Abstract
The patient in our case presented with progressive dyspnoea and cough. Chest radiograph reveals complete opacification of the hemithorax. Complete lung consolidation was not seen on chest CT. The patient in this case had extensive pulmonary and endobronchial Kaposi sarcoma (KS) that led to complete consolidation of the right lung that was diagnosed via bronchoscopy. After diagnosis, he was restarted on antiretroviral therapy and single-agent chemotherapy for treatment of pulmonary KS.
Background
Pulmonary Kaposi sarcoma (KS) is a vascular tumour that is associated with human herpes virus 8 (HHV-8). KS usually occurs in immunocompromised hosts such as patients with HIV. In those with HIV, the CD4 count is usually <150 cells/mm3. The widespread use of antiretroviral therapy (ART) has led to a striking decrease in the incidence of KS and also improved prognosis in affected patients. The clinical presentation varies greatly depending on the degree of lung involvement. Pulmonary KS can involve the pulmonary parenchyma, airway mucosa, pleura and/or mediastinal lymph nodes.1 2 The following case report presents an unusual case of pulmonary KS with extensive involvement of the pulmonary parenchyma and airways presenting as complete opacification of the hemithorax.
Case presentation
Our patient is a man aged 50 years with a history of HIV for 10 years, non-compliant with ART who presented to our hospital with progressive dyspnoea for the past 6 months. On further review of systems, he also reported a constant dry cough, unintentional weight loss of 35 pounds over the past 6 months and new skin lesion on his chest and back. Otherwise, he denied any fevers, chills, chest pain, haemoptysis, nausea, vomiting or diarrhoea.
Review of the vital signs showed that the patient was afebrile, normotensive, tachycardic to mid-90s, respiratory rate of 18 and an oxygen saturation of 100% on room air. The patient was not in any apparent respiratory distress and was able to speak in full sentences. Oral examination showed thrush but no other lesions. Lung examination revealed decreased breath sounds in the right lung, and dullness to percussion; left lung examination was normal. On skin examination, there were multiple non-blanching purple lesions on the chest and back. The rest of physical examination was unremarkable.
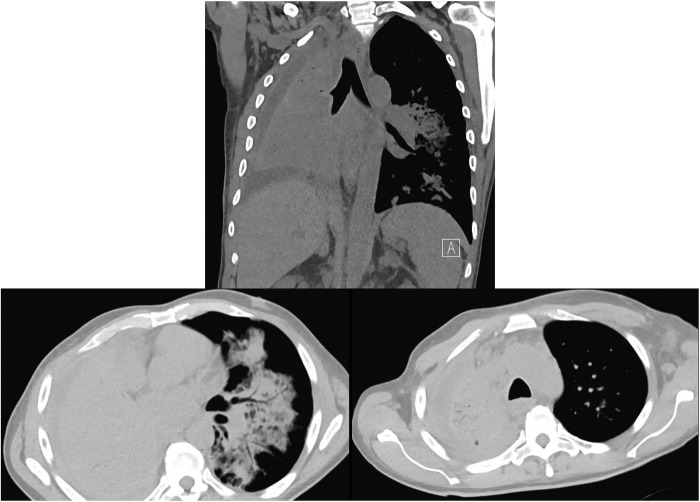
Initial laboratory examination showed a white cell count (WCC) of 9.0K/mL, haemoglobin of 8.5 g/dL and platelet count of 195K/mL. The CD4 count was 12 cells/mL. Electrolytes, kidney function and liver function panels were within normal limits. Chest X-ray is shown in figure 1. Complete opacification of the right hemithorax was noted. A CT of the chest without contrast was obtained for better characterisation of the anatomy. CT chest is shown in figure 2. Complete consolidation of the right lung was noted, with evidence of airbronchograms, narrowing of the right lower lobe airway with a cut-off sign, a right small pleural effusion and nodular consolidative process around the bronchovascular bundles in the left lung.
Figure 1.
