Abstract
The tetrasaccharide structures Siaα2,3Galβ1,3(Fucα1,4)GlcNAc and Siaα2,3Galβ1,4(Fucα1,3)GlcNAc constitute the epitopes of the carbohydrate antigens sialyl-Lewis a (sLea) and sialyl-Lewis x (sLex), respectively, and are the minimal requirement for selectin binding to their counter-receptors. Interaction of sLex expressed on the cell surface of leucocytes with E-selectin on endothelial cells allows their arrest and promotes their extravasation. Similarly, the rolling of cancer cells ectopically expressing the selectin ligands on endothelial cells is potentially a crucial step favoring the metastatic process. In this review, we focus on the biosynthetic steps giving rise to selectin ligand expression in cell lines and native tissues of gastrointestinal origin, trying to understand whether and how they are deregulated in cancer. We also discuss the use of such molecules in the diagnosis of gastrointestinal cancers, particularly in light of recent data questioning the ability of colon cancers to express sLea and the possible use of circulating sLex in the early detection of pancreatic cancer. Finally, we reviewed the data dealing with the mechanisms that link selectin ligand expression in gastrointestinal cells to cancer malignancy. This promising research field seems to require additional data on native patient tissues to reach more definitive conclusions.
Keywords: carbohydrate antigens, glycosylation, cancer diagnosis, cancer malignancy
1. Introduction
The three members of the selectin family bind carbohydrate structures (and are therefore referred to as lectins) through a Ca++-dependent domain. They are transmembrane type I proteins with a short cytoplasmic tail and the long N-terminal portion containing the carbohydrate recognition domain protruding in the extracellular space. The three selectins differ in their structure and pattern of cell type expression. L-selectin is expressed on lymphocytes, monocytes and granulocytes, and is responsible for their homing through the binding of specific carbohydrate structures expressed by the high endothelial venules in lymph nodes. P-selectin is expressed by platelets and endothelial cells and is stored in membranes of α granules of platelets and in Weibel-Palade bodies of endothelial cells. It is involved in the earlier steps of the adhesion process since its mobilization to the cell surface of activated endothelial cells from the stores occurs within minutes upon the activation stimuli. E-selectin is constitutively expressed on the cell surface of venular endothelia of bone marrow and skin, while in other organs it is expressed upon stimulation by TNF-α, IL-1β, or LPS. The interaction between E-selectin and its ligands expressed on the cell surface allows leukocyte rolling, a preliminary step leading to leukocyte adhesion to endothelial cells and extravasation [1].
The minimal sugar structure required for selectin binding is constituted by an α2,3-sialylated and α1,3/4 fucosylated tetrasaccharide based on a type 1 (Galβ1,3GlcNAc) or a type 2 (Galβ1,4GlcNAc) chain. Two isomers, known as sialyl-Lewis a (sLea: Siaα2,3Galβ1,3[Fucα1,4]GlcNAc) and sialyl-Lewis x (sLex: Siaα2,3Galβ1,4[Fucα1,3]GlcNAc) fulfill this basic requirement. The addition of a sulfate group at the 6 position of GlcNAc generates 6-sulfo-sLex, which is considered the physiologic ligand for L-selectin [2]. This epitope is carried by mucin-type N- or O-linked oligosaccharides [3].
Although selectins were originally described in the context of inflammation and immunological response, their role in cancer progression soon became evident in consideration of the role played by cancer cell adhesion to endothelia in metastasis. Concurrently, many reports were describing various glycan alterations associated with cancer, including the expression of sLea and sLex as a hallmark of the malignant phenotype [4]. Interestingly, sLea is the epitope of the CA19.9 antigen, one of the most widely used tumor markers in the clinical management of pancreatic and other gastrointestinal cancers, as well as for research purposes. Recently, a role of circulating sLex has been proposed as a marker for monitoring the metastatic progression of breast cancer [5] and has been associated with tumor invasion by a meta-analysis study [6].
In the present article, we review recent data on the biosynthesis and role of selectin ligands in gastrointestinal cancers. In particular, we will discuss how the mechanisms operating in the immunological response and inflammation are recapitulated in cancer cells, and their significance in gastrointestinal cancer patients. We will also highlight the still controversial points requiring further experimental studies.
2. Biosynthesis of sLex and sLea
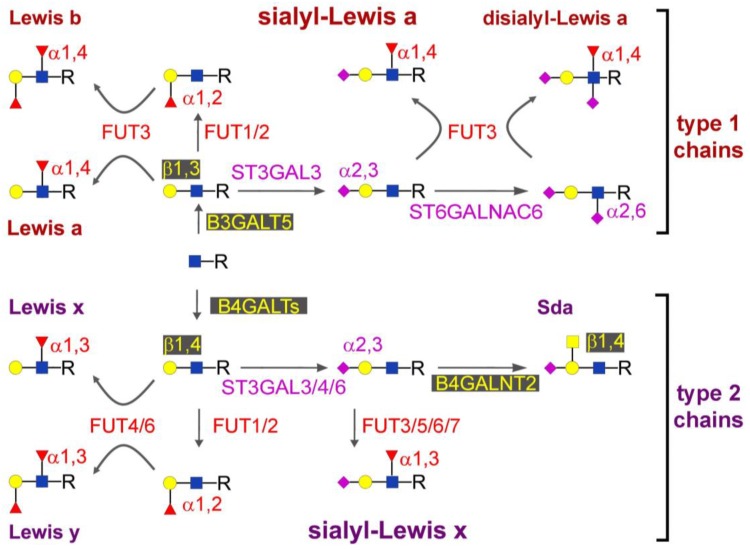
The biosynthesis process of sLea/sLex (Figure 1) has been known since the nineties [7]. However, the actual contribution of the different α2,3 sialyltransferases and α1,3/4 fucosyltransferases to the biosynthesis of selectin ligands in different healthy and cancer gastrointestinal tissues is far from established. This is due to the complex tissue-specific pattern of expression of these enzymes and the difficult use of the mouse model [7,8]. Moreover, the genetic manipulation of cell lines by cDNA transfection or gene silencing [9,10,11,12,13] provides mainly indications on the potential role of a glycosyltransferase in elaborating a sugar epitope in an experimental system, rather than on its real role in normal or cancer tissues. For example, in pancreatic cancer cell lines, both ST3GAL3 and ST3GAL4 are able to induce sLex biosynthesis [10,14], although the over-expression of sialyl-Lewis (sLe) antigens in surgical specimens is not correlated with either gene [15]. In normal and cancer colonic tissues, the α2,3-sialylation step of sLex biosynthesis appears to be mediated by ST3GAL4, owing to the very low expression of ST3GAL3 [16], while the α1,3-fucosylation step is mediated mainly by FUT6 [17]. However, the overexpression of sLex in colon cancer tissues cannot be explained by the up-regulation of any single glycosyltransferase [16,18,19]. In a recent review [20], we have proposed that the right question to answer is not “why are sLex levels high in colon cancer?” but rather “why are sLex levels low in the normal colon?” Competition between FUT6 and enzymes synthesizing alternative structures, such as Siaα2,3(GalNAcβ1,4)Galβ1,3/4GlcNAc (the Sda antigen) [21,22] or the sialyl 6-sulfo-Lewis x antigen [23] has been proposed. The Sda antigen is the product of β1,4 N-acetylgalactosaminyltransferase-II (B4GALNT2) [24,25]. Both the Sda antigen and B4GALNT2 are highly expressed in normal colon and down-regulated in colon cancer [26,27]. The expression of B4GALNT2 induces down-regulation of sLex in colonic and gastric cancer cell line [21,22] and probably also in normal colonic tissues.
Figure 1.
Structure and biosynthesis of Lewis antigens. Monosaccharides are depicted. Blue square: N-acetylglucosamine (GlcNAc); yellow square: N-acetylgalactosamine (GalNAc); yellow circle: galactose (Gal); red triangle: fucose (Fuc); pink diamond: sialic acid (Sia). Anomers, linkage positions, and enzymes involved in the reactions are indicated. All enzymes known to be able to perform a reaction are listed, note that only some of them are proven to be expressed in gastrointestinal tissues, as detailed in the text.
In fact, while in colon cancer tissues (where B4GALNT2 levels are low) sLex levels correlate with FUT6 expression [17], in normal colon (where B4GALNT2 is high), they correlate with the FUT6/B4GALNT2 ratio [28].
The branching point and the possible competition between the biosynthesis of type 1 and 2 chains, (and consequently between sLea and sLex) is mainly controlled by the relative levels of expression of β1,3- and β1,4-galactosyltransferases. Seven β1,4-galactosyltransferase enzymes (B4GALT1–7) are known in humans [29]. B4GALT1, the first cloned glycosyltransfease and one of the most deeply investigated, is the one forming with lactalbumin the lactose synthase complex [30]. Association of B4GALT1 with sLex expression has been reported in cancer [31] but little data on the other members of the family are available. Up-regulation of type 2 chains [32], of B4GALT1 [33], and B4GALT4 [34] has been reported in colon cancer and is associated with malignancy.
Four β1,3-galactosyltransferases (B3GALTs) have been described in humans: B3GALT1–2 and B3GALT4–5. Much less data are available on B3GALTs than on B4GALTs. Sometimes they are limited to a single report, as in the case of B3GALT2 [35], whose function is totally unknown. The role of B3GALT5 in gastrointestinal tissues is rather well defined. In vivo and in cell models its expression is closely associated with that of Lewis type 1 antigens, including sLea [36,37]. B3GALT5 acts on N- and O-linked chains of glycoproteins, on glycolipids, and on soluble type 1 chain oligosaccharides [37,38]. In glycolipids, it is specifically responsible for globo-series elongation [39]. In Chinese hamster ovary (CHO) cells, it competes with B4GALTs, blocking poly-N-acetyllactosamine chain elongation and sLex synthesis [37], while its silencing in the pancreatic cancer cell line BxPC-3 reduces sLea expression and increases sLex secretion [38]. B3GALT5 is strongly expressed in the normal colonic mucosa, resulting in Lewis a and, to a much lower extent, in sLea expression. Conversely, the B3GALT5 transcripts are down-regulated in colon cancer, where type 1 Lewis antigens are scarcely or not detectable [37,40,41]. In normal and pancreatic cancer tissues the amount of B3GALT5 mRNA is similar, and lower than in the colon mucosa. In both cases, type 1 chain Lewis antigens are well detectable, but only in the glandular ducts or in the ductal-like structures present in the relatively well differentiated adenocarcinomas [40]. In CHO cells, B3GALT1 appeared to be able to synthesize sLea [37] and it was recently proposed as the enzyme responsible for sLea synthesis in other tissues, such as the prostate [42]. Notably, in cell lines derived from gastrointestinal cancers, the expression of sLea and/or sLex is rather infrequent. The cell lines COLO-205, SW-1116 and LS-174T express sLex, and the first two surprisingly express also high amounts of sLea. This finding apparently contradicts the sLex/sLea competition revealed by the studies in CHO and BxPC3 cells above reported, and suggests that multiple variables affect the relative expression of such antigens in vivo.
