Abstract
Cushing's syndrome (CS) can be classified as adrenocorticotropic hormone (ACTH)-dependent or ACTH-independent depending on the ACTH levels. However, 30% of the patients with CS have ACTH levels in the ‘grey zone’ (5–20 pg/mL), thereby posing a challenge in establishing the aetiological diagnosis. In a patient with full-blown features of Cushing's syndrome with equivocal ACTH levels, and a pituitary microadenoma on contrast-enhanced MRI sella, can falsely lead to a diagnosis of Cushing's disease. Pituitary microadenoma, if <6 mm in size, may be an incidental finding (incidentaloma) in this scenario and can be present in ∼3–27% of the healthy population. Therefore, in a patient with CS with equivocal ACTH levels and a pituitary microadenoma, multiple samplings for ACTH and adrenal imaging should be performed to exclude ACTH-independent CS and if required, bilateral inferior petrosal sinus sampling to determine the source of ACTH excess.
Background
In patients with Cushing's syndrome there is a considerable overlap in the adrenocorticotropic hormone (ACTH) levels. A patient with overt features of Cushing's syndrome with equivocal ACTH levels and pituitary microadenoma of <6 mm in size poses a diagnostic dilemma, as the incidence of pituitary incidentaloma is around 3–27% in the general population.1 In such a scenario ACTH dependency should be established by multiple sampling of ACTH or by intravenous corticotropin releasing hormone (CRH) stimulation test. Further, to localise the source of ACTH excess, bilateral inferior petrosal sinus sampling (BIPSS) should be performed to avoid inadvertent pituitary surgery in such cases. We report a case of Cushing's syndrome with ACTH levels in the ‘grey zone’ and pituitary microadenoma of <6 mm size, who underwent trans-sphenoidal pituitary surgery. However, the patient's clinical features did not resolve after surgery with persistent biochemical abnormalities, and subsequently on re-evaluation was found to have an adrenocortical carcinoma.
Case presentation
A 25-year-old man presented with weight gain, rounding of face, irritability, facial plethora, swelling of legs and generalised weakness of 6 months duration. On examination, he had facial plethora, moon facies, acne, acanthosis nigricans (grade III), violaceous purplish striae (>1 cm) over axilla, centripetal obesity, cuticular atrophy, pulp atrophy and proximal muscle weakness. Systemic examination was unremarkable. Investigations showed 8:00 hours serum cortisol at 800 nmol/L, 16:00 hours serum cortisol at 665 nmol/L, overnight dexamethasone suppression test (ONDST) 670 nmol/L (N<50 nmol/L), midnight ACTH 15.8 pg/mL (single value), and midnight cortisol 590 nmol/L and T4 4.0 μg/dL. However, other pituitary functions were not assessed. Contrast-enhanced MRI (CEMRI) of sella showed hypointense lesion on the left side of the pituitary measuring 5.5×4.3 mm size (Figure 1). A diagnosis of pituitary ACTH-dependent Cushing's syndrome was made, and replacement with levothyroxine was started. The patient was subjected to trans-sphenoidal surgery with resection of pituitary microadenoma. Histopathology of the resected tissue was reported as a pituitary adenoma with focal ACTH positivity on immunohistochemistry. Postoperatively, the patient did not improve symptomatically and morning serum cortisol was still 612 nmol/L. ONDST was non-suppressible with a serum cortisol value of 714 nmol/L. The patient was referred to our institute in view of persistent signs and symptoms, and hypercortisolaemia. On examination, he had clinical features of hypercortisolism suggestive of persistent disease. Investigations showed 8:00 hours serum cortisol at 978 nmol/L, 23:00 hours serum cortisol at 958 nmol/L, 8:00 hours ACTH<1 pg/mL (multiple values), ONDST 933 nmol/L, low-dose dexamethasone suppression test 585 nmol/L (N<50 nmol/L), dehydroepiandrosterone Sulphate (DHEAS) 1021 μg/dL (N 160–449), luteinising hormone (LH) 0.2 mIU/mL (N 1.7–8.6), follicle stimulating hormone (FSH) 3.0 mIU/mL (N 1.5–12.4), testosterone 5.8 nmol/L (N 9.9–27.8), oestradiol 31.7 ng/mL (N 7.6–42.6), prolactin 18.4 ng/mL (N 4.0–15.2), T3 1.1 ng/mL (N 0.8–2.0), T4 7.4 μg/dL (N 4.8–12.7), thyroid-stimulating hormone (TSH) 1.7 μIU/mL (N 0.27–4.2) and glycated haemoglobin 5.9%. Dual energy X-ray absorptiometry (DXA) scan showed T score of −0.2 at lumbar spine and −0.9 at femoral neck. Review of the pituitary histopathology was carried out in our institute, which showed normal pituitary gland; however, immunohistochemistry for pituitary hormones was not possible due to paucity of tissue. Contrast enhanced computerised tomography (CECT) abdomen showed heterogeneous enhancing mass lesion with arterial hypervascularity in the left suprarenal area measuring 10.7×8.6×11.3 cm (figure 2). 18Fludeoxyglucose (FDG) positron emission tomography-computerised tomography (PET-CT) showed heterogeneous FDG uptake (standardised uptake value (SUV) maximum 14.0) in a well-defined soft tissue mass of size 11.5×9.5×11.7 cm in the left suprarenal area with no hypermetabolic tissue elsewhere in the body. Diagnosis of ACTH-independent Cushing's syndrome due to adrenocortical carcinoma with pituitary incidentaloma was considered and the patient was subjected to left-sided open adrenalectomy. The resected mass measured 10×8×5 cm and weighed 556 g with no capsular breach (European Network for Study of Adrenal Tumour, Stage II). Histopathological examination was suggestive of adrenocortical carcinoma with modified Weiss score of 4/7.