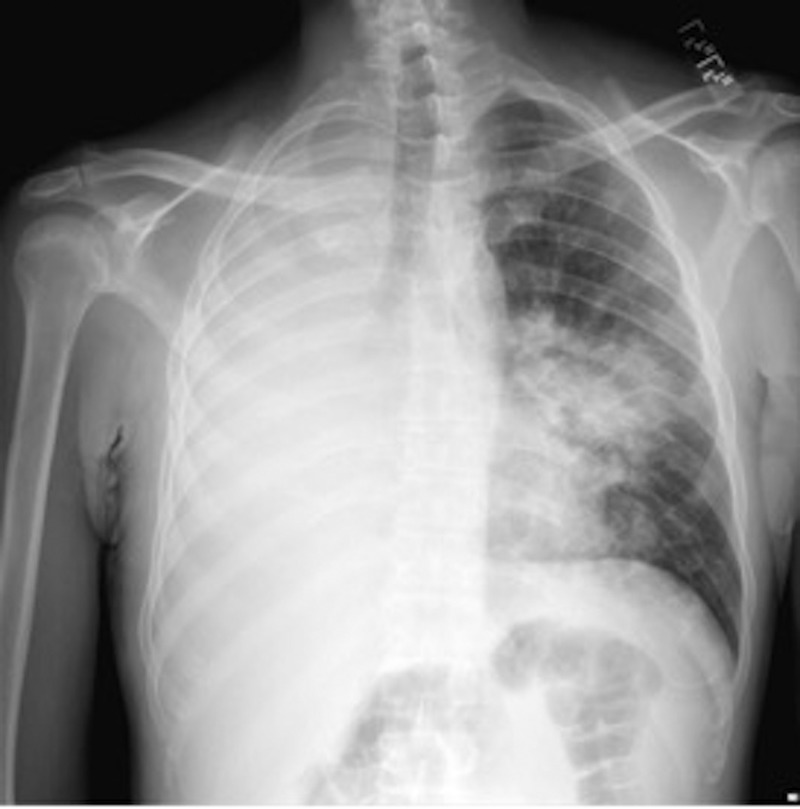
Chest radiograph with complete opacification of the right hemithorax, and multiple nodular opacities in the left lung field.
Figure 2.
CT chest showing complete consolidation of the right lung with airbronchograms, right pleural effusion and multiple nodular opacities in the left lung centred around the bronchovascular bundles.
The patient was admitted to medical floor with subsequent pulmonary consultation requested for evaluation of the radiological findings. Given the low CD4 count, multiple infectious and non-infectious possibilities were considered, including mycobacterial infections, fungal infections, lymphoma and KS, given the skin findings and pulmonary lesions in the left lung. A bronchoscopy was performed; the image is shown in figure 3. The airway mucosa was irregular, narrowed and with innumerable red vascular lesions diffusely on the right side that easily bled. The right upper, middle and lower lobe bronchi were severely narrowed not allowing advancement of the bronchoscope into those segments. The left side was not examined due to poor tolerance of the procedure. A bronchial wash and endobronchial biopsy was performed. Results of the bronchial wash revealed a bloody fluid with an RBC count of 400 K, WCC of 700 with 75% neutrophils,+1 Candida albicans, acid-fast bacilli and Pneumocystis pneumonia stains were negative. A biopsy of the skin lesions performed was consistent with KS.
Figure 3.
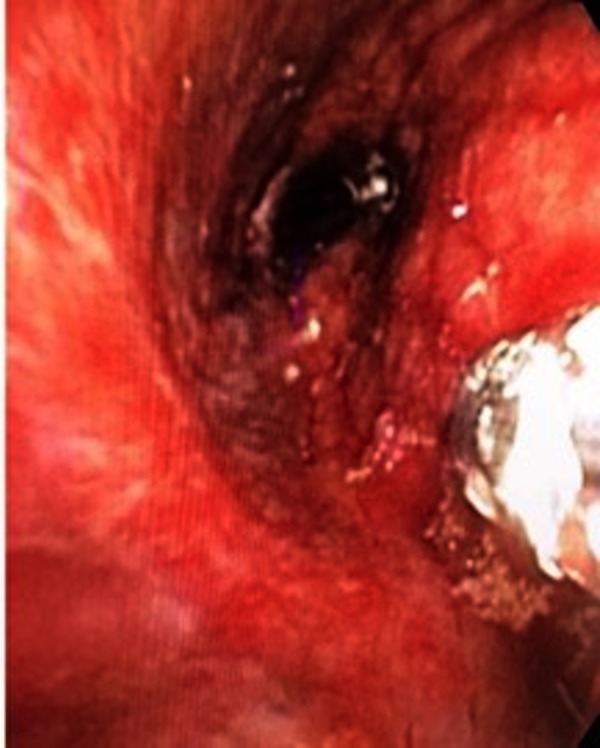
Bronchoscopic view showing narrowed right middle and lower lobe bronchi with diffuse red vascular lesions, biopsy forceps are in the right side of the image.