Another important biosynthetic step of selectin ligand biosynthesis is represented by the addition of the GlcNAc residue preceding the galactosyl residues. The GlcNAc transferases involved differ depending on the nature of the carbohydrate chain. On N-linked chains, the elongation of the polylactosaminic chains bearing sLex occurs mainly in the β-1,6 branching, whose biosynthesis is controlled by the metastasis-associated enzyme GnT5 [43]. On the O-linked chains of glycoproteins, sLex/sLea expression is strongly dependent on Core 2 GlcNAc transferase (C2GnT) [44], while in glycolipids a pivotal role in their biosynthesis appears to be played by a β1,3GlcNAc transferase which synthesizes a common precursor of both type 1 and 2 chain Lewis structures [45]. Interestingly, this enzyme is activated by Helicobacter pylori infection, leading in stomach cells to increased expression of sLex, which is a ligand for H. pylori adhesin SabA [46].
3. Regulation of sLea and sLex
Unfortunately, the knowledge of glycosyltransferase gene regulations and its relation to cancer is still preliminary. Epigenetic control is supposed to be relevant in many cases [47,48], while other emerging mechanisms controlling gene expression, such as the presence of regulatory RNA sequence and or RNA binding proteins [49], are, as yet, unexplored. At present, data are available about promoter sequences and potential regulatory elements operating in various tissues [50,51,52,53,54,55,56,57], while those actually involved in gastrointestinal cancer are described in very few cases [43,58,59,60,61]. This is a very promising field of research since a bidirectional relationship may exist between the expression of sLe antigens and the mechanisms controlling cell growth. For instance, the expression of the metastasis-suppressive gene Nm23-H1 in a human hepatocarcinoma cell line induces down-regulation of both FUTs and ST3GALs, resulting in reduced sLex expression [62]. On the other hand, sLe antigens expressed on the TGF-β receptor enhance colon cancer cell migration through promotion of the epithelial to mesenchymal transition [63], which instead was found associated to the activity of the ST6GAL1 gene in other cancer cell lines [64].
It is well known that oncogenes are able to activate glycosyltransferases involved in the biosynthesis of cancer-related carbohydrate structures. Good examples are provided by the MGAT5 gene which is up-regulated by src [65], ErbB2 [66], v-sis [67] and Ras [68,69] oncogenes, through Ets-1 transcription factor [70,71] and by sialyltransferase ST6GAL1, which is regulated by both N-ras and H-ras through RalGEF signaling [72,73,74,75,76]. Also, the biosynthesis of Lewis type structures is affected by oncogenes. In gastric cancer cells, the forced expression of ST3GAL4 led to increased invasive potential through the biosynthesis of sLex, which, in turn, activates the c-Met pathway [77]. In addition, the expression of the Lewis y antigen, induced by the forced expression of FUT4 [78,79] or of FUT2 [80], led to increased cell growth through activation of a signal transduction pathway generated at the cell membrane level. Colon cancer cells induced to epithelial to mesenchymal transition by EGF and/or bFGF, displayed increased sLea/sLex expression because of up-regulation of ST3GAL1, -3 and -4 and of FUT3, with a concomitant decrease of FUT2. These effects were dependent on c-myc expression [81]. Altogether, these data indicate that both sialylated (sLex/sLea) and non-sialylated (Lewis y) Lewis type antigens, when expressed on cell membrane receptors, can activate an “outside-in” (from the cell membrane to the nucleus) signal transduction pathways leading to increased growth and malignancy. On the other hand, expression of activated oncogenes leads to overexpression of Lewis-type antigens through glycosyltransferase stimulation, generating an “inside-out” (from the nucleus to the cell membrane) flow of information. It can be hypothesized that these two opposite flows of information generate a loop self-fueling invasive cell growth.
Some reports also suggested that sLe expression is regulated by mechanisms alternative to glycosyltransferase regulation. They include the cancer-associated expression of nucleotide-donor transporters [82,83,84] and hypoxia [85], sometimes working in tandem [83]. These data were not further developed and need to be evaluated on the light of recent papers dealing with the derangement of fucosylation occurring in cancer. A general loss of the fucosylation potential has been reported in fact in up to 13% of colorectal cancers due to mutations of an enzyme involved in the biosynthesis of GDP-Fuc [86,87], the mandatory donor of fucose in all fucosyltransferase-catalyzed reactions. Such mutation and the consequent loss of fucosylation was very recently reported to enhance inflammation and tumors in the mouse [88]. On the other side, a general increase of fucosylation was also reported in colorectal cancer, as suggested by the binding of Aleuria aurantia lectin [89], that preferentially recognizes fucose α1,2/3/6 linked to lactosamine units [90]. Increased fucosylation levels were also reported in cancer stem cells of the pancreas and associated to chemoresistance in pancreatic cell lines [91].
4. Plasma Carriers of sLex or sLea
Owing to the role of sLe antigens in cancer diagnosis, the nature of their carrier molecules in plasma is of crucial importance. In fact, the global level of expression of these antigens in plasma does not always provide the sufficient sensitivity and/or specificity to discriminate cancer from non-malignant conditions, nor to follow disease progression, while the presence of sLe epitopes on specific glycoproteins can provide the necessary sensitivity and/or specificity. For example, in pancreatic cancer (Table 1), the CA19.9 antigen has been reported to be carried by MUC1, MUC5AC and MUC16 [92,93], and also by apolipoprotein B-100, kininogen and apolipoprotein E [94]. However, while on MUC16 the up-regulation of CA19.9 parallels that of the protein carrier (indicating that no real change of glycosylation occurred), on MUC1 and MUC5A the elevation of CA19.9 was not due to the increased expression of the protein carrier [93]. The protein carrier may reflect the disease state.
Table 1.
Plasma protein-carrying sialyl-Lewis (sLe) antigens in pancreatic diseases. sLea: sialyl-Lewis a; sLex: sialyl-Lewis x.
| Selectin Ligand | Carrier Molecule | Disease Involved | Reference |
|---|---|---|---|
| sLea | MUC1 | cancer > chronic pancreatitis | [92,93] |
| sLea | MUC5AC | cancer > chronic pancreatitis | [92,93] |
| sLea | MUC16 | chronic pancreatitis > cancer | [92,93] |
| sLea | Apo-B-100 | cancer = chronic pancreatitis | [94] |
| sLea | Apo-E | cancer = chronic pancreatitis | [94] |
| sLea | Kininogen | cancer = chronic pancreatitis | [94] |
| sLex | α1-acid glycoprotein | cancer > chronic pancreatitis | [95] |
| sLex | Ceruloplasmin | cancer > chronic pancreatitis | [95] |
| sLex | Haptoglobin | chronic pancreatitis > cancer | [95] |
| sLex | Fetuin | chronic pancreatitis > cancer | [95] |
| sLex | Antitrypsin | chronic pancreatitis > cancer | [95] |
| sLex | Transferrin | chronic pancreatitis > cancer | [95] |
In fact, MUC16 is the most prevalent CA19.9 carrier in pancreatitis, while MUC5AC and MUC1 are the preferential carriers in cancer. Acute phase proteins are carriers of sLex in pancreatic diseases [95]. In particular, increased sLex expression on α1-acid glycoprotein occurs in both cancer and chronic pancreatitis, while increased expression on haptoglobin, fetuin, antitrypsin and transferrin is associated only with chronic pancreatitis, not with pancreatic cancer [95]. Ceruloplasmin is a carrier of sLex in pancreatic diseases; an increase of the sLex/ceruloplasmin ratio is present in pancreatic cancer [96]. In plasma of pancreatic cancer patients, CA19.9 is carried also by glycolipids associated with the bile globular membrane, a membrane vesicle secreted by normal bile [97]. In some colorectal cancer patients, sLex is carried by α1-acid glycoprotein in both tissues and plasma [98]. By using high density antibody arrays, a variety of glycoproteins expressing sLea and/or sLex were identified in plasma of colon cancer patients [99]. SPARC, coagulation factor V, neuropeptide Y, complement C2 and α2-macroglobulin were among the glycoproteins more strongly expressing sLea. These glycoproteins (and many others) also expressed sLex. In pancreatic and colon cancer cell lines, MUC1 is a carrier of various Lewis type antigens, including sLea and sLex [100]. In gastric cancer, an increased expression of sLex, carried by acute response proteins released by the liver, is probably dependent on the systemic inflammatory status associated with cancer [101].
Interestingly, the fucosylation pattern of some serum proteins is specifically affected in gastrointestinal cancer patients. For example, pancreatic ductal adenocarcinomas are associated with increased α1,3fucosylation of α1-acid glycoprotein, which is of hepatic origin [102], while colorectal cancers are associated with altered core-fucosylation of IgG glycans, which are produced by plasma cells [103]. In particular, the core-fucosylation was increased in non-sialylated IgG glycans and decreased in those sialylated. Since the glycosylation of IgG glycans is strongly dependent on the inflammatory status (reviewed in [104]), it is possible that the cancer-associated systemic inflammation affects the glycosylation machinery of distant tissues. Pro-inflammatory cytokines have been reported to regulate the expression of glycosyltransferases involved in the biosynthesis of disease-associated glycans. In particular, TNF was found able to up-regulate ST3GAL4 in human lung increasing sLex expression [105]. It is worth recalling that sLe antigens can be carried by a glycosphingolipid backbone, forming gangliosides. sLe gangliosides potentially circulate in the bloodstream in the form of membrane vescicles [97], and are actually effective selectin ligands [106,107]. In colon cancer cells, sLea ganglioside was found able to bind E-selectin and promote cell adhesion upon removal of mucin carrying sLea [108], suggesting a potential cooperation between the various carrier structures.
