Abstract
Objective
Neurodegenerative diseases, such as dementia, have a profound impact on those with the conditions and their family carers. Consequently, the accurate measurement of family carers' quality of life (QOL) is important. Generic measures may miss key elements of the impact of these conditions, so using disease-specific instruments has been advocated. This systematic review aimed to identify and examine the psychometric properties of disease-specific outcome measures of QOL of family carers of people with neurodegenerative diseases (Alzheimer's disease and other dementias; Huntington's disease; Parkinson's disease; multiple sclerosis; and motor neuron disease).
Design
Systematic review.
Methods
Instruments were identified using 5 electronic databases (PubMed, PsycINFO, Web of Science, Scopus and the International Bibliography of the Social Sciences (IBSS)) and lateral search techniques. Only studies which reported the development and/or validation of a disease-specific measure for adult family carers, and which were written in English, were eligible for inclusion. The methodological quality of the included studies was evaluated using the COnsensus based Standards for the selection of health Measurement Instruments (COSMIN) checklist. The psychometric properties of each instrument were examined.
Results
676 articles were identified. Following screening and lateral searches, a total of 8 articles were included; these reported 7 disease-specific carer QOL measures. Limited evidence was available for the psychometric properties of the 7 instruments. Psychometric analyses were mainly focused on internal consistency, reliability and construct validity. None of the measures assessed either criterion validity or responsiveness to change.
Conclusions
There are very few measures of carer QOL that are specific to particular neurodegenerative diseases. The findings of this review emphasise the importance of developing and validating psychometrically robust disease-specific measures of carer QOL.
Keywords: Neurodegenerative diseases, quality of life, psychometrics, measurement, carers
Strengths and limitations of this study.
This study provides the first comprehensive review of disease-specific instruments measuring quality of life of family carers of people with neurodegenerative diseases.
The findings of the review offer guidance to researchers and clinicians in the selection of appropriate and psychometrically strong disease-specific instruments.
Only instruments specific to neurodegenerative diseases were examined.
We did not review the performance of generic measures of quality of life.
The instruments identified in this review were developed specifically for family carers rather than professional (ie, paid) carers of people with neurodegenerative diseases.
Introduction
Neurodegenerative diseases have a profound impact as they lead to a prolonged and irreversible decline in global intellectual, social and physical functioning. People living with neurodegenerative diseases tend to progressively lose their independence and require increased levels of care and support. Dementia is the most common type of neurodegenerative disease, affecting 800 000 people in the UK alone,1 and costing £26.3 billion to society each year.2 Family members, friends and neighbours who act as carers are a vital determinant of positive outcomes for people with neurodegenerative diseases such as dementia. They provide a wide range of practical and emotional support, social care, and assistance with activities of daily living.3 These caregiving tasks include, for example, help with personal care, managing finances and legal affairs, social activities, mobility, and administering and coordinating medication.3
Caring for a person with a neurodegenerative disease may result in multiple negative outcomes, including increased anxiety and depression, stress, exhaustion, social isolation, and concerns about the future.1 Nevertheless, caring can also have positive effects such as greater closeness to the person with the condition, reciprocity and spiritual growth.4–6 In recent years, there has been an increasing research focus on assessing quality of life (QOL) as an informative and important patient-reported outcome measure in the person with the disease and their carers. Although QOL is subjective in regard to how it is perceived by the individual, there is growing consensus that it represents a multidimensional construct encompassing various domains such as physical health, socioeconomic status; psychological, emotional and social well-being, and that it is a useful way of capturing the broad impacts of complex disorders such as neurodegenerative disease.7 In this paper, following the recommendations of our carer consultation, the term ‘family carer’ is used to refer to all ‘informal’ carers (ie, not paid carers), including family members, neighbours and friends of a person with a neurodegenerative disease. This also includes people who are not, in fact, family members, since the term ‘informal’ was seen as minimising and trivialising the nature of the care provided, and the term ‘carer’ by itself was seen as too imprecise.
Given the important role of family carers, it is essential for the person with the illness as well as for the carer, that carers of people with neurodegenerative diseases maintain a good QOL. Carer QOL is likely to be associated with patient QOL, and when carer QOL deteriorates, there is likely to be a higher risk that the person with dementia will need admission into a care home, so driving lower life quality and higher societal cost. It is also important to measure the broad QOL carer impacts of interventions for the person with dementia and for carers. To ensure this can be monitored effectively, it is necessary to measure carer QOL accurately using psychometrically robust measures. To date, QOL of affected individuals and their carers has been predominantly assessed using generic health status instruments such as the SF-368 and the EQ-5D.9 Disease-specific instruments have been used much less frequently. This is problematic as generic instruments miss issues that are pertinent to specific conditions and, consequently, are less responsive to detecting changes in carer QOL over time.10 11 For example, an increased level of support received as a carer has been associated with better carer QOL in dementia,4 but neither the EQ-5D nor the SF-36 capture such a construct.
An earlier review has examined the effects (eg, physical, social, emotional, financial) of caring for an elderly family member with dementia on carer QOL.11 Disease-specific and generic instruments were identified that assess QOL of either patients with dementia or their carers. However, the psychometric properties of these measures were not reported or discussed, and the review focused exclusively on dementia and Alzheimer's disease. Building on this, we therefore completed a systematic review to identify and examine the disease-specific instruments that measure QOL of family carers of people with a neurodegenerative disease. Here, we examine the psychometric properties of these instruments.
