Abstract
Membrane gas separation has potential for the recovery and purification of helium, because the majority of membranes have selectivity for helium. This review reports on the current state of the research and patent literature for membranes undertaking helium separation. This includes direct recovery from natural gas, as an ancillary stage in natural gas processing, as well as niche applications where helium recycling has potential. A review of the available polymeric and inorganic membranes for helium separation is provided. Commercial gas separation membranes in comparable gas industries are discussed in terms of their potential in helium separation. Also presented are the various membrane process designs patented for the recovery and purification of helium from various sources, as these demonstrate that it is viable to separate helium through currently available polymeric membranes. This review places a particular focus on those processes where membranes are combined in series with another separation technology, commonly pressure swing adsorption. These combined processes have the most potential for membranes to produce a high purity helium product. The review demonstrates that membrane gas separation is technically feasible for helium recovery and purification, though membranes are currently only applied in niche applications focused on reusing helium rather than separation from natural sources.
Keywords: helium, membranes, polymeric, selectivity, process
1. Introduction
Helium is a noble gas that has a wide range of applications in important scientific, medical and industrial applications [1,2]. These industries take advantage of helium’s very low boiling temperature and chemically inert nature [3]. Helium’s largest usage is as a coolant in magnetic resonance imaging (MRIs) in hospitals, as well as an inert gas in welding, a carrier gas in analytical and scientific equipment, helium/oxygen mixtures for deep-sea SCUBA divers and in pressurizing and purging of pressure vessels, such as in rocket technology. Only ~8% of the global helium market is for party balloons [1].
The global demand for helium is growing, driven mainly by demand in Asia, especially in the developing economics of China and India [1]. The global usage of helium has grown by 990 million cubic feet from 2000 to 2015, and as such the helium price has risen from US$50 to US$104 per thousand cubic feet in that time [4,5]. To meet this demand new helium production facilities have recently been commissioned in Qatar and Australia [6]. However, global production is anticipated to fall short of global demand over the coming decades [2]. The increase in the helium price is expected to make low quality helium reserves attractive and drive further interest in helium recovery and recycling in existing industries. However, conventional technologies for helium recovery and purification rely on cryogenic liquefaction followed by pressure swing adsorption (PSA), both of which are energy intensive, especially as the helium concentration decreases in the feed [7]. As such, alternative technologies that can separate and purify helium will increasingly be investigated because of the economic advantage they might potentially have over the conventional approach. Membrane gas separation is one such technology that has significant potential in processing and purifying helium. This review discusses the sources of helium that are currently used, as well as potential recycling applications, which present niche opportunities for membranes. The current state of polymeric and inorganic membranes for helium separation is presented and analyzed. A major focus of the review is the discussion of membrane process strategies for helium recovery, including those presented in the patent literature, given the importance of the membrane process in gas separation economics [8]. Hence, this review aims to inform the membrane research community on the potential application of helium separation.
2. Helium Sources
Natural gas is the primary source of helium for commercial purposes, where it has accumulated over eons as the result of radioactive decay of uranium and thorium in the Earth’s interior [3]. The largest reserves of helium-rich natural gas fields exist in Western USA, where helium is commercially separated through cryogenic liquefaction and pressure swing adsorption [3]. The helium concentrations are generally <1%, but high quality fields in New Mexico and Alaska USA have helium compositions of 4.05 and 2.54% respectively [3]. Other helium-rich commercial natural gas fields exist in Qatar, Australia, Poland and Algeria, and potential fields exist in Russia, Iran, Italy, Tanzania and India, with a limited list of fields around the world provided in Table 1. The most important fields in the USA are the Hugoton-Panhandle field located across Texas, Oklahoma and Kansas as well as the LaBarge field in Wyoming [9]. Separation of helium from air has only been suggested under the most extreme situations, because the concentration is 5.2 ppm [1].
Table 1.
| Natural Gas Field | He | CH4 | N2 | CO2 | C2+ |
|---|---|---|---|---|---|
| New Mexico, USA | 4.05 | 49 | 45 | 0.90 | 1.05 |
| Alaska, USA | 2.54 | 90.2 | 6.8 | 0.3 | – |
| Texas, USA | 1.17 | 66.2 | 31.1 | 0.10 | 1.43 |
| Alberta, Canada | 0.53 | 93 | 6 | 0.50 | – |
| Ostrow, Poland | 0.40 | 56 | 46 | 0.30 | 0.30 |
| North Field, Qatar | 0.03 | 79.5 | 5.19 | 3.68 | 8.85 |
| Palm Valley, Australia | 0.21 | 97.5 | 2.3 | 0.10 | – |
Recycling sources of helium are associated with leakages and waste gas from applications such as pressure tank testing [1]. These generally consist of helium with a mixture of air, with a much higher helium concentration than found in natural gas. The helium concentrations in these applications can be up to 99%, but is often diluted with air due to direct exposure to the environment. However, the quantity of gas to be treated is generally very small and present only in niche industries. For example, helium leakage from modern MRIs is very low and helium pressure tank testing is intermittent. Hence, in many of these industries the focus is on reducing helium wastage.
The separation of helium from natural gas using membranes has been discussed and demonstrated since membranes were first commercially proven as a technology [11]. However, to the best of the authors’ knowledge, no large scale membrane plant currently exists that separates and purifies helium from natural gas. In part this is because pressure swing adsorption (PSA) dominates the industry [6]. Similarly, the recycling and purification of helium from industrial processes, such as coolant leakage and waste gas from pressure vessels is technically possible through membrane separation. The advantage of membranes in recycling processes is clearly demonstrated by the range of commercial membrane processes currently available for helium recovery from localized sources [12,13,14].
