Abstract
Introduction
Oral steroids induce remission in about 90% of children with idiopathic nephrotic syndrome (INS), which is characterised by severe proteinuria and hypoalbuminaemia. Some children become steroid-dependent (SD) and require addition of calcineurin inhibitors (CNI) to maintain remission. Since these oral agents are toxic, alternative interventions are needed for long-term treatment. The anti-CD20 antibody rituximab has shown promising steroid-sparing properties in clinical trials, but benefits are less convincing in complicated forms of SD-INS. Ofatumumab, a new anti-CD20 antibody with stronger affinity to CD20, may be superior to rituximab in maintaining oral steroid-free and CNI-free disease remission in children with SD-INS.
Methods and analysis
This open-label, two-parallel-arm, controlled, phase II randomised clinical trial will enrol children with SD-INS maintained in remission with oral steroids and CNI. Children will be randomised to either ofatumumab or rituximab infusion. After infusion of either antibody, steroids will be maintained for 30 days and then tapered off by 0.3 mg/kg/week until complete withdrawal. 1 week after complete steroid withdrawal, CNI will be decreased by 50% and withdrawn within 2 additional weeks. We will enrol 140 children to detect as significant at the 2-sided p value of 0.01 with a power of >0.8, a reduction in the risk of 1-year relapse (primary end point) of at least 0.3 (ie, from 0.65 to 0.35; (risk ratio 0.54)) in the ofatumumab arm when compared with the rituximab arm. We will compare the amount of steroids required to maintain complete disease remission at 6 and 24 months, relapse-free period, relapse rate per year as secondary end points. Circulating cell populations will be studied as biomarkers or predictors of the anti-CD20 response.
Ethics and dissemination
The trial received ethics approval from the local ethics board. We will publish study results and present them at international scientific meetings.
Trial registration numbers
NCT02394119; 2015-000624-28; Pre-results.
Strengths and limitations of this study.
This study will be conducted as a multicentre randomised controlled trial coordinated by a large national referral centre for paediatric nephrology.
It will provide patients and healthcare providers with important information on benefits and harms of two anti-CD20 antibodies in idiopathic nephrotic syndrome (INS).
Disease relapse is an important outcome for children with INS for its social, psychological and clinical implications.
More important outcomes such as end-stage kidney failure or death are difficult to include in randomised controlled trials in children as they occur relatively late in the disease course.
Limitations related to lack of blinding are mitigated by the objective outcomes chosen and blinding of outcome assessors.
Introduction
Idiopathic nephrotic syndrome (INS) is a disease characterised by episodes of severe proteinuria and hypoalbuminaemia (serum albumin <2.5 g/dL), often associated with dyslipidaemia and hypercoagulability. In Western countries, INS affects 2–2.7 new children per 100 000 children per year and has a prevalence of 16 cases per 100 000.1 Oral corticosteroids are the cornerstone of therapy, inducing remission of INS in ∼90% of children.2 However, up to 85% of cases relapse within 5 years3 and many will develop steroid dependence (SD). SD-INS relapses within 2 weeks of steroid withdrawal and requires continuation of treatment. Clinical practice guidelines suggest using low-dose prednisone to maintain remission in SD-INS (evidence 2C-D), and use of steroid-sparing agents (ie, calcineurin inhibitors; CNI) for children who develop steroid-related adverse effects (evidence 1B).4 Given the toxicity of steroids and CNI, there is a need to investigate alternative treatment options.
The interest in the anti-CD20 chimeric antibody rituximab followed the observation of a dramatic reduction in proteinuria in children with nephrotic syndrome who received rituximab to treat idiopathic thrombocytopenic purpura5 or a post-transplant lymphoproliferative disorder.6 7 More recent observational studies8–17 confirmed potential benefits in mixed populations with nephrotic syndrome, but clinical trials did not confirm expected benefits in steroid-resistant (SR) forms of INS.18 Although recent randomised controlled trials support the use of rituximab in SD-INS, benefits may be suboptimal, especially in complicated forms of the disease. While a single infusion of rituximab allowed steroid withdrawal and was non-inferior to steroids in maintaining remission in a trial of children with uncomplicated forms of SD-INS,19 in children with frequently relapsing and complicated forms of SD-INS requiring the use of CNI, benefits of rituximab are less convincing.20 21
Ofatumumab is a fully humanised anti-CD20 monoclonal IgG1(k) antibody of last generation. Ofatumumab binds with more affinity to CD20, potentially leading to more efficient complement-dependent cytotoxicity. In small series, ofatumumab induced remission in children with SR-INS who did not respond to rituximab.22 23 From an economic point of view, ofatumumab is not superior to rituximab as the cost of the two drugs for square metres of body surface is similar. Ofatumumab is currently in phase III clinical trials for the treatment of chronic lymphocytic leukaemia, relapsing lymphoma and rheumatoid arthritis.24 Owing to its stronger affinity for the CD20 and its fully humanised structure, ofatumumab may be used in larger doses with minimal risk of side effects leading to potentially larger benefits, that is, longer remission period, than the chimeric antibody rituximab. Some studies support this conclusion.25 26 The aim of this trial is to test whether ofatumumab is superior to rituximab in maintaining complete disease remission in children with steroid and CNI-dependent INS.