Methods
The systematic review was conducted and reported in accordance with the recommendations from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.12
Inclusion criteria
Instruments were included in the review if they satisfied the following criteria: (1) the study described the development (inauguration paper) and/or the evaluation of the psychometric properties and relationships with other relevant constructs (validation paper) of a measure of carer QOL; (2) the study population were adult (aged 18 years or above) family (defined as family members, neighbours or friends) carers of people with a neurodegenerative disease (dementia, Alzheimer's disease, Parkinson's disease (PD), Huntington's disease (HD), multiple sclerosis (MS) and motor neuron disease); (3) the instrument was designed to be disease-specific and not a generic measure of QOL; (4) studies only documented instruments that were self-report in their design. Any studies that reported an eligible measure (eg, as an outcome in a clinical trial) without any explicit validation were excluded; and (5) studies were written in English.
Search strategy
Articles were identified from initial searches of five electronic databases: PubMed, PsycINFO, Scopus, Web of Science and the International Bibliography of the Social Sciences (IBSS). The searches were conducted from inception until 8 February 2016. All articles identified up until this time were screened regardless of their publication date. We used the following five combined sets of search terms: (1) ‘quality of life’; AND (2) caregiver* OR carer*; AND (3) informal OR unpaid OR spous* OR family; AND (4) dementia OR alzheimer* OR parkinson* OR huntington* OR multiple sclerosis OR motor neurone disease; AND (5) valid* OR reliab* OR development OR psychometric. The search terms were intentionally broad and sensitive enough to ensure that all potentially relevant articles were identified (please see online supplementary material file for full details of search strategy). Six additional articles were obtained using lateral searching techniques.13 These searches involved the manual checking of reference lists of included studies (snowballing), citation searches using the ‘Cited by’ option on Web of Science, Google Scholar, and Scopus, and the ‘Related articles’ option on PubMed and Web of Science (lateral searching). The grey literature (ie, unpublished studies) was also searched using the following databases: OpenGrey and Patient-Reported Outcome and Quality of Life Instruments Database (PROQOLID). We attempted to make direct contact (via email) with the authors of a manuscript when no published psychometric data were available for the instrument being reported. Two independent reviewers (TEP and NF) screened article titles and abstracts against the predefined inclusion criteria. Full-text articles were sought for all potentially relevant studies. Any disagreements concerning inclusion were resolved through discussion and advice from a third reviewer (SD).
bmjopen-2016-013611supp.pdf (88KB, pdf)
Data extraction
Two reviewers (TEP and NF) extracted, independently, the following data for studies that met the inclusion criteria: name of the instrument, country, sample characteristics (gender, age), study design/setting, measurement domains, number of items, response format, evidence of reliability and validity.
Quality assessment
The methodological quality of the studies was assessed using the COnsensus based Standards for the selection of health Measurement Instruments (COSMIN) checklist.14–16 This is a standardised tool which assesses the measurement properties of health-related instruments across nine domains (internal consistency, reliability, measurement error, content validity (including face validity), construct validity (subdivided into structural validity, hypotheses testing and cross-cultural validity), criterion validity and responsiveness) with each domain rated using 5–18 items. Each item is rated as ‘excellent’, ‘good’, ‘fair’ or ‘poor’ quality. A methodological quality score for each measurement property is obtained by taking the lowest rating of any item in that box (worst score counts). Two independent reviewers (TEP and NF) assessed the methodological quality of the included studies using the checklist. Any disagreements in scoring were resolved through discussion and advice from a third reviewer (SD).
Results
Search results
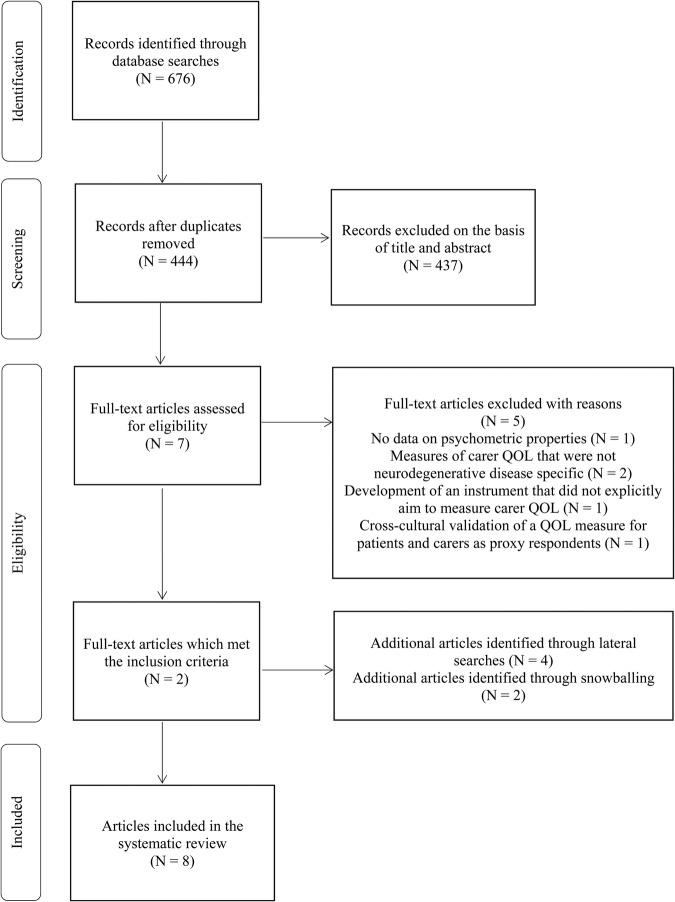
The initial database searches identified 676 articles, of which 232 were deleted because of duplicates. After title and abstract screening, we assessed seven articles as potentially relevant and obtained full texts. From reviewing the full texts of these remaining articles, we found two studies that met inclusion criteria (figure 1). Six additional articles were identified using lateral search techniques.