3. Polymeric Membranes
Gas separation through non-porous polymeric membranes is dependent on concentration of gas within the polymeric matrix, which is influenced by favorable intermolecular interactions between the gas molecule and polymer chain. The gas permeability (P) through a range of polymeric membranes has been widely measured, with permeability described through the solution-diffusion model, and dependent on the diffusivity (D) and solubility (S) of the gas within the polymer [15]:
| (1) |
For rubbery polymers the solubility of a gas corresponds to the Henry’s Law constant (kD) [15]:
| (2) |
The Henry’s law constant can be determined from Flory-Huggins theory of mixing, as it is related to the volume fraction of the amorphous polymer (φp), the partial molar volume of the gas (VR), the molar volume of an ideal gas at STP (VS = 22,410 cm3/mol) and the Flory-Huggins interaction parameter between gas and polymer (χ). Hence, the Henry’s law constant (kD) can be expressed as [16]:
| (3) |
For glassy polymers, the free volume between polymeric chains can accommodate additional gas sorption, and hence the solubility to that region is described by the dual-sorption theory. This is modelled through a Langmuir isotherm, dependent on the Langmuir affinity constant (b) and maximum capacity (C’p) of the free volume. Hence, the solubility for glassy polymers is modelled by [15]:
| (4) |
For helium permeability, the inert nature of the gas means that the solubility within polymeric membranes will be significantly lower compared to other gases. The only interaction between helium and the polymer is through dispersion forces, and as such the dual-sorption model cannot be applied. For the Henry’s law region it can be argued that the Flory-Huggins interaction parameter (χ) is zero, as there will be no intermolecular interaction between helium and the polymer chain. Hence, the Henry’s Law constant of helium in any polymeric membrane will merely be a function of the volume fraction of the polymer. This argument is used in the sorption measurements of other gases in polymers undertaken by gravimetric analysis, where helium is used as a reference gas to correct for buoyancy [17]. Kamiya et al. [18] report χ values for helium in a range of rubbery polymeric membranes. These values are significantly higher than those reported for other simple gases, such as H2, N2, and CH4, because of the need to ensure the calculated Henry’s law constants is very low (Equation (3)). Kamiya et al. state that there is considerable error in their calculations because of the hypothetical value they assumed for the vapor pressure of the liquefied helium at the measurement temperature (p0). For glassy polymeric membranes, a similar argument can be made for helium solubility associated with the free volume region; in that the Langmuir affinity constant (b) will be zero and there will be no preferential sorption of helium in the free volume. Hence, the amount of helium within the polymeric membrane would be equal to that of helium in the gas surrounding the material, and the excess sorbed amount is zero [19]. Rather, helium sorption within the free volume region will be based on size exclusion alone. Hence, helium permeability is dependent on diffusivity through the polymeric membrane, rather than solubility.
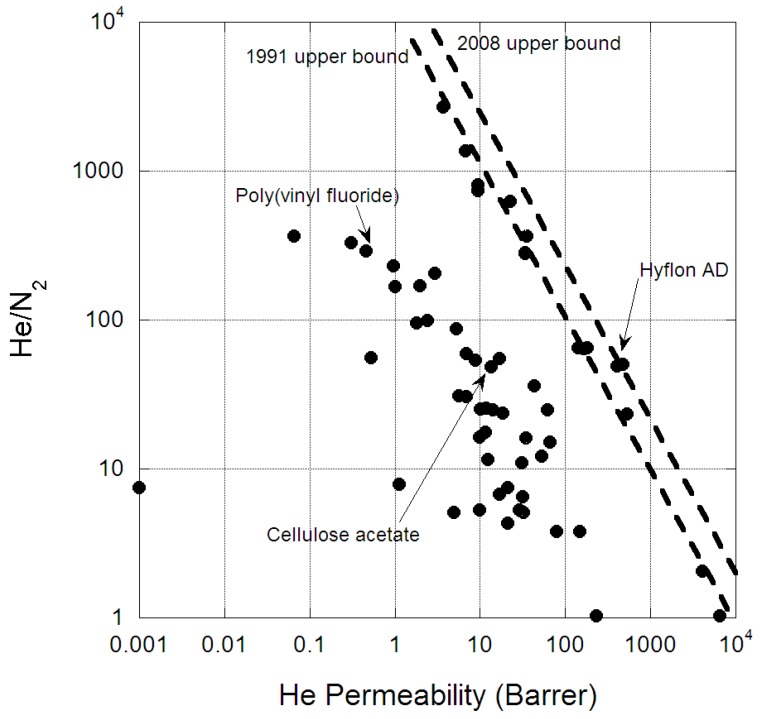
The helium permeability in a list of polymeric membranes is provided in Table 2. The Robeson’s plot of He against N2 is provided in Figure 1 and this has also been well reported in the literature [20,21]. All reported membranes have selectivity for He over N2, in part because of the smaller kinetic diameter of He compared to N2, ensuring He has a higher diffusivity through polymeric membranes [22]. Similarly, all glassy polymeric membranes have selectivity for He over CH4, however some rubbery polymeric membranes have selectivity for CH4. This is because of the strong solubility of CH4 in silicone-based polymers which dominates permeability through this class of polymer [23]. For both gas pairs, the highest permeable polymers are poly (trimethylsilylpropyne) (PTMSP) and substituted polyacetylenes, which are well known to have high fractional free volumes, which are almost microporous in structure [24,25]. Alternatively, high selectivity for both gas pairs is achieved for dense polymers such as polypyrrolone. For helium there is the standard trade-off between permeability and selectivity, with the upper bound clearly present for He/N2 separation (Figure 1). The upper bound for He/N2 gas pair has only improved slightly since Robeson first reported the behavior [20,21]; this may be an indication of the little research interest in helium separation from nitrogen. Furthermore, the gradient (λ) of these upper bounds corresponds well to established theory [26], based on the ratio of kinetic diameters (d):
| (5) |
Table 2.
He permeability (Barrer), He/N2 and He/CH4 selectivities in a range of polymeric membranes.
