Abstract
Objective
To present information on the quality of newborn care services and health facility readiness to provide newborn care in 6 African countries, and to advocate for the improvement of providers' essential newborn care knowledge and skills.
Design
Cross-sectional observational health facility assessment.
Setting
Ethiopia, Kenya, Madagascar, Mozambique, Rwanda and Tanzania.
Participants
Health workers in 643 facilities. 1016 health workers were interviewed, and 2377 babies were observed in the facilities surveyed.
Main outcome measures
Indicators of quality of newborn care included (1) provision of immediate essential newborn care: thermal care, hygienic cord care, and early and exclusive initiation of breast feeding; (2) actual and simulated resuscitation of asphyxiated newborn infants; and (3) knowledge of health workers on essential newborn care, including resuscitation.
Results
Sterile or clean cord cutting instruments, suction devices, and tables or firm surfaces for resuscitation were commonly available. 80% of newborns were immediately dried after birth and received clean cord care in most of the studied facilities. In all countries assessed, major deficiencies exist for essential newborn care supplies and equipment, as well as for health worker knowledge and performance of key routine newborn care practices, particularly for immediate skin-to-skin contact and breastfeeding initiation. Of newborns who did not cry at birth, 89% either recovered on their own or through active steps taken by the provider through resuscitation with initial stimulation and/or ventilation. 11% of newborns died. Assessment of simulated resuscitation using a NeoNatalie anatomic model showed that less than a third of providers were able to demonstrate ventilation skills correctly.
Conclusions
The findings shared in this paper call attention to the critical need to improve health facility readiness to provide quality newborn care services and to ensure that service providers have the necessary equipment, supplies, knowledge and skills that are critical to save newborn lives.
Keywords: neonatal resuscitation, essential newborn care, newborn care, breastfeeding, cross-sectional observational assessment
Strengths and limitations of this study.
The study helps close the evidence gap on the quality of newborn care.
The study team collected and analysed directly observed data during childbirth.
The study was conducted in multiple countries, which allowed the researchers to examine newborn care practices across these countries.
The country samples are neither representative at the national level, nor are they similar across countries.
No data on user perspectives regarding quality of care, which is an essential component of quality, were collected because it was outside the scope of this assessment.
Introduction
Globally, 2.7 million newborns die each year, largely as a result of birth asphyxia, complications of preterm birth and infections.1 This burden of death is disproportionately concentrated in low-income and middle-income countries (LMICs). Sub-Saharan Africa has the highest neonatal mortality rates (NMRs),1 experiencing a mortality reduction of 28% since 1990.2 Despite the impressive efforts undertaken around the Millennium Development Goals (MDG) 4 to reduce child mortality, the decline of neonatal mortality has been much slower than for mortality among children aged 1–59 months; consequently, neonatal deaths now contribute to about 45% of total under-5 deaths in 2015.1 Recognising the importance of addressing this problem, in 2014 the World Health Assembly endorsed the Every Newborn Action Plan (ENAP)—a road map for ending preventable newborn deaths and stillbirths—with a target for all countries to attain 12 or fewer neonatal deaths per 1000 live births by 2030.3 This target is included also in the Sustainable Development Goals.4 Additionally, the ENAP's first two strategic objectives focus on strengthening and investing in quality of care (QoC) around the time of birth as mechanisms to achieving the mortality target.3
Evidence suggests that the majority of these deaths can be prevented, particularly those occurring at birth and immediately thereafter, with simple, evidence-based essential newborn care (ENC) interventions conducted by skilled providers and supported with available commodities.5 These immediate interventions include ensuring that the newborn is kept warm with immediate drying and skin-to-skin contact, giving appropriate stimulation for newborns unable to breathe, providing additional neonatal resuscitation measures as necessary, and ensuring infection prevention, early initiation of exclusive breast feeding and hygienic cord care.2 5 6 Timeliness of service provision and quality of interventions are essential to prevent newborn deaths and consequent disability.7 8
However, shortfalls in the quality of maternal and child health in LMICs9–12 have resulted from a serious lack of attention to the evaluation and quality improvement of facility newborn care.13–15 Health facilities play a crucial role in reducing neonatal mortality, and thus it is critical that they have the capacity and QoC to meet demand and move towards greater improvements in health and survival for newborn infants. However, evidence suggests that provider skills and competencies within health facilities are insufficient to increase neonatal survival.16
Intrapartum complications, including birth asphyxia, account for nearly a quarter of all neonatal deaths.17 Up to 10% of babies require support to initiate breathing in the first minutes of life.2 6 18 Stimulation, including drying and rubbing, is required to initiate breathing in ∼10 million babies at birth globally every year. This technology-free practice should be adequately performed by all skilled birth attendants. Approximately six million babies require resuscitation with bag and mask ventilation.18 Basic resuscitation, including bag and mask ventilation with room air, can have a big impact on survival and can be achieved with basic training and competencies.4 6 18 Given proper training and adequately reprocessed functional equipment, neonatal resuscitation can be performed in low-resource settings.19 Basic resuscitation is adequate for most babies to survive; neonatal resuscitation training can avert 30% of deaths of full-term babies and 5–10% of preterm babies, saving hundreds of thousands of newborn lives. Less than 1% of asphyxiated babies require more advanced resuscitation.18
An increased focus on quality improvement for patient care within the health system and in formulation and implementation of health policies is critically important.12 20 Identifying gaps in and barriers to quality newborn care in facilities is one of the critical steps in the improvement process. To guide QoC improvement activities for maternal and newborn care at health facilities in selected countries, the Maternal and Child Health Integrated Program (MCHIP), with funding from the US Agency for International Development, conducted a multicountry health facility assessment that included actual observations of clinical care for mothers and newborn infants at health facilities in six sub-Saharan African countries: Ethiopia, Kenya, Madagascar, Mozambique, Rwanda and Tanzania (including Zanzibar). This paper presents information on key aspects of health facility readiness to provide ENC services, including resuscitation and the QoC at the assessed facilities.
Methods
Setting and sample
The facility surveys were conducted in the six countries with ongoing MCHIP-supported projects. Health facility surveys were conducted between 2009 and 2012 in Ethiopia, Kenya, Madagascar, Mozambique, Rwanda and Tanzania (including Zanzibar). MCHIP collaborated with the Ministry of Health and other relevant stakeholders in each country to determine appropriate sampling for participating facilities based on the purpose of the survey in that country. Facility sampling took into account the number of facilities offering antenatal care and delivery services, facility client caseloads, and the fieldwork logistics involved. Surveys were conducted as part of a cross-sectional assessment or an existing survey or process. In Kenya, the survey was integrated into the 2010 Demographic Health Survey (DHS) Service Provision Assessment (SPA). Higher level facilities, such as district and regional hospitals, were generally selected through a census approach, whereas lower level facilities, such as health centres, were selected through random sampling procedures.21
Study participants included women in labour, newborns and healthcare providers working in the labour and delivery ward on the day of the assessment. The sample size for the required number of deliveries was estimated for individual countries to be at least 250 based on 50% prevalence for study indicators of interest, with 80% power and 95% precision, assuming a design effect of 2 for clustering of observations by provider and health facility, with adequate power to measure at least 18% change in key variables over time. Since the prevalence of the indicators of interest in the study population was unknown, 50% was used to generate the largest sample size.