Methods and design
Rationale and justification of the active comparator
Owing to its fully humanised structure and stronger affinity for the CD20 antigen, ofatumumab can be used in larger doses than the chimeric antibody rituximab with minimal risk of side effects. Knowledge generation to support the use of new steroid-sparing agents is an important unmet healthcare need in paediatric nephrology, especially for children with complicated forms of SD-INS requiring steroids and CNI (tacrolimus or ciclosporin). In the present study, CNI have been chosen among steroid-sparing agents because they are more effective than other drugs and therefore more commonly used.27
Head-to-head comparison between rituximab and ofatumumab is justified on the basis of available trials supporting the use of anti-CD20 antibodies to maintain remission of INS without steroids and CNI.19–21
Objectives
This trial will test whether ofatumumab is superior to rituximab in maintaining oral drug-free disease remission (complete or partial remission) at 12 months (primary objective) and reduce the risk of relapse in a longer follow-up of 24 months (secondary objective) in children with SD and CNI-dependent INS. This study will also collect information on occurrence of side effects (ie, acute and long-term drug-related adverse events) and need to restart the use of steroids or CNI following the infusion of the trial interventions.
Design
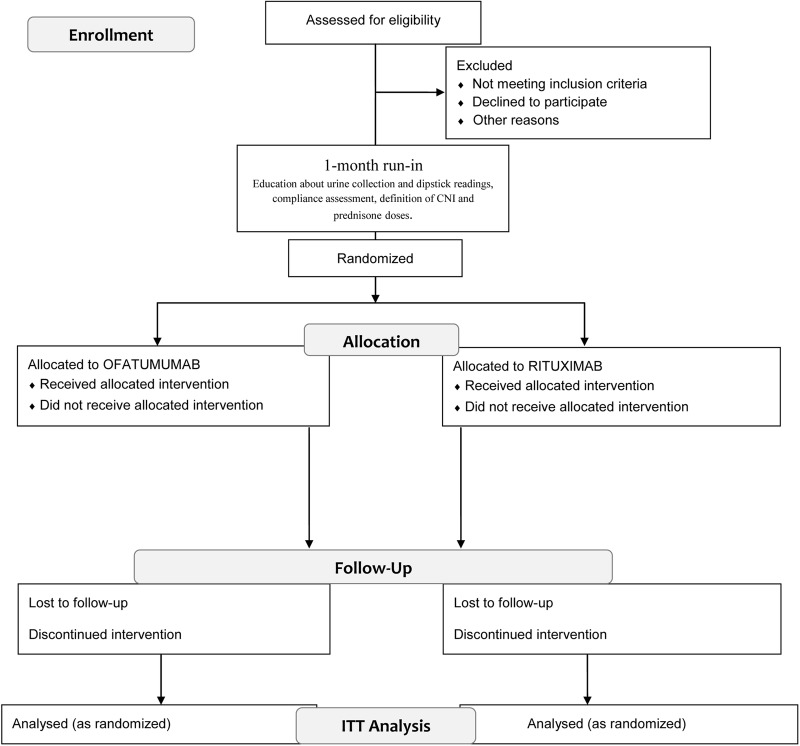
This is an open-label, two-parallel-arm, controlled, phase II randomised clinical trial testing the superiority of ofatumumab over rituximab in maintaining steroid-free and CNI-free disease remission in children with SD-INS (figure 1). Eligible participants will enter a 1-month run-in period, during which instruction on urine collection and dipstick readings will be carefully reviewed, compliance assessed and CNI and prednisone doses will be reduced to the minimum doses required to maintain complete remission (usually the minimum doses used in the previous 6 months). After run-in, children will be randomised in a 1:1 ratio to either the intervention arm (ofatumumab) or the active comparator arm (rituximab).
Figure 1.
Schematic view of trial design. CNI, calcineurin inhibitors; ITT, intention to treat.
