Abstract
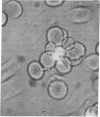
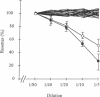
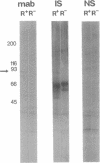
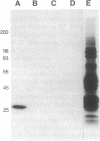
Cerebral involvement in Plasmodium falciparum malaria is associated with sequestration of infected red blood cells and occlusion of cerebral vessels. Adhesion of infected erythrocytes along the vascular endothelium as well as binding of uninfected erythrocytes to cells infected with late-stage asexual parasites (rosetting) may be important in erythrocyte sequestration. We report that the recently discovered rosetting phenomenon shares characteristics with other human cell-cell interactions (heparin sensitivity, temperature independence, Ca2+/Mg2+ and pH dependence). Mono- and polyclonal antibodies specific for PfHRP1, a histidine-rich protein present in the membrane of P. falciparum-infected erythrocytes, disrupt rosettes but do not affect attachment of infected erythrocytes to endothelial cells. The inhibitory anti-PfHRP1 antibodies reacted with rosetting parasites in indirect immunofluorescence and with P. falciparum polypeptides of Mr 28,000 and Mr 90,000 in immunoprecipitation and immunoblotting, respectively. No inhibitory effects on erythrocyte rosetting were obtained with antibodies to related histidine-rich or other antigens of P. lophurae or P. falciparum. Whether the epitope that mediates rosetting, and is recognized by the anti-PfHRP1 antibodies, is located on PfHRP1 or on a crossreactive antigen remains to be established. The results suggest that endothelial cytoadherence and erythrocyte rosetting involve different molecular mechanisms.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aikawa M. Human cerebral malaria. Am J Trop Med Hyg. 1988 Jul;39(1):3–10. doi: 10.4269/ajtmh.1988.39.3. [DOI] [PubMed] [Google Scholar]
- Anders R. F., Coppel R. L., Brown G. V., Kemp D. J. Antigens with repeated amino acid sequences from the asexual blood stages of Plasmodium falciparum. Prog Allergy. 1988;41:148–172. [PubMed] [Google Scholar]
- Berzins K., Perlmann H., Wåhlin B., Carlsson J., Wahlgren M., Udomsangpetch R., Björkman A., Patarroyo M. E., Perlmann P. Rabbit and human antibodies to a repeated amino acid sequence of a Plasmodium falciparum antigen, Pf 155, react with the native protein and inhibit merozoite invasion. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1065–1069. doi: 10.1073/pnas.83.4.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs B. A., Culvenor J. G., Ng J. S., Kemp D. J., Brown G. V. Plasmodium falciparum: cytoadherence of a knobless clone. Exp Parasitol. 1989 Aug;69(2):189–197. doi: 10.1016/0014-4894(89)90187-2. [DOI] [PubMed] [Google Scholar]
- David P. H., Handunnetti S. M., Leech J. H., Gamage P., Mendis K. N. Rosetting: a new cytoadherence property of malaria-infected erythrocytes. Am J Trop Med Hyg. 1988 Mar;38(2):289–297. doi: 10.4269/ajtmh.1988.38.289. [DOI] [PubMed] [Google Scholar]
- David P. H., Hommel M., Miller L. H., Udeinya I. J., Oligino L. D. Parasite sequestration in Plasmodium falciparum malaria: spleen and antibody modulation of cytoadherence of infected erythrocytes. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5075–5079. doi: 10.1073/pnas.80.16.5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley T. J., Leech J. H., Green T. J., Daniel W. A., Wahlgren M., Miller L. H., Howard R. J. A comparison of knobby (K+) and knobless (K-) parasites from two strains of Plasmodium falciparum. Mol Biochem Parasitol. 1983 Nov;9(3):271–278. doi: 10.1016/0166-6851(83)90102-0. [DOI] [PubMed] [Google Scholar]
- Handunnetti S. M., David P. H., Perera K. L., Mendis K. N. Uninfected erythrocytes form "rosettes" around Plasmodium falciparum infected erythrocytes. Am J Trop Med Hyg. 1989 Feb;40(2):115–118. doi: 10.4269/ajtmh.1989.40.115. [DOI] [PubMed] [Google Scholar]
- Hemler M. E. Adhesive protein receptors on hematopoietic cells. Immunol Today. 1988 Apr;9(4):109–113. doi: 10.1016/0167-5699(88)91280-7. [DOI] [PubMed] [Google Scholar]
- Holmquist G., Udomsangpetch R., Berzins K., Wigzell H., Perlmann P. Plasmodium chabaudi antigen Pch105, Plasmodium falciparum antigen Pf155, and erythrocyte band 3 share cross-reactive epitopes. Infect Immun. 1988 Jun;56(6):1545–1550. doi: 10.1128/iai.56.6.1545-1550.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard R. J. Malarial proteins at the membrane of Plasmodium falciparum-infected erythrocytes and their involvement in cytoadherence to endothelial cells. Prog Allergy. 1988;41:98–147. doi: 10.1159/000415221. [DOI] [PubMed] [Google Scholar]
- Igarashi I., Oo M. M., Stanley H., Reese R., Aikawa M. Knob antigen deposition in cerebral malaria. Am J Trop Med Hyg. 1987 Nov;37(3):511–515. doi: 10.4269/ajtmh.1987.37.511. [DOI] [PubMed] [Google Scholar]