Data collection
Data collection included observations of normal and complicated births, an inventory of labour and delivery supplies, and interviews with and knowledge tests for health workers attending deliveries. Data were collected on the quality of services and facility readiness to deliver routine and emergency care for pregnant and delivering women and for newborns. However, this paper only reports on the ENC component of the study. A series of five data collection tools were used to gather information on the quality of immediate newborn care. A clinical checklist was used to observe deliveries within 1 hour after birth to evaluate the quality of immediate ENC and newborn resuscitation. Provider interviews included questions on appropriate equipment needed for immediate newborn care after birth, components of immediate newborn care, signs and symptoms of newborn sepsis, and actions to be taken to care for low birthweight (LBW; <2.5 kg) babies. Each question had multiple answers, so respondents were asked to provide as many answers as came to mind and were probed if necessary. Given the limited number of actual asphyxiated babies, NeoNatalie anatomic models were used to assess providers' decision-making and ventilation skills in newborn resuscitation in a subset of countries: Madagascar, Rwanda and Tanzania. It is used to create scenarios of a baby with various breathing conditions. The chest of the simulator rises when the appropriate bag and mask ventilation technique is applied. A facility inventory was also conducted, and observations were made of key infrastructural components, storage capabilities, supplies and equipment essential for newborn healthcare services. Box 1 further describes the assessment tools and their purpose.
Box 1. Survey tools and their purpose.
1. Facility inventory checklist: assessed conditions of infrastructure, and availability and condition of commodities, supplies, and equipment.
2–3. Clinical practice observation tools for antenatal care (2) and labour and delivery (3): examined provider implementation of the evidence-based practices for routine care and screening, prevention, and management of major obstetric and newborn complications at the time of birth, including postpartum haemorrhage, pre-eclampsia/eclampsia, newborn asphyxia and respectful provider/client interactions.
4. Health worker interview and knowledge test: assessed health worker knowledge of how to identify, manage and treat common maternal and newborn health (MNH) complications, including newborn resuscitation skills.
5. Key informant interview and document review: reviewed national policies related to MNH care included the country's essential drug lists or formularies, clinical practice policies and guidelines, and curricula/syllabi on relevant topics for both preservice and in-service education for health professions.
The antenatal care, labour and delivery tools were adapted from existing tested obstetric tools.22 Additional items were added based on the contents of the WHO's Managing Complications in Pregnancy and Childbirth (MCPC) document.23 The newborn resuscitation observation items were based on the Helping Babies Breathe algorithm (http://www.helpingbabiesbreathe.org). The tools were pretested at health facilities, and the validity and reliability of the tools were assessed during each of the data collectors training conducted in the study countries. Observation scores by trained observers were compared with trainers' scores, and inter-rater reliability was examined. The inventory tool was adapted from the DHS SPA tool, a well-tested tool.24 All the study tools can be accessed at http://www.mchip.net/QoCSurveys.
Data collectors were trained to observe the entire labour and birthing process, starting from the first stage of labour through the first hour postdelivery for normal birth or until completion of the management of birth asphyxia or any maternal complication beyond the first hour. If data collectors did not arrive until the second stage of labour or during a newborn resuscitation, they were required to record from the time of their arrival to the same end point as stated above. This was an observational study only and no intervention was expected according to the study protocol. However, observers, who were all clinicians, were trained to first call for the service providers' supervisor if and when they witnessed any life-threatening actions by a health worker. If there was no supervisor or any other senior health worker available, they had the personal choice to step out of their data collector role and assist the provider in their professional role as a licenced and practising clinician in their respective countries. The decision was the data collector's and not a required intervention mandated by the study protocol. It was discussed with and accepted by the Johns Hopkins University Institutional Review Board (IRB).
A local researcher supervised the survey in each country. Staff from MCHIP headquarters collaborated with the local researchers, local MCHIP staff, the Ministry of Health and other relevant country-specific organisations for planning and implementation. Skilled birth attendants, including doctors, nurses and midwives, were trained as data collectors by MCHIP staff who were doctors, nurses, midwives, and monitoring and evaluation specialists. During the 1–2 weeks training period, data collectors were introduced to the aim and objectives of the study, research tools, standardisation of clinical observation skills, and use of HTC smartphones or Samsung Galaxy tablets, including hands-on practice using one of these tools in the field. Data collectors worked in teams of three to four people over 2–4 days to collect data in each facility. To minimise bias, the data collectors did not visit health facilities where they were employed or supervised.
Data were transmitted daily to a cloud-based, password-protected server, with data monitored and cleaned on an ongoing basis. The data entry system incorporated forced data quality checks before transmission to the central server. Both on-site and off-site monitoring of data flow was conducted given the two-directional capability of the electronic data processing system.
Indicators and data analysis
Evidence-based, globally accepted guidelines for clinical practice for immediate newborn care, described in the WHO's MCPC, were used as the gold standard for QoC.23 Indicators of quality of newborn care included (1) observation of ENC: dry baby with towel/cloth immediately, discard wet towel/cloth and cover with dry towel/cloth, place newborn skin-to-skin, tie or clamp cord when pulsations stop or 2–3 min after birth, cut cord with clean blade or scissors, help mother initiate breast feeding within first hour of birth, and practice infection prevention measures (hand washing and use of sterile gloves); (2) observations and/or simulations of neonatal resuscitation: dry, position, ventilate and adjust mask if needed; and (3) health worker knowledge of newborn care: immediate newborn care, signs of sepsis, and care of LBW babies and associated equipment and supplies. Indicators of facility readiness to provide newborn care included the percentage of facilities with the following equipment necessary for immediate newborn care: disposable cord ties/clamps, clean dry towel or blanket to wrap the baby, sterile/clean scissors or blade, bag and mask, suction device, and resuscitation table or firm surface for conducting newborn resuscitation.
Descriptive analyses were conducted, including frequencies, means, and simple and cross-tabulations of quality and process indicators. The facility's readiness, mainly availability of equipment and supplies needed for immediate newborn care and resuscitation, was assessed. Data were disaggregated by type of facility to assess potential differences, which were displayed if significant. All analyses were conducted using Stata V.12 (StataCorp, College Station, Texas, USA).
Ethical approval and informed consent
Verbal informed consent was obtained from all study participants. As this was a minimal risk observational study conducted in settings with low literacy and obtained from women in active labour, verbal rather than written consent was used. In cases where a woman was ill or unable to give consent due to an emergency obstetric complication, the next of kin provided informed consent on her behalf.
Results
Characteristics of sampled health facilities and healthcare providers
The assessments were conducted in a total of 643 health facilities with varying proportions of hospitals (referral level) and health centres. Table 1 shows the proportion of hospitals versus health centres sampled in each of the countries. There was a slightly bigger proportion of hospitals than health centres in the overall sample, ranging from 28% in Tanzania to 100% in Ethiopia. More health centres were sampled in Tanzania because of prioritisation of MCHIP project areas and focus on support to health centres with high delivery caseloads.