| Polymer | He Permeability | He/N2 | He/CH4 | Ref. | Citations |
|---|---|---|---|---|---|
| Poly(trimethylsilylpropyne) | 4100 | 2.05 | 0.98 | [30] | 5 |
| Poly(trimethylsilylpropyne) | 6500 | 1.03 | 0.433 | [31] | 74 |
| Substituted Poly(diphenylacetylene) | 11200 | 0.97 | 0.38 | [25] | 33 |
| Substituted Poly(diphenylacetylene) | 15800 | 1.01 | 0.46 | [25] | 33 |
| Substituted Poly(diphenylacetylene) | 12800 | 1.07 | 0.46 | [25] | 33 |
| Substituted Poly(diphenylacetylene) | 17800 | 1.07 | 0.51 | [25] | 33 |
| Substituted Poly(diphenylacetylene) | 13700 | 1.05 | 0.47 | [25] | 33 |
| Isotactic poly(methyl methacrylate) (PMMA) | 3.75 | 2679 | – | [32] | 51 |
| Atactic PMMA | 9.43 | 806 | – | [32] | 51 |
| Syndiotactic PMMA | 9.57 | 736 | – | [32] | 51 |
| Poly(trichloromonochloroethylene) poly diacetylene (PDA) | 34.1 | 284 | – | [15] | 1384 |
| Nafion 117 | 40.9 | – | 401 | [33] | 93 |
| Poly(trichloromonochloroethylene) | 34.1 | – | 406 | [15] | 1384 |
| Tetramethyl bis polycarbonate | 206 | – | 43.8 | [15] | 1384 |
| Poly(vinyl alcohol) | 0.0071 | – | – | [34] | 67 |
| Poly(vinyl alcohol) | 0.052 | – | – | [15] | 1384 |
| 6FDA-DAF polyimide | 98.5 | – | 156 | [35] | 73 |
| 6FDA/tetramethyl PDA polyimide | 530 | 23.2 | – | [15] | 19 |
| Polyimide (6FDA-6FpDA:DABA (2:1)) | 142 | 65 | – | [36] | 31 |
| Polyimide | 396 | – | – | [15] | 1384 |
| Polypyrrolone (6FDA/PMDA (10/90)-TAB) | 22.5 | 622 | 3041 | [37] | 55 |
| Polypyrrolone (6FDA/PMDA (25/75)-TAB) | 35.7 | 364 | 1594 | [37] | 55 |
| Polypyrrolone (6FDA-TAB) | 166 | 64.4 | 184 | [37] | 55 |
| Polyarylate (TMHFBPA I/T) | 182 | 64.8 | – | [38] | 6 |
| Hyflon AD | 405 | 48.8 | 167 | [16] | 1186 |
| Hyflon AD60X | 476 | 50.3 | 157 | [39] | 43 |
| Teflon AF-2400 | 3600 | – | 6 | [40] | 153 |
| Teflon FEP | 62 | 25 | 44 | [7] | 70 |
| Viton E60 fluoroelastomer | 30.5 | – | – | [41] | 19 |
| Viton fluoroelastomer | 43.9 | – | – | [41] | 19 |
| Cytop | 170 | – | – | [16] | 1186 |
| Fluorinated polynorbornene | 185 | – | – | [42] | 20 |
| Hostaflon perfluoroalkoxy alkane (PFA) | 43.9 | 35.9 | 41.8 | [43] | – |
| Poly(tetrafluoroethylene-co-ethylene) | 5.63 | 30.9 | – | [43] | – |
| Poly(trifluorochloroethylene-co-ethylene) | 5.33 | 87.5 | – | [43] | – |
| Polyvinyl fluoride | 1.8 | 95 | 280 | [7] | 70 |
| Poly(vinyl fluoride) | 0.46 | 289 | – | [43] | – |
| Low density polyethylene (LDPE) | 4.92 | 5.06 | 1.68 | [41] | 552 |
| High density polyethylene (HDPE) | 1.14 | 7.8 | 2.97 | [41] | 552 |
| Poly(ethylene-co-propylene) | 31.9 | 6.49 | – | [42] | 14 |
| Poly(ethylene-co-propylene) | 29 | 5.31 | – | [42] | 14 |
| Poly(ethylene-co-propylene) | 21.3 | 4.32 | – | [42] | 14 |
| Poly(propylene) | 0.373 | 0.85 | – | [43] | – |
| Trespaphan | 14.1 | 25 | – | [44] | – |
| Trespaphan | 11.96 | 25.3 | – | [44] | – |
| Trespaphan | 10.25 | 25.2 | – | [44] | – |
| Trespaphan | 11.6 | 17.6 | – | [44] | – |
| Poly(styrene) | 18.64 | 23.73 | – | [45] | 17 |
| Polystyrene | 35 | 16 | 15 | [7] | 70 |
| Poly(ethyl methacrylate) | 6.9 | 30.5 | – | [43] | – |
| Poly(vinyl acetate) | 12.57 | – | 398 | [46] | 418 |
| Poly(trifluorochloroethylene) | 6.79 | 1360 | – | [47] | – |
| Poly(vinyl alcohol) | 0.001 | 7.5 | – | [47] | – |
| Poly(vinyl benzoate) | 8.88 | 53.79 | – | [48] | 95 |
| Poly(vinyl chloride) | 2 | 168.5 | 71.4 | [43] | – |
| Saran | 0.31 | 330 | 260 | [21,47] | 47 |
| Poly(butadiene) | 32.6 | 5.06 | – | [42] | 14 |
| Poly(butadiene-co-acryonitrile) | 16.9 | 6.7 | – | [49] | 213 |
| Poly(butadiene-co-acryonitrile) | 12.3 | 11.5 | – | [49] | 213 |
| Poly(butadiene-co-acryonitrile) | 9.85 | 16.3 | – | [49] | 213 |
| Poly(oxydimethylsilylene) | 233 | 1.03 | – | [43] | – |
| Nylon 6 | 0.53 | 55.8 | – | [47] | – |
| Cellulose acetate | 13.6 | 48.6 | – | [47] | – |
| Cellulose nitrate | 6.9 | 59.5 | – | [50] | 16 |
| Ethyl cellulose | 53.4 | 12.1 | – | [50] | 16 |
| Polyvinyl fluoride | 0.97 | 231 | – | [51] | 16 |
| Polyvinylidene chloride | 0.066 | 366 | – | [51] | 16 |
| Nylon 6 | 2.43 | 98.8 | – | [51] | 16 |
| Mylar | 1.002 | 167 | 170 | [21,51] | 16 |
| Polyethylene terephthalate | 2.967 | 206 | – | [51] | 16 |
| Cellulose acetate | 1990 | – | 11.8 | [26] | 13 |
| Silicone rubber | 356 | – | 0.34 | [26] | 13 |
| Phenylene silicone rubber | 150 | 3.8 | 0.75 | [7] | 70 |
| Nitrile silicone rubber | 79 | 3.8 | 0.79 | [7] | 70 |
| Polycarbonate | 67 | 15 | 19 | [7] | 70 |
| Trithene B | 34 | 280 | 400 | [7] | 70 |
| Ethyl cellulose | 31 | 11 | 4.9 | [7] | 70 |
| Ethylene-vinyl acetate | 21 | 7.5 | 1.9 | [7] | 70 |
| Viton A | 17 | 55 | 110 | [7] | 70 |
| Polyvinyl chloride | 14 | – | 7 | [7] | 70 |
Figure 1.
Permeability (Barrer) versus selectivity for polymeric membranes separating helium from nitrogen.