- Kilejian A. Characterization of a protein correlated with the production of knob-like protrusions on membranes of erythrocytes infected with Plasmodium falciparum. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4650–4653. doi: 10.1073/pnas.76.9.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijnen H. R., Hoylaerts M., Collen D. Heparin binding properties of human histidine-rich glycoprotein. Mechanism and role in the neutralization of heparin in plasma. J Biol Chem. 1983 Mar 25;258(6):3803–3808. [PubMed] [Google Scholar]
- Luse S. A., Miller L. H. Plasmodium falciparum malaria. Ultrastructure of parasitized erythrocytes in cardiac vessels. Am J Trop Med Hyg. 1971 Sep;20(5):655–660. [PubMed] [Google Scholar]
- MacPherson G. G., Warrell M. J., White N. J., Looareesuwan S., Warrell D. A. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol. 1985 Jun;119(3):385–401. [PMC free article] [PubMed] [Google Scholar]
- Morgan W. T. Human serum histidine-rich glycoprotein. I. Interactions with heme, metal ions and organic ligands. Biochim Biophys Acta. 1978 Aug 21;535(2):319–333. doi: 10.1016/0005-2795(78)90098-3. [DOI] [PubMed] [Google Scholar]
- Oo M. M., Aikawa M., Than T., Aye T. M., Myint P. T., Igarashi I., Schoene W. C. Human cerebral malaria: a pathological study. J Neuropathol Exp Neurol. 1987 Mar;46(2):223–231. doi: 10.1097/00005072-198703000-00009. [DOI] [PubMed] [Google Scholar]
- Raventos-Suarez C., Kaul D. K., Macaluso F., Nagel R. L. Membrane knobs are required for the microcirculatory obstruction induced by Plasmodium falciparum-infected erythrocytes. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3829–3833. doi: 10.1073/pnas.82.11.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson K. J., Hall J. R., Jennings M. W., Harris T. J., Marsh K., Newbold C. I., Tate V. E., Weatherall D. J. A highly conserved amino-acid sequence in thrombospondin, properdin and in proteins from sporozoites and blood stages of a human malaria parasite. Nature. 1988 Sep 1;335(6185):79–82. doi: 10.1038/335079a0. [DOI] [PubMed] [Google Scholar]
- Rock E. P., Marsh K., Saul A. J., Wellems T. E., Taylor D. W., Maloy W. L., Howard R. J. Comparative analysis of the Plasmodium falciparum histidine-rich proteins HRP-I, HRP-II and HRP-III in malaria parasites of diverse origin. Parasitology. 1987 Oct;95(Pt 2):209–227. doi: 10.1017/s0031182000057681. [DOI] [PubMed] [Google Scholar]
- Rock E. P., Saul A. J., Taylor D. W., Leech J. H., Sherwood J. A., Howard R. J. Expression of the histidine-rich protein PfHRP1 by knob-positive Plasmodium falciparum is not sufficient for cytoadherence of infected erythrocytes. Infect Immun. 1988 Dec;56(12):3301–3304. doi: 10.1128/iai.56.12.3301-3304.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley M. E., Abdalla S., Brown J. The distribution of Plasmodium falciparum in the peripheral blood and bone marrow of Gambian children. Trans R Soc Trop Med Hyg. 1981;75(1):103–105. doi: 10.1016/0035-9203(81)90019-5. [DOI] [PubMed] [Google Scholar]
- Smitskamp H., Wolthuis F. H. New concepts in treatment of malignant tertian malaria with cerebral involvement. Br Med J. 1971 Mar 27;1(5751):714–716. doi: 10.1136/bmj.1.5751.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. W., Parra M., Chapman G. B., Stearns M. E., Rener J., Aikawa M., Uni S., Aley S. B., Panton L. J., Howard R. J. Localization of Plasmodium falciparum histidine-rich protein 1 in the erythrocyte skeleton under knobs. Mol Biochem Parasitol. 1987 Sep;25(2):165–174. doi: 10.1016/0166-6851(87)90005-3. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Trager W., Rudzinska M. A., Bradbury P. C. The fine structure of Plasmodium falciparum and its host erythrocytes in natural malarial infections in man. Bull World Health Organ. 1966;35(6):883–885. [PMC free article] [PubMed] [Google Scholar]
- Udomsangpetch R., Aikawa M., Berzins K., Wahlgren M., Perlmann P. Cytoadherence of knobless Plasmodium falciparum-infected erythrocytes and its inhibition by a human monoclonal antibody. Nature. 1989 Apr 27;338(6218):763–765. doi: 10.1038/338763a0. [DOI] [PubMed] [Google Scholar]
- Udomsangpetch R., Wåhlin B., Carlson J., Berzins K., Torii M., Aikawa M., Perlmann P., Wahlgren M. Plasmodium falciparum-infected erythrocytes form spontaneous erythrocyte rosettes. J Exp Med. 1989 May 1;169(5):1835–1840. doi: 10.1084/jem.169.5.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernot-Hernandez J. P., Heidrich H. G. The relationship to knobs of the 92,000 D protein specific for knobby strains of Plasmodium falciparum. Z Parasitenkd. 1985;71(1):41–51. doi: 10.1007/BF00932917. [DOI] [PubMed] [Google Scholar]
- Wahlgren M., Aslund L., Franzén L., Sundvall M., Wåhlin B., Berzins K., McNicol L. A., Björkman A., Wigzell H., Perlmann P. A Plasmodium falciparum antigen containing clusters of asparagine residues. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2677–2681. doi: 10.1073/pnas.83.8.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlgren M., Carlson J., Udomsangpetch R., Perlmann P. Why do Plasmodium falciparumm-infected erythrocytes form spontaneous erythrocyte rosettes? Parasitol Today. 1989 Jun;5(6):183–185. doi: 10.1016/0169-4758(89)90141-5. [DOI] [PubMed] [Google Scholar]