Table 1.
Number of facilities sampled per country
| Sample | Ethiopia* | Kenya | Madagascar | Mozambique | Rwanda | Tanzania | Total |
|---|---|---|---|---|---|---|---|
| Facilities visited | 19 | 409 | 36 | 46 | 72 | 61 | 643 |
| Hospitals | 100% | 52% | 75% | 46% | 58% | 28% | 53% |
| Health centres | 0% | 48% | 25% | 54% | 42% | 72% | 47% |
*Only hospitals were sampled in Ethiopia due to low numbers of deliveries in lower level health facilities.
A total of 2689 births were observed across the six countries. Of these, the study team observed newborn care for 2377 newborns and actual resuscitation of 230 newborns through the first hour after birth. Newborn care was not observed for some births for various reasons, including births that occurred when the observer was with another mother or had left the labour ward or facility. A total of 514 newborn resuscitation simulations were conducted. A knowledge test was administered to a total of 1016 healthcare providers to assess information used for decision-making related to newborn care. Information on their clinical qualifications, training and experience providing newborn care and supervision was also gathered (table 2).
Table 2.
Number of observations, knowledge tests and simulations per country
| Sample | Ethiopia* | Kenya | Madagascar | Mozambique | Rwanda | Tanzania | Total |
|---|---|---|---|---|---|---|---|
| Observations of care | |||||||
| Deliveries | 192 | 626 | 347 | 525 | 293 | 706 | 2689 |
| Newborn and postnatal care for mother and baby | 115 | 571 | 336 | 508 | 225 | 622 | 2377 |
| Newborn resuscitation | 18 | 44 | 48 | 22 | 43 | 49 | 224 |
| Knowledge and skills test administered | |||||||
| Health workers interviewed | 79 | 210 | 139 | 186 | 145 | 257 | 1016 |
| Newborn resuscitation simulations | NA† | NA† | 132 | NA† | 137 | 245 | 514 |
*Only hospitals were sampled in Ethiopia due to low numbers of deliveries in lower level health facilities.
†Newborn resuscitation simulations were not done in Kenya, Ethiopia and Mozambique.
NA, not available.
Table 3 illustrates the characteristics of 1016 service providers who were interviewed, by country. They comprised 93% skilled birth attendants, the majority of whom were nurses or midwives, with predominantly female interviewees in all countries.
Table 3.
Characteristics of interviewed health workers
| Interviewed health worker characteristics | Ethiopia | Kenya | Madagascar | Mozambique | Rwanda | Tanzania | Pooled total |
|---|---|---|---|---|---|---|---|
| Cadre* | |||||||
| Physician† | 7.6% | 4.3% | 25.2% | 0.5% | 13.1% | 3.5% | 7.7% |
| Nurse/midwife‡ | 78.5% | 94.8% | 69.8% | 84.4% | 86.2% | 84.4% | 85.1% |
| Student/unskilled§ | 7.6% | 1.0% | 0.7% | 9.7% | 0.7% | 6.2% | 3.9% |
| Other/missing | 6.3% | 0.0% | 4.3% | 5.4% | 0.0% | 5.8% | 3.2% |
| Gender | |||||||
| Male | 32.9% | 33.8% | 13.7% | 0.5% | 26.2% | 5.4% | 17.7% |
| Female | 67.1% | 66.2% | 86.3% | 98.9% | 73.8% | 91.8% | 81.6% |
| Missing | 0.0% | 0.0% | 0.0% | 0.5% | 0.0% | 2.7% | 0.7% |
| Total interviews | 79 | 210 | 139 | 186 | 145 | 257 | 1016 |
*Health cadres' definitions were provided by each country.
†Doctor: general practitioners, obstetricians, gynaecologists and other specialists such as paediatricians, residents or assistant medical officers.
‡Nurse/midwife: Bachelor of Science and Diploma in Nursing/Midwifery, registered and enrolled nurses/midwives, nursing officers, maternal and child health aides, paramedics, or health officers.
§Student/unskilled: medical attendants, health assistants or traditional birth attendants.
Immediate ENC practices
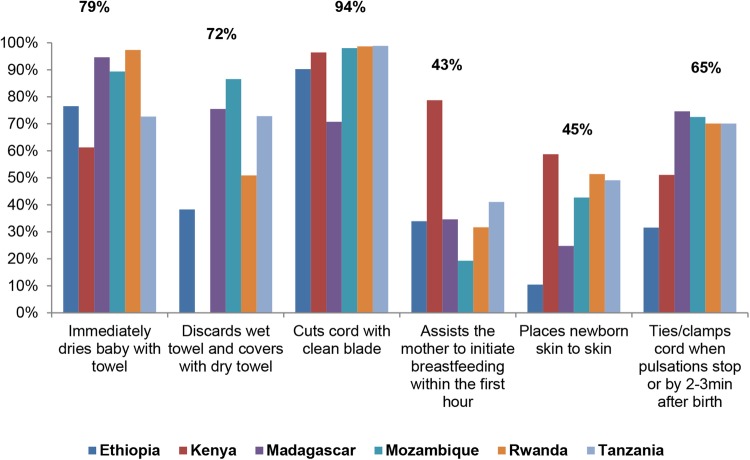
Results shown in figure 1 reveal areas of strength and weakness in performance of ENC practices by providers caring for the 2377 babies observed in the facilities surveyed. Coverage differed between and within countries. The most frequently observed practice, performed for 94% of newborns observed, was cutting the cord with a clean blade or scissors, followed by immediate drying with a towel/cloth (79%). However, only 43% of all mothers (ranging from 19% to 79%) were provided assistance to initiate breast feeding within the first hour of life, and only 45% of newborns (ranging from 10% to 59%) were placed skin-to-skin immediately after delivery.
Figure 1.
Observations of immediate essential newborn care.
Use of high-level disinfected or sterile gloves for vaginal examinations during labour was high (93% of all observations) but hand washing prior to delivery was observed for only 37% of cases; providers were more likely to wash their hands after delivery (78%). The majority of facilities had soap and water (89%) and clean or sterile gloves (73%) available in the delivery area.