5. Role in Diagnosis
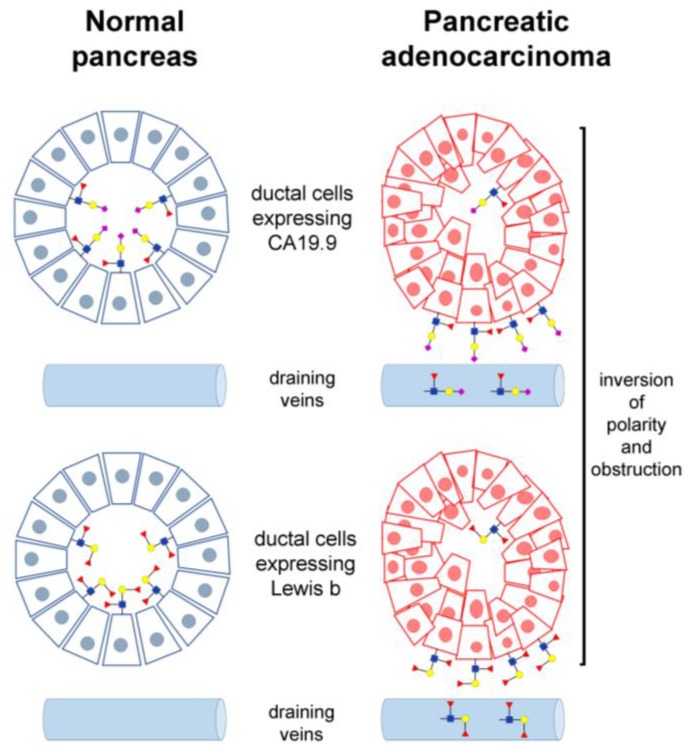
Due to the general derangement of the glycosylation machinery associated with cancer [109], many carbohydrate structures are potential markers for early diagnosis of gastrointestinal cancers. However, sLea remains the most consolidated and used carbohydrate tumor marker. As the epitope of CA19.9 antigen, it represents the target of the monoclonal antibody 1116-NS-19-9 developed 30 years ago immunizing mice with the colon cancer cell line SW-1116. At present, the antigen is determined for multiple purposes in the serum of patients suffering various gastrointestinal diseases, leading to an enormous number of clinical determinations whose rationale is questioned [110,111]. According to the American Society of Clinical Oncology [112] and the European Group on Tumor Markers [113], determination of CA19.9 is recommended only for the follow-up, surveillance, and response to therapy of pancreatic cancer. Taking into account the prevalence of pancreatic cancer, the expected number of CA19.9 determinations was calculated to be 10-fold lower than the number of tests actually performed in a western country [111].This is probably due to the assumption that serum CA19.9 values may help the differential diagnosis of several gastrointestinal diseases, including cancers arising not only in the pancreas [114,115,116], but in the colon [117], stomach [118,119], and bile ducts [120]. In this regard, it is important to recall that the synthesis, expression, and secretion of CA19.9 was demonstrated in many healthy tissues, including the pancreas [121] and the bile ducts [122]. In fact, both the pancreatic juice and the bile physiologically contain relevant amounts of the antigen. The question is why blood CA19.9 increases in some patients but not in others, and why elevated levels of serum CA19.9 are found in cancer patients and in patients affected by non-malignant diseases as well. The highest CA19.9 serum values are reported in severely jaundiced patients [123], while the elevation in chronic pancreatitis is lower and overlapping that of pancreatic cancer patients [124]. To explain these observations, several years ago an hypothesis was formulated proposing that in pancreatic cancer the increased serum CA19.9 levels were due to the inversion of polarity of transformed ductal cells [125] and to the obstruction of the ducts, which in turn give rise to the abundant reabsorption of the antigen in the blood [124]. On the other hand, immunohistochemical detection suggested an abundant and rather specific expression of CA19.9 in pancreas and colon cancer, and not in the surrounding normal tissue [126,127,128]. Normal colon was rather considered to express the structurally related antigens Lewis a and Lewis b [129]. These observations were further consolidated by the discovery of disialyl-Lewis a in healthy colon mucosa, due to the activity of the sialyltranferase ST6GALNAC6 (Figure 1). It was proposed that sLea expression in colon cancer was due the loss of disialyl-Lewis a because of the down-regulation of ST6GALNAC1 [130]. However, differential expression of disialyl-Lewis a is not known in organs other than the colon, and colon cancer is associated with a strong down-regulation of B3GALT5 [37,41,131], the key enzyme in the synthesis of all Lewis type 1 antigens, including sLea (Figure 1). Coherently, CA19.9 was scarcely detected in colon cancer by dot-blotting [40,132] or western blotting [16]. This apparent contradiction was recently explained by the finding that using the 1116-NS-19-9 anti-CA19.9 antibody by immunohistochemistry generates artifacts [133]. In contrast, the use of the same antibody by immunofluorescence correctly confirmed the absence of CA19.9 in colon cancer, suggesting that the antigen circulating in such patients may originate outside the colon, i.e. from the bile [40]. Immunofluorescence also showed that in normal pancreas, CA19.9 expression is restricted to the ducts, and in cancer it is restricted to ductal like structures, while the bulk of the malignant cells lacks expression. Altogether, these data suggest a re-evaluation of the hypothesis, based on the obstruction of the ducts and the reverse of polarity, which provides a more satisfactory explanation for serum CA19.9 elevation. This hypothesis predicts that other carbohydrate antigens synthesized and secreted by pancreatic ductal cells may undergo reabsorption in the blood due to obstruction and reverse of polarity occurring in cancer (Figure 2). Recently, Lewis b was actually found expressed in a pancreatic cancer patient lacking CA19.9 [40], suggesting that such antigen may turn useful for monitoring CA19.9-negative patients.
Figure 2.
Proposed model of the elevation of circulating CA19.9 in pancreatic cancer patients. The inversion of polarity occurring in malignant ductal cells [125] together with the obstruction of the cancerous ducts determine reabsorption by the blood of the molecules normally secreted by the organ [121,124]. According to recent data [40], the expression of type 1 chain Lewis antigens in the pancreas is regulated by the individual glycosyltransferase pattern, which is not deregulated in cancer. Consequently, serum CA19.9 levels increase only in those patients actually expressing the antigen in their normal ducts. Non-malignant pancreatic diseases causing obstruction, such as chronic pancreatitis, are expected to determine partially overlapping elevation of circulating antigens. Sugars are depicted as in Figure 1.
In the future, it would be advisable to detect all Lewis antigens in tissue sections of surgical specimens by immunofluorescence and compare the results with the clinical records of the patients, including their serum CA19.9 levels. In fact, a relevant issue to be addressed concerns the potential expression of sLex in conjunction or alternative to CA19.9. Recently, it was proposed that CA19.9-negative cancer patients may be monitored through circulating sLex [134], and the expression of sLex in distinct glycans appears to be independent of that of sLea, potentially improving the diagnostic accuracy over CA19.9 [135]. The expression of sLex on plasma protein seems to be actually associated with gastrointestinal cancers, as above reported [96].
6. Role in Malignancy
sLe antigens may promote metastasis by forming emboli of cancer cells and platelets, which favor their arrest on endothelia [136,137,138,139]. In colon cancer patients, increased expression of sLex and sLea correlated with metastasis and poor survival [140,141,142,143]. Down-regulation of sLe expression by knock-down of key glycosyltransferases in cancer cell lines resulted in reduced selectin binding and reduced metastatic ability [11,12,144], while forced expression of sLe antigens by gene transfer increased adhesion to selectins in vitro and metastatic ability in vivo [10]. The role of selectins in the metastatic process was confirmed by the finding that the formation of experimental pulmonary metastases could be inhibited by the use of peptides mimicking sLea and was inhibited in E-selectin- knock-out mice [145]. Owing to the fact that mesothelial cells express, like endothelial cells, E- and P-selectins, the two adhesion molecules are crucial also for peritoneal spread of pancreatic carcinoma [146].
Beside adhesion, sLe antigens influence also angiogenesis and immune recognition of cancer cells. The role of sLex in angiogenesis is indicated by the observation that the formation of tube-like networks of endothelial cells induced by the co-culture with cancer cells can be inhibited by antibodies against sLex [147]. Moreover, the ability of hepatocarcinoma cells to promote angiogenesis was inhibited by blocking sLex biosynthesis [148]. On the other hand, forced expression of sLea and type 1 Lewis antigens in a colon cancer cell line by transfection with B3GALT5 also resulted in increased angiogenesis and tumor growth [133]. Altogether, these results suggest that angiogenesis is stimulated by sLe antigens, regardless of the underlying carbohydrate structure (sLea or sLex). Cells expressing high levels of sLex are a better target of natural killer cells and thus less metastatic than those expressing moderate levels of the antigen [149,150].
Many of the experimental models reported above utilized subsets of cancer cell lines or engineered cell clones differing in the expression of the terminal sLe epitopes, which are seeded in contact with endothelial cells in vitro, or inoculated in nude mice to form xenografts. Although their presence is the basic requirement for selectin binding, the mere over-expression of sLea and/or sLex does not necessarily imply an increase of the adhesion properties of the cells. Conversely, a shift from the expression of a particular carrier molecule to another has the potential to alter more deeply the adhesion properties of the cells. At present, the actual expression of sLe antigens in native gastrointestinal cancers is not adequately known because of the little available data [151,152], potentially affected by artifacts [127,128,141]. Particularly, in colon cancer evidence of the expression of genuine CA19.9 is missing, and elevation of the circulating antigen is reported mainly in advanced stages of the disease [153,154]. In none of cases reported in vivo was any information available about the nature of the carrier molecules.
7. Concluding Remarks
A large number of clinical and experimental data is now available through many papers dealing with the relationship between the expression of sLe antigens and the phenotype of various cancer types, including those of gastrointestinal origin. The biosynthesis of sLe antigens is mainly controlled through the regulation of several glycosyltransferase genes, which has been elucidated only for few of them. Consequently, it is not yet possible to link sLe expression to the mechanisms controlling cell growth. It is well established that sLe expression has the potential to deeply influence the phenotype of cancer cells by modulating the adhesion properties, the angiogenic ability, and the immune recognition. However, the actual and reciprocal expression of sLea or sLex in native gastrointestinal cancers is still questioned and needs to be unambiguously confirmed in the near future. In particular, recent data indicated that the detection by histochemistry of sLea/CA19.9 by the NS-1116-19-9 antibody provided artifacts, suggesting re-evaluation of Lewis antigen expression by immunofluorescence. Moreover, the nature of the carrier molecules and their ability to constitute the proper scaffold promoting cell adhesion in vivo requires further attention. In particular, the peculiar role of gangliosides as selectin ligands deserves dedicated experiments. The presence of these antigens has been identified on a variety of protein and non-protein carriers in the plasma. Selectin ligands associated with specific carrier molecules represent a class of novel markers potentially useful in early diagnosis, as they are able to discriminate neoplastic and non-neoplastic diseases. At present, the simple detection of sLe antigens fails in targeting such clinical aims. In several cases, these antigens are carried by molecules that presumably have not been synthesized by the cancer cells, indicating an indirect mechanism for their overexpression. The proposed biliary origin of circulating CA19.9 in colon cancer patients [40,133] needs further investigation but provides a paradigm of the indirect mechanisms of production of carbohydrate markers circulating in some cancer patients.
Acknowledgments
This work was supported by grants from the University of Insubria to Marco Trinchera. Adele Aronica was supported by the Ph.D. Program in Molecular and Translational Medicine of the University of Milan.