Bedside ultrasound scan of the right pleural effusion revealed a small pleural effusion with loculations. Thoracentesis was performed with 100 cc of bloody fluid removed. Analysis showed an exudative fluid by all criteria used, with a pH of 7.16, and cell count of 12 405 cells/mL with 58% lymphocytes and 155 non-RBC cells/mL. Microbiological analysis showed no organisms, and cytology revealed no malignant cells.
Histological examination of the endobronchial biopsy is shown in figure 4. Figure 4A shows infiltration of bronchial wall with spindle-shaped cells and multiple abnormal ectatic blood vessels. Immunohistochemical stain for CD31 is shown in figure 4B, CD34 in figure 4C and HHV-8 in figure 4D. Results were consistent with pulmonary KS.
Figure 4.
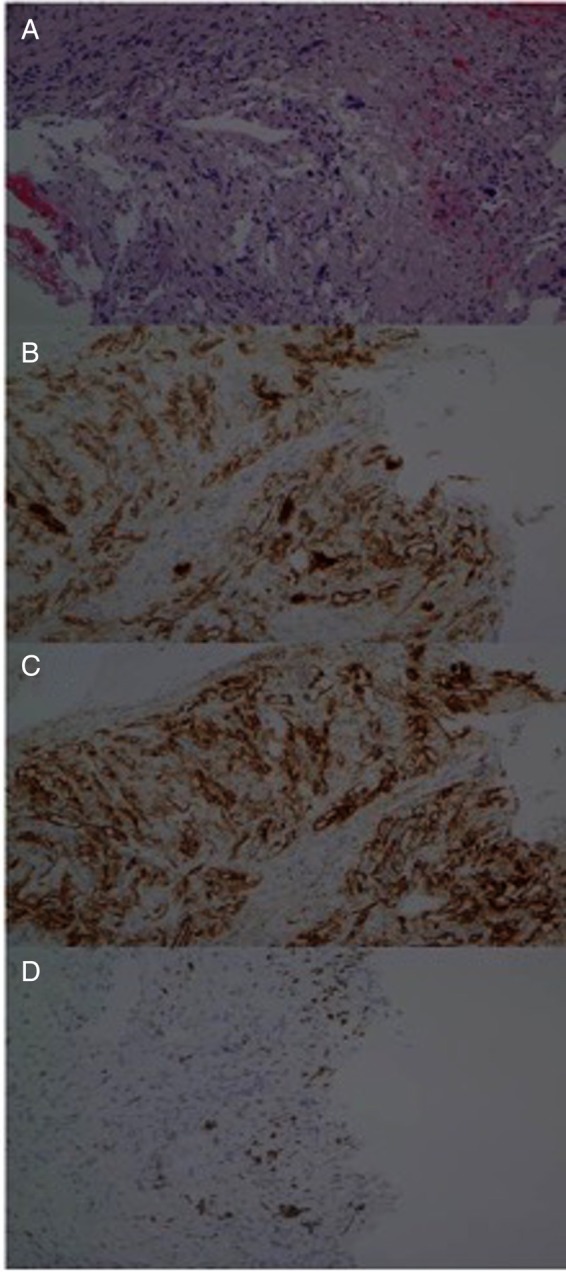
Histology of the endobronchial biopsy sample. (A) Spindle cells noted with ectatic blood vessels. (B) Spindle cells stain positive for CD31, CD34 in (C) and HHV-8 in (D).