Neonatal resuscitation
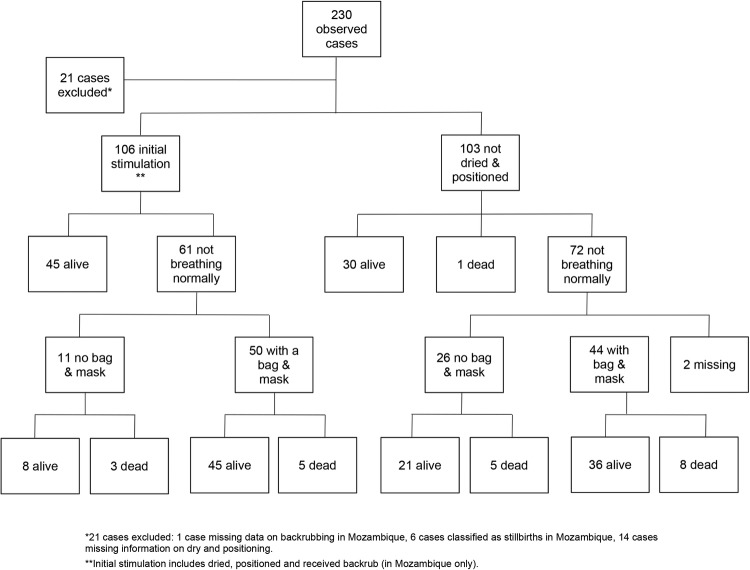
Figure 2 illustrates providers' observed actions for 209 asphyxiated babies; 21 babies (9% of total cases) were excluded because they were classified as stillbirths or had missing data. Of the 209 asphyxiated babies with data, 106 received stimulation (ie, drying and/or back rubbing) and positioning of their heads to ensure the patency of their airways (left side of figure 2). The remaining 103 babies were not dried immediately, did not receive back rubs, or did not have their heads positioned to ensure their airways were opened. Forty-five out of the 106 (42%) babies who received stimulation and proper positioning of their necks recovered, while 61 were still not breathing normally. Fifty of these 61 received ventilation with bag and mask; of these, 45 recovered and 5 were declared dead. The remaining 11 (of 61) did not receive bag and mask ventilation; 8 started breathing spontaneously and 3 were declared dead.
Figure 2.
Neonatal resuscitation management tree for all countries.
Thirty of the 103 babies who were not dried and/or received no back rubs or whose necks were not extended to open their airways (right side of figure 2) initiated breathing spontaneously, 1 was declared dead, and 72 continued to be asphyxiated. Forty-four of the 72 asphyxiated babies received bag and mask ventilation, and 36 recovered while 8 died. Of the remaining 26 who received no bag and mask ventilation, 21 recovered and 5 died. Overall, out of the 209 observed asphyxiated babies, 185 (89%) newborns recovered, 22 died and 2 had missing outcome data.
Availability of ENC equipment and supplies
Table 4 presents the availability of equipment needed for ENC and management of birth asphyxia. The presence of disposable cord ties or clamps was below 70% when pooled across all countries, with a range between 36.4% and 99.5%. The largest gaps were found for towels and blankets necessary to dry and wrap newborns (or cover them if placed skin-to-skin with their mothers) and promote thermoregulation, which were present in only 40.5% of all facilities, with a great deal of variability across countries (8.0–53.2%), apart from Rwanda, which proved to have a greater supply (80.6%). The majority of facilities were equipped with sterile scissors or blades crucial to maintaining hygienic cord care and preventing infections. Overall, the availability of all three ENC supplies was poor, at only 30.8% of all facilities (ranging from 8.2% to 52.7%). Results were similar at hospital and health centre levels.
Table 4.
Availability of selected equipment/supplies for immediate ENC and newborn resuscitation
| ENC and newborn resuscitation equipment/supplies | Ethiopia |
Kenya |
Madagascar |
Mozambique |
Rwanda |
Tanzania |
Total |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hospital | Health centre | Hospital | Health centre | Hospital | Health centre | Hospital | Health centre | Hospital | Health centre | Hospital | Health centre | Hospital | Health centre | Pooled | |
| Disposable cord ties or clamps | 70% | NA | 99% | 100% | 39% | 25% | 73% | 77% | 59% | 51% | 46% | 79% | 68% | 74% | 70% |
| Towel or blanket to wrap baby | 46% | NA | 59% | 15% | 59% | 28% | 31% | 30% | 82% | 71% | 9% | 6% | 47% | 22% | 41% |
| Sterile scissors or blade | 94% | NA | 98% | 100% | 80% | 100% | 94% | 89% | 100% | 100% | 88% | 85% | 93% | 90% | 92% |
| Bag and mask | 90% | NA | NA | NA | 50% | 13% | 82% | 80% | 89% | 68% | 100% | 55% | 83% | 61% | 57% |
| Suction device | 88% | NA | 95% | 71% | 93% | 53% | 100% | 100% | 94% | 83% | 91% | 76% | 94% | 82% | 91% |
| Resuscitation table for newborn | 100% | NA | 65% | 37% | 43% | 39% | 94% | 77% | 74% | 74% | 88% | 73% | 75% | 66% | 72% |
NA, not available.
Facilities exhibited high availability of suction devices (pooled mean=90.7%). Availability of resuscitation tables averaged 72.4% but varied across countries. Meanwhile, newborn-specific bag and mask availability was high in most facilities, particularly hospitals (81.9–100%)—apart from Madagascar, with a low of 13% for health centres—which brought the pooled average to 57.1%. It should be noted that differences were observed by the type of facility. Availability of resuscitation equipment was higher in hospitals in Tanzania and Madagascar than in health centres. All countries apart from Ethiopia also reported having tube and masks, although at a slightly lower availability than bag and masks (43.5%).
The study assessed the availability of other newborn-related equipment and supplies, including the availability of weighing scales and antibiotics for newborn infection treatment. Almost all the facilities had weighing scales (pooled mean=95.4%), but only about 50% and 60% of facilities across all countries had adequate supplies of injectable ampicillin and gentamicin, and oral amoxicillin, respectively.
Health worker knowledge
Table 5 illustrates health workers' knowledge on four key areas related to newborn care. Gaps in knowledge on newborn care were observed in all countries and varied considerably for each indicator and country. Mean percentage scores for knowledge on basic equipment and supplies needed to provide immediate care after birth ranged from 25% to 50%, with great variations by country and type of equipment. Only 11% of respondents in Kenya mentioned all of the essential equipment, the highest percentage of all countries. A range of 36–62% in mean scores was found for health worker knowledge related to immediate newborn care practices. For knowledge on identification of newborn sepsis and care of LBW babies, the mean scores ranged from 27% to 65% and 32% to 54%, respectively. Most providers knew that LBW babies needed thermal protection but were less knowledgeable about the importance of feeding and ensuring infection prevention measures. For immediate newborn care, sepsis, and care of LBW babies, a higher percentage of health workers in Rwanda mentioned all relevant indicators (24%, 18% and 11%), although the percentages were still very low. Health workers in Kenya displayed greater overall knowledge in each category compared with other countries.
Table 5.
