Abstract
Non-recurrence and extralaryngeal branching are 2 of the more frequently encountered anomalies of the recurrent laryngeal nerve. If not anticipated intraoperatively, these abnormalities can put the nerve at risk, with subsequent vocal cord palsy. It is therefore important to report on and understand these abnormalities. We present a unique case of a non-recurrent laryngeal nerve with a coexisting contralateral nerve demonstrating extralaryngeal branching. This case allows us to demonstrate the importance of arteria lusoria in head and neck surgery, and to conclude that non-recurrence and extralaryngeal branching can occur separately within individual nerves in the same patient. The case also highlights the importance of a systematic intraoperative approach to the identification of every recurrent laryngeal nerve, especially in bilateral procedures having already exposed an anomalous nerve on one side.
Background
The recurrent laryngeal nerve (RLN) is a cervical branch of the vagus nerve, providing motor, sensory and parasympathetic nerve fibres to the larynx.1–6 Therefore, maintaining its integrity during surgery is of vital importance and depends substantially on the surgeon's knowledge of its anatomy. Unfortunately, the RLN's anatomy can be highly variable. The two most commonly reported abnormalities are non-recurrence of the nerve, and extralaryngeal branching (ELB) patterns.1 3 5 According to Ling and Smoll's3 recent systematic review, the RLN is ‘non-recurrent’ in an estimated 0.57% of cases. ELB patterns are more prevalent with quoted rates ranging from 33% to 72%.1–5 7–9 Two previous reports estimate bilateral bifurcations of the nerves to occur in ∼7–9% of cases.6 7 No cases of bilateral trifurcations have been reported. Furthermore, no cases of a single ipsilateral nerve demonstrating both non-recurrence and ELB have been previously described.
There has been no previous report of a non-RLN (NRLN) and a coexisting contralateral nerve with ELB, in the same patient. Here, we report one such a case, in order to raise awareness for this potential combination of anomalies. In doing so, we provide key learning points, which will prove useful for all surgeons operating in the neck.
Case presentation
This case concerns that of a 73-year-old woman who presented with an asymptomatic neck mass. She was a fully independent, a non-smoker, with no notable medical history. Physical examination demonstrated a right-sided, level II neck mass, with features indicative of an enlarged cervical lymph node.
Investigations
The ultrasound scan of the patient's neck revealed a neck mass to be a probable pathological lymph node, with no other abnormalities seen. Fine-needle aspiration cytology inconclusively suggested a metastatic papillary thyroid carcinoma (PTC), level IIb lymph node. An incisional biopsy was subsequently arranged, which confirmed metastatic PTC (pT1aN1b). The patient's CT of the neck and thorax revealed an aberrant right-sided subclavian artery also known as arteria lusoria (AL; figure 1). However, the AL was not described in the radiologist's report. The patient had no further imaging or other investigations, and proceeded to surgery.
Figure 1.