Oncology specialists were consulted, and the patient was initiated on a single-agent chemotherapy with liposomal doxorubicin 36 mg in dextrose 5% intravenous (dosed every 3 weeks), in addition to ART (abacavir, dolutegravir and lamivudine) which were started early in the hospital course. The patient did not receive granulocyte colony-stimulating factor for myelosuppression prophylaxis with doxorubicin.
Follow-up CT chest 4 weeks after initiation of doxorubicin is shown in figure 5 for comparison. As shown, there was significant improvement after treatment, and the patient subjectively felt better from a pulmonary prospective.
Figure 5.
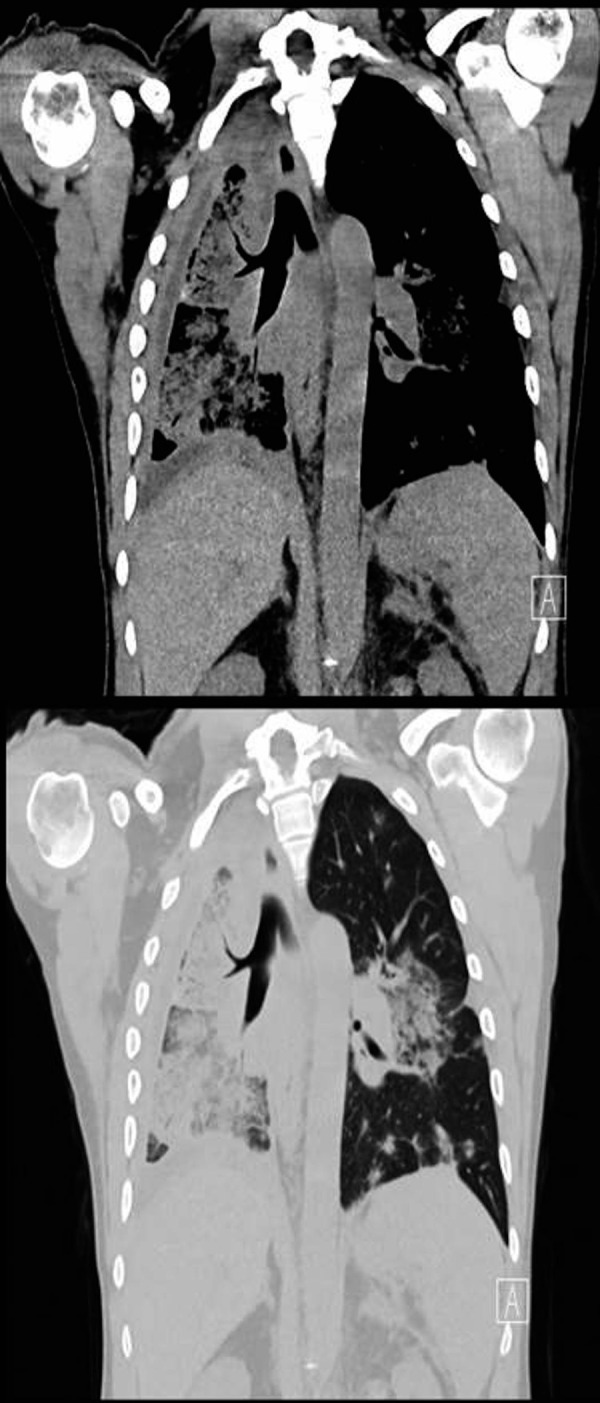
CT chest 4 weeks after initiation of chemotherapy.
Investigations
CBC, BMP, CD4 count
Chest radiograph
CT chest
Bronchoscopy with biopsy, stains for CD31, CD34 and HHV-8
BAL
Thoracentesis
Pleural fluid culture and cytology
Differential diagnosis
Pulmonary KS
Bacterial pneumonia
Fungal pneumonia
Tuberculosis
Lung cancer
Lymphoma
Treatment
Antiretroviral therapy
Chemotherapy with doxorubicin
Outcome and follow-up
The patient was seen in clinic 4 weeks after initiation of therapy and he reported that his dyspnoea and cough were much improved. In addition, CT chest showed significant improvement as well.