Individual components of health worker knowledge on newborn equipment and care
| Knowledge category and key equipment/practice | Country (% health workers with number of correct answers) |
||||
|---|---|---|---|---|---|
| Basic equipment and supplies needed for immediate care after birth | Ethiopia (N=76) | Kenya (N=248) | Mozambique (N=186) | Rwanda (N=144) | Tanzania (N=179) |
| Two dry warm towels or cloths | 55.0 | 74.0 | 78.0 | 36.0 | 52.0 |
| Flat surface with warm cloth | 17.0 | 75.0 | 22.0 | 29.0 | 12.9 |
| Source of warmth—heat lamp | 25.0 | 66.4 | 40.3 | 59.0 | 12.9 |
| Self-inflating ventilation bag | 49.0 | 28.4 | 29.0 | 55.0 | 29.1 |
| Newborn face mask size 1 | 57.0 | 8.2 | 59.0 | 34.1 | |
| Newborn face mask size 0 | 38.0 | 43.0 | 21.8 | ||
| Mucus extractor/suction/bulb syringe | 88.0 | 41.8 | 43.5 | 76.0 | 44.7 |
| Sterile or disinfected clamps, scissors, and cord ties | 25.0 | 74.5 | 22.0 | 17.3 | |
| Sterile blade or scissors | 66.7 | ||||
| Sterile or disposable cord ties/clamps | 30.6 | ||||
| Clock or watch with seconds hand | 13.0 | 26.4 | 20.4 | 14.0 | 3.4 |
| Mean percentage score for category | 41.0 | 49.3 | 38.0 | 44.0 | 25.3 |
| Immediate newborn care | Ethiopia (N=76) | Kenya (N=248) | Mozambique (N=186) | Rwanda (N=144) | Tanzania (N=250) |
| Wipe face after birth | 64.0 | 77.0 | 57.5 | 64.6 | 81.6 |
| Conduct dry cord care (sterile cut, applying nothing to stump) | 83.0 | 59.0 | 91 | 71.6 | |
| Cut cord with sterile blade/scissors | 22.6 | ||||
| Conduct dry cord care (nothing applied to stump) | 12.4 | ||||
| Ensure baby was breathing | 36.0 | 49.0 | 38.2 | 50.7 | 47.6 |
| Provide thermal protection | 78.0 | 82.0 | 60.8 | 75 | 63.2 |
| Initiate breast feeding within 1 hour | 51.0 | 66.0 | 44.6 | 41.7 | 43.6 |
| Assess/examine newborn within 1 hour | 18.0 | 51.0 | 14.5 | 40.3 | 24.8 |
| Provide eye prophylaxis | 58.0 | 48.0 | 39.2 | 45.8 | 8.8 |
| Mean percentage score for category | 55.0 | 61.7 | 36.0 | 58.0 | 48.7 |
| Signs of sepsis | Ethiopia (N=78) | Kenya (N=248) | Mozambique (N=185) | Rwanda (N=144) | Tanzania (N=250) |
| Poor/no breast feeding | 67.0 | 70.7 | 48.6 | 57.6 | 62.4 |
| Hypothermia/hyperthermia | 81.0 | 88.5 | 88.9 | 69.6 | |
| Hypothermia | 15.1 | ||||
| Hyperthermia | 82.2 | ||||
| Restlessness/irritability | 44.0 | 72.1 | 39.5 | 36.1 | 55.6 |
| Breathing difficulties | 45.0 | 29.8 | 41.1 | 49.3 | 49.2 |
| Mean percentage score for category | 59.0 | 65.3 | 27.0 | 58.0 | 59.2 |
| Care of LBW newborns | Ethiopia (N=78) | Kenya (N=248) | Mozambique (NA) | Rwanda (N=144) | Tanzania (N=248) |
| Provide thermal protection | 82.0 | 85.1 | 76.4 | 76.2 | |
| Provide extra support to mother to establish and maintain breast feeding | 15.0 | 74.0 | 34.7 | 27.4 | |
| Monitor sucking capability | 29.0 | 38.5 | 44.4 | 44.0 | |
| Monitor newborn closely for first 24 hours | 15.0 | 33.2 | 36.1 | 33.5 | |
| Ensure infection prevention | 17.0 | 48.2 | 31.3 | 24.2 | |
| Mean percentage score for category | 32.0 | 54.0 | 45.0 | 41.0 | |
The grey shaded boxes mean the question/item was not asked.
LBW, low birthweight.
In addition, due to limited numbers of actual asphyxiated newborn infants, NeoNatalie models were used to assess providers' skills in newborn resuscitation (n=514) in Madagascar, Rwanda and Tanzania. Demonstration of correct initial steps—including drying, rubbing of the back and extension of the neck—ranged from 52% to 72% of providers. Less than one-third (26–31%) of providers performed all steps without error to ventilate the NeoNatalie model using a bag and mask. Another 55–74% of providers readjusted the mask, checked airway positioning, or took further correct steps to improve ventilation.
Discussion
This assessment in six sub-Saharan African countries shows serious gaps in health facility readiness to provide quality newborn health services. The three main components of service delivery examined were (1) performance of key immediate ENC practices at hospitals and health centres, (2) health worker knowledge, and (3) the equipment and supplies to implement them. The study included an actual clinical observation of care provided to newborns during and immediately after birth. Almost all of the babies observed had their umbilical cord cut with clean or sterile scissors or blade, and about four out of five were dried immediately after birth. However, other essential care practices, including early initiation of breast feeding, skin-to-skin contact and delayed cord clamping, were performed for less than three-quarters of the observed deliveries. Studies have shown that early initiation of breast feeding and early skin-to-skin contact both contribute significantly to the maintenance of adequate thermal control and infection prevention in newborn infants.25 26 The earlier that breast feeding is initiated for a newborn, the lower the risk of newborn death.27 However, at an average of 45%, this was one of the least-performed newborn care practices by health workers in all the countries studied. A similar protective effect on mortality has been shown for hand washing by birth attendants.28 Unfortunately, hand washing in this study was also found to be low despite adequate availability of soap and water in delivery areas.
Other studies have reported a much higher level of practice of some of these essential care practices in the study countries. The 2014–2015 Tanzania SPA reported skin-to-skin contact, early initiation of breast feeding, and drying and wrapping the baby as routine practice in over 90% of health facilities assessed.29 A similar finding was reported in Kenya in the 2010 SPA.30 However, the information was obtained through interviews with health facility staff and not actual clinical observation of their care practices. In addition to the routine newborn care practices reported by health facility staff in Tanzania and Kenya, the Kenya SPA reported on observed clinical newborn practices, information collected in collaboration with MCHIP reported in this paper. No SPA with information on maternal and newborn care services has been conducted for any of the remaining four study countries. The WHO's Service Availability and Readiness Assessment (SARA) tool does not include actual clinical observation for newborn services.31
Previous clinical observational studies in Ghana,15 the Philippines,8 Nepal32 and Bangladesh33 have also identified gaps in the quality of newborn healthcare. A health facility assessment in rural Ghana highlighted major gaps in newborn care equipment and quality of newborn care as well as the importance of quality improvement.15 A recent study in the Philippines also found that bathing of the baby in the first 24 hours was common and placing the baby skin-to-skin was infrequent.8 However, initiation of breast feeding was lower in this study (apart from in Kenya) than found in studies in the Philippines8 and Nepal.32 In addition, poor quality of neonatal care, specifically poor hygiene practices, lack of appropriate thermal care practices and substandard care of LBW babies were identified through a hospital survey in 18 hospitals in Bangladesh.33
Intrapartum-related complications is the second leading global cause of newborn deaths.1 Evidence shows that the majority of babies who experience difficulty in the initiation of breathing at birth can be saved with simple stimulation, such as drying and/or rubbing the baby's back.18 Of those newborns identified as requiring some form of resuscitation, 22% and 39% of them recovered through initial stimulation and bag and mask ventilation, respectively. Additionally, 28% recovered on their own without any intervention, and 11% died. Almost half of all of the observed babies who had difficulty initiating breathing at birth and required some form of resuscitation were not immediately dried, which could cause hypothermia and less responsiveness to resuscitation. When assessing the quality of resuscitation using the NeoNatalie model overall, less than a third of providers ventilated without error. The percentage of providers who correctly stimulated and made proper adjustments including repositioning the neck and the mask to facilitate breathing ranged from 50% to 75%. Every birth that occurs at a health facility that provides delivery services should have staff competent to resuscitate newborn infants who do not breathe at birth.