Author Contributions
The three authors together planned the overall scope and organization of the text. Marco Trinchera wrote the initial version of the article that was completed by Fabio Dall’Olio. All three authors read and edited the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.McEver R.P. Selectins: Initiators of leucocyte adhesion and signalling at the vascular wall. Cardiovasc. Res. 2015;107:331–339. doi: 10.1093/cvr/cvv154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kawashima H., Petryniak B., Hiraoka N., Mitoma J., Huckaby V., Nakayama J., Uchimura K., Kadomatsu K., Muramatsu T., Lowe J.B., et al. N-acetylglucosamine-6-O-sulfotransferases 1 and 2 cooperatively control lymphocyte homing through L-selectin ligand biosynthesis in high endothelial venules. Nat. Immunol. 2005;6:1096–1104. doi: 10.1038/ni1259. [DOI] [PubMed] [Google Scholar]
- 3.Kawashima H., Fukuda M. Sulfated glycans control lymphocyte homing. Ann. N. Y. Acad. Sci. 2012;1253:112–121. doi: 10.1111/j.1749-6632.2011.06356.x. [DOI] [PubMed] [Google Scholar]
- 4.Vajaria B.N., Patel P.S. Glycosylation: A hallmark of cancer? Glycoconj. J. 2016 doi: 10.1007/s10719-016-9755-2. [DOI] [PubMed] [Google Scholar]
- 5.Fujita T., Murayama K., Hanamura T., Okada T., Ito T., Harada M., Komatsu A., Koyama H., Kanai T., Maeno K., et al. CsLex (Sialyl Lewis X) is a Useful Tumor Marker for Monitoring of Breast Cancer Patients. Jpn. J. Clin. Oncol. 2011;41:394–399. doi: 10.1093/jjco/hyq190. [DOI] [PubMed] [Google Scholar]
- 6.Liang J.X., Liang Y., Gao W. Clinicopathological and prognostic significance of sialyl Lewis X overexpression in patients with cancer: A meta-analysis. OncoTargets. Ther. 2016;9:3113–3125. doi: 10.2147/OTT.S102389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lowe J.B. Glycosylation in the control of selectin counter-receptor structure and function. Immunol. Rev. 2002;186:19–36. doi: 10.1034/j.1600-065X.2002.18603.x. [DOI] [PubMed] [Google Scholar]
- 8.Dupuy F., Germot A., Marenda M., Oriol R., Blancher A., Julien R., Maftah A. α1,4-Fucosyltransferase Activity: A Significant Function in the Primate Lineage has Appeared Twice Independently. Mol. Biol. Evol. 2002;19:815–824. doi: 10.1093/oxfordjournals.molbev.a004138. [DOI] [PubMed] [Google Scholar]
- 9.Carvalho A.S., Harduin-Lepers A., Magalhaes A., Machado E., Mendes N., Costa L.T., Matthiesen R., Almeida R., Costa J., Reis C.A. Differential expression of α-2,3-sialyltransferases and α-1,3/4-fucosyltransferases regulates the levels of sialyl Lewis a and sialyl Lewis x in gastrointestinal carcinoma cells. Int. J. Biochem. Cell Biol. 2010;42:80–89. doi: 10.1016/j.biocel.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Perez-Garay M., Arteta B., Pages L., De Llorens R., de Bolos C., Vidal-Vanaclocha F., Peracaula R. α2,3-sialyltransferase ST3Gal III modulates pancreatic cancer cell motility and adhesion in vitro and enhances its metastatic potential in vivo. PLoS ONE. 2010;5:e12524. doi: 10.1371/journal.pone.0012524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiller K.M., Mayben J.P., Bendt K.M., Manousos G.A., Senger K., Cameron H.S., Weston B.W. Transfection of α1,3 fucosyltransferase antisense sequences impairs the proliferative and tumorigenic ability of human colon carcinoma cells. Mol. Carcinog. 2000;27:280–288. doi: 10.1002/(SICI)1098-2744(200004)27:4<280::AID-MC6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 12.Weston B.W., Hiller K.M., Mayben J.P., Manousos G.A., Bendt K.M., Liu R., Cusack J.C., Jr. Expression of human α1,3 fucosyltransferase antisense sequences inhibits selectin-mediated adhesion and liver metastasis of colon carcinoma cells. Cancer Res. 1999;59:2127–2135. [PubMed] [Google Scholar]
- 13.Beum P.V., Singh J., Burdick M., Hollingsworth M.A., Cheng P.W. Expression of core 2 β1,6-N-acetylglucosaminyltransferase in a human pancreatic cancer cell line results in altered expression of MUC1 tumor-associated epitopes. J. Biol. Chem. 1999;274:24641–24648. doi: 10.1074/jbc.274.35.24641. [DOI] [PubMed] [Google Scholar]
- 14.Perez-Garay M., Arteta B., Llop E., Cobler L., Pages L., Ortiz R., Ferri M.J., de Bolos C., Figueras J., De Llorens R., et al. α2,3-Sialyltransferase ST3Gal IV promotes migration and metastasis in pancreatic adenocarcinoma cells and tends to be highly expressed in pancreatic adenocarcinoma tissues. Int. J. Biochem. Cell Biol. 2013;45:1748–1757. doi: 10.1016/j.biocel.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 15.Nakamori S., Nishihara S., Ikehara Y., Nagano H., Dono K., Sakon M., Narimatsu H., Monden M. Molecular mechanism involved in increased expression of sialyl Lewis antigens in ductal carcinoma of the pancreas. J. Exp. Clin. Cancer Res. 1999;18:425–432. [PubMed] [Google Scholar]
- 16.Kudo T., Ikehara Y., Togayachi A., Morozumi K., Watanabe M., Nakamura M., Nishihara S., Narimatsu H. Up-regulation of a set of glycosyltransferase genes in human colorectal cancer. Lab. Investig. 1998;78:797–811. [PubMed] [Google Scholar]
- 17.Trinchera M., Malagolini N., Chiricolo M., Santini D., Minni F., Caretti A., Dall’Olio F. The biosynthesis of the selectin-ligand sialyl Lewis x in colorectal cancer tissues is regulated by fucosyltransferase VI and can be inhibited by an RNA interference-based approach. Int. J. Biochem. Cell Biol. 2011;43:130–139. doi: 10.1016/j.biocel.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Dohi T., Hashiguchi M., Yamamoto S., Morita H., Oshima M. Fucosyltransferase-producing sialyl Lea and sialyl Lex carbohydrate antigen in benign and malignant gastrointestinal mucosa. Cancer. 1994;73:1552–1561. doi: 10.1002/1097-0142(19940315)73:6<1552::AID-CNCR2820730605>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Ito H., Hiraiwa N., Sawada-Kasugai M., Akamatsu S., Tachikawa T., Kasai Y., Akiyama S., Ito K., Takagi H., Kannagi R. Altered mRNA expression of specific molecular species of fucosyl- and sialyl-transferases in human colorectal cancer tissues. Int. J. Cancer. 1997;71:556–564. doi: 10.1002/(SICI)1097-0215(19970516)71:4<556::AID-IJC9>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 20.Dall’Olio F., Malagolini N., Chiricolo M., Trinchera M., Harduin-Lepers A. The expanding roles of the Sda/Cad carbohydrate antigen and its cognate glycosyltransferase B4GALNT2. Biochim. Biophys. Acta. 2014;1840:443–453. doi: 10.1016/j.bbagen.2013.09.036. [DOI] [PubMed] [Google Scholar]
- 21.Kawamura Y.I., Kawashima R., Fukunaga R., Hirai K., Toyama-Sorimachi N., Tokuhara M., Shimizu T., Dohi T. Introduction of Sda carbohydrate antigen in gastrointestinal cancer cells eliminates selectin ligands and inhibits metastasis. Cancer Res. 2005;65:6220–6227. doi: 10.1158/0008-5472.CAN-05-0639. [DOI] [PubMed] [Google Scholar]
- 22.Malagolini N., Santini D., Chiricolo M., Dall’Olio F. Biosynthesis and expression of the Sda and sialyl Lewis x antigens in normal and cancer colon. Glycobiology. 2007;17:688–697. doi: 10.1093/glycob/cwm040. [DOI] [PubMed] [Google Scholar]
- 23.Izawa M., Kumamoto K., Mitsuoka C., Kanamori C., Kanamori A., Ohmori K., Ishida H., Nakamura S., Kurata-Miura K., Sasaki K., et al. Expression of sialyl 6-sulfo Lewis X is inversely correlated with conventional sialyl Lewis X expression in human colorectal cancer. Cancer Res. 2000;60:1410–1416. [PubMed] [Google Scholar]
- 24.Lo Presti L., Cabuy E., Chiricolo M., Dall’Olio F. Molecular Cloning of the Human b1,4 N-Acetylgalactosaminyltransferase Responsible for the Biosynthesis of the Sda Histo-Blood Group Antigen: The Sequence Predicts a Very Long Cytoplasmic Domain. J. Biochem. 2003;134:675–682. doi: 10.1093/jb/mvg192. [DOI] [PubMed] [Google Scholar]
- 25.Montiel M.D., Krzewinski-Recchi M.A., Delannoy P., Harduin-Lepers A. Molecular cloning, gene organization and expression of the human UDP-GalNAc:Neu5Aca2-3Galb-R β1,4-N-acetylgalactosaminyltransferase responsible for the biosynthesis of the blood group Sda/Cad antigen: Evidence for an unusual extended cytoplasmic domain. Biochem. J. 2003;373:369–379. doi: 10.1042/bj20021892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dohi T., Yuyama Y., Natori Y., Smith P.L., Lowe J.B., Oshima M. Detection of N-acetylgalactosaminyltransferase mRNA which determines expression of Sda blood group carbohydrate structure in human gastrointestinal mucosa and cancer. Int. J. Cancer. 1996;67:626–631. doi: 10.1002/(SICI)1097-0215(19960904)67:5<626::AID-IJC6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 27.Malagolini N., Dall’Olio F., Di Stefano G., Minni F., Marrano D., Serafini-Cessi F. Expression of UDP-GalNAc:NeuAc α2,3Gal b-R beta 1,4(GalNAc to Gal) N-acetylgalactosaminyltransferase involved in the synthesis of Sda antigen in human large intestine and colorectal carcinomas. Cancer Res. 1989;49:6466–6470. [PubMed] [Google Scholar]
- 28.Groux-Degroote S., Wavelet C., Krzewinski-Recchi M.A., Portier L., Mortuaire M., Mihalache A., Trinchera M., Delannoy P., Malagolini N., Chiricolo M., et al. B4GALNT2 gene expression controls the biosynthesis of Sda and sialyl Lewis X antigens in healthy and cancer human gastrointestinal tract. Int. J. Biochem. Cell Biol. 2014;53:442–449. doi: 10.1016/j.biocel.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 29.Furukawa K., Sato T. Beta-1,4-galactosylation of N-glycans is a complex process. Biochim. Biophys. Acta. 1999;1473:54–66. doi: 10.1016/S0304-4165(99)00169-5. [DOI] [PubMed] [Google Scholar]