To the best of the authors’ knowledge no polymeric membrane has been successfully commercialized for He recovery and purification from natural gas directly or as part of a natural gas processing plant. However, a range of commercial membranes exist for acid gas removal which are used in natural gas processing, and these may have the capability for helium recovery. Asymmetric cellulose acetate is reported to have a He permeance of 106 GPU, a He/N2 selectivity of 34 and a He/CH4 selectivity of 31 [27], which places it in the middle of the Robeson plots. Hence, cellulose acetate-based commercial Cynara (Cameron) and Separex (Honeywell UOP) membranes, currently used in the removal of H2S and CO2 from natural gas, may be viable in helium recovery applications. Cellulose acetate membranes have been used commercially for hydrogen recovery [28], which can be comparable to helium recovery given the similarity in size of these two molecules. Indeed, other commercial polymeric gas separation membranes, such as Prism (Air Products), Medal (Air Liquide) and Ube Industries, have been commercialized for hydrogen separation [28] and hence there is opportunity in helium recovery. It is expected that polymeric membranes for helium separation will be an active research and commercialization area in the future. In particular, research is expected to focus on improvements in He/N2 and He/CH4 selectivity, as the low concentration in natural gas favors higher selectivity membranes to achieve the desired recovery and purification [29]. Critically, helium solubility within polymeric membranes cannot be altered and so improvements to the selectivity are better achieved through changing the respective diffusivity. This would favor polymeric membrane materials that are denser and have lower fractional free volume, because this morphology would impact the diffusivity of the smaller He atom to a lesser degree than the diffusivity of N2 and CH4.
4. Inorganic Membranes
Inorganic membranes have also been investigated for helium separation; the performance of a number of them is provided in Table 3. Inorganic membranes have a number of advantages over polymeric membranes, for example their ability to withstand harsher conditions such as high temperatures and corrosive gases [43]. However, the focus in the literature is on hydrogen separation, with helium permeation being one of the gases used in characterization rather than as a specific application. All of the inorganic membranes presented here are porous and hence it is difficult to achieve the selectivities observed for polymeric membranes (Table 2). The inert nature of helium means that surface diffusion and capillary condensation will not occur for porous membranes. However, many of the reported inorganic membranes have selectivities above the Knudsen diffusion selectivity (He/N2 is 1.9, He/CH4 is 2) [44] indicating that molecular sieving is occurring, where the kinetic diameter of He is 2.6 Å, N2 is 3.64 Å and CH4 is 3.8 Å [45].
Table 3.
He permeance (GPU), He/N2 and He/CH4 selectivities in a range of inorganic membranes.
| Material | He Permeance | He/N2 | He/CH4 | Ref. | Citations |
|---|---|---|---|---|---|
| Ni doped silica | 3466 | – | 600 (300 °C) | [46] | 31 |
| Porous Alumina | 86,190 | – | – | [47] | – |
| Isoreticular Metal-Organic framework (IRMOF-3) | 2986 | 2.5 | 1.6 | [48] | 44 |
| IRMOF-3 and -6 | 2389 | 2.6 | 1.3 | [48] | 44 |
| Metal-Organic framework (MMOF) | 32.9 | 3.5 | [49] | 176 | |
| [Cu2(bza)4(pyz)]n | 8.1 | 3.9 | 7.3 | [50] | 32 |
| [Cu2(bza)4(pyz)]n | 1.76 | – | – | [50] | 32 |
| Cu-BTC | 4181 | 2.6 | 2.07 | [51] | 19 |
| Hydroxy sodalite | – | 8.8 | 5 | [52] | – |
| Vycor Glas | 4.8 Barrer | 7619 | – | [11] | 70 |
| Microporous Silica | 2933 | 31 | 147 | [53] | 84 |
| Microporous Silica | 6570 | 560 | – | [54] | 65 |
| Microporous Silica | 89.6 | – | 5000 | [55] | 44 |
Metal organic framework (MOF) membranes provide high selectivities for inorganic-based membranes, in part because the framework morphology can be specially designed to enable helium diffusivity while hindering other gases permeation. Similarly, microporous silica prepared by silica sol deposition on alumina support layer membranes achieve very high helium permeances and selectivities [53,54]. This is because the resulting silica pores’ diameters are of molecular size and hence excellent molecular sieving is achieved, while the deposition technique is able to fabricate a consistent layer without any defects. Thus, there is potential for microporous silica membranes to also produce high purity helium product from many of the aforementioned sources, however fabrication on large scales remains an issue. To the best of the authors’ knowledge no commercial inorganic membrane module exists for helium separation. Inorganic membranes will be an active area of research into the future, however it is expected that helium separation will be considered a minor application.
5. Membrane Processes
For membranes, both polymeric and inorganic, to be viable for helium recovery, the process design needs to be economically competitive against conventional technology. Therefore a major focus of membrane research in helium recovery will be on developments to the process designs that achieve the aims of low energy duty and minimizing equipment sizing, including membrane area. In other gas separation applications, it has been demonstrated that improvements in process design have a stronger influence on membranes’ economic competitiveness than just increasing the permselectivity of the membrane [56], and this argument also holds for helium recovery.