Maintenance of resuscitation skills requires ongoing practice and periodic refresher training through on-site and off-site courses and mentorship. However, many service providers do not receive this type of support. In the Tanzania 2014–2015 SPA, only 33% of staff reported having received refresher training on newborn resuscitation in the 24 months prior to the assessment, and 42% have ever received training on newborn resuscitation.29 Data from the most recent SPA health facility surveys conducted in Ghana,34 Rwanda35 and Uganda36 indicate that only 2–12% of those responsible for conducting deliveries were trained in neonatal resuscitation, and only 8–22% of the facilities where deliveries were performed had the proper equipment for resuscitation. A study in the Philippines found that <50% of staff were trained in neonatal and paediatric resuscitation.8 With the exception of thermal care, health workers' knowledge related to immediate newborn care and associated equipment was poor and differed greatly among countries; mean knowledge per country for each category ranged roughly from about a quarter to two-thirds of providers. A study in 21 hospitals in 7 countries in sub-Saharan Africa and South Asia also found inadequate knowledge among doctors, nurses and medical assistants, particularly with regard to sepsis.9
Availability of essential newborn health equipment and other supplies, including bags and masks, was less of an issue in the assessed facilities. Most facilities had some supplies of newborn resuscitation equipment: suction, face mask and ventilation bag. The majority of facilities were equipped with sterile scissors or blades crucial to maintaining hygienic cord care and preventing infections, but, overall, the availability of all ENC supplies was poor. The largest gaps were found in the supply of towels and blankets necessary to dry and wrap newborns and promote thermoregulation.
A study in Kenya14 that surveyed eight large district hospitals to assess the structural components of QoC found that equipment was not always available and that even large hospitals experienced poor QoC. Similarly, this study found that gaps were observed irrespective of the type of facility. Lack of infrastructural components and resources can negatively influence community perceptions of quality and usage of care, as found in Nigeria,37 and can further weaken linkages between the community and facility.14
Strengths and limitations of the study
This paper describes directly observed quality of newborn care at hospitals and health centres through a facility assessment using a robust methodology in six countries in sub-Saharan Africa. It provides new information on the frequency with which evidence-based practices are performed according to international standards and guidelines. The use of direct observation in this study is a major strength. Direct observations of labour and delivery care are considered the gold standard in low-income and middle-income settings and more reliable than chart reviews, provider interviews or client exit interviews for assessing provider performance. However, due to cost and time requirements, observations are rarely conducted as part of health facility assessments, especially observation of labour and delivery care, and even more infrequently for management of newborn complications such as birth asphyxia.
However, the study had limitations. Settings and facility samples differed greatly across countries, making it difficult to make comparisons. However, the purpose of the study was to inform country-specific quality improvement and policy development efforts, not to make cross-country comparisons. At times as the study evolved, survey questions differed slightly between countries, which limited samples for certain analyses. The availability of equipment was based on point prevalence at the time of observation and does not necessarily imply a constant supply, but this was the best possible assessment given the resources. Simulated resuscitation results may have been affected by previous exposure or non-exposure of health workers to the NeoNatalie model. Observations could have been biased as a result of the Hawthorne Effect, meaning that providers could have changed their behaviours because they knew that they were being watched. Additionally, observational data are based on the judgements of observers and could therefore result in some biases. The study did not include any tools to assess user perspectives on QoC, such as facility exit interviews or focus groups with recently delivered women in the community, as this was beyond the scope of the current study. Women's perceptions of QoC at facilities and their satisfaction with services are important determinants of whether or not women deliver at a facility, relevant for future investigation.
Implications and next steps
Many countries are implementing strategies to increase the percentage of pregnant women who deliver in health facilities as an intervention crucial to reducing maternal and newborn mortality. However, as this assessment shows, the care provided at most facilities is not optimal. These study findings will contribute to the evidence base on the quality of newborn facility care, calling attention to the issue and helping policymakers and stakeholders make informed decisions on the type of support needed for provision and maintenance of quality ENC services in health facilities. The hope is that such a study can help in closing the gap between policy and implementation.
In order for countries to achieve the targets of the Sustainable Development Goals for ending preventable newborn and maternal deaths, it is essential that health workers at health facilities have the competencies required to provide quality ENC in a timely manner, with systems in place to facilitate implementation and maintain quality. Health facility assessments using tools such as the SPA and SARA are crucial first steps in identifying and then closing existing gaps in the quality of newborn care at health facilities. However, these assessments are usually conducted without observation of the actual provision of care by service providers, which might lead to some gaps being missed. Clinical observation data could be collected as part of such formal assessments or periodically as part of ongoing regular supportive supervision or at sentinel surveillance sites.
Whether identified through interviews with health workers or observations of actual performance of ENC practices by health workers, gaps in the quality of newborn health services have to be closed to ensure that newborn infants receive optimal care during delivery. Comparison of the MDG 2010 and 2015 reports shows significant reduction in NMRs for all the six countries assessed (Ethiopia: 39–28 per 1000 live births, Kenya: 33–22 per 1000, Madagascar: 35–20 per 1000, Mozambique: 43–27 per 1000, Rwanda: 35–19 per 1000 and Tanzania: 33–19 per 1000).38 39 The contribution of actual improvement in newborn care practices by service providers in health facilities to the NMR reduction in these countries is unknown since the clinical observational assessments have not been repeated.
Many countries already recognise the need to address the quality of maternal and newborn care in health facilities and are taking actions to address these concerns. There are also existing global initiatives, such as the ENAP,3 the Global Strategy for Women's, Children's and Adolescents' Health40 and the Mother-Baby Friendly Birthing Facilities Initiative,41 as well as new initiatives to encourage government accountability; these are not limited to increasing supply and demand for care but also focus on ensuring equitable care, an often neglected yet a critical objective of accountability.42 These initiatives provide frameworks for countries to assess gaps in their maternal, newborn and child health policies; service guidelines; and implementation plans, and to examine how they help health workers acquire, maintain and use their competencies. To further help countries improve the quality of their maternal and newborn health (MNH) services, the WHO recently released its Standards for improving quality of maternal and newborn care in health facilities.43 This document provides a framework with eight domains of QoC and associated performance indicators that countries could use to assess, improve and monitor the MNH services provided at health facilities. The implementation of this framework has been supported by a strong advocacy effort calling for quality, equity and dignity for all women and newborns.44
It is essential that quality improvement approaches be endorsed by national health authorities, integrated into existing systems and supported with strong policies in order to be effective, efficient and aligned with global initatives.45 46 The findings and lessons learnt from this study need to be taken forward as part of this WHO-led initiative and will be critical for advancing and supporting ministries of health to harmonise and lead quality improvement approaches across the health system. We hope that this paper will influence this important mechanism.