- 30.Qasba P.K., Ramakrishnan B., Boeggeman E. Structure and function of β-1,4-galactosyltransferase. Curr. Drug Targets. 2008;9:292–309. doi: 10.2174/138945008783954943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Obaide M.A., Alobydi H., Abdelsalam A.G., Zhang R., Srivenugopal K.S. Multifaceted roles of 5′-regulatory region of the cancer associated gene B4GALT1 and its comparison with the gene family. Int. J. Oncol. 2015;47:1393–1404. doi: 10.3892/ijo.2015.3136. [DOI] [PubMed] [Google Scholar]
- 32.Holmes E.H., Ostrander G.K., Clausen H., Graem N. Oncofetal expression of Lex carbohydrate antigens in human colonic adenocarcinomas. Regulation through type 2 core chain synthesis rather than fucosylation. J. Biol. Chem. 1987;262:11331–11338. [PubMed] [Google Scholar]
- 33.Ichikawa T., Nakayama J., Sakura N., Hashimoto T., Fukuda M., Fukuda M.N., Taki T. Expression of N-acetyllactosamine and β1,4-galactosyltransferase (b4GalT-I) during adenoma-carcinoma sequence in the human colorectum. J. Histochem. Cytochem. 1999;47:1593–1602. doi: 10.1177/002215549904701211. [DOI] [PubMed] [Google Scholar]
- 34.Chen W.S., Chang H.Y., Li C.P., Liu J.M., Huang T.S. Tumor b-1,4-galactosyltransferase IV overexpression is closely associated with colorectal cancer metastasis and poor prognosis. Clin. Cancer Res. 2005;11:8615–8622. doi: 10.1158/1078-0432.CCR-05-1006. [DOI] [PubMed] [Google Scholar]
- 35.Kolbinger F., Streiff M.B., Katopodis A.G. Cloning of a human UDP-galactose:2-acetamido-2-deoxy-D-glucose 3b- galactosyltransferase catalyzing the formation of type 1 chains. J. Biol. Chem. 1998;273:433–440. doi: 10.1074/jbc.273.1.433. [DOI] [PubMed] [Google Scholar]
- 36.Isshiki S., Togayachi A., Kudo T., Nishihara S., Watanabe M., Kubota T., Kitajima M., Shiraishi N., Sasaki K., Andoh T., et al. Cloning, expression, and characterization of a novel UDP-galactose:b- N-acetylglucosamine b1,3-galactosyltransferase (b3Gal-T5) responsible for synthesis of type 1 chain in colorectal and pancreatic epithelia and tumor cells derived therefrom. J. Biol. Chem. 1999;274:12499–12507. doi: 10.1074/jbc.274.18.12499. [DOI] [PubMed] [Google Scholar]
- 37.Salvini R., Bardoni A., Valli M., Trinchera M. β1,3-Galactosyltransferase b3Gal-T5 acts on the GlcNAcb1-->3Galb1-->4GlcNAcb1-->R sugar chains of carcinoembryonic antigen and other N-linked glycoproteins and is down-regulated in colon adenocarcinomas. J. Biol. Chem. 2001;276:3564–3573. doi: 10.1074/jbc.M006662200. [DOI] [PubMed] [Google Scholar]
- 38.Mare L., Trinchera M. Suppression of β1,3galactosyltransferase β3Gal-T5 in cancer cells reduces sialyl-Lewis a and enhances poly N-acetyllactosamines and sialyl-Lewis x on O-glycans. Eur. J. Biochem. 2004;271:186–194. doi: 10.1046/j.1432-1033.2003.03919.x. [DOI] [PubMed] [Google Scholar]
- 39.Cheung S.K., Chuang P.K., Huang H.W., Hwang-Verslues W.W., Cho C.H., Yang W.B., Shen C.N., Hsiao M., Hsu T.L., Chang C.F., et al. Stage-specific embryonic antigen-3 (SSEA-3) and beta3GalT5 are cancer specific and significant markers for breast cancer stem cells. Proc. Natl. Acad. Sci. USA. 2016;113:960–965. doi: 10.1073/pnas.1522602113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aronica A., Avagliano L., Caretti A., Tosi D., Bulfamante G.P., Trinchera M. Unexpected distribution of CA19.9 and other type 1 chain Lewis antigens in normal and cancer tissues of colon and pancreas: Importance of the detection method and role of glycosyltransferase regulation. Biochim. Biophys. Acta. 2017;1861:3210–3220. doi: 10.1016/j.bbagen.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 41.Isshiki S., Kudo T., Nishihara S., Ikehara Y., Togayachi A., Furuya A., Shitara K., Kubota T., Watanabe M., Kitajima M., et al. Lewis type 1 antigen synthase (b3Gal-T5) is transcriptionally regulated by homeoproteins. J. Biol. Chem. 2003;278:36611–36620. doi: 10.1074/jbc.M302681200. [DOI] [PubMed] [Google Scholar]
- 42.Chachadi V.B., Ali M.F., Cheng P.W. Prostatic cell-specific regulation of the synthesis of MUC1-associated sialyl Lewis a. PLoS ONE. 2013;8:e57416. doi: 10.1371/journal.pone.0057416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kizuka Y., Taniguchi N. Enzymes for N-Glycan Branching and Their Genetic and Nongenetic Regulation in Cancer. Biomolecules. 2016 doi: 10.3390/biom6020025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimodaira K., Nakayama J., Nakamura N., Hasebe O., Katsuyama T., Fukuda M. Carcinoma-associated expression of core 2 β1,6-N-acetylglucosaminyltransferase gene in human colorectal cancer: Role of O-glycans in tumor progression. Cancer Res. 1997;57:5201–5206. [PubMed] [Google Scholar]
- 45.Holmes E.H., Hakomori S., Ostrander G.K. Synthesis of type 1 and 2 lacto series glycolipid antigens in human colonic adenocarcinoma and derived cell lines is due to activation of a normally unexpressed b1,3N-acetylglucosaminyltransferase. J. Biol. Chem. 1987;262:15649–15658. [PubMed] [Google Scholar]
- 46.Marcos N.T., Magalhaes A., Ferreira B., Oliveira M.J., Carvalho A.S., Mendes N., Gilmartin T., Head S.R., Figueiredo C., David L., et al. Helicobacter pylori induces β3GnT5 in human gastric cell lines, modulating expression of the SabA ligand sialyl-Lewis x. J. Clin. Investig. 2008;118:2325–2336. doi: 10.1172/JCI34324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lauc G., Vojta A., Zoldoš V. Epigenetic regulation of glycosylation is the quantum mechanics of biology. Biochim. Biophys. Acta. 2014;1840:65–70. doi: 10.1016/j.bbagen.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 48.Nakamura K., Yamashita K., Sawaki H., Waraya M., Katoh H., Nakayama N., Kawamata H., Nishimiya H., Ema A., Narimatsu H., et al. Aberrant methylation of GCNT2 is tightly related to lymph node metastasis of primary CRC. Anticancer Res. 2015;35:1411–1421. [PubMed] [Google Scholar]
- 49.Pichon X., Wilson L.A., Stoneley M., Bastide A., King H.A., Somers J., Willis A.E. RNA binding protein/RNA element interactions and the control of translation. Curr. Protein Pept. Sci. 2012;13:294–304. doi: 10.2174/138920312801619475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mardahl M., Schröter M.F., Engelbert D., Pink M., Sperandio M., Hamann A., Syrbe U. Core 2 ß1,6-N-acetylglucosaminyltransferase-I, crucial for P-selectin ligand expression is controlled by a distal enhancer regulated by STAT4 and T-bet in CD4+ T helper cells 1. Mol. Immunol. 2016;77:132–140. doi: 10.1016/j.molimm.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 51.Ebel M.E., Kansas G.S. Defining the Functional Boundaries of the Murine a1,3-Fucosyltransferase Fut7 Reveals a Remarkably Compact Locus. J. Biol. Chem. 2014;289:6341–6349. doi: 10.1074/jbc.M113.511790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shan X., Aziz F., Tian L.L., Wang X.Q., Yan Q., Liu J.W. Ginsenoside Rg3-induced EGFR/MAPK pathway deactivation inhibits melanoma cell proliferation by decreasing FUT4/LeY expression. Int. J. Oncol. 2015;46:1667–1676. doi: 10.3892/ijo.2015.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bobowski M., Vincent A., Steenackers A., Colomb F., Van Seuningen I., Julien S., Delannoy P. Estradiol represses the G(D3) synthase gene ST8SIA1 expression in human breast cancer cells by preventing NFκB binding to ST8SIA1 promoter. PLoS ONE. 2013;8:e62559. doi: 10.1371/journal.pone.0062559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Teylaert B., Meurice E., Bobowski M., Harduin-Lepers A., Gaucher C., Fontayne A., Jorieux S., Delannoy P. Molecular cloning, characterization, genomic organization and promoter analysis of the α1,6-fucosyltransferase gene (fut8) expressed in the rat hybridoma cell line YB2/0. BMC Biotechnol. 2011 doi: 10.1186/1472-6750-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lehoux S., Groux-Degroote S., Cazet A., Dhaenens C.M., Maurage C.A., Caillet-Boudin M.L., Delannoy P., Krzewinski-Recchi M.A. Transcriptional regulation of the human ST6GAL2 gene in cerebral cortex and neuronal cells. Glycoconj. J. 2010;27:99–114. doi: 10.1007/s10719-009-9260-y. [DOI] [PubMed] [Google Scholar]
- 56.Choi H.J., Chung T.W., Kang N.Y., Kim K.S., Lee Y.C., Kim C.H. Involvement of CREB in the transcriptional regulation of the human GM3 synthase (hST3Gal V) gene during megakaryocytoid differentiation of human leukemia K562 cells. Biochem. Biophys. Res. Commun. 2004;313:142–147. doi: 10.1016/j.bbrc.2003.11.103. [DOI] [PubMed] [Google Scholar]
- 57.Appenheimer M.M., Huang R.Y., Chandrasekaran E.V., Dalziel M., Hu Y.P., Soloway P.D., Wuensch S.A., Matta K.L., Lau J.T. Biologic contribution of P1 promoter-mediated expression of ST6Gal I sialyltransferase. Glycobiology. 2003;13:591–600. doi: 10.1093/glycob/cwg066. [DOI] [PubMed] [Google Scholar]
- 58.Taniguchi A. Promoter structure and transcriptional regulation of human b-galactoside a2, 3-sialyltransferase genes. Curr. Drug Targets. 2008;9:310–316. doi: 10.2174/138945008783954998. [DOI] [PubMed] [Google Scholar]
- 59.Zulueta A., Caretti A., Signorelli P., Dall’Olio F., Trinchera M. Transcriptional control of the B3GALT5 gene by a retroviral promoter and methylation of distant regulatory elements. FASEB J. 2014;28:946–955. doi: 10.1096/fj.13-236273. [DOI] [PubMed] [Google Scholar]