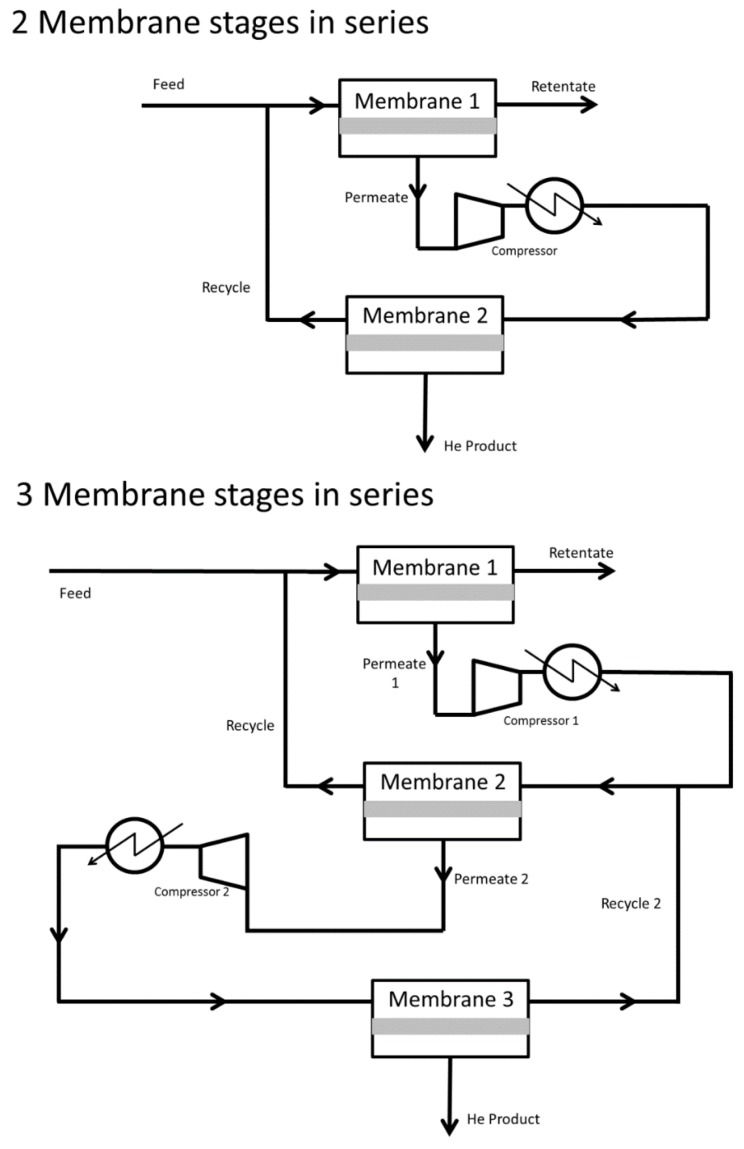
There has been a range of membrane processes designed for the recovery of helium from different sources, both in the research and patent literature. For the recovery and purification of helium directly from natural gas, membranes have demonstrated that this separation is possible when combined as two and three stages in series, with recycle streams (Figure 2) [29,57,58]. These designs can enable natural gas with 1 mol % helium and greater to be purified to a very high concentration, while utilizing existing polymeric membranes that have high He/CH4 selectivities. For example, the Alaska field (Table 1—2.54 mol %) can be purified to 99% through a three stage process with a membrane based on Teflon FEP (He/CH4 selectivity of 44) operating with pressures of 10 MPa, or with a poly vinylchloride-based membrane (He/CH4 selectivity of 71.4) when the pressure is 5 MPa. In contrast, a Hyflon AD-based membrane (He/CH4 selectivity of 157) will be able to process the Alaska field through a three stage process with membrane pressures of 625 kPa [29]. However, to achieve this high separation and purification the stage-cut of the second or third membrane stage is marginal and the required pressure driving force across the membranes is substantial. Hence, this requires significant compression energy and is the reason that direct recovery of helium from natural gas through membranes has not been commercialized.
Figure 2.
Process diagram example of two and three membrane stages processes, with recycle streams, recovering helium.
Membranes inclusion in natural gas processing for helium recovery is a more viable option, and there are a range of different processes in the patent literature. Two and three membrane stages in series, with recycles, have been demonstrated to recover and purify helium from the exit gas off the nitrogen rejection unit (NRU) [29]. This is because the helium concentration is higher than in natural gas, and separation is mainly from nitrogen. This separation can be achieved with He/N2 selectivities above 20 and reasonable compression ratios. Commercial cellulose acetate-based membranes should be able to undertake this separation as well as commercial polyimide membranes [27]. As such, there is little need for the development of novel membranes in this application, and rather the focus should be on fabrication of low cost modules and ultra-thin layers to enhance flux. Indeed, processing NRU exit gas should be a more straightforward operation than acid gas removal from natural gas, because water and higher hydrocarbons have been removed from the gas, which are known to effect membrane performance in acid gas removal [59].
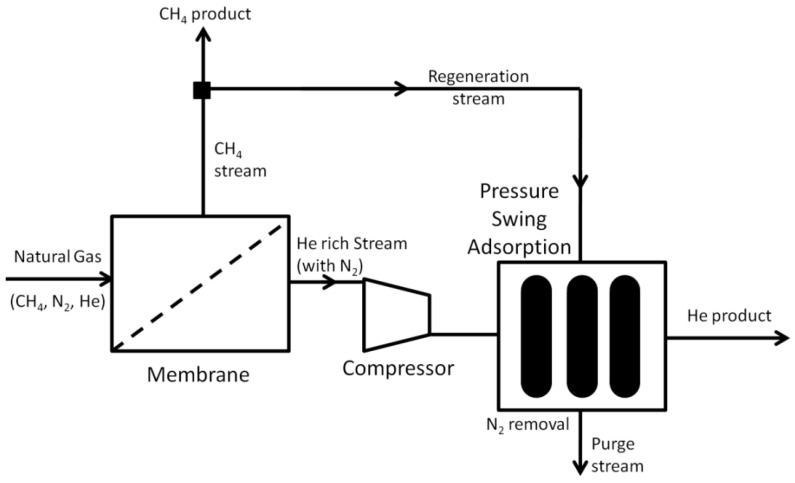
The most viable membrane process for helium is the upgrading of crude helium to 90 mol % purity, which has energy duties comparable with other separation technologies. This requires a He/N2 selectivity of 10 for a 60 mol % feed, increasing to a He/N2 selectivity of 17 for a 50 mol % feed. This can be achieved with a variety of polymeric membranes (Table 2), including those membranes commercialized for natural gas sweetening. However, this process requires PSA to undertake the final purification stage and achieve ultra-high purity helium. Indeed, the majority of membrane designs processing helium consist of membranes used in series with another separation technology, generally PSA. This can consist of the membrane stage undertaking the first recovery stage and then sending the helium-rich stream to undergo final purification through another technology [60,61,62,63], as demonstrated in Figure 3. Or it can consist of another separation technology, such as PSA, undertaking the recovery of helium before the membrane stage achieves final purity specifications [64]. For PSA, the need to regenerate the adsorbent beds is critical, and a part of the novelty in the patent literature is to use the membrane’s retenate gas stream as the regeneration gas [65,66]. Additional novelty in many of these processes is the stream switching between the membrane unit and the PSA beds, and how they are interconnected to reduce compression requirements, recycle helium and regeneration of the adsorbent beds. Many of the patented systems of these hybrid membrane–PSA systems for natural gas are not specific for helium, but are rather broad in the gases discussed. This enables them to also cover hydrogen recovery from natural and refinery gas [67], which behaves very similar to helium recovery.
Figure 3.
Process combining membrane separation with pressure swing adsorption (PSA) for the recovery and purification of helium.
It is also possible to position membrane separation between two alternative separation technologies. United States Patent 5224350 describes such an invention [68], where a physical solvent absorption process is used to remove acidic gas from a natural gas stream. The off gas of this is rich in helium and nitrogen and therefore undergoes separation through a membrane unit to recover the helium. This helium product then undergoes PSA to produce the final purified helium product. It is therefore clear that standard membrane gas separation can be incorporated with a range of other separation technologies to achieve the required helium recovery and purification from natural gas. The process designs are achievable with polymeric membranes having reasonable He/N2 and He/CH4 selectivities, which are already present in commercialized membranes for natural gas sweetening. However, to the best of the authors’ knowledge no large scale implementation of these membrane processes has occurred for helium recovery from natural gas processing; though with continual interest in lower grade helium reserves there will be future interest in membrane process design developments in this area. It is expected that the continual improvements in membrane process design in other gas processing applications will also be applied to helium recovery. In particular, a focus on stronger integration with PSA is expected.