After infusion of intervention or comparator, steroids will be maintained at the baseline (post-run-in) dose for 30 days and then tapered off by 0.3 mg/kg/week until complete withdrawal. One week after the steroid withdrawal, CNI will be decreased by 50% and withdrawn within 2 additional weeks. All children will be followed for up to 24 months. In the case of relapses during the study (see outcome section) children will be treated with oral prednisone (60 mg/m2), in order to achieve remission. At remission, children will be treated with another infusion of either ofatumumab or rituximab, according to the allocation arm. After infusion of intervention or comparator, steroids will be maintained at initial dose for 30 days and then tapered off by 0.3 mg/kg/week until complete withdrawal. One week after the steroid withdrawal, CNI will be decreased by 50% and withdrawn within 2 additional weeks. Inability to withdraw CNI or need to restart CNI (development of steroid resistance) will be considered a relapse (primary end point) and a reason to terminate the study. In this case, patients should be treated at the discretion of the investigator, according to the best clinical practice guidelines. Study enrolment started in June 2015 and is expected to be complete by the end of 2017.
Setting
Interventions will be administered during an inpatient setting at the Nephrology Unit of the Giannina Gaslini Children's Hospital, Genoa. Follow-up visits will be performed at the same institution or by local nephrologists if travel to Genoa is not possible.
Eligibility criteria
Patients aged 2–18 years, in complete disease remission and with a proven clinical history of SD and CNI-dependent INS are eligible for inclusion in the study. SD-INS will be defined by two consecutive relapses during corticosteroid therapy tapering or within 14 days of steroid withdrawal; CNI-dependent INS will be defined by relapse following ciclosporin or tacrolimus discontinuation.
Exclusion criteria include: positivity to autoimmunity tests (ANA, nDNA, ANCA); reduction in C3 levels; steroid resistant forms of INS; eGFR<90/mL/min/1.73 m2 valuated according to revised Bedside Schwartz Formula for patients between 2 and 17 years and with CKD-EPI Creatinine 2009 Equation for 18 years old patients; pregnancy; neoplasm; previous or actual HBV (with HBeAb positivity) or HCV infection; CD20 B lymphocytes count <2.5% and treatment with rituximab or steroid-sparing agents other than CNI in the past 6 months.
Participant identification process
All patients affected by SD and CNI-dependent INS followed up in the nephrology outpatient clinic at the Giannina Gaslini Children's Hospital will be evaluated for recruitment as they return to clinic. A preliminary interview for clinical and pharmacological history will be performed in order to verify the eligibility criteria. A study coordinator will illustrate the project, deliver the information material (information sheet and informed consent form) and collect written informed consent in order to complete the screening. Parents'/guardian's written informed consent, and child's assent will be collected before any study-related procedure not part of the participant's normal medical care. Participants or their families will be able to withdraw consent at any time.
Randomisation
Participants will be randomised 1:1 to the intervention or active comparator arm. A distant site with no clinical involvement in the trial will generate two randomisation lists (for age ≥9 years and <9 years) using permuted blocks of variable size. Stratification by age is motivated by the need to maximise the likelihood of balancing factors potentially affecting the effects of the intervention on outcomes, which are associated with age (disease duration, age at onset, relapse history and disease severity).20 Assignments will be notified electronically after obtaining signed consent. A study coordinator responsible for recruitment will obtain signed consent (and assent for participants capable of assenting), assign a unique participant study number and request randomisation. An analyst from a distant site not involved in patient care, where the randomisation lists have been generated and kept concealed from the clinical investigators, will communicate the allocation arm to the study coordinator (based on the participant's study number).
Blinding
The following reasons justify the lack of participant and investigator blinding:
Ofatumumab and rituximab require different methods for infusion; for example, the ofatumumab requires a filter that cannot be blinded. According to the producer indications, the same filter may alter/reduce the availability/stability rituximab; and
Ofatumumab must be diluted in 1000 mL of normal saline and infused at a fixed high rate; the producer does not guarantee rituximab stability using the same dilution/infusion strategy.
Treatment arms
Intervention
Children randomised to the intervention arm will receive the fully humanised monoclonal anti-CD20 antibody ofatumumab. Participants assigned to the intervention arm will receive a premedication with methylprednisolone (2 mg/kg infused in 30′ intravenous diluted in 100 mL of normal saline), oral cetirizine (0.2 mg/kg) and oral paracetamol (15 mg/kg), in order to reduce common reactions. Registered nurses will deliver the premedication and the intervention drug in the Nephrology Department of Giannina Gaslini Children's Hospital. Ofatumumab will be infused intravenous, at a dose of 1500 mg/1.73 m2, diluted in 1000 mL of normal saline, at a rate of 12 mL/hour in the first 30′. Thereafter, the infusion rate will be doubled every 30 min up to a maximum of 200 mL/hour.