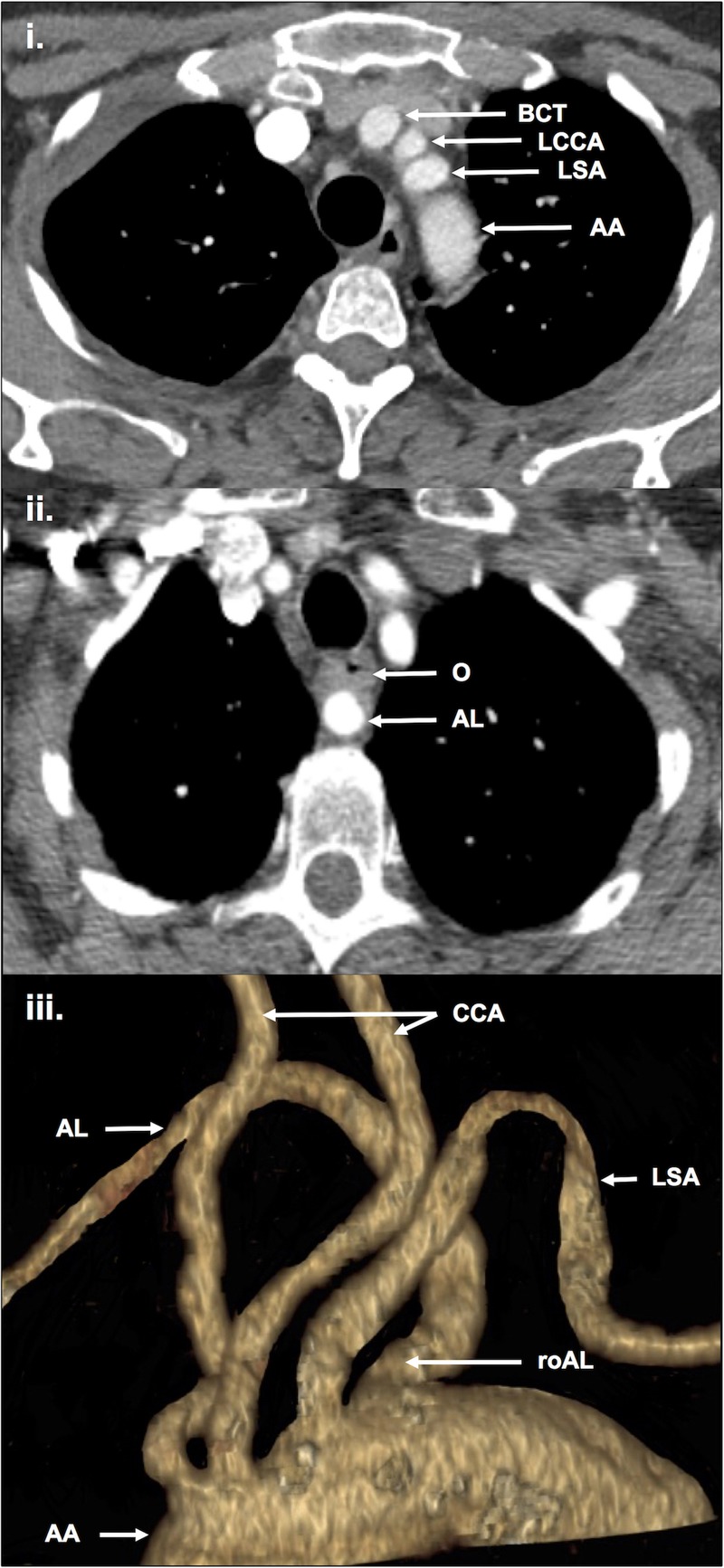
CT thorax: (i) normal anatomy for comparison in axial plane; (ii) the patient's AL in axial plane; (iii) 3D reconstruction of the patient's AL. 3D; AA, aortic arch; AL, arteria lusoria; BCT, brachiocephalic trunk; CCA, common carotid arteries; LCCA, left common carotid artery; LSA, left subclavian artery; O, oesophagus; roAL, root of arteria lusoria.
Treatment
The patient had a total thyroidectomy with central compartment (level VI) and right-selective neck dissections (levels II–IV). Intraoperatively, a right-sided NRLN was dissected and preserved, in combination with a left-sided nerve demonstrating bifid ELB (also preserved; figure 2). Surgical histopathology confirmed multifocal, right lobar PTC, and six positive lymph nodes.
Figure 2.