- 60.Dabrowska A., Baczyńska D., Widerak K., Laskowska A., Ugorski M. Promoter analysis of the human alpha1,3/4-fucosyltransferase gene (FUT III) Biochim. Biophys. Acta. 2005;1731:66–73. doi: 10.1016/j.bbaexp.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 61.Taniuchi F., Higai K., Tanaka T., Azuma Y., Matsumoto K. Transcriptional regulation of fucosyltransferase 1 gene expression in colon cancer cells. Sci. World J. 2013 doi: 10.1155/2013/105464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Duan L.L., Guo P., Zhang Y., Chen H.L. Regulation of metastasis-suppressive gene Nm23-H1 on glycosyl-transferases involved in the synthesis of sialyl Lewis antigens. J. Cell Biochem. 2005;94:1248–1257. doi: 10.1002/jcb.20346. [DOI] [PubMed] [Google Scholar]
- 63.Hirakawa M., Takimoto R., Tamura F., Yoshida M., Ono M., Murase K., Sato Y., Osuga T., Sato T., Iyama S., et al. Fucosylated TGF-beta receptors transduces a signal for epithelial-mesenchymal transition in colorectal cancer cells. Br. J. Cancer. 2014;110:156–163. doi: 10.1038/bjc.2013.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lu J., Isaji T., Im S., Fukuda T., Hashii N., Takakura D., Kawasaki N., Gu J. β-Galactoside α2,6-Sialyltranferase 1 Promotes Transforming Growth Factor-beta-mediated Epithelial-Mesenchymal Transition. J. Biol. Chem. 2014;289:34627–34641. doi: 10.1074/jbc.M114.593392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buckhaults P., Chen L., Fregien N., Pierce M. Transcriptional regulation of N-acetylglucosaminyltransferase V by the src oncogene. J. Biol. Chem. 1997;272:19575–19581. doi: 10.1074/jbc.272.31.19575. [DOI] [PubMed] [Google Scholar]
- 66.Chen L., Zhang W., Fregien N., Pierce M. The her-2/neu oncogene stimulates the transcription of N-acetylglucosaminyltransferase V and expression of its cell surface oligosaccharide products. Oncogene. 1998;17:2087–2093. doi: 10.1038/sj.onc.1202124. [DOI] [PubMed] [Google Scholar]
- 67.Guo H.B., Zhang Q.S., Chen H.L. Effects of H-ras and v-sis overexpression on N-acetylglucosaminyltransferase V and metastasis-related phenotypes in human hepatocarcinoma cells. J. Cancer Res. Clin. Oncol. 2000;126:263–270. doi: 10.1007/s004320050341. [DOI] [PubMed] [Google Scholar]
- 68.Lu Y., Chaney W. Induction of N-acetylglucosaminyltransferase V by elevated expression of activated or proto-Ha-ras oncogenes. Mol. Cell Biochem. 1993;122:85–92. doi: 10.1007/BF00925741. [DOI] [PubMed] [Google Scholar]
- 69.Wojciechowicz D.C., Park P.Y., Datta R.V., Paty P.B. CEA is the major PHA-L-reactive glycoprotein in colon carcinoma cell lines and tumors: Relationship between K-ras activation and β1-6 branching of N-linked carbohydrate on CEA. Biochem. Biophys. Res. Commun. 2000;273:147–153. doi: 10.1006/bbrc.2000.2906. [DOI] [PubMed] [Google Scholar]
- 70.Kang R., Saito H., Ihara Y., Miyoshi E., Koyama N., Sheng Y., Taniguchi N. Transcriptional regulation of the N-acetylglucosaminyltransferase V gene in human bile duct carcinoma cells (HuCC-T1) is mediated by Ets-1. J. Biol. Chem. 1996;271:26706–26712. doi: 10.1074/jbc.271.43.26706. [DOI] [PubMed] [Google Scholar]
- 71.Ko J.H., Miyoshi E., Noda K., Ekuni A., Kang R., Ikeda Y., Taniguchi N. Regulation of the GnT-V promoter by transcription factor Ets-1 in various cancer cell lines. J. Biol. Chem. 1999;274:22941–22948. doi: 10.1074/jbc.274.33.22941. [DOI] [PubMed] [Google Scholar]
- 72.Dalziel M., Dall’Olio F., Mungul A., Piller V., Piller F. Ras oncogene induces b-galactoside α2,6-sialyltransferase (ST6Gal I) via a RalGEF-mediated signal to its housekeeping promoter. Eur. J. Biochem. 2004;271:3623–3634. doi: 10.1111/j.1432-1033.2004.04284.x. [DOI] [PubMed] [Google Scholar]
- 73.Delannoy P., Pelczar H., Vandamme V., Verbert A. Sialyltransferase activity in FR3T3 cells transformed with ras oncogene: Decreased CMP-Neu5Ac:Gal b1-3GalNAc α-2,3-sialyltransferase. Glycoconj. J. 1993;10:91–98. doi: 10.1007/BF00731192. [DOI] [PubMed] [Google Scholar]
- 74.Easton E.W., Bolscher J.G., van den Eijnden D.H. Enzymatic amplification involving glycosyltransferases forms the basis for the increased size of asparagine-linked glycans at the surface of NIH 3T3 cells expressing the N-ras proto-oncogene. J. Biol. Chem. 1991;266:21674–21680. [PubMed] [Google Scholar]
- 75.Le Marer N., Laudet V., Svensson E.C., Cazlaris H., van Hille B., Lagrou C., Stehelin D., Montreuil J., Verbert A., Delannoy P. The c-Ha-ras oncogene induces increased expression of b-galactoside α-2,6-sialyltransferase in rat fibroblast (FR3T3) cells. Glycobiology. 1992;2:49–56. doi: 10.1093/glycob/2.1.49. [DOI] [PubMed] [Google Scholar]
- 76.Seales E.C., Jurado G.A., Singhal A., Bellis S.L. Ras oncogene directs expression of a differentially sialylated, functionally altered b1 integrin. Oncogene. 2003;22:7137–7145. doi: 10.1038/sj.onc.1206834. [DOI] [PubMed] [Google Scholar]
- 77.Gomes C., Osorio H., Pinto M.T., Campos D., Oliveira M.J., Reis C.A. Expression of ST3GAL4 leads to sLex expression and induces c-Met activation and an invasive phenotype in gastric carcinoma cells. PLoS ONE. 2013;8:e66737. doi: 10.1371/journal.pone.0066737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang X.S., Liu S., Liu Y.J., Liu J.W., Liu T.J., Wang X.Q., Yan Q. Overexpression of fucosyltransferase IV promotes A431 cell proliferation through activating MAPK and PI3K/Akt signaling pathways. J. Cell Physiol. 2010;225:612–619. doi: 10.1002/jcp.22250. [DOI] [PubMed] [Google Scholar]
- 79.Shaper N.L., Mann P.L., Shaper J.H. Cell surface galactosyltransferase: Immunochemical localization. J. Cell Biochem. 1985;28:229–239. doi: 10.1002/jcb.240280305. [DOI] [PubMed] [Google Scholar]
- 80.Liu J.J., Lin B., Hao Y.Y., Li F.F., Liu D.W., Qi Y., Zhu L.C., Zhang S.L., Iwamori M. Lewis(y) antigen stimulates the growth of ovarian cancer cells via regulation of the epidermal growth factor receptor pathway. Oncol. Rep. 2010;23:833–841. [PubMed] [Google Scholar]
- 81.Sakuma K., Aoki M., Kannagi R. Transcription factors c-Myc and CDX2 mediate E-selectin ligand expression in colon cancer cells undergoing EGF/bFGF-induced epithelial-mesenchymal transition. Proc. Natl. Acad. Sci. USA. 2012;109:7776–7781. doi: 10.1073/pnas.1111135109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kumamoto K., Goto Y., Sekikawa K., Takenoshita S., Ishida N., Kawakita M., Kannagi R. Increased expression of UDP-galactose transporter messenger RNA in human colon cancer tissues and its implication in synthesis of Thomsen- Friedenreich antigen and sialyl Lewis A/X determinants. Cancer Res. 2001;61:4620–4627. [PubMed] [Google Scholar]
- 83.Yin J., Hashimoto A., Izawa M., Miyazaki K., Chen G.Y., Takematsu H., Kozutsumi Y., Suzuki A., Furuhata K., Cheng F.L., et al. Hypoxic culture induces expression of sialin, a sialic acid transporter, and cancer-associated gangliosides containing non-human sialic acid on human cancer cells. Cancer Res. 2006;66:2937–2945. doi: 10.1158/0008-5472.CAN-05-2615. [DOI] [PubMed] [Google Scholar]
- 84.Yusa A., Miyazaki K., Kimura N., Izawa M., Kannagi R. Epigenetic silencing of the sulfate transporter gene DTDST induces sialyl Lewisx expression and accelerates proliferation of colon cancer cells. Cancer Res. 2010;70:4064–4073. doi: 10.1158/0008-5472.CAN-09-2383. [DOI] [PubMed] [Google Scholar]
- 85.Koike T., Kimura N., Miyazaki K., Yabuta T., Kumamoto K., Takenoshita S., Chen J., Kobayashi M., Hosokawa M., Taniguchi A., et al. Hypoxia induces adhesion molecules on cancer cells: A missing link between Warburg effect and induction of selectin-ligand carbohydrates. Proc. Natl. Acad. Sci. USA. 2004;101:8132–8137. doi: 10.1073/pnas.0402088101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moriwaki K., Noda K., Furukawa Y., Ohshima K., Uchiyama A., Nakagawa T., Taniguchi N., Daigo Y., Nakamura Y., Hayashi N., et al. Deficiency of GMDS leads to escape from NK cell-mediated tumor surveillance through modulation of TRAIL signaling. Gastroenterology. 2009;137:188–198. doi: 10.1053/j.gastro.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 87.Nakayama K., Moriwaki K., Imai T., Shinzaki S., Kamada Y., Murata K., Miyoshi E. Mutation of GDP-Mannose-4,6-Dehydratase in Colorectal Cancer Metastasis. PLoS ONE. 2013;8:e70298. doi: 10.1371/journal.pone.0070298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Y., Huang D., Chen K.Y., Cui M., Wang W., Huang X., Awadellah A., Li Q., Friedman A., Xin W.W., et al. Fucosylation Deficiency in Mice Leads to Colitis and Adenocarcinoma. Gastroenterology. 2017;152:193–205. doi: 10.1053/j.gastro.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Osuga T., Takimoto R., Ono M., Hirakawa M., Yoshida M., Okagawa Y., Uemura N., Arihara Y., Sato Y., Tamura F., et al. Relationship Between Increased Fucosylation and Metastatic Potential in Colorectal Cancer. J. Natl. Cancer Inst. 2016 doi: 10.1093/jnci/djw210. [DOI] [PubMed] [Google Scholar]
- 90.Romano P.R., Mackay A., Vong M., Desa J., Lamontagne A., Comunale M.A., Hafner J., Block T., Lec R., Mehta A. Development of recombinant Aleuria aurantia lectins with altered binding specificities to fucosylated glycans. Biochem. Biophys. Res. Commun. 2011;414:84–89. doi: 10.1016/j.bbrc.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sawanobori A., Moriwaki K., Takamatsu S., Kamada Y., Miyoshi E. A glycoproteomic approach to identify novel glycomarkers for cancer stem cells. Proteomics. 2016;16:3073–3080. doi: 10.1002/pmic.201500472. [DOI] [PubMed] [Google Scholar]