Conclusion
Results of the health facility assessments presented in this paper have highlighted major gaps in facility readiness and quality of immediate newborn care in six sub-Saharan African countries through observations of clinical care provided. There is a need for such studies to establish a better understanding of QoC practices for women and newborns at health facilities as countries seek to end preventable maternal and child deaths. Better understanding of the QoC will lead to improved supportive and enabling strategies and actions, such as on-site mentorship and skills updates, to close the gap in the delivery of optimal facility newborn care as women respond to the call to deliver in health facilities. It will require the continued leadership from ministries of health at all levels (national, regional and district) and collaboration with donors, international and local implementing partners, professional associations, preservice institutions, beneficiaries, and other stakeholders to ensure health workers are competent in knowledge and skills and that essential equipment and supplies are available to guarantee all newborns receive the essential care needed to enable their survival.
Acknowledgments
The authors wish to thank all the interviewers, observers, service providers and patients in the six study countries for their work and contribution to making the data available.
Footnotes
Contributors: BR designed the study, and BR, RK, AG, AT, JPR and GT conducted the study. The newborn component of the study was conceived by BR, JdG-J, HER, BR, SA and GM. The paper was conceptualised by LV, JdG-J, HER, BR, SA, NK, GM, RB and WM. LV drafted the paper. All authors reviewed the manuscript multiple times, provided feedback and gave final approval before submission.
Funding: The study was funded by United States Agency for International Development, under the terms of the Leader with Associates Cooperative Agreement Number GHS-00-08-00002-00. The corresponding author had full access to all data and, together with the second author, the final responsibility to submit for publication.
Competing interests: None declared.
Ethics approval: The study was reviewed and approved by the Institutional Review Board (IRB) at Johns Hopkins Bloomberg School of Public Health (IRB number: 00002549) as well as by the ethical review boards in all study countries and by all partners.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Data are available on request.
References
- 1.UNICEF. Child Mortality Estimates. Secondary Child Mortality Estimates 2015. http://www.childmortality.org/index.php?r=site/index.
- 2.Save the Children. Ending newborn deaths: ensuring every baby survives. London, UK: Save the Children, 2014. [Google Scholar]
- 3.WHO, UNICEF. Every newborn: an action plan to end preventable deaths. Geneva: World Health Organization, 2014. [Google Scholar]
- 4.Chou D, Daelmans B, Jolivet RR et al. Ending preventable maternal and newborn mortality and stillbirths. BMJ 2015;351:h4255 10.1136/bmj.h4255 [DOI] [PubMed] [Google Scholar]
- 5.Darmstadt GL, Bhutta ZA, Cousens S et al. Evidence-based, cost-effective interventions: how many newborn babies can we save? Lancet 2005;365:977–88. 10.1016/S0140-6736(05)71088-6 [DOI] [PubMed] [Google Scholar]
- 6.Wall SN, Lee AC, Niermeyer S et al. Neonatal resuscitation in low-resource settings: what, who, and how to overcome challenges to scale up? Int J Gynaecol Obstet 2009;107(Suppl 1):S47–62, S63–4 10.1016/j.ijgo.2009.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Partnership for Maternal, Newborn and Child Health. A global review of the key interventions related to reproductive, maternal, newborn and child health. Geneva: PMNCH, 2011. [Google Scholar]
- 8.Sobel HL, Silvestre MA, Mantaring JB III et al. Immediate newborn care practices delay thermoregulation and breastfeeding initiation. Acta Paediatr 2011;100:1127–33. 10.1111/j.1651-2227.2011.02215.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nolan T, Angos P, Cunha AJ et al. Quality of hospital care for seriously ill children in less-developed countries. Lancet 2001;357:106–10. 10.1016/S0140-6736(00)03542-X [DOI] [PubMed] [Google Scholar]
- 10.Althabe F, Bergel E, Cafferata ML et al. Strategies for improving the quality of health care in maternal and child health in low- and middle-income countries: an overview of systematic reviews. Paediatr Perinat Epidemiol 2008;22(Suppl 1):42–60. 10.1111/j.1365-3016.2007.00912.x [DOI] [PubMed] [Google Scholar]
- 11.Fauveau V, de Bernis L. “Good obstetrics” revisited: too many evidence-based practices and devices are not used. Int J Gynaecol Obstet 2006;94:179–84. 10.1016/j.ijgo.2006.05.020 [DOI] [PubMed] [Google Scholar]
- 12.van den Broek NR, Graham WJ. Quality of care for maternal and newborn health: the neglected agenda. BJOG 2009;116(Suppl 1):18–21. 10.1111/j.1471-0528.2009.02333.x [DOI] [PubMed] [Google Scholar]
- 13.English M, Ntoburi S, Wagai J et al. An intervention to improve paediatric and newborn care in Kenyan district hospitals: understanding the context. Implement Sci 2009;4:42 10.1186/1748-5908-4-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Opondo C, Ntoburi S, Wagai J et al. Are hospitals prepared to support newborn survival? An evaluation of eight first-referral level hospitals in Kenya. Trop Med Int Health 2009;14:1165–72. 10.1111/j.1365-3156.2009.02358.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vesel L, Manu A, Lohela TJ et al. Quality of newborn care: a health facility assessment in rural Ghana using survey, vignette and surveillance data. BMJ Open 2013;3:e002326 10.1136/bmjopen-2012-002326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dickson KE, Simen-Kapeu A, Kinney MV et al. Every newborn: health-systems bottlenecks and strategies to accelerate scale-up in countries. Lancet 2014;384:438–54. 10.1016/S0140-6736(14)60582-1 [DOI] [PubMed] [Google Scholar]
- 17.Liu L, Oza S, Hogan D et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systemic analysis with implications for the Sustainable Development Goals. Lancet 2016;388:3027–35. 10.1016/S0140-6736(16)31593-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee AC, Cousens S, Wall SN et al. Neonatal resuscitation and immediate newborn assessment and stimulation for the prevention of neonatal deaths: a systematic review, meta-analysis and Delphi estimation of mortality effect. BMC Public Health 2011;11(Suppl 3):S12 10.1186/1471-2458-11-S3-S12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newton O, English M. Newborn resuscitation: defining best practice for low-income settings. Trans R Soc Trop Med Hyg 2006;100:899–908. 10.1016/j.trstmh.2006.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duysburgh E, Zhang WH, Ye M et al. Quality of antenatal and childbirth care in selected rural health facilities in Burkina Faso, Ghana and Tanzania: similar finding. Trop Med Int Health 2013;18:534–47. 10.1111/tmi.12076 [DOI] [PubMed] [Google Scholar]
- 21.USAID/MCHIP. Introduction to the maternal and newborn quality of care surveys. Baltimore, MA: Jphieg, 2013. [Google Scholar]
- 22.Columbia University. AMDD Needs assessment of emergency obstetric and newborn care. Obstetric facility assessment tool. New York: 2010. [Google Scholar]
- 23.WHO. Integrated management of pregnancy and childbirth. Managing complications in pregnancy and childbirth: a guide for midwives and doctors. Geneva, Switzerland: World Health Organization, 2007. [Google Scholar]
- 24.ICF/Macro. Service Provision Assessment survey tool. http://www.measuredhs.com/What-We-Do/Survey-Types/SPA.cfm.