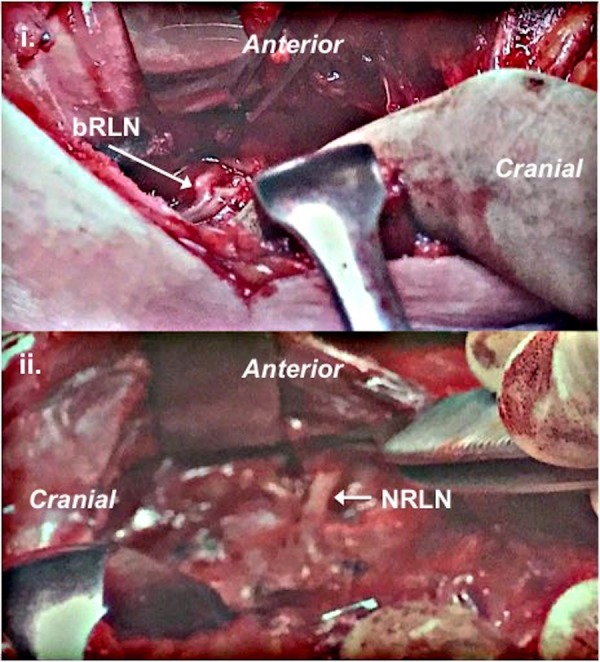
Intraoperative photographs (same patient): (1) extralaryngeal bifurcation of the left recurrent laryngeal nerve; (2) right-sided non-recurrent laryngeal nerve. bRLN, bifid recurrent laryngeal nerve; NRLN, non-recurrent laryngeal nerve.
Outcome and follow-up
This woman received postoperative radio-iodine ablation, and has had no signs of recurrence 1 year post-treatment.
Discussion
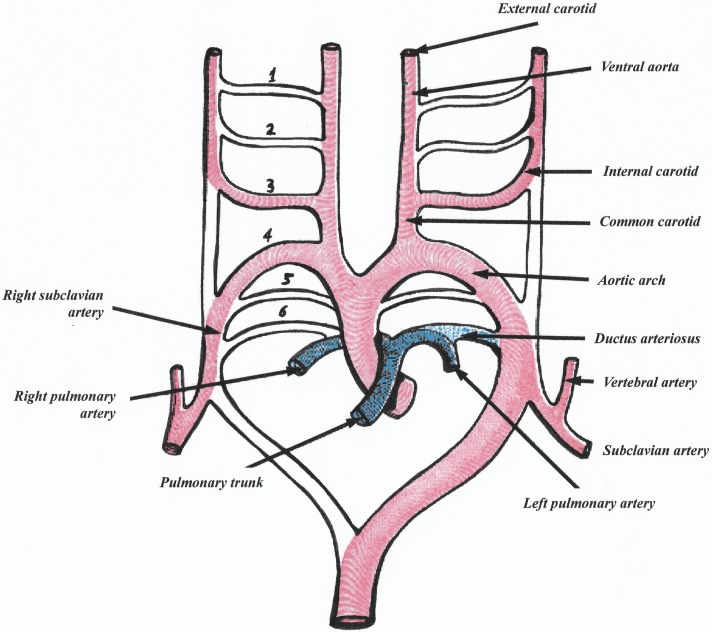
AL, and its relevance to the RLN: The prevalence of NRLNs is estimated to be ∼0.57%.3 It is widely agreed that the NRLN takes its more direct course to the larynx due to variations during embryological development.10–12 The normally RLNs are the nerves of the paired sixth branchial arches. The ventral portions of these arches become the pulmonary arteries, leaving the nerves restrained only by the remaining dorsal parts. However, these dorsal parts regress, as do the fifth branchial arches, leaving the nerves to recur and ascend from underneath the fourth arches. The right and left fourth arches become the right subclavian artery and the aortic arch, respectively; this is considered normal embryological development (figure 3).13
Figure 3.
The aortic arches in embryo (modified illustration from Gray's ‘Anatomy of the Human Body’, figure 47313).
However, in the case of our patient and her NRLN, the right-sided fourth branchial arch also degenerated and was replaced by an aberrant right subclavian artery arising from the left-sided aortic arch; this anomalous artery is termed AL. The absent right-sided fourth branchial arch allows the nerve to take a more direct course to the larynx, that is, non-recurrent. It is agreed that a NRLN is associated with AL in all cases.1 3 10 12 Therefore, previous reports of left-sided NRLNs have been reviewed as being ‘true’ only in the presence of situs inversus/dextrocardia, or ‘false’ where the aortic arch remains left-sided and a cervical, sympathetic, nervous-communicating branch was presumably misidentified as a NRLN.11 12 Although not impossible, the combination of a left-sided NRLN and situs inversus is extremely rare, and so we can conclude that NRLNs are always associated with AL, and are nearly always right-sided. AL is the only constant here, and so its preoperative diagnosis has been considered in an effort to indirectly identify the presence of a NRLN.