Active comparator
Children randomised to the active comparator will receive the chimeric monoclonal anti-CD20 antibody rituximab. In order to reduce common reactions, children will receive the same premedication as described above for the ofatumumab arm. As for Ofatumumab, registered expert nurses will deliver the premedication and the active comparator in the Nephrology Department of Giannina Gaslini Children's Hospital. Rituximab will be administered at a dose of 375 mg/m2. For doses between 100 and 250 mg, rituximab will be diluted in 100 mL of normal saline and administered at 2 mL/hour for the first 30′; 3 mL/hour for the second 30′; 6 mL/hour for the third 30′; 15 mL/hour until the end of the infusion. For doses between 260 and 500 mg, rituximab will be diluted in 250 mL of normal saline and administered at 6 mL/hour for the first 30′; 9 mL/hour for the second 30′; 18 mL/hour for the third 30′; 36 mL/hour until the end. For doses between 510 and 1000 mg, rituximab will be diluted in 500 mL of normal saline and administered at 9 mL/hour for the first 30′; thereafter, the infusion rate will be doubled every 30 min up to a maximum of 72 mL/hour.
Since ofatumumab and rituximab will be administered during a hospitalisation, the protocol does not include strategies to improve adherence to therapy.
Relevant concomitant care and interventions that are permitted or prohibited during the trial
Any medications not listed in the exclusion criteria may be given at the discretion of the investigator. The investigator will record all concomitant medications taken by the participant during the study from the date of informed consent, in the appropriate section of the case report form. In view of a possible role in the reduction in proteinuria and worsening of glomerular filtration rate, the use of ACE inhibitors/ARBs will not be allowed during the study. In hypertensive patients, these drugs will be replaced with other antihypertensive agents: α-adrenergic or β-adrenergic blockers (eg, carvedilol 0.2–1 mg/kg/day b.i.d., up to a maximum of 50 mg/day) will be administered as first-line drugs; in patients with intolerance or contraindication to β blockers (ie, children with asthma), calcium channel blockers (eg, amlodipine 0.06–0.3 mg/kg/day, up to a maximum of 10 mg/day) will be used.
Outcomes
The primary end point will be the occurrence of disease relapse or need of steroids or CNI to maintain complete remission within 12 months of randomisation. Relapse is defined by uPCR ≥2000 mg/g (≥200 mg/mmol) or >3+ protein on urine dipstick for 3 consecutive days.24 Failure to achieve full steroid and CNI withdrawal will be considered a relapse. The secondary end points will be relapse-free period, relapse rate per year and the amount of steroids required to maintain complete and partial disease remission at 6 and 24 months. Complete remission is defined by uPCR <200 mg/g (<20 mg/mmol) or 1+ protein on urine dipstick for 3 consecutive days.24 Partial remission is defined by proteinuria reduction of 50% or greater from the presenting value and absolute uPCR between 200 and 2000 mg/g (20–200 mg/mmol).28 Secondary aims will be to tests circulating cell populations as biomarkers or predictors of the anti-CD20 response. Safety end points will be frequency and severity of adverse events and abnormal values in biochemical tests and haematology assessments.
Safety data
We will collect any untoward medical occurrence in the form of signs, symptoms, abnormal laboratory findings or diseases that emerges or worsens relative to baseline (ie, present at the initial study visit). Policy and approach to define adverse events is reported in the online supplementary appendix.
bmjopen-2016-013319supp_appendix.pdf (88KB, pdf)
Data collection, management and analysis
Data collection methods and adherence during follow-up
Study visits will occur at baseline, after 1 month and every 3 months thereafter, unless complications or relapses occur. Determination of 24 hours proteinuria at baseline and after 6, 12 and 24 months will be performed at a central laboratory (in order to assess the primary and secondary outcome). Dipstick for proteinuria determination will be evaluated daily. In the case of dipstick positivity, the presence of proteinuria will be confirmed with 24-hour urine collection. Complete blood count, kidney function, plasma proteins, immunoglobulins, lipid status (cholesterol and triglycerides), albumin and lymphocyte subpopulations (for CD20 lymphocytes B count) will be obtained at 1, 3, 6, 9, 12, 15, 18, 21 and 24 months during protocol visits. Time schedule of enrolment, interventions assessments and visits for participants are shown in table 1. At discharge from Nephrology Unit, each patient will receive a clinical diary form, to be filled with proteinuria levels at dipstick, body weight, current treatment (steroid and CNI dosage) and monthly send to our centre via fax or email. A study coordinator will maintain ongoing contact with the children, their families and the family physician to collect clinical data including blood pressure and potential adverse events also in order to minimise loss to follow-up/dropout.