Figure 1.
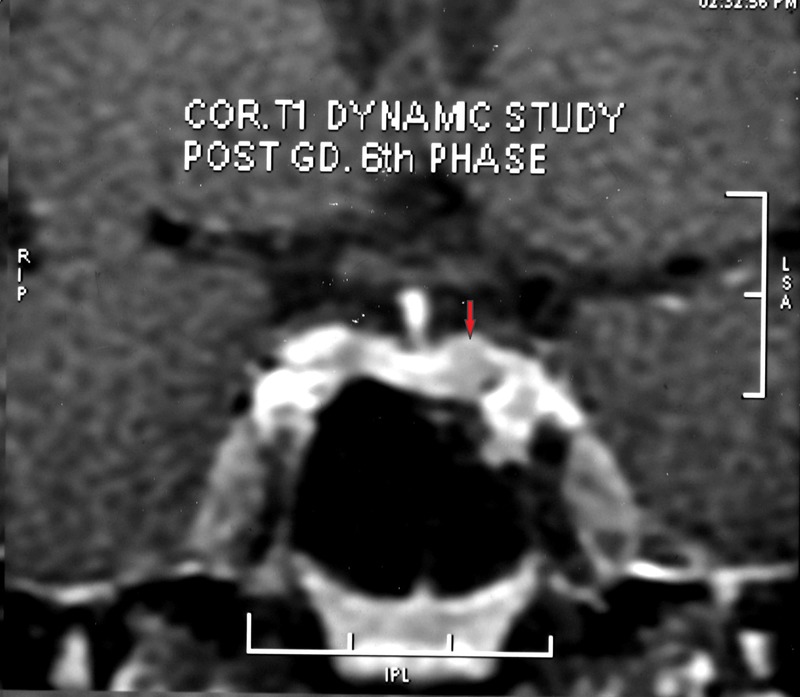
Contrast-enhanced MRI sella showing hypointense lesion on the left side of the pituitary measuring 5.5×4.3 mm.
Figure 2.
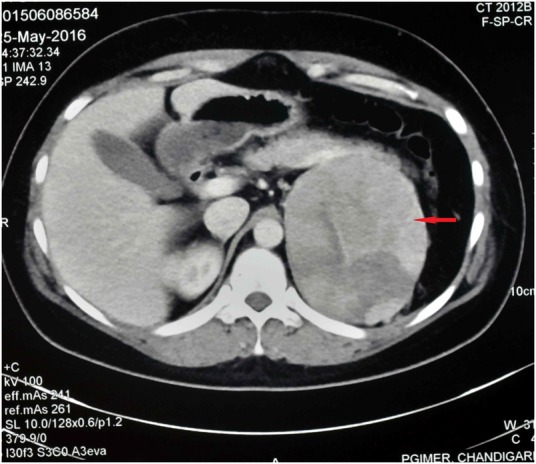
Contrast enhanced computerised tomography (CECT) of abdomen showing heterogeneous enhancing mass lesion with arterial hypervascularity in the left suprarenal area measuring 10.7×8.6×11.3 cm.
Outcome and follow-up
The patient underwent open left adrenalectomy and postoperatively the patient was started on hydrocortisone supplementation and mitotane therapy (1000 mg/day in divided doses). Postoperative hormonal evaluation showed 24-hour urine free cortisol at 27 μg/day (off hydrocortisone for 24 hours), testosterone at 4.98 nmol/L and DHEAS at 5.7 μg/dL. Repeat CECT abdomen did not show any residual lesion and he was subjected to tumour-bed radiotherapy. 18FDG PET-CT is planned on follow-up.