Detecting AL: Detection of AL may be incidental, following investigation for dysphagia. The clinical syndrome of ‘dysphagia lusoria’ is where the AL causes oesophageal compression, which may be demonstrated on initial contrast swallow and/or oesphagoscopy.14 However, these investigations are limited by low sensitivity, specificity, variable AL course (retro-oesophageal=85%; preoesophageal=15%; pretracheal=5%), and the need for reported dysphagia to prompt their use.14 15 Similarly, a plain chest radiograph may reveal a linear, oblique, mediastinal shadow, but only in 20% of cases.14 15 Cross-sectional studies (ie, CT or MRI±angiography) are more promising as they can directly demonstrate an AL in ∼100% of cases.14 15 Furthermore, preoperative CT is now commonplace in head and neck surgery. However, it involves radiation exposure, there needs to be an initial indication for its request, and AL may be under-reported by radiologists.15
Ultrasonography is often a favourable imaging modality in general, and has been shown to have great potential for detecting AL. Iacobone et al's15 study examined the effect of detecting AL using ‘on-table ultrasonography’, on intraoperative and postoperative outcomes. They describe intraoperative ultrasound examination of the neck, whereby the right common carotid artery (RCCA) is followed down as far as the sternal notch.15 At the sternal notch, the ultrasound probe is angled to follow the RCCA to its origin.15 If the RCCA comes from the bifurcation of the brachiocephalic trunk (as normally expected), then this is seen as the ‘Y-sign’.15 In this situation, the other limb of the bifurcation is presumed to be a normal right subclavian artery, and therefore associated with a normally right RLN.15 If however, the RCCA comes directly from the aortic arch, then the right subclavian artery is presumed to be abberant (ie, AL) and thus associated with a right NRLN.15 This is indeed the only study to examine the effect of any form of preoperative imaging on intraoperative NRLN identification and outcomes. In the test group, Iacobone et al15 reported a correctly predicted NRLN rate of 100%, NRLN palsy rate of 0% (p<0.05), and significant reductions in the intraoperative identification time for NRLNs and RLNs, inclusive of time for the ultrasound scanning (p<0.01). On retrospective review of our patient's ultrasound scan, her AL could not be seen. However, this was expected as the scan was never originally intended to detect AL, but rather to characterise the neck mass and to guide fine-needle aspiration.
ELB of the RLN: A number of published series have endeavoured to define the prevalence of ELB of the RLN. The wide range quoted for this prevalence is ∼33–72%.1–5 7–9 The importance of identifying ELB is to prevent any inadvertent damage to the nerve with subsequent vocal cord palsy. As well as providing sensory supply to the larynx and subglottis, the RLN supplies motor innervation to various intrinsic laryngeal muscles. These can be categorised into adductors and abductors of the vocal cords, although this may be a simplistic view.16 Various papers have looked into the distinct function of anterior versus posterior branches in the case of bifurcation, which is the most common number of nerve branches encountered;4–9 17–19 however, there is no agreement. For example, there are reports that conclude that the anterior branch supplies both the vocal cord adductors and abductors, while the posterior branch is purely sensory.5 6 Conversely, other reports suggest that the adductor fibres are within the anterior branch, while the abductor fibres are within the posterior branch.17–19 There has been no such attempt of assigning function to branches in the case of nerve trifurcation. We believe this functional uncertainty in fact further emphasises the importance of identifying and preserving all branches of the RLN.