- 92.Yue T., Maupin K.A., Fallon B., Li L., Partyka K., Anderson M.A., Brenner D.E., Kaul K., Zeh H., Moser A.J., et al. Enhanced discrimination of malignant from benign pancreatic disease by measuring the CA 19-9 antigen on specific protein carriers. PLoS ONE. 2011;6:e29180. doi: 10.1371/journal.pone.0029180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yue T., Goldstein I.J., Hollingsworth M.A., Kaul K., Brand R.E., Haab B.B. The prevalence and nature of glycan alterations on specific proteins in pancreatic cancer patients revealed using antibody-lectin sandwich arrays. Mol. Cell Proteom. 2009;8:1697–1707. doi: 10.1074/mcp.M900135-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yue T., Partyka K., Maupin K.A., Hurley M., Andrews P., Kaul K., Moser A.J., Zeh H., Brand R.E., Haab B.B. Identification of blood-protein carriers of the CA 19-9 antigen and characterization of prevalence in pancreatic diseases. Proteomics. 2011;11:3665–3674. doi: 10.1002/pmic.201000827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sarrats A., Saldova R., Pla E., Fort E., Harvey D.J., Struwe W.B., De Llorens R., Rudd P.M., Peracaula R. Glycosylation of liver acute-phase proteins in pancreatic cancer and chronic pancreatitis. Proteom. Clin. Appl. 2010;4:432–448. doi: 10.1002/prca.200900150. [DOI] [PubMed] [Google Scholar]
- 96.Balmana M., Sarrats A., Llop E., Barrabes S., Saldova R., Ferri M.J., Figueras J., Fort E., De Llorens R., Rudd P.M., et al. Identification of potential pancreatic cancer serum markers: Increased sialyl-Lewis X on ceruloplasmin. Clin. Chim. Acta. 2015;442C:56–62. doi: 10.1016/j.cca.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 97.Uozumi N., Gao C., Yoshioka T., Nakano M., Moriwaki K., Nakagawa T., Masuda T., Tanabe M., Miyoshi E. Identification of a novel type of CA19-9 carrier in human bile and sera of cancer patients: An implication of the involvement in nonsecretory exocytosis. J. Proteome Res. 2010;9:6345–6353. doi: 10.1021/pr100600u. [DOI] [PubMed] [Google Scholar]
- 98.Croce M.V., Salice V.C., Lacunza E., Segal-Eiras A. Alpha 1-acid glycoprotein (AGP): A possible carrier of sialyl lewis X (slewis X) antigen in colorectal carcinoma. Histol. Histopathol. 2005;20:91–97. doi: 10.14670/HH-20.91. [DOI] [PubMed] [Google Scholar]
- 99.Rho J.H., Mead J.R., Wright W.S., Brenner D.E., Stave J.W., Gildersleeve J.C., Lampe P.D. Discovery of sialyl Lewis A and Lewis X modified protein cancer biomarkers using high density antibody arrays. J. Proteom. 2014;96:291–299. doi: 10.1016/j.jprot.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Burdick M.D., Harris A., Reid C.J., Iwamura T., Hollingsworth M.A. Oligosaccharides expressed on MUC1 produced by pancreatic and colon tumor cell lines. J. Biol. Chem. 1997;272:24198–24202. doi: 10.1074/jbc.272.39.24198. [DOI] [PubMed] [Google Scholar]
- 101.Bones J., Byrne J.C., O’Donoghue N., McManus C., Scaife C., Boissin H., Nastase A., Rudd P.M. Glycomic and glycoproteomic analysis of serum from patients with stomach cancer reveals potential markers arising from host defense response mechanisms. J. Proteome Res. 2011;10:1246–1265. doi: 10.1021/pr101036b. [DOI] [PubMed] [Google Scholar]
- 102.Balmana M., Gimenez E., Puerta A., Llop E., Figueras J., Fort E., Sanz-Nebot V., de B.C., Rizzi A., Barrabes S., et al. Increased a1-3 fucosylation of a-1-acid glycoprotein (AGP) in pancreatic cancer. J. Proteom. 2016;132:144–154. doi: 10.1016/j.jprot.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 103.Vuckovic F., Theodoratou E., Thaci K., Timofeeva M., Vojta A., Stambuk J., Pucic-Bakovic M., Derek L., Servis D., Rudd P., et al. IgG glycome in colorectal cancer. Clin. Cancer Res. 2016;22:3078–3086. doi: 10.1158/1078-0432.CCR-15-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dall’Olio F., Vanhooren V., Chen C.C., Slagboom P.E., Wuhrer M., Franceschi C. N-glycomic biomarkers of biological aging and longevity: A link with inflammaging. Ageing Res. Rev. 2013;12:685–698. doi: 10.1016/j.arr.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 105.Dewald J.H., Colomb F., Bobowski-Gerard M., Groux-Degroote S., Delannoy P. Role of Cytokine-Induced Glycosylation Changes in Regulating Cell Interactions and Cell Signaling in Inflammatory Diseases and Cancer. Cells. 2016 doi: 10.3390/cells5040043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yamaguchi M., Ishida H., Galustian C., Feizi T., Kiso M. Synthesis and selectin-binding activity of N-deacetylsialyl Lewis X ganglioside. Carbohydr. Res. 2002;337:2111–2117. doi: 10.1016/S0008-6215(02)00274-4. [DOI] [PubMed] [Google Scholar]
- 107.Lopez P.H., Schnaar R.L. Gangliosides in cell recognition and membrane protein regulation. Curr. Opin. Struct. Biol. 2009;19:549–557. doi: 10.1016/j.sbi.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Laskowska A., Dolińiska-Krajewska B., Zabel M., Ugorski M. Sialosyl Le(a)-carrying gangliosides present on the surface of colon carcinoma cells are not directly involved in adhesion to E-selectin. Eur. J. Cell Biol. 2001;80:784–791. doi: 10.1078/0171-9335-00211. [DOI] [PubMed] [Google Scholar]
- 109.Dall’Olio F., Malagolini N., Trinchera M., Chiricolo M. Mechanisms of cancer-associated glycosylation changes. Front. Biosci. 2012;17:670–699. doi: 10.2741/3951. [DOI] [PubMed] [Google Scholar]
- 110.Accordino M.K., Wright J.D., Vasan S., Neugut A.I., Tergas A., Hu J.C., Hershman D.L., Accordino M.K., Wright J.D., Vasan S., et al. Serum Tumor Marker Use in Patients With Advanced Solid Tumors. J. Oncol. Pract. 2016;12:65–66. doi: 10.1200/JOP.2015.005660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gion M., Peloso L., Trevisiol C., Squarcina E., Zappa M., Fabricio A.S. An epidemiology-based model as a tool to monitor the outbreak of inappropriateness in tumor marker requests: A national scale study. Clin. Chem. Lab. Med. 2016;54:473–482. doi: 10.1515/cclm-2015-0329. [DOI] [PubMed] [Google Scholar]
- 112.Locker G.Y., Hamilton S., Harris J., Jessup J.M., Kemeny N., Macdonald J.S., Somerfield M.R., Hayes D.F., Bast R.C., Jr. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J. Clin. Oncol. 2006;24:5313–5327. doi: 10.1200/JCO.2006.08.2644. [DOI] [PubMed] [Google Scholar]
- 113.Duffy M.J., Sturgeon C., Lamerz R., Haglund C., Holubec V.L., Klapdor R., Nicolini A., Topolcan O., Heinemann V. Tumor markers in pancreatic cancer: A European Group on Tumor Markers (EGTM) status report. Ann. Oncol. 2010;21:441–447. doi: 10.1093/annonc/mdp332. [DOI] [PubMed] [Google Scholar]
- 114.Huang Z., Liu F. Diagnostic value of serum carbohydrate antigen 19-9 in pancreatic cancer: A meta-analysis. Tumour. Biol. 2014;35:7459–7465. doi: 10.1007/s13277-014-1995-9. [DOI] [PubMed] [Google Scholar]
- 115.Fritz S., Hackert T., Hinz U., Hartwig W., Buchler M.W., Werner J. Role of serum carbohydrate antigen 19-9 and carcinoembryonic antigen in distinguishing between benign and invasive intraductal papillary mucinous neoplasm of the pancreas. Br. J. Surg. 2011;98:104–110. doi: 10.1002/bjs.7280. [DOI] [PubMed] [Google Scholar]
- 116.Kanda M., Fujii T., Takami H., Suenaga M., Inokawa Y., Yamada S., Nakayama G., Sugimoto H., Koike M., Nomoto S., et al. The combination of the serum carbohydrate antigen 19-9 and carcinoembryonic antigen is a simple and accurate predictor of mortality in pancreatic cancer patients. Surg. Today. 2014;44:1692–1701. doi: 10.1007/s00595-013-0752-9. [DOI] [PubMed] [Google Scholar]
- 117.Kaprio T., Satomaa T., Heiskanen A., Hokke C.H., Deelder A.M., Mustonen H., Hagstrom J., Carpen O., Saarinen J., Haglund C. N-glycomic Profiling as a Tool to Separate Rectal Adenomas from Carcinomas. Mol. Cell Proteom. 2015;14:277–288. doi: 10.1074/mcp.M114.041632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Choi A.R., Park J.C., Kim J.H., Shin S.K., Lee S.K., Lee Y.C., Chung J.B. High level of preoperative carbohydrate antigen 19-9 is a poor survival predictor in gastric cancer. World J. Gastroenterol. 2013;19:5302–5308. doi: 10.3748/wjg.v19.i32.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Han E.S., Lee H.H., Lee J.S., Song K.Y., Park C.H., Jeon H.M. At which stage of gastric cancer progression do levels of carcinoembryonic antigen and carbohydrate antigen 19-9 increase? Application in advanced gastric cancer treatment. J. Gastric. Cancer. 2014;14:123–128. doi: 10.5230/jgc.2014.14.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lin M.S., Huang J.X., Yu H. Elevated serum level of carbohydrate antigen 19-9 in benign biliary stricture diseases can reduce its value as a tumor marker. Int. J. Clin. Exp. Med. 2014;7:744–750. [PMC free article] [PubMed] [Google Scholar]
- 121.Kalthoff H., Kreiker C., Schmiegel W.H., Greten H., Thiele H.G. Characterization of CA 19-9 bearing mucins as physiological exocrine pancreatic secretion products. Cancer Res. 1986;46:3605–3607. [PubMed] [Google Scholar]
- 122.Baeckstrom D., Karlsson N., Hansson G.C. Purification and characterization of sialyl-Le(a)-carrying mucins of human bile; evidence for the presence of MUC1 and MUC3 apoproteins. J. Biol. Chem. 1994;269:14430–14437. [PubMed] [Google Scholar]
- 123.La Greca G., Sofia M., Lombardo R., Latteri S., Ricotta A., Puleo S., Russello D. Adjusting CA19-9 values to predict malignancy in obstructive jaundice: Influence of bilirubin and C-reactive protein. World J. Gastroenterol. 2012;18:4150–4155. doi: 10.3748/wjg.v18.i31.4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Furuya N., Kawa S., Hasebe O., Tokoo M., Mukawa K., Maejima S., Oguchi H. Comparative study of CA242 and CA19-9 in chronic pancreatitis. Br. J. Cancer. 1996;73:372–376. doi: 10.1038/bjc.1996.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kloppel G., Lingenthal G., von B.M., Kern H.F. Histological and fine structural features of pancreatic ductal adenocarcinomas in relation to growth and prognosis: Studies in xenografted tumours and clinico-histopathological correlation in a series of 75 cases. Histopathology. 1985;9:841–856. doi: 10.1111/j.1365-2559.1985.tb02870.x. [DOI] [PubMed] [Google Scholar]