Discussion
The patient in this case had extensive pulmonary and endobronchial KS that led to complete consolidation of the right lung. This presentation of KS has never been reported to date to the best of our knowledge. Pulmonary KS usually occurs concomitantly with the presence of mucocutaneous involvement in about 90% of cases, which is the case in our patient. Less commonly pulmonary KS is the initial presentation of KS occurring in the absence of mucocutaneous involvement in 15% of cases.3–5
Diagnosis of pulmonary KS maybe made based on classic bronchoscopic appearance which was present in our case; however, given the unusual presentation and extent of disease, other aetiologies were considered and a biopsy was performed to confirm the diagnosis. The visualisation of spindle cell proliferation, ectatic blood vessels and positive immunohistochemical stains for CD31, CD34 and HHV-8 helps confirm the diagnosis.3–7
The patient in our case likely had multiple involvements of pulmonary KS. On the left lung, as evident on the CT chest, there were nodular opacities centred around the bronchovascular, bundles which are classically described with parenchymal KS.6–9 The right lung, however, had a much greater extent of involvement. The right lung was noted to be completely atelectatic/consolidated on CT chest, and on bronchoscopy, there were extensive endobronchial KS lesions causing narrowing of the airways. The differential for the total consolidation/atelectasis in the right lung included: complete atelectasis solely due to extensive endobronchial KS, postobstructive pneumonia with consolidation again as a result of the endobronchial lesions, and diffuse parenchymal KS. Given our patient lacked signs of infection, postobstructive pneumonia was felt to be less likely. The complete consolidation of the right lung can be explained by the extensive endobronchial lesions that caused severe narrowing of the airways resulting in atelectasis; however, given the developing parenchymal opacities in the left which are classic for KS, and the bloody exudative pleural effusion on the right side, the patient likely had parenchymal KS on the right side as well. Furthermore, the patient did not seek medical attention early, which likely contributed to the progression of the KS to this extent.
Our patient also was noted to have a pleural effusion. On thoracentesis, the effusion was noted to be bloody, and analysis revealed that it is an exudative effusion. The differential diagnosis for the pleural effusion included: pleural effusion secondary to KS, parapneumonic effusion and effusion as a result of atelectasis. The patient did not show signs of infection, and pleural fluid analysis showed a lymphocytic predominant bloody effusion with a negative Gram stain and no WCC were seen, making a parapneumonic effusion less likely. In addition, since the pleural fluid was a bloody exudate, it was felt to be less likely caused by atelectasis which normally causes a simple transudative effusion. Pleural effusions are well described with visceral pleural KS; however, this is usually diagnosed with a pleural biopsy.10 11
The treatment of mild cases of KS involves initiation of ART which is usually sufficient. However, chemotherapy is indicated for patients with extensive or rapidly progressive mucocutaneous KS, symptomatic visceral disease or pulmonary disease. Extensive organ involvement as in our patient usually requires the addition of single-agent chemotherapy such as doxorubicin, which was used in this case.12 13
Prognosis is variable in patients with KS; however, in one study of KS patients, the 5-year survival was 92% for those with early-stage KS compared with 83% for those with advanced-stage KS; for the 140 patients with advanced-stage KS that were treated with combination ART and liposomal anthracycline chemotherapy, the 5-year overall survival was 85%.14
Learning points.
Pulmonary Kaposi sarcoma (KS) involvement can be extensive to cause complete lung consolidation.
Pulmonary KS should be included in the differential diagnosis of HIV patients who have similar presentation.
With atypical presentation as in our case, biopsy is sometimes necessary to establish diagnosis and allow for institution of therapy.
With extensive KS involvement, single-agent chemotherapy is needed in addition to antiretroviral therapy as in our case.