As there are no reported methods of diagnosing ELB preoperatively, the challenge is therefore in the intraoperative identification of all extralaryngeal branches. This identification can be aided with knowledge of its branching patterns and occurrence rates. It is possible to measure the distance between the inferior border of the cricoid cartilage, and the ELB point. This distance has been shown to be predictable by numerous previous studies, with a quoted range of ∼0.3–4.5 cm for a total of 2256 nerves.1–7 These reports agree that the vast majority of ELB points are found within ≤2.0 cm of the cricoid cartilage, as was the case for our patient.1–7 We believe this knowledge, with reference to the easily identifiable cricoid cartilage, to be very useful when the surgeon tries to satisfy the recognition and preservation of all the branches. Regarding occurrence rates, we know that bilateral bifurcations of the nerves are estimated to occur in ∼7–9%.6–7 Furthermore, previous studies have made observations regarding the prevalence of ELB depending on laterality. While the proportions reported vary for right-sided versus left-sided ELB, they all agree that there is only a slight preponderance for right-sided ELB (right-sided proportion range 51–64%).1–8 No cases of a single ipsilateral nerve demonstrating both non-recurrence and ELB have been previously described.
Our patient's bifid RLN was in fact left-sided. Moreover, this is the first reported case of a RLN with ELB, in combination with a coexisting, contralateral NRLN. We therefore conclude that a single patient can have a non-RLN with a coexisting contralateral nerve demonstrating ELB. To the best of our knowledge, this has not been reported previously. This shows the importance of approaching both sides of the neck equally when considering the possibility of ELB. Notably, however, this differs for NRLNs in that they will only be found on the right side of the neck, unless the patient has situs inversus/dextrocardia. A systematic intraoperative approach to every RLN is of utmost importance, especially in bilateral procedures having already exposed an anomalous nerve on one side. While ELB cannot be reliably predicted, NRLNs are almost always right-sided, and are always present with the readily detectable AL. Neck ultrasound scans are very often routinely performed prior to neck surgery. With this in mind, further ultrasonographic training in the detection of AL (and therefore NRLN) should be considered for ultrasonographers, radiologists and surgeons. Furthermore, radiologists should be encouraged to report AL when present on cross-sectional imaging, given the importance it has for intraoperative NRLN detection. Collectively, this could help to reduce postoperative vocal cord palsy rates following neck surgery.
Learning points.
The anomalies of a non-recurrent laryngeal nerve (NRLN), and a coexisting, contralateral nerve with extralaryngeal branching (ELB), can occur in the same patient.
NLRNs are always associated with arteria lusoria (AL), and are almost always right-sided.
NRLN can be reliably predicted preoperatively, while ELB cannot.
A systematic intraoperative approach to every recurrent laryngeal nerve is essential.
Further training in the preoperative detection of AL could improve both intraoperative NRLN identification, and vocal cord palsy rates.
Acknowledgments
The authors thank Dr Jagrit Shah (Consultant Head and Neck Radiologist, Queen's Medical Centre) for kindly providing the CT vascular reconstruction in figure 1.