Figure 1.
PRISMA flow diagram of study selection. PRISMA,Preferred Reporting Items for Systematic Reviews and Meta-Analyses; QOL, quality of life.
In total, we included eight studies in the systematic review and these reported seven carer QOL instruments across the neurodegenerative diseases. All of them were self-report measures and consisted of the HD quality-of-life battery for carers (HDQoL-C);17 HD quality-of-life battery for carers-short form (HDQoL-C-SF);18 CAREQOL-MS for MS;19 Alzheimer's Carers Quality of Life Instrument (ACQLI);20 Parkinson Disease Questionnaire for Carers (PDQ-Carer);21 Parkinsonism Carers QoL (PQoL Carers);22 and the Caregiver Quality Of Life for dementia (CGQOL).6 We did not identify any instruments designed to measure the QOL of carers of people with motor neuron disease.
Study characteristics
The sample characteristics for the eight studies assessing the carer QOL instruments are presented in table 1.
Table 1.
Characteristics of the included studies
| Carer QOL Instrument | Author(s) | Country | Sample Total N (% female) Mean age years (SD) |
Study design/setting | Measurement domains | Number of items/response format |
|---|---|---|---|---|---|---|
| HDQoL-C | Aubeeluck and Buchanan17 | UK | 87 (62.1) 58.2 (14.2) |
Longitudinal/cohort: spousal carers recruited through the Huntington's Disease Association UK | Demographic and objective information; practical aspects of caregiving; satisfaction with life; feelings about living with Huntington's disease | 34 items 11-point Likert scale |
| HDQoL-C-SF | Aubeeluck et al18 | France Italy |
301 (60.5) 57.1 (13.1) |
Cross-sectional survey: family or friend carers of people with Huntington's disease | Satisfaction with life; feelings about living with Huntington's disease | 20 items 11-point Likert scale |
| CAREQOL-MS | Benito-Leon et al19 | Spain | 276 (56.5) 50.2 (12.6) |
Longitudinal/cohort: family carers of people with MS recruited from 19 Spanish outpatient clinics | Physical burden and global health; social impact; emotional impact; need of support; emotional reactions to patient's psychic status | 24 items 5-point Likert scale |
| ACQLI | Doward20 | UK France Germany Italy Spain |
192 (72.3) 60.0 (12.2) |
Longitudinal/cohort: family carers of people with dementia | Single domain of carer QOL | 30 items Dichotomous (true/not true) |
| PDQ-Carer | Jenkinson et al21 | UK | 236 (63.5) 68.2 (9.5) |
Cross-sectional survey: family carers of people with Parkinson's disease who were members of Parkinson's UK | Social and personal activities; anxiety and depression; self-care; stress | 29 items 5-point Likert scale |
| PDQ-Carer-SI | Morley et al23 | UK | 236 (63.5) 68.2 (9.5) |
Cross-sectional survey: family carers of people with Parkinson's disease who were members of Parkinson's UK | Single summary index score computed using the four subscales of the PDQ-Carer | 29 items 5-point Likert scale |
| PQoL Carers | Pillas et al22 | UK | 430 (62.4) 66.2 (8.5) |
Cross-sectional survey: family carers of people with MSA and PSP | Single domain of carer QOL | 26 items 5-point Likert scale |
| CGQOL | Vickrey et al6 | USA | 200 (79.0) 61.5 (13.5) |
Longitudinal/cohort: family carers of people with dementia | Assistance with instrumental activities of daily living; assistance with activities of daily living; role limitations due to caregiving; personal time; family interaction; demands of caregiving; worry; spirituality and faith; benefits of caregiving; caregiver feelings | 80 items 3-point and 5-point Likert scales specific to groups of items |
ACQLI, Alzheimer's Carers Quality of Life Instrument; CGQOL, Caregiver Quality Of Life; HDQoL-C, Huntington's disease quality-of-life battery for carers; HDQoL-C-SF, Huntington's disease quality-of-life battery for carers short form; MS, multiple sclerosis; MSA,multiple system atrophy; PDQ-Carer, Parkinson Disease Questionnaire for Carers; PDQ-Carer-SI, Parkinson Disease Questionnaire for Carers Summary Index; PQoL Carers, Parkinsonism Carers QoL; PSP,progressive supranuclear palsy; QOL, quality of life.
The total sample sizes of these studies ranged from 8717 to 430.22 The studies recruited participants from the UK,17 21–23 Spain19 and the USA.6 The remaining two studies recruited participants across multiple countries which included France, Italy and Germany.18 20 The mean age of participants across the eight studies was 61.2 years. The number of measurement domains of the instruments ranged from 1 (ACQLI, PQoL Carers) to 10 (CGQOL). The number of items of these instruments ranged from 20 (HDQoL-C-SF) to 80 (CGQOL). All measures used Likert-type rating scales (ranging from 3-point to 11-point), except for the ACQLI which used a dichotomous (true/not true) response format.
