cebe P, Kaplan DL. Water-insoluble Silk Films with Silk I Structure. Acta Biomater. 2010;6:1380–1387. doi: 10.1016/j.actbio.2009.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim S, Marelli B, Brenckle MA, Mitropoulos AN, Gil ES, Tsioris K, Tao H, Kaplan DL, Omenetto FG. All-water-based Electron-beam Lithography Using Silk as a Resist. Nat Nanotechnol. 2014;9:306–310. doi: 10.1038/nnano.2014.47. [DOI] [PubMed] [Google Scholar]
- 19.Hu X, Shmelev K, Sun L, Gil ES, Park SH, Cebe P, Kaplan DL. Regulation of Silk Material Structure by Temperature-Controlled Water Vapor Annealing. Biomacromolecules. 2011;12:1686–1696. doi: 10.1021/bm200062a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang C, Wang X, Gunawidjaja R, Lin YH, Gupta MK, Kaplan DL, Naik RR, Tsukruk VV. Mechanical Properties of Robust Ultrathin Silk Fibroin Films. Adv Funct Mater. 2007;17:2229–2237. [Google Scholar]
- 21.Dunn SD. Effects of The Modification of Transfer Buffer Composition and The Renaturation of Proteins in Gels on The Recognition of Proteins on Western Blots by Monoclonal Antibodies. Anal Biochem. 1986;157:144–153. doi: 10.1016/0003-2697(86)90207-1. [DOI] [PubMed] [Google Scholar]
- 22.Lu SZ, Wang XQ, Lu Q, Zhang XH, Kluge JA, Uppal N, Omenetto F, Kaplan DL. Insoluble and Flexible Silk Films Containing Glycerol. Biomacromolecules. 2010;11:143–150. doi: 10.1021/bm900993n. [DOI] [PubMed] [Google Scholar]
- 23.Thygesen K, et al. Third Universal Definition of Myocardial Infarction. J Am Coll Cardiol. 2012;60:1–18. doi: 10.1016/j.jacc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 24.Thygesen K, et al. Third Universal Definition of Myocardial Infarction. Circulation. 2012;126:2020–2033. doi: 10.1161/CIR.0b013e31826e1058. [DOI] [PubMed] [Google Scholar]
- 25.Debold EP. Recent Insights into the Molecular Basis of Muscular Fatigue. Med Sci Sports Exercise. 2012;44:1440–1452. doi: 10.1249/MSS.0b013e31824cfd26. [DOI] [PubMed] [Google Scholar]
- 26.Tang L, Casas J. Quantification of Cardiac Biomarkers Using Label-free and Multiplexed Gold Nanorod Bioprobes for Myocardial Infarction Diagnosis. Biosens Bioelectron. 2014;61:70–75. doi: 10.1016/j.bios.2014.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tadepalli S, Kuang ZF, Jiang QS, Liu KK, Fisher MA, Morrissey JJ, Kharasch ED, Slocik JM, Naik RR, Singamaneni S. Peptide Functionalized Gold Nanorods for the Sensitive Detection of a Cardiac Biomarker Using Plasmonic Paper Devices. Sci Rep. 2015;5:16206. doi: 10.1038/srep16206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao Z, Fu JL, Dhakal S, Johnson-Buck A, Liu MH, Zhang T, Woodbury NW, Liu Y, Walter NG, Yan H. Nanocaged Enzymes with Enhanced Catalytic Activity and Increased Stability against Protease Digestion. Nat Commun. 2016;7:10619. doi: 10.1038/ncomms10619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sutherland TD, Sriskantha A, Church JS, Strive T, Trueman HE, Kameda T. Stabilization of Viruses by Encapsulation in Silk Proteins. ACS Appl Mater Interfaces. 2014;6:18189–18196. doi: 10.1021/am5051873. [DOI] [PubMed] [Google Scholar]
- 30.Fernandez A, Scheraga HA. Insufficiently Dehydrated Hydrogen Bonds as Determinants of Protein Interactions. Proc Natl Acad Sci U S A. 2003;100:113–118. doi: 10.1073/pnas.0136888100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mao JS, Liu HF, Yin YJ, Yao KD. The Properties of Chitosan-gelatin Membranes and Scaffolds Modified with Hyaluronic Acid by Different Methods. Biomaterials. 2003;24:1621–1629. doi: 10.1016/s0142-9612(02)00549-5. [DOI] [PubMed] [Google Scholar]
- 32.Vepari C, Kaplan DL. Silk as a Biomaterial. Prog Polym Sci. 2007;32:991–1007. doi: 10.1016/j.progpolymsci.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stein WH, Moore S. Amino Acid Composition of Beta-lactoglobulin and Bovine Serum Albumin. J Biol Chem. 1949;178:79–91. [PubMed] [Google Scholar]
- 34.Wolfenden R, Andersson L, Cullis PM, Southgate CCB. Affinities of Amino-Acid Side Chains For Solvent Water. Biochemistry. 1981;20:849–855. doi: 10.1021/bi00507a030. [DOI] [PubMed] [Google Scholar]
- 35.Rockwood DN, Preda RC, Yucel T, Wang XQ, Lovett ML, Kaplan DL. Materials Fabrication from Bombyx mori Silk Fibroin. Nat Protoc. 2011;6:1612–1631. doi: 10.1038/nprot.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FTIR spectra of silk film at 40 °C; AFM scratch experiments measuring silk and HA film thickness; LSPR spectra of control experiments; HRP activity measurement (PDF)