Footnotes
Contributors: JDC is the lead author. SB and JAM were involved in identification of case, contributions to discussion and key learning points. JDC and JJA were involved in literature review for discussion, and preparation of case presentation including figures.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Ardito G, Revelli L, D'Alatri L et al. Revisited anatomy of the recurrent laryngeal nerves. Am J Surg 2004;187:249–53. 10.1016/j.amjsurg.2003.11.001 [DOI] [PubMed] [Google Scholar]
- 2.Katz AD. Extralaryngeal division of the recurrent laryngeal nerve. Report of 400 patients and the 721 nerves measured. Am J Surg 1986;152:407–10. 10.1016/0002-9610(86)90313-2 [DOI] [PubMed] [Google Scholar]
- 3.Ling XY, Smoll NR. A systematic review of variations of the recurrent laryngeal nerve. Clin Anat 2016;29:104–10. 10.1002/ca.22613 [DOI] [PubMed] [Google Scholar]
- 4.Nemiroff PM, Katz AD. Extralaryngeal divisions of the recurrent laryngeal nerve: surgical and clinical significance. Am J Surg 1982;144:466–9. 10.1016/0002-9610(82)90425-1 [DOI] [PubMed] [Google Scholar]
- 5.Rustad WH. Revised anatomy of the recurrent laryngeal nerve: surgical importance based on the dissection of 100 cadavers. J Clin Endocrinol Metab 1954;14:87–96. 10.1210/jcem-14-1-87 [DOI] [PubMed] [Google Scholar]
- 6.Serpell JW, Yeung MJ, Grodski S. The motor fibres of the recurrent laryngeal nerve are located in the anterior extralaryngeal branch. Ann Surg 2009;249:648–52. 10.1097/SLA.0b013e31819ed9a4 [DOI] [PubMed] [Google Scholar]
- 7.Beneragama T, Serpell JW. Extralaryngeal bifurcation of the recurrent laryngeal nerve: a common variation. ANZ J Surg 2006;76:928–31. 10.1111/j.1445-2197.2006.03899.x [DOI] [PubMed] [Google Scholar]
- 8.Hisham AN, Lukman MR. Recurrent laryngeal nerve in thyroid surgery: a critical appraisal. ANZ J Surg 2002;72:887–9. 10.1046/j.1445-2197.2002.02578.x [DOI] [PubMed] [Google Scholar]
- 9.Katz AD, Nemiroff P. Anastamosis and bifurcations of the recurrent laryngeal nerve—report of 1177 nerves visualized. Am Surg 1993;59:188–91. [PubMed] [Google Scholar]
- 10.Cannon CR. The anomaly of nonrecurrent laryngeal nerve: Identification and management. Otolaryngol Head Neck Surg 1999;120:769–71. 10.1053/hn.1999.v120.a84675 [DOI] [PubMed] [Google Scholar]
- 11.Henry JF, Audiffret J, Denizot A et al. The nonrecurrent laryngeal nerve: a review of 33 cases, including two on the left side. Surgery 1988;104:977–84. [PubMed] [Google Scholar]
- 12.Raffaelli M, Iacobone M, Henry JF. The “false” nonrecurrent inferior laryngeal nerve. Surgery 2000;128:1082–7. 10.1067/msy.2000.109966 [DOI] [PubMed] [Google Scholar]
- 13.Gray H. Anatomy of the human body. 20th edn Philadelphia: Lea & Febiger, 1918. Fig 473. http://www.bartleby.com/107/135.html (accessed 19.07.16). [Google Scholar]
- 14.Materazzi G, Berti P, Iacconi P et al. Non-recurrent laryngeal nerve predicted before thyroidectomy by preoperative imaging. J Am Coll Surg 2000;191:580 10.1016/S1072-7515(00)00692-X [DOI] [PubMed] [Google Scholar]
- 15.Iacobone M, Viel G, Zanella S et al. The usefulness of preoperative ultrasonographic identification of nonrecurrent inferior laryngeal nerve in neck surgery. Langenbecks Arch Surg 2008;393:633–8. 10.1007/s00423-008-0372-9 [DOI] [PubMed] [Google Scholar]
- 16.Chitkara AE. Neuroanatomy of the larynx. In: Sulica E, Blitzer A, eds. Vocal fold paralysis. Berlin: Springer, 2006:3–16. [Google Scholar]
- 17.King BT, Gregg RL. An anatomical reason for various behaviours of paralyzed vocal cords. Ann Otol Rhinol Laryngol 1948;57:925–44. 10.1177/000348944805700401 [DOI] [PubMed] [Google Scholar]
- 18.Morrison LF. Recurrent laryngeal nerve paralysis; revised conception based on the dissection of one hundred cadavers. Ann Otol Rhinol Laryngol 1952;61:567–92. 10.1177/000348945206100228 [DOI] [PubMed] [Google Scholar]
- 19.Schweizer V, Dörfl J. The anatomy of the inferior laryngeal nerve. Clin Otolaryngol Allied Sci 1997;22:362–9. 10.1046/j.1365-2273.1997.00028.x [DOI] [PubMed] [Google Scholar]