Table 1.
Participants timeline
| Study period |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Enrolment | Allocation and treatment | Follow-up |
Close-out | |||||||
| Timepoint | −1 | 0 | t1 | t3 | t6 | t9 | t12 | t15 | t18 | t24 |
| ENROLMENT | ||||||||||
| Relevant medical history | X | |||||||||
| Eligibility screen | X | |||||||||
| Project illustration | X | |||||||||
| Informed consent | X | |||||||||
| Instruction about immunosuppressive drugs tapering | X | X | ||||||||
| Allocation | X | |||||||||
| INTERVENTIONS | ||||||||||
| Ofatumumab/rituximab administration | X | |||||||||
| ASSESSMENTS | ||||||||||
| Dosage on 24-hours urine collection | X | X | X | X | X | X | X | X | X | X |
| Physical examination and vital signs | X | X | X | X | X | X | X | X | X | X |
| Haematology and biochemistry (complete blood count, kidney function, plasma proteins immunoglobulins, lipid status—cholesterol and triglycerides—, albumin, lymphocyte subpopulations—for CD20 lymphocytes B count) | X | X | X | X | X | X | X | X | X | X |
| Adverse events data records | X | X | X | X | X | X | X | X | X | |
Data management
The investigators, or designees, will be responsible for recording study data in the case report form and entering study data in the electronic database prepared by the study coordinating centre. Coordinating centre data management at the G. Gaslini Institute, Genoa, will be responsible for data processing, in accordance with G. Gaslini's data management procedures. Database will be locked once quality assurance procedures have been completed.
Statistical methods
We will use standard statistical methods to summarise the sample characteristics overall and by arm assignment, using statistics for quantitative and qualitative data as appropriate. For the analysis of the primary outcome, we will use logistic regression to compare the risk of relapse at 12 months with a two-sided p value of <0.05 as level of statistical significance. The comparison will be repeated at 6 months (secondary outcome). As in previous studies, we will use survival methods in secondary analyses to assess the risk of repeated relapses and estimate the average time to relapse by treatment group.29 In all analyses, we will use an intention-to-treat approach, whereby participants will be analysed as randomised regardless of protocol adherence. Missing data will be replaced in a way that reflects the worst-case scenario: missing data in the active comparator group will be considered as successes and missing data in the active intervention will be considered failures.
Sample size
We will enrol 140 children in this study, and follow them for at least 1 year. Assuming a baseline risk of relapse at 1 year of 0.65 among children assigned to rituximab, this sample size will allow to detect as significant at the two-sided p value of 0.01 with a power of >0.8, a reduction in the risk of 1 -year relapse of at least 0.3 (ie, from 0.65 to 0.35; risk reduction by 0.46). This sample size accounts for a total proportion of drop-out and drop-in <10%.
Our centre is a national referral centre for paediatric nephrology. We treat 50 new cases of INS per year, of which 80% are SD-INS. Our records demonstrate that we were able to successfully recruit participants in similar studies.18–20 29
Study termination
Participants will be informed that they have the right to withdraw from the study at any time, without prejudice to their medical care, and that they are not obliged to state their reasons. Any withdrawal must be fully documented in the case report form and source documents, and will be followed up by the investigator. Follow-up will be considered complete when the participant has completed all study procedures and assessments up to the month-12 visit (primary end point) and month-24 visit (secondary end point). The investigator may withdraw a participant at any time if this is considered to be in the participant's best interest. Study termination will be mandatory in the following situations: development of steroid resistance disease requiring other steroid-sparing agents, pregnancy, significant worsening of renal function, onset of malignancy, serious hypersensitivity or allergic reaction, any other serious adverse event, serious intercurrent illness, administrative reasons, or investigator's or participant's or parents'/legal tutor's request.
We have no plans for interim analyses or prespecified stopping rules. The data safety and monitoring board will decide if the study is safe when 50% of the participants will be enrolled (without comparisons). The sponsor may temporarily or permanently discontinue the study for safety, ethical, compliance or other reasons. If the study is suspended or discontinued, the investigator will be responsible for promptly informing the Independent Ethics Committee. Where required by local regulations, the sponsor will be responsible for informing the Independent Ethics Committee of study discontinuation.