Methodological quality of studies
Table 2 provides a summary of the scores from the COSMIN checklist. Six of the eight studies (75%) included in the review had at least one methodological domain rated as ‘poor’ quality.6 17–21 Across all studies, the measurement property that received the highest number of ‘poor’ ratings was content validity (4/8 studies). Five of the eight studies (63%) had at least one methodological domain rated as of ‘fair’ quality.6 18 21–23 The measurement property that received the highest number of ‘fair’ ratings was internal consistency (5/8 studies). Of the 8 studies, 2 (26%) had at least one area of methodological quality rated as ‘good’ or ‘excellent’.19 22 The measurement properties that received ‘good’ ratings were reliability and measurement error.19 The measurement properties that received ‘excellent’ ratings were internal consistency, structural validity19 and content validity.22
Table 2.
Results of COSMIN checklist
| Carer QOL instrument | Study reference | Internal consistency | Reliability | Measurement error | Content validity | Structural validity | Hypotheses testing | Cross-cultural validity | Criterion validity | Responsiveness |
|---|---|---|---|---|---|---|---|---|---|---|
| HDQoL-C | Aubeeluck and Buchanan17 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| HDQoL-C-SF | Aubeeluck et al18 | 2 | 0 | 0 | 0 | 2 | 2 | 1 | 0 | 0 |
| CAREQOL-MS | Benito-Leon et al19 | 4 | 3 | 3 | 1 | 4 | 0 | 0 | 0 | 0 |
| ACQLI | Doward20 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| PDQ-Carer | Jenkinson et al21 | 2 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 |
| PDQ-Carer-SI | Morley et al23 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| PQoL Carers | Pillas et al22 | 2 | 0 | 0 | 4 | 2 | 0 | 0 | 0 | 0 |
| CGQOL | Vickrey et al6 | 2 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 |
Key: 4: excellent, 3: good, 2: fair, 1: poor, 0: no information available.
ACQLI, Alzheimer's Carers Quality of Life Instrument; CGQOL, Caregiver Quality Of Life; COSMIN,COnsensus-based Standards for the selection of health Measurement Instruments;HDQoL-C, Huntington's disease quality-of-life battery for carers; HDQoL-C-SF, Huntington's disease quality-of-life battery for carers short form; MS, multiple sclerosis; PDQ-Carer-SI, Parkinson Disease Questionnaire for Carers Summary Index; PQoL Carers, Parkinsonism Carers QoL; QOL, quality of life.
Psychometric properties of the carer QOL instruments
The instruments that had allowed the most comprehensive evaluation of psychometric properties across the 9 domains of the COSMIN checklist were the CAREQOL-MS (5/9 domains assessed) and the CGQOL (5/9 domains assessed). The HDQoL-C, HDQoL-C-SF and ACQLI each had four of the nine domains evaluated. The measures that had the least evidence available for their psychometric properties were the PQoL Carers (3/9 domains assessed), the PDQ-Carer (3/9 domains assessed) and the summary index of the PDQ-Carer (2/9 domains assessed). Internal consistency was the most widely assessed measurement property, and was examined in all seven instruments. Cronbach's α coefficients for the total scale and instrument subscales ranged from 0.75 to 0.94 (see table 3 for an overview of the psychometric properties of each carer QOL instrument).
Table 3.
Evidence of the reliability and validity of the carer QOL instruments
| Reliability | Validity | |||||
|---|---|---|---|---|---|---|
| Carer QOL instrument | Author(s) | Internal consistency | Test–retest | Measurement error | Content validity | Construct validity |
| HDQoL-C | Aubeeluck and Buchanan17 | Not provided for total scale. Subscale α: 0.80, 0.84, 0.89 | Test–retest (N=10) over 2 weeks. Pearson correlation: 0.78, 0.86, 0.90 for subscales | Not assessed | Two experts in the field of QOL and two experts in the field of HD commented on item content | Convergent validity: positive correlations of HDQoL-C subscales with WHOQOL-BREF, Satisfaction with Life Scale, and Perceived Health Visual Analogue Scale |
| HDQoL-C-SF | Aubeeluck et al18 | Not provided for total scale. Subscale α: 0.88, 0.80 | Not assessed | Not assessed | Not assessed | Known groups analyses showed that HDQoL-C-SF scores were higher (better carer QOL) for carers of patients in the low disease severity group compared with the moderate and high severity groups |
| CAREQOL-MS | Benito-Leon et al19 | Not provided for total scale. Subscale α: 0.90, 0.85, 0.81, 0.78, 0.75 | Test–retest (N=276) over 2 weeks. ICC for total scale score=0.96 and ranged from 0.75 to 0.95 for subscales. Cohen's κ ranged from 0.46 to 0.93. One item had κ<0.60 and 15 items had κ≥0.80. | SE of measurement ranged from 0.91 to 2.43 across the five subscales over 2 weeks time interval. | Item content analysed by MS experts. Focus groups of MS carers and patients discussed item pool. Items rated for clarity and meaning by MS experts and a separate carer sample | Convergent validity: positive correlations of CAREQOL-MS subscales with Zarit Burden Interview. Moderate-to-high negative correlations of CAREQOL-MS subscales with physical and mental components of SF-36 |
| ACQLI | Doward20 | α ranged between 0.87 and 0.95 across the UK, France, Germany, Italy and Spain for times 1 and 2 administrations of the ACQLI. | Test–retest over 2 weeks. Spearman correlations were 0.93, 0.92, 0.95, 0.94, 0.90 for UK, France, Germany, Italy and Spain, respectively. Based on very small N per country | Not assessed | Field test of items with samples of carers. States that carers found the ACQLI to be understandable, acceptable and relevant across all countries | Convergent validity: positive spearman correlations of ACQLI with General Well-being Index in UK and Italy. Known groups analysis demonstrated that the ACQLI can distinguish between carers based on their current health status. |