For other helium-rich applications, membranes are used to recover and recycle the helium product and there are a number of commercial units that can be purchased [69,70]. For example, Innovative Gas Systems (IGS) have their GENERON membrane for the recovery of helium from cryogenic, controlled atmosphere and electronic applications [13]. Their membrane process claims to produce a product purity of >90% and helium recovery >98%. Evonik Industries also markets their Sepuran membranes for the recovery and recycling of helium from a similar range of applications [12]. In atmosphere-helium mixtures, such as that required for deep sea diving, Divex Global markets their Helipure system, which uses a membrane to recover the helium from air mixtures and recompresses for recycling [14]. Their process claims a single membrane pass is able to reduce the nitrogen amount by a third and lower the oxygen amount to three quarters of their original concentration. Hence, there are clearly commercial opportunities for membranes in these niche industries and with the rising helium price it is anticipated that industry usages with recycle opportunities will also be commercially explored.
6. Conclusions
This review examines the current state of the literature for helium recovery and purification by membrane gas separation. The major developments in membranes technology will be in process developments, as currently available polymeric and inorganic membranes have the required permselectivity to achieve the desired separation in many of the potential helium recovery situations. Helium can be sourced from natural gas and related gas processing, as well as from the recycle and recovery associated with niche industrial applications. A wide range of polymeric membranes have been investigated for helium separation, with standard Robeson plot behavior observed against nitrogen and methane. Inorganic membranes have also been reported for helium separation, though their selectivities are lower than polymeric membranes because of their porous nature. A range of membrane process designs have been reported for recovery and purification of helium. Two and three stage membrane processes can recover helium directly from natural gas as well as from the nitrogen rejection unit exhaust gas, though they require considerable compression duties. The patent literature has a strong focus on combined processes where membranes are in a series with another separation technology to achieve both high helium recovery and high helium purity in the final product. This other separation technology is most often pressure swing adsorption, and the gas stream switching and connectivity between the PSA unit and membrane are of particular interest to reduce the energy duty of helium recovery and increase the viability of the process design.
Author Contributions
Colin A. Scholes and Ujjal K. Ghosh reviewed and analyzed the literature equally, as well as wrote the paper together.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Clarke R., Nuttall W., Glowacki B. Endangered helium: Bursting the myth. Chem. Eng. 2013;870:32–36. [Google Scholar]
- 2.Scholes C.A. Helium: Is the party really over? Chem. Aust. 2011;78:18–20. [Google Scholar]
- 3.Haussinger P., Glathaar R., Rhode W., Kick H., Benkmann C., Weber J., Wunschel H.-J., Stenke V., Leicht E., Stenger H. Ullmann’s Encyclopedia of Industrial Chemistry. John Wiley and Sons; Hoboken, NJ, USA: 2011. Noble Gases; pp. 392–448. [Google Scholar]
- 4.Peterson J.B. Helium Mineral Commodity Summaries. U.S. Geological Survey; Reston, VA, USA: 2001. [Google Scholar]
- 5.Hamak J.E. Helium Mineral Commodity Summaries. U.S. Geological Survey; Reston, VA, USA: 2016. [Google Scholar]
- 6.Rufford T.E., Chan K.I., Huang S.H., May E.F. A review of conventional and emerging process technologies for the recovery of helium from natural gas. Adsorpt. Sci. Technol. 2014;32:49–72. doi: 10.1260/0263-6174.32.1.49. [DOI] [Google Scholar]
- 7.Kerry F.G. Industrial Gas Handbook Gas Separation and Purification. CRC Press; Boca Raton, FL, USA: 2007. Rare (Noble) Gases. [Google Scholar]
- 8.Spillman R. Economics of Gas Separation Membrane Processes. In: Noble R.D., Stern S.A., editors. Membrane Separations Technology. Elsevier Science; Eastbourne, UK: 1995. pp. 589–667. [Google Scholar]
- 9.Committee on the impact of selling the Federal helium reserve . The Impact of Selling the Federal Helium Reserve. National Academy Press; Washington, DC, USA: 2000. [Google Scholar]
- 10.Zartman R.E., Wasserburg G.J., Reynolds J.H. Helium, argon and carbon in some natural gases. J. Geophys. Res. 1961;66:277–306. doi: 10.1029/JZ066i001p00277. [DOI] [Google Scholar]
- 11.Stern S.A., Sinclair T.F., Gareis P.J., Vahldieck N.P., Mohr P.H. Helium recovery by permeation. Ind. Eng. Chem. 1965;57:49–60. doi: 10.1021/ie50662a008. [DOI] [Google Scholar]
- 12.Industries E. Sepuran Noble. [(accessed on 25 September 2016)]. Available online: http://www.sepuran.com.
- 13.Systems I.G. Helium Recovery. Generon Membrane Technology. [(accessed on 25 September 2016)]. Available online: http://www.igs-global.com.
- 14.Divex Helipure Membrane Gas Separation System. [(accessed on 25 September 2016)]. Available online: http://www.divexglobal.com.