Data safety and monitoring board, study monitoring and end point adjudication committee
The members of DSMB will include experts in methodology and clinical nephrology; the sponsor will be responsible for study monitoring. The investigator or his/her delegate will ensure that the case report forms are completed in a timely manner to allow a study monitor to periodically access them and all study-related materials. The frequency of monitoring visits will be determined by the site enrolment rate. On study completion, the study monitor will visit the site to conduct a study termination visit. This will involve collection of any outstanding documentation. A blinded end point adjudication committee will ensure an ongoing review of all of the relevant data relating to each participant and to establish whether or not an end point has occurred.
Ethics approval and trial status
Before initiation of the study, we obtained written approval of the protocol, informed consent form and any information presented to potential participants from the local Independent Ethics Committee (Comitato Etico Regione Liguria). We also obtained approval from the Italian Drug Agency (Agenzia Italiana del FArmaco, AIFA). If any amendments to the protocol occur during the study, written approval must be obtained prior to its implementation. The Investigator is responsible for ensuring that these actions occur. Where required by local regulations, the Sponsor is responsible for ensuring Independent Ethics Committee approval of the study.
The study was registered at https://clinicaltrials.gov (study number: NCT02394119) and https://eudract.ema.europa.eu (study number 2015-000624-28). The trial is currently recruiting: study enrolment started on 10 June 2015.
Dissemination
The completed randomised clinical trial study will be summarised in a final report that accurately and completely presents the study objectives, methods, results, limitations of the study and interpretation of findings. The authors of this study protocol will inform the contributing investigators (primary healthcare providers of study participants) in advance about any plans to publish or present data from this randomised controlled clinical trial. Any publications and presentations of the results (abstract in journals or newspapers, oral presentations, etc), either in whole or in part, by investigators or their representatives will require presubmission review by the authors of this study protocol and all coauthors.
Discussion
SD nephrotic syndrome carries a high risk of toxicity from steroids or standard steroid-sparing agents. Therefore, alternative treatment options are needed. Although recent studies support the use of rituximab as a steroid-sparing and CNI-sparing agent in SD-INS, benefits may be suboptimal, especially in complicated forms of the disease. The new anti-CD20 ofatumumab may be more effective than rituximab in controlling the disease, due to its stronger affinity for the CD20. Moreover, due to its fully humanised structure, it may be used in larger doses with minimal risk of side effects. These considerations motivated the use of an active comparator to test the effects of different agents blocking the CD20 antigen pathway.
This is the first randomised controlled trial comparing the effects of two anti-CD20 antibodies on the risk of relapse of INS following steroid and CNI withdrawal. Strengths in the design of this trial include: objective and clinical important outcomes, identification of a high-risk population (SD-INS maintained in complete remission with steroids and CNI), methods to reduce bias (allocation concealment, objective outcomes, steps to ensure complete follow-up and blinding of outcome adjudicators to treatment assignment), careful collection of safety data and large catchment area of the study centre with clinical and research expertise in paediatric nephrology.
The design of this trial has limitations. First, interventions are not blinded because ofatumumab and rituximab require different methods for infusion. Although strategies to mask the two interventions could have been designed, they would have been expensive. In addition and most importantly, the end point adjudication committee reviewing the clinical and laboratory data will be blinded to intervention assignments. Second, the primary end point is an intermediate rather than a final outcome. However, the laboratory-based measures we adopted to define disease relapse are objective and more distant outcomes, including cardiovascular or infectious complications of nephrotic syndrome, or progression of kidney disease to kidney failure are rarely observed during childhood. A very large multinational trial would be necessary to study these hard end points. On the other hand, relapse of nephrotic syndrome is an important outcome for children and their families, often requiring travel to the nephrology centre in order to access urgent care. Third, this trial will compare the risk of relapse at 1 year based on the first event that occurs. While we planned to maintain participants into the assigned arm for follow-up studies by treating repeated relapses with the same antibody they receive, comparison of the effect of these antibodies on the risk of repeated relapses is not included in the present trial.
In summary, this study addresses an intervention question that is relevant to children with INS and their families. Results from this study may impact the management of paediatric SD-INS in the near future and provide information on safety of these agents more and more often used in clinical practice. In these children, ofatumumab may allow maintenance of long-term remission of INS without steroids and CNI therapy. Improvement in quality of life, reduction in hospitalisation rates and use of healthcare resources are other important expected benefits.