- 25.Conde-Agudelo A, Diaz-Rossello JL. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst Rev 2014;(4):CD002771 10.1002/14651858.CD002771.pub3 [DOI] [PubMed] [Google Scholar]
- 26.Edmond KM, Zandoh C, Quigley MA et al. Delayed breastfeeding initiation increases risk of neonatal mortality. Pediatrics 2006;117:e380–6. 10.1542/peds.2005-1496 [DOI] [PubMed] [Google Scholar]
- 27.NEOVITA Study Group. Timing of initiation, patterns of breastfeeding, and infant survival: prospective analysis of pooled data from three randomised trials. Lancet Glob Health 2016;4:e266–75. 10.1016/S2214-109X(16)00040-1 [DOI] [PubMed] [Google Scholar]
- 28.Rhee V, Mullany LC, Khatry SK et al. Impact of maternal and birth attendant hand-washing on neonatal mortality in Southern Nepal. Arch Pediatr Adolesc Med 2008;162:603–8. 10.1001/archpedi.162.7.603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ministry of Health and Social Welfare (MoHSW). [Tanzania Mainland], Ministry of Health (MoH) [Zanzibar], National Bureau of Statistics (NBS), Office of the Chief Government Statistician (OCGS), and ICF International 2015. Tanzania Service Provision Assessment Survey 2014–15. Rockville, MA, USA: MoHSW, MoH, NBS, OCGS, and ICF International; http://dhsprogram.com/publications/publication-SPA22-SPA-Final-Reports.cfm. [Google Scholar]
- 30.National Coordinating Agency for Population and Development. (NCAPD) [Kenya], Ministry of Medical Services (MOMS) [Kenya], Ministry of Public Health and Sanitation (MOPHS) [Kenya], Kenya National Bureau of Statistics (KNBS) [Kenya], ICF Macro. 2011. Kenya Service Provision Assessment Survey 2010. Nairobi, Kenya: National Coordinating Agency for Population and Development, Ministry of Medical Services, Ministry of Public Health and Sanitation, Kenya National Bureau of Statistics, and ICF Macro; http://dhsprogram.com/publications/publication-SPA17-SPA-Final-Reports.cfm. [Google Scholar]
- 31.Service Availability and Readiness Assessment. (SARA): an annual monitoring system for service delivery reference manual. Version 2.2 2015. http://www.who.int/healthinfo/systems/sara_reference_manual/en/.
- 32.Khanal V, Gavidia T, Adhikari M et al. Poor thermal care practices among home births in Nepal: further analysis of Nepal Demographic and Health Survey 2011. PLoS ONE 2014;9:e89950 10.1371/journal.pone.0089950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoque DM, Rahman M, Billah SM et al. An assessment of the quality of care for children in eighteen randomly selected district and sub-district hospitals in Bangladesh. BMC Pediatr 2012;12:197 10.1186/1471-2431-12-197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghana Statistical Service (GSS), Health Research Unit, Ministry of Health, and ORC Macro. Ghana Service Provision Assessment Survey 2002. Calverton, MA: Ghana Statistical Service and ORC Macro, 2003. http://dhsprogram.com/publications/publication-SPA6-SPA-Final-Reports.cfm [Google Scholar]
- 35.National Institute of Statistics. (NIS) [Rwanda], Ministry of Health (MOH) [Rwanda], and Macro International Inc. 2008. Rwanda Service Provision Assessment Survey 2007. Calverton, MA, USA: NIS, MOH, and Macro International Inc; http://dhsprogram.com/publications/publication-SPA15-SPA-Final-Reports.cfm [Google Scholar]
- 36.Ministry of Health. (MOH) [Uganda] and Macro International Inc. 2008. Uganda Service Provision Assessment Survey 2007. Kampala, Uganda: Ministry of Health and Macro International Inc; http://dhsprogram.com/publications/publication-SPA13-SPA-Final-Reports.cfm. [Google Scholar]
- 37.Uzochukwu BS, Onwujekwe OE, Akpala CO. Community satisfaction with the quality of maternal and child health services in southeast Nigeria. East Afr Med J 2004;81:293–9. 10.4314/eamj.v81i6.9178 [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization and UNICEF. Countdown to 2015 decade report (2000–2010): taking stock of maternal, newborn and child survival. Geneva, Switzerland: World Health Organization, 2010. http://www.who.int/pmnch/topics/child/CountdownReportOnly.pdf?ua=1. [Google Scholar]
- 39.World Health Organization and UNICEF. Countdown to 2015: a decade of tracking progress for maternal, newborn and child survival. The 2015 Report. Geneva, Switzerland: World Health Organization, 2015. http://countdown2015mnch.org/documents/2015Report/Countdown_to_2015_final_report.pdf. [Google Scholar]
- 40.United Nations. Survive, thrive, transform. The global strategy for women's, children's and adolescent's health. New York: United Nations, 2015. [Google Scholar]
- 41.Lalonde AB, Miller S. Mother-baby friendly birthing facilities initiative. Int J Gynecol Obstet 2015;128:93–4. 10.1016/j.ijgo.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 42.Barroso C, Lichuma W, Mason E et al. Accountability for women's, children's and adolescents’ health in the Sustainable Development Goal era. BMC Public Health 2016;16(Suppl 2):799 10.1186/s12889-016-3399-9 [DOI] [Google Scholar]
- 43.World Health Organization. Standards for improving quality of maternal and newborn care in health facilities 2016. http://www.who.int/maternal_child_adolescent/documents/improving-maternal-newborn-care-quality/en/.
- 44.Kinney MV, Boldosser-Boesch A, McCallon B. Quality, equity, and dignity for women and babies. Lancet 2016;388:2066–8. 10.1016/S0140-6736(16)31525-2. [DOI] [PubMed] [Google Scholar]
- 45.Milner KM, Duke T, Bucens I. Reducing newborn mortality in the Asia-Pacific region: quality hospital services and community-based care. J Paediatr Child Health 2013;49:511–18. 10.1111/jpc.12249 [DOI] [PubMed] [Google Scholar]
- 46.Tamburlini G, Yadgarova K, Kamilov A et al. Improving the quality of maternal and neonatal care: the role of standard based participatory assessments. PLoS ONE 2013;8:e78282 10.1371/journal.pone.0078282 [DOI] [PMC free article] [PubMed] [Google Scholar]