- 15.Petropoulos J.H. Mechanisms and Theories for Sorption and Diffusion of Gases in Polymers. In: Paul D.R., Yampol’skii Y.P., editors. Polymeric Gas Separation. CRC Press; Boca Raton, FL, USA: 1994. pp. 17–81. [Google Scholar]
- 16.Flory P. Principles of Polymer Chemistry. Cornell University Press; Ithaca, NY, USA: 1953. [Google Scholar]
- 17.Scholes C.A., Tao W.X., Stevens G.W., Kentish S.E. Sorption of methane, nitrogen, carbon dioxide and water in matrimid 5218. J. Appl. Polym. Sci. 2010;117:2284–2289. doi: 10.1002/app.32148. [DOI] [Google Scholar]
- 18.Kamiya Y., Naito Y., Mizoguchi K., Terada K., Moreau J. Thermodynamic intereactions in rubbery polymer/gas systems. J. Polym. Sci. Polym. Phys. B. 1997;35:1049–1053. doi: 10.1002/(SICI)1099-0488(199705)35:7<1049::AID-POLB4>3.0.CO;2-R. [DOI] [Google Scholar]
- 19.Brandani S., Mangano E., Sarkisov L. Net, excess and absolute adsorption and adsorption of helium. Adsorption. 2016;22:261–276. doi: 10.1007/s10450-016-9766-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robeson L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991;62:165–185. doi: 10.1016/0376-7388(91)80060-J. [DOI] [Google Scholar]
- 21.Robeson L.M. The upper bound revisited. J. Membr. Sci. 2008;320:390–400. doi: 10.1016/j.memsci.2008.04.030. [DOI] [Google Scholar]
- 22.Scholes C.A., Kentish S.E., Stevens G.W. Effects of minor components in carbon dioxide capture using polymeric gas separation membranes. Sep. Purif. Rev. 2009;38:1–44. doi: 10.1080/15422110802411442. [DOI] [Google Scholar]
- 23.Scholes C.A., Kentish S.E., Stevens G.W. The effect of hydrogen sulfide, carbon monoxide and water on the performance of a pdms membrane in carbon dioxide/nitrogen separation. J. Membr. Sci. 2010;350:189–199. doi: 10.1016/j.memsci.2009.12.027. [DOI] [Google Scholar]
- 24.Budd P.M., Makhseed S.M., Ghanem B.S., Msayib K.J., Tattershall C.E., McKeown N.B. Microporous polymeric materials. Mater. Today. 2004;7:40–46. doi: 10.1016/S1369-7021(04)00188-9. [DOI] [Google Scholar]
- 25.Hu Y., Shiotsuki M., Sanda F., Freeman B.D., Masuda T. Synthesis and properties of indan-based polyacetylenes that feature the highest gas permeability among all the existing polymers. Macromolecules. 2008;41:8525–8532. doi: 10.1021/ma801845g. [DOI] [Google Scholar]
- 26.Freeman B.D. Basis of permeability/selectivity tradeoff relations in polymeric gas separation membranes. Macromolecules. 1999;32:375–380. doi: 10.1021/ma9814548. [DOI] [Google Scholar]
- 27.Gantzel P.K., Merten U. Gas separations with high-flux cellulose acetate membranes. Ind. Eng. Chem. Process Des. Dev. 1970;9:331–332. doi: 10.1021/i260034a028. [DOI] [Google Scholar]
- 28.Baker R.W. Membrane Technology and Applications. John Wiley & Sons; Chichester, UK: 2004. [Google Scholar]
- 29.Scholes C.A., Ghosh U. Helium separation through polymeric membranes: Selectivity targets. J. Membr. Sci. 2016;520:221–230. doi: 10.1016/j.memsci.2016.07.064. [DOI] [Google Scholar]
- 30.Takada K., Matsuya H., Masuda T., Higashimura T. Gas permeability of polyacetylenes carrying substituents. J. Appl. Polym. Sci. 1985;30:1605–1616. doi: 10.1002/app.1985.070300426. [DOI] [Google Scholar]
- 31.Toy L.G., Nagai K., Freeman B.D., Pinnau I., He Z., Masuda T., Teraguchi M., Yampolskii Y.P. Pure gas and vapor permeation and sorption properties of poly[1-phenyl-2-[p-(trimethylsilyl)phenyl]acetylene] (ptmsdpa) Macromolecules. 2000;33:2516–2524. doi: 10.1021/ma991566e. [DOI] [Google Scholar]
- 32.Min K.E., Paul D.R. Effect of tacticity on permeation properites of poly(methyl metacrylate) J. Polym. Sci. B Polym. Phys. 1988;26:1021–1033. doi: 10.1002/polb.1988.090260507. [DOI] [Google Scholar]
- 33.Chiou J.S., Paul D.R. Gas permeation in a dry nafion membrane. Ind. Eng. Chem. Res. 1988;27:2161–2164. doi: 10.1021/ie00083a034. [DOI] [Google Scholar]
- 34.Pye D.G., Hoehn H.H., Panar M. Measurement of gas permeability of polymers. I. Permeabilities in constant volume/variable pressure apparatus. J. Appl. Polym. Sci. 1976;20:1921–1931. doi: 10.1002/app.1976.070200719. [DOI] [Google Scholar]
- 35.Kim T.-H., Koros W.J., Husk G.R. Advanced gas separation membrane materials: Rigid aromatic polyimides. Sep. Sci. Technol. 1988;23:1611–1626. doi: 10.1080/01496398808075652. [DOI] [Google Scholar]
- 36.Kim J.H., Koros W.J., Paul D.R. Effects of CO2 exposure and physical aging on the gas permeability of thin 6fda-based polyimide membranes: Part 1. Without crosslinking. J. Membr. Sci. 2006;282:21–31. doi: 10.1016/j.memsci.2006.05.004. [DOI] [Google Scholar]
- 37.Zimmerman C.M., Koros W.J. Polypyrrolones for membrane gas separations. I. Structural comparison of gas transport and sorption properties. J. Polym. Sci. B Polym. Phys. 1999;37:1235–1249. doi: 10.1002/(SICI)1099-0488(19990615)37:12<1235::AID-POLB5>3.0.CO;2-J. [DOI] [Google Scholar]
- 38.Guzman-Gutierrez M.T., Ruiz-Trevino F.A., Zolutukhin M., Hernandez-Lopez S., Scherf U. Gas transport properties of high free volume polyarylates based on isophthalic/terephthalic acid chloride mixtures. J. Membr. Sci. 2007;305:347–352. doi: 10.1016/j.memsci.2007.08.026. [DOI] [Google Scholar]
- 39.Macchione M., Jansen J.C., Luca G.D., Tocci E., Longeri M., Drioli E. Experimental analysis and simulation of the gas transport in dense hyflon® AD60X membranes: Influence of residual solvent. Polymer. 2007;48:2619–2635. doi: 10.1016/j.polymer.2007.02.068. [DOI] [Google Scholar]
- 40.Pinnau I., Toy L.G. Gas and vapor transport properties of amorphous perfluorinated copolymer membranes based on 2,2-bistrifluoromethyl-4,5-difluoro-1,3-dioxole/tetrafluoroethylene. J. Membr. Sci. 1996;109:125–133. doi: 10.1016/0376-7388(95)00193-X. [DOI] [Google Scholar]