- 126.Itai S., Nishikata J., Yoneda T., Ohmori K., Yamabe H., Arii S., Tobe T., Kannagi R. Tissue distribution of 2-3 and 2-6 sialyl Lewis A antigens and significance of the ratio of two antigens for the differential diagnosis of malignant and benign disorders of the digestive tract. Cancer. 1991;67:1576–1587. doi: 10.1002/1097-0142(19910315)67:6<1576::AID-CNCR2820670620>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 127.Ohshio G., Ogawa K., Kudo H., Yamabe H., Nakashima Y., Kim Y.C., Endo K., Watanabe Y., Manabe T., Tobe T. Immunohistochemical studies on the localization of cancer associated antigens DU-PAN-2 and CA19-9 in carcinomas of the digestive tract. J. Gastroenterol. Hepatol. 1990;5:25–31. doi: 10.1111/j.1440-1746.1990.tb01764.x. [DOI] [PubMed] [Google Scholar]
- 128.Portela S.V., Martin C.V., Romay L.M., Cuevas E., Martin E.G., Briera A.F. sLea and sLex expression in colorectal cancer: Implications for tumourigenesis and disease prognosis. Histol. Histopathol. 2011;26:1305–1316. doi: 10.14670/HH-26.1305. [DOI] [PubMed] [Google Scholar]
- 129.Wolf B.C., Salem R.R., Sears H.F., Horst D.A., Lavin P.T., Herlyn M., Itzkowitz S.H., Schlom J., Steele G.D., Jr. The expression of colorectal carcinoma-associated antigens in the normal colonic mucosa. An immunohistochemical analysis of regional distribution. Am. J. Pathol. 1989;135:111–119. [PMC free article] [PubMed] [Google Scholar]
- 130.Miyazaki K., Ohmori K., Izawa M., Koike T., Kumamoto K., Furukawa K., Ando T., Kiso M., Yamaji T., Hashimoto Y., et al. Loss of disialyl Lewisa the ligand for lymphocyte inhibitory receptor sialic acid-binding immunoglobulin-like lectin-7 (Siglec-7) associated with increased sialyl Lewisa expression on human colon cancers. Cancer Res. 2004;64:4498–4505. doi: 10.1158/0008-5472.CAN-03-3614. [DOI] [PubMed] [Google Scholar]
- 131.Caretti A., Sirchia S.M., Tabano S., Zulueta A., Dall’Olio F., Trinchera M. DNA methylation and histone modifications modulate the b1,3 galactosyltransferase b3Gal-T5 native promoter in cancer cells. Int. J. Biochem. Cell Biol. 2012;44:84–90. doi: 10.1016/j.biocel.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 132.Mare L., Caretti A., Albertini R., Trinchera M. CA19.9 antigen circulating in the serum of colon cancer patients: Where is it from? Int. J. Biochem. Cell Biol. 2013;45:792–797. doi: 10.1016/j.biocel.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 133.Terraneo L., Avagliano L., Caretti A., Bianciardi P., Tosi D., Bulfamante G.P., Samaja M., Trinchera M. Expression of carbohydrate-antigen sialyl-Lewis a on colon cancer cells promotes xenograft growth and angiogenesis in nude mice. Int. J. Biochem. Cell Biol. 2013;45:2796–2800. doi: 10.1016/j.biocel.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 134.Tang H., Singh S., Partyka K., Kletter D., Hsueh P., Yadav J., Ensink E., Bern M., Hostetter G., Hartman D., et al. Glycan motif profiling reveals plasma sialyl-Lewis X elevations in pancreatic cancers that are negative for CA 19-9. Mol. Cell Proteom. 2015;14:1323–1333. doi: 10.1074/mcp.M114.047837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tang H., Partyka K., Hsueh P., Sinha J.Y., Kletter D., Zeh H., Huang Y., Brand R.E., Haab B.B. Glycans related to the CA19-9 antigen are elevated in distinct subsets of pancreatic cancers and improve diagnostic accuracy over CA19-9. Cell Mol. Gastroenterol. Hepatol. 2016;2:201–221. doi: 10.1016/j.jcmgh.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Laubli H., Borsig L. Selectins promote tumor metastasis. Semin. Cancer Biol. 2010;20:169–177. doi: 10.1016/j.semcancer.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 137.Paschos K.A., Canovas D., Bird N.C. The engagement of selectins and their ligands in colorectal cancer liver metastases. J. Cell Mol. Med. 2010;14:165–174. doi: 10.1111/j.1582-4934.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Witz I.P. The selectin-selectin ligand axis in tumor progression. Cancer Metastasis Rev. 2008;27:19–30. doi: 10.1007/s10555-007-9101-z. [DOI] [PubMed] [Google Scholar]
- 139.Renkonen R., Mattila P., Majuri M.L., Rabina J., Toppila S., Renkonen J., Hirvas L., Niittymaki J., Turunen J.P., Renkonen O., et al. In vitro experimental studies of sialyl Lewis x and sialyl Lewis a on endothelial and carcinoma cells: Crucial glycans on selectin ligands. Glycoconj. J. 1997;14:593–600. doi: 10.1023/A:1018536509950. [DOI] [PubMed] [Google Scholar]
- 140.Ichikawa D., Kitamura K., Tani N., Nishida S., Tsurutome H., Hakomori S.I., Ikeda E., Mutoh F., Kurioka H., Yamagishi H. Molecular detection of disseminated cancer cells in the peripheral blood and expression of sialylated antigens in colon cancers. J. Surg. Oncol. 2000;75:98–102. doi: 10.1002/1096-9098(200010)75:2<98::AID-JSO5>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 141.Nakamori S., Kameyama M., Imaoka S., Furukawa H., Ishikawa O., Sasaki Y., Kabuto T., Iwanaga T., Matsushita Y., Irimura T. Increased expression of sialyl Lewisx antigen correlates with poor survival in patients with colorectal carcinoma: Clinicopathological and immunohistochemical study. Cancer Res. 1993;53:3632–3637. [PubMed] [Google Scholar]
- 142.Nakamori S., Kameyama M., Imaoka S., Furukawa H., Ishikawa O., Sasaki Y., Izumi Y., Irimura T. Involvement of carbohydrate antigen sialyl Lewisx in colorectal cancer metastasis. Dis. Colon Rectum. 1997;40:420–431. doi: 10.1007/BF02258386. [DOI] [PubMed] [Google Scholar]
- 143.Yamada N., Chung Y.S., Maeda K., Sawada T., Ikehara T., Nishino H., Okuno M., Sowa M. Increased expression of sialyl Lewis A and sialyl Lewis X in liver metastases of human colorectal carcinoma. Invasion Metastasis. 1995;15:95–102. [PubMed] [Google Scholar]
- 144.Opolski A., Laskowska A., Madej J., Wietrzyk J., Klopocki A., Radzikowski C., Ugorski M. Metastatic potential of human CX-1 colon adenocarcinoma cells is dependent on the expression of sialosyl Lea antigen. Clin. Exp. Metastasis. 1998;16:673–681. doi: 10.1023/A:1006502009682. [DOI] [PubMed] [Google Scholar]
- 145.Thurin M., Kieber-Emmons T. SA-Lea and Tumor Metastasis: The Old Prediction and Recent Findings. Hybrid Hybridomics. 2002;21:111–116. doi: 10.1089/153685902317401708. [DOI] [PubMed] [Google Scholar]
- 146.Gebauer F., Wicklein D., Stubke K., Nehmann N., Schmidt A., Salamon J., Peldschus K., Nentwich M.F., Adam G., Tolstonog G., et al. Selectin binding is essential for peritoneal carcinomatosis in a xenograft model of human pancreatic adenocarcinoma in pfp--/rag2-- mice. Gut. 2012;62:741–750. doi: 10.1136/gutjnl-2011-300629. [DOI] [PubMed] [Google Scholar]
- 147.Tei K., Kawakami-Kimura N., Taguchi O., Kumamoto K., Higashiyama S., Taniguchi N., Toda K., Kawata R., Hisa Y., Kannagi R. Roles of cell adhesion molecules in tumor angiogenesis induced by cotransplantation of cancer and endothelial cells to nude rats. Cancer Res. 2002;62:6289–6296. [PubMed] [Google Scholar]
- 148.Mathieu S., Gerolami R., Luis J., Carmona S., Kol O., Crescence L., Garcia S., Borentain P., El Battari A. Introducing a1,2-linked fucose into hepatocarcinoma cells inhibits vasculogenesis and tumor growth. Int. J. Cancer. 2007;121:1680–1689. doi: 10.1002/ijc.22797. [DOI] [PubMed] [Google Scholar]
- 149.Ohyama C., Tsuboi S., Fukuda M. Dual roles of sialyl Lewis X oligosaccharides in tumor metastasis and rejection by natural killer cells. EMBO J. 1999;18:1516–1525. doi: 10.1093/emboj/18.6.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ohyama C., Kanto S., Kato K., Nakano O., Arai Y., Kato T., Chen S., Fukuda M.N., Fukuda M. Natural killer cells attack tumor cells expressing high levels of sialyl Lewis x oligosaccharides. Proc. Natl. Acad. Sci. USA. 2002;99:13789–13794. doi: 10.1073/pnas.212456599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Doekhie F.S., Morreau H., de Bock G.H., Speetjens F.M., Dekker-Ensink N.G., Putter H., van de Velde C.J., Tollenaar R.A., Kuppen P.J. Sialyl Lewis X expression and lymphatic microvessel density in primary tumors of node-negative colorectal cancer patients predict disease recurrence. Cancer Microenviron. 2008;1:141–151. doi: 10.1007/s12307-008-0014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Futamura N., Nakamura S., Tatematsu M., Yamamura Y., Kannagi R., Hirose H. Clinicopathologic significance of sialyl le(x)expression in advanced gastric carcinoma. Br. J. Cancer. 2000;83:1681–1687. doi: 10.1054/bjoc.2000.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Vukobrat-Bijedic Z., Husic-Selimovic A., Sofic A., Bijedic N., Bjelogrlic I., Gogov B., Mehmedovic A. Cancer Antigens (CEA and CA 19-9) as Markers of Advanced Stage of Colorectal Carcinoma. Med. Arch. 2013;67:397–401. doi: 10.5455/medarh.2013.67.397-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yamashita K., Watanabe M. Clinic al significance of tumor markers and an emerging perspective on colorectal cancer. Cancer Sci. 2009;100:195–199. doi: 10.1111/j.1349-7006.2008.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]