| PDQ-Carer | Jenkinson et al21 | Not provided for total scale. Subscale α: 0.92, 0.87, 0.86, 0.83 | Not assessed | Not assessed | Very limited assessment of content validity. Items were evaluated by focus groups of researchers and a pilot sample of carers. | Convergent validity: moderate-to-high negative correlations of PDQ-Carer subscales with physical and mental components of SF-36 |
| PDQ-Carer-SI | Morley et al23 | α=0.94 | Not assessed | Not assessed | Not assessed | Convergent validity: moderate-to-high negative correlations of PDQ-Carer-SI score with physical and mental components of SF-36 |
| Parkinsonism Carers QoL (PQoL Carers) | Pillas et al22 | α=0.96 | Not assessed | Not assessed | Very limited assessment of content validity. The questionnaire was pilot tested in a small group of carers | Convergent validity: Subscales of PQoL Carers correlated with Caregiver Burden Inventory, Hospital Anxiety and Depression Scale and the EQ-5D. ANOVA revealed that PQoL Carers scores differentiate between carers based on their current health status. |
| Caregiver Quality Of Life (CGQOL) | Vickrey et al6 | Not provided for total scale. Subscale α: 0.88, 0.93, 0.78, 0.83, 0.86, 0.86, 0.82, 0.94, 0.92, 0.89 | Test-retest (N=71) between 11 and 63 days following first administration (75% within 21 days). ICC ranged from 0.53 to 0.89, exceeding 0.70 for 6 of the 10 subscales | Not assessed | Not assessed | Convergent validity: Regression and correlation analyses of CGQOL subscales with a range of patient and carer characteristics (e.g. number of hours spent caregiving, duration of being a carer) |
ACQLI, Alzheimer's Carers Quality of Life Instrument; CGQOL, Caregiver Quality Of Life; HD, Huntington's disease; HDQoL-C, Huntington's disease quality-of-life battery for carers; HDQoL-C-SF, Huntington's disease quality-of-life battery for carers short form; ICC, intraclass correlation coefficient; MS, multiple sclerosis; PDQ-Carer-SI, Parkinson Disease Questionnaire for Carers Summary Index; PQoL Carers, Parkinsonism Carers QoL; QOL, quality of life.
Reliability of the measures was reported in 4/8 studies.6 17 19 20 These studies computed test–retest reliability over 2–3 weeks time interval through calculation of Pearson or Spearman correlations. Intraclass correlation coefficients (ICCs) were also used to assess the test–retest reliability of individual subscales in two instruments: the CAREQOL-MS and CGQOL. ICCs ranged between 0.53 and 0.95 for all subscales across these two measures. The CAREQOL-MS was the only instrument to calculate measurement error.
Content validity was evaluated in 5/8 studies.17 19 20–22 Structural validity was assessed in 7/8 studies.6 17–19 21–23 Principal components analysis (PCA) was the most frequently employed method of statistical analysis for examining structural validity. No studies included the use of confirmatory factor analysis (CFA) with either continuous or categorical variables (also known as item response theory (IRT)) to assess factorial structure.
Correlational and known group analyses were most commonly used to assess convergent and discriminant validity. However, hypotheses testing was carried out for only two instruments; the HDQoL-C-SF18 and the CGQOL.6 Cross-cultural validity was evaluated for three measures: the HDQoL-C-SF,18 ACQLI20 and CGQOL.6 None of the instruments assessed criterion validity or responsiveness.
Constructs assessed by the carer quality of life instruments
Huntington's disease
As displayed in table 3, the HDQoL-C consists of four measurement domains (demographic and objective information; practical aspects of caregiving; satisfaction with life; feelings about living with HD) which assess the QOL of spousal carers of people with HD. It is a 34-item instrument which employs an 11-point Likert-type response scale. PCA using varimax rotation was performed separately on each set of items for three of the four domains (not the component of demographic and objective information). This identified three subcomponents for the first component of ‘practical aspects of care giving’ (levels of support and access to professionals; long-term and genetic issues; daily hassles). PCA on the third domain of ‘satisfaction with life’ extracted two subcomponents (overall QOL issues; personal issues). Finally, PCA on the fourth domain of ‘feelings about living with HD’ resulted in the identification of two subcomponents (negative feelings about life; positive feelings about life).
The short form of the HDQoL-C measures two of the four domains of the original instrument (satisfaction with life; feelings about living with HD). It is a 20-item measure using an 11-point Likert-type response scale. Exploratory factor analysis (EFA) using varimax rotation was performed on the 17 items of the ‘feelings about living with HD’ subscale. This resulted in the extraction of the two subcomponents of ‘negative feelings about life’ and ‘positive feelings about life’ which accounted for 84% of the total variance. Factor analysis was not performed on the ‘satisfaction with life’ subscale as only three items were retained from the full-length measure. In addition to the English language version of the HDQoL-C, the measure was translated into French and Italian.
