Abstract
Alcohol use disorders are chronically relapsing conditions that pose significant health challenges for our society. Stress is a prevalent trigger of relapse, particularly for women, yet the mechanisms by which alcohol and stress interact, and how this differs between males and females, remain poorly understood. The glutamatergic circuit connecting the basolateral (BLA) and central (CeA) nuclei of the amygdala is a likely locus for such adaptations, yet the impact of alcohol, corticosterone and their interaction on this circuit has been understudied. In particular, no studies have addressed sex differences in these effects or potential differential responses between the lateral and medial subdivisions of the central nucleus. Thus, we assessed the effects of alcohol and corticosterone treatments on BLA-evoked compound glutamatergic responses in medial and lateral CeA neurons from male and female rats. We observed minimal differences between medial and lateral CeA responses to alcohol and corticosterone in male rats, which were primarily sensitive to alcohol-induced inhibition of glutamatergic postsynaptic potentials. Unlike male neurons, cells from female rats displayed reduced sensitivity to alcohol’s inhibitory effects. In addition, female neurons diverged in their sensitivity to corticosterone, with lateral CeA neuronal responses significantly blunted following corticosterone treatment and medial CeA neurons largely unchanged by corticosterone or subsequent co-application of alchol. Together these data highlight striking differences in how male and female amygdala respond to alcohol and the stress hormone corticosterone, factors which may impact differential susceptibility of the sexes to alcohol- and stress-related disorders.
Keywords: sex differences, electrophysiology, ethanol, amygdala, stress
1. Introduction
Alcohol use disorders, which affect 5–10% of the population worldwide (World Health Organization, 2014), are characterized by their chronically relapsing nature. Stress is a primary trigger of alcohol craving and relapse (Seo and Sinha, 2014), and females display stronger relationships between stress, craving in heavy drinkers and relapse in former drinkers than males (Hartwell and Ray, 2013; Heffner et al., 2011). Females are more likely to develop anxiety-related disorders (McLean et al., 2011) and show a stronger relationship between lifetime trauma and alcohol relapse propensity (Heffner et al., 2011). Post-traumatic stress disorder (PTSD) more often precedes the development of alcohol use disorders in females than in males (Sonne et al., 2003), and women with PTSD and alcohol use disorders are more likely to drink alcohol as a stress coping mechanism than men with the same diagnoses (Lehavot et al., 2014). These data suggest females have an enhanced sensitivity to the impact of stress on alcohol use, yet the neural mechanisms underlying this sexual dimorphism remain poorly understood.
The close relationship between stress and alcohol use implicates common underlying neurocircuitry, and the greater susceptibility of females to stress-related disorders suggests enhanced stress-related adaptation of this common circuitry in females, relative to males. One brain region particularly suited to support stress-alcohol interactions is the amygdala, a group of interconnected nuclei centrally involved in reward processing, anxiety-like behavior and aversive conditioning (Aggleton, 2000). In particular, the intra-amygdala circuit connecting the basolateral nucleus (BLA) to the central nucleus (CeA) participates in the neural patterning of behavioral responses and adaptations to both positive and negative reinforcers (Janak and Tye, 2015). This circuit presents an intriguing locus for cross-sensitization to stress and alcohol (ethanol), since both independently alter neuronal activity in the BLA and the CeA. Ethanol acutely potentiated GABAergic responses in both amygdala subdivisions (Roberto et al., 2003; Silberman et al., 2008; Zhu and Lovinger, 2006), but modulation of glutamatergic activity differed by region. Ethanol significantly reduced the amplitude of locally evoked excitatory postsynaptic potentials (EPSPs) in the CeA of ethanol-naïve and chronic ethanol-treated male rats (Roberto et al., 2004), although individual cells varied in the magnitude and direction of ethanol’s effects (Herman et al., 2016). After chronic intermittent ethanol treatment in male rats, postsynaptic alterations were accompanied by increased presynaptic glutamate release in the male rat CeA (Roberto et al., 2004), and one source of this may be the glutamatergic BLA afferents. In support of this, chronic intermittent ethanol exposure increased the amplitude of spontaneous excitatory postsynaptic currents in the BLA of male rats (Lack et al., 2007), an adaptation which could generate the observed increase in glutamate release in the CeA.