Acknowledgments
The Institute Giannina Gaslini (trial sponsor) will provide logistic and financial support to the trial through grants from the ministry of health (‘Cinque per mille of IRPEF-Finanziamento della ricerca sanitaria’), the ‘Fondazione Malattie Renali del Bambino’ and the ‘Fondazione La Nuova Speranza’ (‘Progetto integrato per la definizione dei meccanismi implicati nella glomerulo sclerosi focale’). The sponsor will appoint a study monitor. The authors thank the members of the DSMB (Giovanni Candiano, PhD, Gianluca Caridi and Antonella Trivelli, MD) and end point adjudication committee (Drs Fabrizio Ginevri and Enrico Verrina).
Footnotes
Contributors: PR, AB and GMG were involved in conception and trial design and in drafting of the article.
Disclaimer: The study sponsor had no role in the study design and protocol development of this study. The sponsor will not have any role in the collection, analysis or interpretation of the data, or in the writing of report for publication. The researchers have complete independence from the sources of funding in all aspects of this study.
Funding: Giannina Gaslini Children's Hospital will provide intervention and active comparator drugs (ofatumumab and rituximab, respectively) and the infrastructure for the trial. The ‘Fondazione La Nuova Speranza’ (‘Progetto integrato per la definizione dei meccanismi implicati nella glomerulo sclerosi focale’) founded the insurance coverage.
Competing interests: None declared.
Ethics approval: Local Independent Ethics Committee (Comitato Etico Regione Liguria).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Cameron JS, Turner DR, Ogg CS et al. The nephrotic syndrome in adults with ‘minimal change’ glomerular lesions. Q J Med 1974;43:461–88. [PubMed] [Google Scholar]
- 2.McEnery PT, Strife CF. Nephrotic syndrome in childhood. Management and treatment in patients with minimal change disease, mesangial proliferation, or focal glomerulosclerosis. Pediatr Clin North Am 1982;29:875–94. [PubMed] [Google Scholar]
- 3.Kyrieleis HA, Löwik MM, Pronk I et al. Long-term outcome of biopsy-proven, frequently relapsing minimal-change nephrotic syndrome in children. Clin J Am Soc Nephrol 2009;4:1593–600. 10.2215/CJN.05691108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hahn D, Hodson EM, Willis NS et al. Corticosteroid therapy for nephrotic syndrome in children. Cochrane Database Syst Rev 2015;18:CD001533 10.1002/14651858.CD001533.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benz K, Dötsch J, Rascher W et al. Change of the course of steroid-dependent nephrotic syndrome after rituximab therapy. Pediatr Nephrol 2004;19:794–7. 10.1007/s00467-004-1434-z [DOI] [PubMed] [Google Scholar]
- 6.Peskovitz MD, Book BK, Sidner RA. Resolution of recurrent focal segmental glomerulosclerosis proteinuria after rituximab treatment. N Engl J Med 2006;354:1961–3. 10.1056/NEJMc055495 [DOI] [PubMed] [Google Scholar]
- 7.Nozu K, Iijima K, Fujisawa M et al. Rituximab treatment for posttransplant lymphoproliferative disorder (PTLD) induces complete remission of recurrent nephrotic syndrome. Pediatr Nephrol 2005;20:1660–3. 10.1007/s00467-005-2013-7 [DOI] [PubMed] [Google Scholar]
- 8.Guigonis V, Dallocchio A, Baudouin V et al. Rituximab treatment for severe steroid- or cyclosporine-dependent nephroticsyndrome: a multicentric series of 22 cases. Pediatr Nephrol 2008;23:1269–79. 10.1007/s00467-008-0814-1 [DOI] [PubMed] [Google Scholar]
- 9.Peters HP, van de Kar NC, Wetzels JF. Rituximab in minimal change nephropathy and focal segmental glomerulosclerosis: report of four cases and review of the literature. Neth J Med 2008;66: 408–15. [PubMed] [Google Scholar]
- 10.Kamei K, Ito S, Nozu K et al. Single dose of rituximab for refractory steroid-dependent nephrotic syndrome in children. Pediatr Nephrol 2009;24:1321–8. 10.1007/s00467-009-1191-0 [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Fresnedo G, Segarra A, Gonza′lez E et al. Trabajo de Enfermedades Glomerulares de la Sociedad Espa∼nola de Nefrologı′a (GLOSEN): rituximab treatment of adult patients with steroid-resistant focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 2009;4:1317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujinaga S, Hirano D, Nishizaki N et al. Single infusion of rituximab for persistent steroid-dependent minimal-change nephrotic syndrome after long-term cyclosporine. Pediatr Nephrol 2010;25: 539–44. 10.1007/s00467-009-1377-5 [DOI] [PubMed] [Google Scholar]