Multiple sclerosis
The CAREQOL-MS measures QOL in carers of people with MS. It consists of 24 items and uses a five-point Likert-type response format. PCA using orthogonal rotation (unspecified method of rotation) extracted four factors identified as ‘physical burden and global health’, ‘emotional impact’, ‘need of support’ and ‘emotional reactions to patient's psychic status’ explaining 60% of the total variance. The first factor was later subdivided to include a separate subscale of ‘social impact’.
Parkinson's disease
The PDQ-Carer measures QOL in carers of people with PD. This instrument contains 29 items and uses a five-point Likert-type response format. EFA using varimax rotation identified four factors: social and personal activities, anxiety and depression, self-care, and stress. These factors explained 60% of the total variance.
The PQoL Carers is an alternative measure of QOL in family carers of people with PD. This instrument contains 26 items and uses a five-point Likert-type response format. Parallel analysis was performed to assess the dimensionality of the scale which identified a single factor structure (representing overall QOL), explaining 54% of the total variance.
Dementia and Alzheimer's disease
The ACQLI measures QOL in carers of people with Alzheimer's disease and dementia. This instrument consists of 30 items which use a dichotomous (true/not true) response format. Structural validity of the ACQLI has not been evaluated. The items of the measure assess QOL as a unidimensional construct, but no factor analysis was reported in the development of this scale. Five language versions of the ACQLI are available: English, French, German, Italian and Spanish.
Finally, the CGQOL is the most recently developed measure of QOL in carers of people with dementia and Alzheimer's disease. The instrument contains 80 items across 10 domains (assistance with instrumental activities of daily living; assistance with activities of daily living; role limitations due to caregiving; personal time; family interaction; demands of caregiving; worry; spirituality and faith; benefits of caregiving; caregiver feelings) using either a three-point or a five-point Likert-type response format, with categories specific to groups of items. These 10 subscales were categorised under three superordinate factors labelled ‘tangible assistance’ (comprised of assistance (to the person with dementia) with instrumental activities of daily living; assistance (to the person with dementia) with activities of daily living; personal time; role limitations due to caregiving), ‘psychosocial’ (comprised of role limitations due to caregiving (cross-loaded with tangible assistance); family involvement; demands of caregiving; worry; caregiver feelings) and ‘benefits/faith’ (comprised of spirituality and faith, and benefits of caregiving) using EFA with promax rotation. The CGQOL was originally developed in English and was later translated into Spanish. It is to be noted that items were translated into Spanish, reviewed by a second translator and interviews were then conducted with eight Spanish-speaking carers in which to assess and refine item wording.
Discussion
We identified eight studies which report the development of seven carer QOL measures in neurodegenerative diseases (ie, dementia and Alzheimer's disease; HD; PD; MS). No instruments were identified that measure carer QOL in motor neuron disease. The findings of the studies, taken together, are potentially of value in guiding researchers and health professionals in the selection of an appropriate and psychometrically robust disease-specific instrument. The studies demonstrate some key methodological problems in the current instruments available to measure carer QOL in these neurodegenerative diseases and highlight avenues for future research.
Overall, limited information was available concerning the psychometric properties of the instruments that were identified. Collectively, the studies have shown that many important elements of psychometric evaluation were either absent or not sufficiently tested during the development phases of these disease-specific measures. This review found that the CAREQOL-MS19 and the CGQOL6 received the most comprehensive psychometric evaluation, whereas the PQoL Carers22 and the PDQ-Carer21 (including the validation of the summary index23) had the least information available on their psychometric properties.
Internal consistency was the most widely reported psychometric property and was available for all seven instruments. This was quite strong across the studies with Cronbach's α coefficients ranging from 0.75 to 0.94. Less information was provided for the temporal stability of the measures. Only four of the instruments reported test–retest reliability (HDQoL-C, CAREQOL-MS, ACQLI, CGQOL), and this ranged from being adequate to excellent over 2–3 weeks period. The CAREQOL-MS was the only instrument to report measurement error, which is the more useful figure than reliability since it controls for the population variance and is therefore more readily generalisable to other populations.24
The assessment of factorial structure was generally limited across all studies. EFA and PCA were the methods predominantly used by the test developers to assess dimensionality. However, no factor analysis was reported for the development of the ACQLI, despite the underlying assumption that it is a unidimensional measure of carer QOL. Moreover, none of the studies had performed CFA as a hypothesis-driven test of structural validity. All identified studies disregarded the categorical nature of responses and treated them as continuous for the purposes of factor analysis. While this approximation may be acceptable for items using five or more categories,25 it is generally untenable for dichotomous items24 such as used in the ACQLI. Future research using these instruments should investigate whether the factor structures that were identified in EFA will replicate in CFA when the fit of alternative measurement models are tested. It would also be advantageous for researchers to explore factorial structures using methods for categorical variables (IRT), such as Rasch analysis.26 A strong advantage of Rasch analysis is that different measures of the same attribute can be calibrated using the same scale and items can be used for computerised adaptive testing.27 IRT methods can thus control for item properties that are difficult in classical measurement, such as the item difficulty or ability to discriminate between varying levels of the attribute.
Interestingly, the factor analyses reported in the studies reviewed suggest that similar constructs are being measured by all carer QOL instruments across the various diseases. These measurement domains include, for example, an appraisal of caring demands, evaluation of support received, positive and negative feelings towards caring, the social impact of caring and negative health effects due to caring, such as anxiety, depression, stress and fatigue. In contrast to the other measures, the CGQOL includes a wider array of QOL domains believed to be affected by the caring role such as role limitations and family interaction.