Discussion
We here describe a case of Cushing's syndrome, which was initially treated as corticotropinoma, as CEMRI sella had shown a microadenoma and ACTH level though a single value, was in the ‘grey zone’. Owing to the persistence of symptoms and hypercortisolaemia after trans-sphenoidal surgery, the patient was re-evaluated and was found to have ACTH-independent Cushing's syndrome.
Incidence of endogenous Cushing's syndrome is 5–10 cases per million per year. Cushing's disease is the most common cause of Cushing's syndrome accounting for 70–80% of the cases and out of these 90% are microadenomas and 10% macroadenomas. Adrenal causes contribute 15–20% of all the patients with Cushing's syndrome including adrenocortical tumour (benign/malignant) or ACTH-independent macronodular adrenal hyperplasia.2 3
Measurement of plasma ACTH levels help in aetiological classification of Cushing's syndrome as ACTH-dependent or ACTH-independent. However, there is a considerable overlap in the ACTH levels, as 50% of the patients with Cushing's disease and 25% of the patients with adrenal Cushing's syndrome have ACTH levels within normal reference range.4 5 The 8:00 hours plasma ACTH value of <5 pg/mL is suggestive of ACTH-independent Cushing's syndrome, while 8:00 hours plasma ACTH value of >20 pg/mL is suggestive of ACTH-dependent, and the levels between 5 and 20 pg/mL are in the ‘grey zone’. Patients in the latter group require ACTH and cortisol responses to intravenous CRH stimulation to establish the ACTH dependency.6The plasma ACTH levels in adrenal Cushing's syndrome may not always be <5 pg/mL because of slow evolution of the disease and acquired enzymatic block in the cortisol biosynthesis in the tumour tissue. Similarly, ACTH levels may be in the normal range in patients with Cushing's disease or sometimes in cyclical Cushing's syndrome, due to diurnal variability and pulsatile ACTH secretion, which may not be altered in the early stages of the disease.7 8 Further, heterophile antibodies may also interfere with ACTH assay measured by the electrochemiluminescence immunoassay method ; however, in such a scenario the ACTH levels are usually very high and polyethylene glycol separation is required for correct estimation. This was not observed in the index case as the ACTH levels were in the normal range.9 In most of the studies on ACTH levels have been carried out in the morning or evening; however, it should be carried out when there is nadir of ACTH between 23:00 and 1:00 hours and ACTH level of >22 pg/mL at midnight has been suggested as a cut-off for ACTH dependency.8
In the index case, ACTH value was 15.8 pg/mL and CEMRI sella showed microadenoma, hence a diagnosis of ACTH-dependent pituitary Cushing's syndrome was made and the patient was subjected to trans-sphenoidal surgery. However, in the presence of short-duration symptoms, male sex and equivocal ACTH level, possibility of aetiologies other than Cushing's disease should have been considered. The right approach in this case would have been to perform multiple ACTH sampling, and if the ACTH levels are still in the ‘grey zone’, an intravenous CRH stimulation test should have been performed. Patients with adrenal Cushing's usually show stimulated ACTH values <20 pg/mL, but the patients with Cushing's disease show a value >20 pg/mL. If the ACTH independency is proved, an imaging for adrenal should be performed. If the ACTH dependency is proved, then BIPSS should be performed as the tumour size was <6 mm. Since 90% of the corticotropinoma are microadenomas, and in general population the incidence of pituitary incidentaloma is up to 3–27%, one should always perform BIPSS in pituitary adenomas of size <6 mm to confirm the ACTH source.9–11
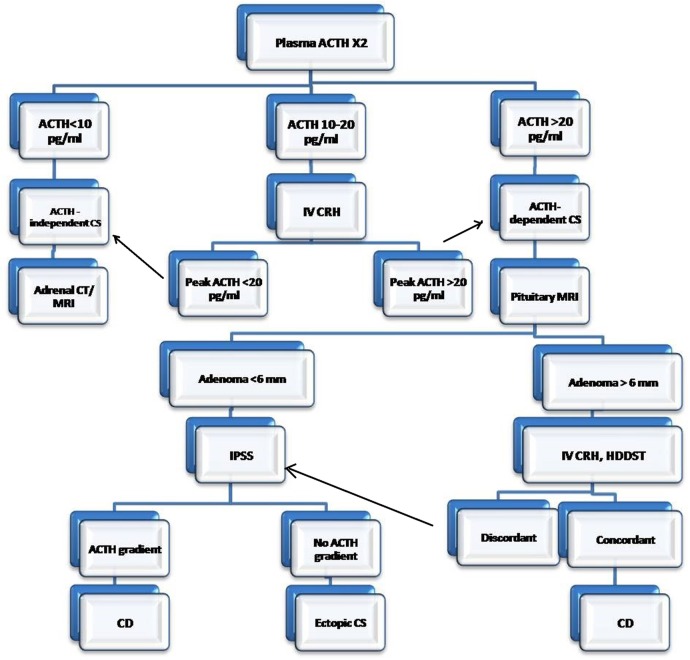
The probability of adrenal incidentaloma in such a scenario should not be considered, as the patient had overt features of Cushing's syndrome. Adrenal incidentalomas occurs in 0.2–7% of patients, depending on age, and may result in diagnostic confusion in presence of equivocal ACTH levels and pituitary microadenoma. Rarely, corticotropinoma may be associated with adrenal nodular enlargement and again ACTH dependency or independency can be judged by ACTH levels. Figure 3 shows a suggested strategy for the differential diagnosis and approach to a patient with Cushing's syndrome.