Footnotes
Contributors: AA was involved in the patient initial work up and follow-up, performing procedure, literature search and drafting/editing of the manuscript. ZA was involved in patient follow-up, performing the procedure, literature search and editing of the manuscript. RK was involved in patient follow-up, literature search and editing of manuscript. SVS was involved in patient follow-up, literature search and editing of manuscript. All authors read and approved the final manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Lodi S, Guiguet M, Costagliola D et al. Kaposi sarcoma incidence and survival among HIV-infected homosexual men after HIV seroconversion. J Natl Cancer Inst 2010;102:784 10.1093/jnci/djq134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joshi M, Markelova N, Palacio D et al. A patient with HIV, dyspnea, and multiple pulmonary nodules: pulmonary Kaposi sarcoma. Chest 2006;130:1924 10.1378/chest.130.6.1924 [DOI] [PubMed] [Google Scholar]
- 3.Haramati LB, Wong J. Intrathoracic Kaposi's sarcoma in women with AIDS. Chest 2000;117:410–14.. 10.1378/chest.117.2.410 [DOI] [PubMed] [Google Scholar]
- 4.Huang L, Schnapp LM, Gruden JF et al. Presentation of AIDS-related pulmonary Kaposi's sarcoma diagnosed by bronchoscopy. Am J Respir Crit Care Med 1996;153:1385 10.1164/ajrccm.153.4.8616570 [DOI] [PubMed] [Google Scholar]
- 5.Pinzone MR, Berretta M, Cacopardo B et al. Epstein-Barr virus- and Kaposi sarcoma-associated herpes virus-related malignancies in the setting of human immunodeficiency virus infection. Semin Oncol 2015;42:258 10.1053/j.seminoncol.2014.12.026 [DOI] [PubMed] [Google Scholar]
- 6.Zibrak JD, Silvestri RC, Costello P et al. Bronchoscopic and radiologic features of Kaposi's sarcoma involving the respiratory system. Chest 1986;90:476 10.1378/chest.90.4.476 [DOI] [PubMed] [Google Scholar]
- 7.Davis SD, Henschke CI, Chamides BK et al. Intrathoracic Kaposi sarcoma in AIDS patients: radiographic-pathologic correlation. Radiology 1987;163:495 10.1148/radiology.163.2.3562832 [DOI] [PubMed] [Google Scholar]
- 8.Sivit CJ, Schwartz AM, Rockoff SD. Kaposi's sarcoma of the lung in AIDS: radiologic-pathologic analysis. AJR Am J Roentgenol 1987;148:25 10.2214/ajr.148.1.25 [DOI] [PubMed] [Google Scholar]
- 9.Meduri GU, Stover DE, Lee M et al. Pulmonary Kaposi's sarcoma in the acquired immune deficiency syndrome. Clinical, radiographic, and pathologic manifestations. Am J Med 1986;81:11–18. [DOI] [PubMed] [Google Scholar]
- 10.O'Brien RF, Cohn DL. Serosanguineous pleural effusions in AIDS-associated Kaposi's sarcoma. Chest 1989;96:460–6. 10.1378/chest.96.3.460 [DOI] [PubMed] [Google Scholar]
- 11.Rubio ER, Chang EE, Kovitz KL. Thoracoscopic management of pleural effusions in Kaposi's sarcoma: a rapid and effective alternative for diagnosis and treatment. South Med J 2002;95:919–21. 10.1097/00007611-200295080-00026 [DOI] [PubMed] [Google Scholar]
- 12.Gill PS, Akil B, Colletti P et al. Pulmonary Kaposi's sarcoma: clinical findings and results of therapy. Am J Med 1989;87:57–61. 10.1016/S0002-9343(89)80483-8 [DOI] [PubMed] [Google Scholar]
- 13.Cadranel JL, Kammoun S, Chevret S et al. Results of chemotherapy in 30 AIDS patients with symptomatic pulmonary Kaposi's sarcoma. Thorax 1994;49:958 10.1136/thx.49.10.958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bower M, Dalla Pria A, Coyle C et al. Prospective stage-stratified approach to AIDS-related Kaposi's sarcoma. J Clin Oncol 2014;32:409–14. 10.1200/JCO.2013.51.6757 [DOI] [PubMed] [Google Scholar]