Correlations with external measures were examined in all seven instruments. As evidence of convergent validity, the disease-specific measures were often found to correlate with generic measures of QOL such as the SF-36 and the EQ-5D.19 21–23 Known group analysis was frequently used to evaluate construct validity. For example, the HDQoL-C-SF differentiated between carers on the basis of the patients' disease severity. As reported previously, it was shown that carers' scores on the HDQoL-C-SF were higher (ie, representing better QOL) when caring for family members whose HD was less severe.18
A limitation of the studies reviewed is that hypotheses were not formulated in the assessment of construct validity. The HDQoL-C-SF and CGQOL were the only two instruments in which hypotheses had been postulated a priori about the expected relationships among measures. As such, it was unclear how researchers had anticipated their measures to be associated with, or distinct from, existing scales that measure similar constructs. Another limitation of the studies included in the review is that criterion-related validity had not been examined or even considered as an important issue. More importantly, none of the instruments that we examined had assessed responsiveness to change. Thus, it is not known whether these instruments are sensitive to detecting changes in carers' QOL over time. Researchers and clinicians need to measure whether interventions and services are effective in improving carer QOL. The lack of known responsiveness to change in these existing measures could, therefore, be problematic for those seeking to administer these measures within intervention programmes and social care settings. The growing use of QOL as a primary outcome measure in interventional studies highlights the importance of conducting responsiveness analysis on these measures and including such analyses in the development of future QOL instruments.
Limitations
There are several limitations of the present review that warrant consideration. First, only articles written in English were sourced, and this may have led to the exclusion of carer QOL measures that were developed and/or validated in other languages. Second, this review only focused on neurodegenerative diseases and did not examine the psychometric properties of carer QOL measures developed for other medical conditions. For example, the Caregiver Quality of Life Index-Cancer (CQOLC);28 scale is a self-report measure of QOL in family carers of patients with cancer. In future research, it would be interesting to compare the psychometric properties of neurodegenerative disease-specific measures with carer QOL instruments across a broader range of disorders. Third, we only examined the measurement of QOL of family carers (defined for our purposes as family members, neighbours or friends) and did not consider professional (paid) carers. It should be noted, however, that no articles in this review were excluded during the screening process on the basis of the carer samples that were reported. It appears that there are no instruments developed to measure the specific QOL impacts on paid carers. Our systematic review appears to have been successful in detecting carer QOL measures that currently exist across the various neurodegenerative diseases.
Conclusions
In this systematic review, we provide a comprehensive overview of disease-specific instruments to measure QOL of family carers of people with a neurodegenerative disease. The included studies had key methodological limitations associated with the measurement of QOL using these disease-specific instruments. These findings indicate that there is a need to further develop and refine these measures and potentially to develop new measures, in order to improve the psychometric quality of the measures available. Given the considerable overlap in the constructs measured by the QOL instruments that we reviewed, it might be useful for researchers to explore whether a single measure of carer QOL for all neurodegenerative diseases would be feasible and valid. However, due to the heterogeneity in symptoms and disease course, a rigorous development process is needed before assuming a single measure would be sufficient. Moreover, it would be advantageous to create shorter and more concise measures of carer QOL. This would offer greater brevity and flexibility to researchers and clinicians who need to administer these instruments in tandem with a number of other scales. Long instruments such as the 80-item CGQOL6 arguably impose a greater burden on respondents. Thus, shorter measures that contain fewer items would benefit respondents by reducing completion time and reporting burden.
Overall, the findings of this review should be helpful in guiding researchers and health professionals in the selection of an appropriate, and psychometrically robust, disease-specific instrument. The accurate assessment of carer QOL is a growing research priority, and the findings of this review are a useful foundation for researchers seeking to develop and validate new measures of carer QOL in neurodegenerative disorders. The development of psychometrically strong, disease-specific measures of QOL is important for the generation of better treatments, services, care and support for people with neurodegenerative disorders and their carers. As demonstrated in this review, there are few instruments that measure carer QOL in neurodegeneration, and the psychometric properties of the few available measures are limited. Further psychometric testing is needed on existing measures; future validation studies should include the use of IRT in conjunction with traditional methods of assessment. There is room for new instruments with stronger psychometric development, evaluation and properties in this important area.
Footnotes
Contributors: All authors were involved in the design of the study. TEP designed the literature search and screened the titles and abstracts of the identified studies in collaboration with NF. TEP and NF performed the data extraction and quality assessment. Preparation of the manuscript was completed by TEP. AB and SB edited and reviewed the manuscript. All authors approved the manuscript. The views expressed are the authors' own.