- 13.Gulati A, Sinha A, Jordan SC et al. Efficacy and safety of treatment with rituximab for difficult steroid-resistant and -dependent nephrotic syndrome: multicentric report. Clin J Am Soc Nephrol 2010;5: 2207–12. 10.2215/CJN.03470410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prytuła A, Iijima K, Kamei K et al. Rituximab in refractory nephrotic syndrome. Pediatr Nephrol 2010;25:461–8. 10.1007/s00467-009-1376-6 [DOI] [PubMed] [Google Scholar]
- 15.Kemper MJ, Gellermann J, Habbig S et al. Long-term follow-up after rituximab for steroid-dependent idiopathic nephrotic syndrome. Nephrol Dial Transplant 2012;27:1910–5. 10.1093/ndt/gfr548 [DOI] [PubMed] [Google Scholar]
- 16.Sellier-Leclerc AL, Baudouin V, Kwon T et al. Rituximab in steroid dependent idiopathic nephrotic syndrome in childhood—follow-up after CD19 recovery. Nephrol Dial Transplant 2012;27: 1083–9. 10.1093/ndt/gfr405 [DOI] [PubMed] [Google Scholar]
- 17.Ito S, Kamei K, Ogura M et al. Survey of rituximab treatment for childhood-onset refractory nephrotic syndrome. Pediatr Nephrol 2013;28:257–64. 10.1007/s00467-012-2319-1 [DOI] [PubMed] [Google Scholar]
- 18.Magnasco A, Ravani P, Edefonti A et al. Rituximab in children with resistant idiopathic nephrotic syndrome. J Am Soc Nephrol 2012;23:1117–24. 10.1681/ASN.2011080775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ravani P, Rossi R, Bonanni A et al. Rituximab in children with steroid-dependent nephrotic syndrome: a multicentre, open-label, non inferiority, randomised controlled trial. J Am Soc Nephrol 2015;26:2259–66. 10.1681/ASN.2014080799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ravani P, Magnasco A, Edefonti A et al. Short-term effects of rituximab in children with steroid- and calcineurin-dependent nephrotic syndrome: a randomised controlled trial. Clin J Am Soc Nephrol 2011;6:1308–15. 10.2215/CJN.09421010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iijima K, Sako M, Nozu K et al. Rituximab for childhood-onset, complicated, frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: a multicentre, double-blind, randomised, placebo controlled trial. Lancet 2014;4:1273–81. 10.1016/S0140-6736(14)60541-9 [DOI] [PubMed] [Google Scholar]
- 22.Basu B. Ofatumumab for rituximab-resistant nephrotic syndrome. N Engl J Med 2014;370:1268–70. 10.1056/NEJMc1308488 [DOI] [PubMed] [Google Scholar]
- 23.Bonanni A, Rossi R, Murtas C et al. Low-dose ofatumumab for rituximab-resistant nephrotic syndrome. BMJ Case Rep 2015;2015:pii: bcr2015210208 10.1136/bcr-2015-210208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robak T. Ofatumumab, a human monoclonal antibody for lymphoid malignancies and autoimmune disorders. Curr Opin Mol Ther 2008;10:292–309. [PubMed] [Google Scholar]
- 25.Vivarelli M, Serafinelli J, Colucci M et al. Ofatumumab in two pediatric nephrotic syndrome patients allergic to Rituximab. Ped Nephrol 2017;32:181–4. [DOI] [PubMed] [Google Scholar]
- 26.Bonanni A, Bertelli E, Moscatelli A et al. Ofatumumab-associated acute respiratory manifestations: clinical characteristics and treatment. Br J Clin Pharmacol 2016;82:1146–8. 10.1111/bcp.13029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gellermann J, Weber L, Pape L et al. Mycophenolate mofetil versus cyclosporine A in children with frequently relapsing nephrotic syndrome. J Am Soc Nephrol 2013;24:1689–97. 10.1681/ASN.2012121200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO Clinical Practice Guideline for Glomerulonephritis. Kidney Int 2012;2(Suppl.):139–274. [Google Scholar]
- 29.Ravani P, Ponticelli A, Siciliano C et al. Rituximab is a safe and effective long-term treatment for children with steroid and calcineurin inhibitor-dependent idiopathic nephrotic syndrome. Kidney Int 2013;84:1025–33. 10.1038/ki.2013.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2016-013319supp_appendix.pdf (88KB, pdf)