- 41.Fitch M.W., Koros W.J., Nolen R.L., Carnes J.R. Permeation of several gases through elastomers, with emphasis on the deuterium/hydrogen pair. J. Appl. Polym. Sci. 1993;47:1033–1046. doi: 10.1002/app.1993.070470610. [DOI] [Google Scholar]
- 42.Teplyakov V.V., Paul D.R., Bespalova N.B., Finkel’shtein E.S. Gas permeation in a fluorine-containing polynorbornene. Macromolecules. 1992;25:4218–4219. doi: 10.1021/ma00042a027. [DOI] [Google Scholar]
- 43.Mottern M.L., Shi J.Y., Shqau K., Yu D., Verweij H. Microstructural Optimization of Thin Supported Inorganic Membranes for Gas and Water Purification. In: Li N.N., Fane A.G., Ho W.S.W., Matsuura T., editors. Advanced Membrane Technology and Applications. Wiley; Hoboken, NJ, USA: 2008. pp. 899–928. [Google Scholar]
- 44.Zolandz R.R., Fleming G.K. Definitions. In: Ho W.S.W., Sirkar K.K., editors. Membrane Handbook. Kluwer Academic Publishers; Norwell, MA, USA: 2001. pp. 19–24. [Google Scholar]
- 45.Breck D.W. Zeolite Molecular Sieves. John Wiley & Sons; New York, NY, USA: 1973. [Google Scholar]
- 46.Kanezashi M., Fujita T., Asaeda M. Nickel-doped silica membranes for separation of helium from organic gas mixtures. Sep. Sci. Technol. 2005;40:225–238. doi: 10.1081/SS-200041989. [DOI] [Google Scholar]
- 47.Van Veen H.M., Tol J.P.B.M., Engelen C.W.R., Veringa H.J. High Temperature Gas Separation with Alumina Membranes; Proceedings of the International Conference on Inorganic Membranes (ICIM2–91); Montpellier, France. 1–4 July 1991. [Google Scholar]
- 48.Yoo Y., Varela-Guerrero V., Jeong H.-K. Isoreticular metal-organic frameworks and their membranes with enhanced crack resistance and moisture stability by surfactant-assisted drying. Langmuir. 2011;27:2652–2657. doi: 10.1021/la104775d. [DOI] [PubMed] [Google Scholar]
- 49.Ranjan R., Tsapatsis M. Microporous metal organic framework membrane on porous support using the seeded growth method. Chem. Mater. 2009;21:4920–4924. doi: 10.1021/cm902032y. [DOI] [Google Scholar]
- 50.Takamizawa S., Takasaki Y., Miyake R. Single-crystal membrane for anisotropic and efficient gas permeation. J. Am. Chem. Soc. 2010;132:2862–2863. doi: 10.1021/ja910492d. [DOI] [PubMed] [Google Scholar]
- 51.Cao F., Zhang C., Xiao Y., Huang H., Zhang W., Liu D., Zhong C., Yang Q., Yang Z., Lu X. Helium recovery by a Cu-BTC metal-organic-framework membrane. Ind. Eng. Chem. Res. 2012;51:11274–11278. doi: 10.1021/ie301445p. [DOI] [Google Scholar]
- 52.Vaezi M.J., Bayat Y., Babaluo A.A., Shafiei S. Separation of helium from gases using the synthesized hydroxy sodalite membrane. Sci. Iran. C. 2016;23:1136–1143. [Google Scholar]
- 53.Asaeda M., Yamasaki S. Separation of inorganic/organic gas mixtures by porous silica membranes. Sep. Purif. Technol. 2001;25:151–159. doi: 10.1016/S1383-5866(01)00099-5. [DOI] [Google Scholar]
- 54.Peters T.A., Fontalvo J., Vorstman M.A.G., Benes N.E., van Dam R.A., Vroon Z.A.E.P., van Soest-Vercammen E.L.J., Keurentjes J.T.F. Hollow fibre microporous silica membranes for gas separation and pervaporation. Synthesis, performance and stability. J. Membr. Sci. 2005;248:73–80. doi: 10.1016/j.memsci.2004.08.023. [DOI] [Google Scholar]
- 55.Araki S., Mohri N., Yoshimitsu Y., Miyake Y. Synthesis, characterization and gas permeation properties of a silica membrane prepared by high-pressure chemical vapor deposition. J. Membr. Sci. 2007;290:138–145. doi: 10.1016/j.memsci.2006.12.034. [DOI] [Google Scholar]
- 56.Ho M.T., Allinson G.W., Wiley D.E. Reducing the cost of CO2 capture from flue gases using membrane technology. Ind. Eng. Chem. Res. 2008;47:1562–1568. doi: 10.1021/ie070541y. [DOI] [Google Scholar]
- 57.Seok D.R., Kang S.G., Hwang S.-T. Separation of helium and hydrocarbon mixtures by a two-membrane column. J. Membr. Sci. 1986;27:1–11. doi: 10.1016/S0376-7388(00)81378-2. [DOI] [Google Scholar]
- 58.Hale P.W., Lokhandwala K.A. Helium Recovery from Gas Streams. 20050217479 A1. U.S. Patent. 2005 Oct 6;
- 59.Scholes C.A., Stevens G.W., Kentish S.E. Membrane gas separation applications in natural gas processing. Fuel. 2012;96:15–28. doi: 10.1016/j.fuel.2011.12.074. [DOI] [Google Scholar]
- 60.Behling R.-D., Peinemann K.V. A Process for the Separation/Recovery of Gases. 6,179,900 B1. U.S. Patent. 2001 Jan 30;
- 61.Choe J.S., Auvil S.R., Agrawal R. Process for Separating Components of a Gas Stream. 4,701,187 A. U.S. Patent. 1987 Oct 20;
- 62.Doshi K.J., Werner R.G., Mitariten M.J. Integrated Membrane/PSA Process and System. 4,863,492. U.S. Patent. 1989 Sep 5;
- 63.Jaynes S.E. System and Process for Gas Recovery. 6,517,791. U.S. Patent. 2003 Feb 11;
- 64.Choe J.S., Agrawal R., Auvil S.R. Process for Recovering Helium from a Multi-Component Gas Stream. 4,717,407. U.S. Patent. 1988 Jan 5;
- 65.Doshi K.J. Enhanced Gas Separation Process. 4,690,695. U.S. Patent. 1987 Sep 1;
- 66.Stoner G., Reingold H.E.I., D’Amico J.S., Knaebel K.S. Enhanced Helium Recovery. 5,632,803. U.S. Patent. 1997 May 27;
- 67.Doshi K.J. Enhanced Hydrogen Recovery from Low Purity Gas Streams. 4,398,926. U.S. Patent. 1983 Aug 16;
- 68.Mehra Y.R. Process for Recovering Helium from a Gas Stream. 5,224,350. U.S. Patent. 1993 Jul 6;
- 69.Schulte T.R. Coolant Recovery Process. 5,377,491. U.S. Patent. 1995 Jan 3;
- 70.Lhote B., Queille P., Zumbrunn J.-P., Duchateau E. Process and Apparatus for Heat Treating Articles While Hardening in Gaseous Medium. 5,158,625. U.S. Patent. 1992 Oct 27;