Funding: This work was supported by an Alzheimer's Society Project Grant (234ASPG14017).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Department of Health. Living well with dementia: a national dementia strategy. London: TSO, 2009. [Google Scholar]
- 2.Prince M, Knapp M, Guerchet M et al. . Dementia UK. 2nd edn London: Alzheimer's Society, 2014. [Google Scholar]
- 3.Manthorpe J, Bowling A. Quality of life measures for carers for people with dementia: measurement issues, gaps in research and promising paths. Res Policy Plan 2016;31:163–78. [Google Scholar]
- 4.Vellone E, Piras G, Talucci C et al. . Quality of life for caregivers of people with Alzheimer's disease. J Adv Nurs 2008;61:222–31. 10.1111/j.1365-2648.2007.04494.x [DOI] [PubMed] [Google Scholar]
- 5.Vellone E, Piras G, Venturini G et al. . Quality of life for caregivers of people with Alzheimer's disease living in Sardinia, Italy. J Transcult Nurs 2012;23:46–55. 10.1177/1043659611414199 [DOI] [PubMed] [Google Scholar]
- 6.Vickrey BG, Hays RD, Maines ML et al. . Development and preliminary evaluation of a quality of life measure targeted at dementia caregivers. Health Qual Life Outcomes 2009;7:1–12. 10.1186/1477-7525-7-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim JW, Zebrack B. Caring for family members with chronic physical illness: a critical review of caregiver literature. Health Qual Life Outcomes 2004;2:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ware JE Jr, Gandek B. The SF-36 Health Survey: development and use in mental health research and the IQOLA Project. Int J Ment Health 1994;23:49–73. 10.1080/00207411.1994.11449283 [DOI] [Google Scholar]
- 9.Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53–72. 10.1016/0168-8510(96)00822-6 [DOI] [PubMed] [Google Scholar]
- 10.Ettema TP, Droes RM, de Lange J et al. . A review of quality of life instruments used in dementia. Qual Life Res 2005;14:675–86. 10.1007/s11136-004-1258-0 [DOI] [PubMed] [Google Scholar]
- 11.Zacharopoulou G, Zacharopoulou V, Lazakidou A. Quality of life for caregivers of elderly patients with dementia and measurement tools: a review. Int J Health Res Innov 2015;3:49–64. [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J et al. . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Ann Intern Med 2009;151:264–9. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 13.Greenhalgh T, Peacock R. Effectiveness and efficiency of search methods in systematic reviews of complex evidence: audit of primary sources. BMJ 2005;331:1064–5. 10.1136/bmj.38636.593461.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mokkink LB, Terwee CB, Patrick DL et al. . The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J Clin Epidemiol 2010a;63:737–45. 10.1016/j.jclinepi.2010.02.006 [DOI] [PubMed] [Google Scholar]
- 15.Mokkink LB, Terwee CB, Patrick DL et al. . The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international Delphi study. Qual Life Res 2010b;19:539–49. 10.1007/s11136-010-9606-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terwee CB, Mokkink LB, Knol DL et al. . Rating the methodological quality in systematic reviews of studies on measurement properties: a scoring system for the COSMIN checklist. Qual Life Res 2012;21:651–7. 10.1007/s11136-011-9960-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aubeeluck A, Buchanan H. The Huntington's disease quality of life battery for carers: reliability and validity. Clin Genet 2007;71:434–45. 10.1111/j.1399-0004.2007.00784.x [DOI] [PubMed] [Google Scholar]
- 18.Aubeeluck A, Dorey J, Squitieri F et al. . Further evidence of reliability and validity of the Huntington's disease quality of life battery for carers: Italian and French translations. Qual Life Res 2012;22:1093–8. 10.1007/s11136-012-0227-2 [DOI] [PubMed] [Google Scholar]
- 19.Benito-Leon J, Rivera-Navarro J, Guerrero AL et al. . The CAREQOL-MS was a useful instrument to measure caregiver quality of life in multiple sclerosis. J Clin Epidemiol 2011;64:675–86. 10.1016/j.jclinepi.2010.08.003 [DOI] [PubMed] [Google Scholar]
- 20.Doward LC. The development of the Alzheimer's carers quality of life instrument. Qual Life Res 1997;6:639. [Google Scholar]
- 21.Jenkinson C, Dummett S, Kelly L et al. . The development and validation of a quality of life measure for the carers of people with Parkinson's disease (the PDQ-Carer). Parkinsonism Relat Disord 2012;18:483–7. 10.1016/j.parkreldis.2012.01.007 [DOI] [PubMed] [Google Scholar]
- 22.Pillas M, Selai C, Quinn NP et al. . Development and validation of a carers quality-of-life questionnaire for parkinsonism (PQoL Carers). Qual Life Res 2016;25:81–8. 10.1007/s11136-015-1071-y [DOI] [PubMed] [Google Scholar]
- 23.Morley D, Dummett S, Kelly L et al. . The PDQ-Carer: development and validation of a summary index score. Parkinsonism Relat Disord 2013;19:448–9. 10.1016/j.parkreldis.2012.11.018 [DOI] [PubMed] [Google Scholar]
- 24.McDonald RP. Test theory. A unified approach. Mahwah, NJ: Lawrence Erlbaum, 1999. [Google Scholar]
- 25.Rhemtulla M, Brosseau-Liard PÉ, Savalei V. When can categorical variables be treated as continuous? A comparison of robust continuous and categorical SEM estimation methods under suboptimal conditions. Psychol Methods 2012;17:354–73. 10.1037/a0029315 [DOI] [PubMed] [Google Scholar]
- 26.Fisher GH, Molenaar IW. Rasch models: foundations, recent developments, and applications. New York: Springer, 1995. [Google Scholar]
- 27.de Boer MR, Moll AC, de Vet HCW et al. . Psychometric properties of vision-related quality of life questionnaires: a systematic review. Ophthalmic Physiol Opt 2004;24:257–73. 10.1111/j.1475-1313.2004.00187.x [DOI] [PubMed] [Google Scholar]
- 28.Weitzner MA, Jacobsen PB, Wagner H Jr, et al. The Caregiver Quality of Life Index-Cancer (CQOLC) scale: development and validation of an instrument to measure quality of life of the family caregiver of patients with cancer. Qual Life Res 1999;8:53–63. 10.1023/A:1026407010614 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2016-013611supp.pdf (88KB, pdf)