Figure 3.
Suggested strategy for the differential diagnosis and approach to a patient with Cushing's syndrome. ACTH, Adrenocorticotropic hormone; CRH, corticotropin releasing hormone; CD, Cushing’s disease; CS, Cushing's syndrome; HDDST, high dose dexamethasone suppression test; IPSS, inferior petrosal sinus sampling; IV, intravenous.
In the index patient, surgery was performed presuming the pituitary incidentaloma to be a corticotropinoma. Pituitary incidentalomas are reported on histopathological examination as pituitary adenomas and focal positivity on immunohistochemistry is due to the presence of normal corticotrope cells, as was seen in the index patient. Further, multiple midnight ACTH measurements on different days may be helpful to determine the ACTH dependency in patients who have ACTH levels in grey zone. Adrenal imaging should be performed in these patients as it is cost-effective in detecting the adrenal pathology.
Learning points.
ACTH values, if in the ‘grey zone’ (10–20 pg/mL), tests should be repeated multiple times in patients with Cushing's syndrome to establish the aetiological diagnosis.
If ACTH levels are in the ‘grey zone’ adrenal imaging should be performed.
Bilateral inferior petrosal sinus sampling should always be carried out if MRI sella shows a microadenoma <6 mm size to confirm the source of ACTH.
Footnotes
Contributors: KDJ wrote the manuscript and managed the patient. RW edited the manuscript. SK performed surgery. AB edited the manuscript and supervised patient management.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Hall WA, Luciano MG, Doppman JL et al. Pituitary magnetic resonance imaging in normal human volunteers: occult adenomas in the general population. Ann Intern Med 1994;120:817–20. 10.7326/0003-4819-120-10-199405150-00001 [DOI] [PubMed] [Google Scholar]
- 2.Nieman LK, Biller BM, Findling JW et al. The diagnosis of Cushing's syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2008;93:1526–40. 10.1210/jc.2008-0125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stratakis CA. Cushing syndrome in pediatrics. Endocrinol Metab Clin North Am 2012;41:793–803. 10.1016/j.ecl.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Findling JW. Clinical application of new immunoradiometric assay for ACTH. Endocrinologist 1992;2:360–5. 10.1097/00019616-199211000-00003 [DOI] [Google Scholar]
- 5.Brossaud J, Bouton M, Gatta B et al. Use of an automated ACTH assay for the diagnosis of pituitary and adrenal related diseases. Clin Biochem 2011;44:1160–2. 10.1016/j.clinbiochem.2011.06.002 [DOI] [PubMed] [Google Scholar]
- 6.Raff H, Findling JW. A physiologic approach to diagnosis of the Cushing syndrome. Ann Intern Med 2003;138:980–91. 10.7326/0003-4819-138-12-200306170-00010 [DOI] [PubMed] [Google Scholar]
- 7.Orth DN. Cushing's syndrome. N Engl J Med 1995;332:791–803. 10.1056/NEJM199503233321207 [DOI] [PubMed] [Google Scholar]
- 8.Melmed S, Williams R. Williams's textbook of endocrinology. Philadelphia: Elsevier/Saunders, 2011:516. [Google Scholar]
- 9.Gulbahar O, Degertekin CK, Akturk M et al. A case with immunoassay interference in the measurement of multiple hormones. J Clin Endocrinol Metab 2015;100:2147–53. 10.1210/jc.2014-4023 [DOI] [PubMed] [Google Scholar]
- 10.Jameson J, De Groot L. Endocrinology adult and pediatric. Elsevier—Health Sciences Division, 2016:241. [Google Scholar]
- 11.Zampetti B, Grossrubatscher E, Ciaramella P et al. Bilateral inferior petrosal sinus sampling. Endocr Connect 2016;5:R12–25. 10.1530/EC-16-0029 [DOI] [PMC free article] [PubMed] [Google Scholar]