Like chronic ethanol, stress enhanced glutamatergic activity in the male mouse BLA, with in vivo stress exposure increasing miniature excitatory postsynaptic current (mEPSC) frequency (Karst et al., 2010) and enhancing long-term potentiation (Sarabdjitsingh et al., 2012). Molecular mimicry of stress-induced HPA axis activation, achieved by acute application of corticosterone to slices, generated similar enhancements of excitability in BLA principal neurons. Corticosterone elevated resting membrane potential and input resistance in BLA neurons from male mice, shifting the population towards slower post-tetanus spike adaptation (Duvarci and Pare, 2007) and increased mEPSC frequency (Karst et al., 2010). Importantly, the BLA neuronal response to a second pulse of corticosterone in stress-naïve mouse slices looked identical to the response to a single corticosterone treatment in slices from mice exposed to stress prior to euthanasia (Karst et al., 2010). Although similar alterations in membrane properties and presynaptic event frequency were not observed in the CeA (Karst et al., 2010), it remains unknown how corticosterone might alter BLA-evoked CeA responses or how female neurons respond to these treatments.
To understand communication between the BLA and CeA, as well as stress and ethanol effects on this communication, it is crucial to consider the architecture of the CeA. Like the amygdala, the CeA can be further reduced into its subdivisions, of which the medial (CeM) and lateral (CeL) subdivisions have long been acknowledged to differ in neuropeptide expression and connectivity (Cassell and Gray, 1989; Cassell et al., 1986). BLA glutamatergic projections terminate on both CeL and CeM GABAergic neurons (Tye et al., 2011), but activating these two CeA subpopulations has opposite effects on CeA output due to the intrinsic circuitry of the CeA, since CeL neurons tonically inhibit CeM neurons (Cassell et al., 1999). Most investigations of functional differences in CeL and CeM neurons have focused on anxiety-like behaviors or stress-induced conditioning. Inhibiting CeL neurons prevented acquisition of fear conditioning in male mice, whereas activation of BLA projections to CeL reduced anxiety-like behavior (Ciocchi et al., 2010; Tye et al., 2011). Conversely, optogenetic activation of BLA projections to CeM generated freezing behavior, while inactivation of CeM neurons blocked expression of fear conditioning in male mice (Ciocchi et al., 2010; Tye et al., 2011). Taken together with the central involvement of CeA neuropeptides in behavioral adaptations accompanying ethanol dependence in male rats (Economidou et al., 2008; Foster et al., 2004; Funk et al., 2006; Gilpin et al., 2011), this intra-amygdala circuit presents a likely locus regulating the interaction between stress and ethanol. Understanding how stress and ethanol similarly, or differentially, regulate the BLA-CeL vs. BLA-CeM circuits is of key importance for understanding how this circuit might adapt to produce stress-related increases in drinking. What remains largely unexplored, however, is whether this circuit differs in its stress- or ethanol-responsiveness in males vs. females.
We set out to elucidate whether sexual dimorphism exists in the glutamatergic circuit connecting the BLA to the CeA, both at baseline and following ethanol or corticosterone treatment. We were particularly interested in whether these effects would differ between CeM and CeL neurons, as well as whether corticosterone alters ethanol’s effects. We hypothesized, based on sexual dimorphism in stress-induced corticosterone release (Rivier, 1993), that female CeA neurons would differ from male neurons in sensitivity to corticosterone. We also hypothesized, based on sex differences in ethanol consumption (Almeida et al., 1998), that ethanol might differentially alter BLA-evoked EPSPs. Finally, we hypothesized that sexual dimorphism in acute ethanol and corticosterone effects would result in sex differences in the interaction between corticosterone and ethanol treatments.
2. Materials and methods
2.1. Animals
Male and female Wistar rats were obtained from Charles River Laboratories (Raleigh, NC, USA), aged approximately 8 weeks old upon arrival. Rats were group housed (2–4 per cage) in Plexiglas cages, with food and water available ad libitum. The temperature- and humidity-controlled housing room was on a 12h:12h light:dark cycle. Following arrival, rats were housed in the facility for a minimum of 1 week prior to experimentation, with recordings performed on slices from 9- to 14-week-old rats. All procedures were approved by The Scripps Research Institute’s Institutional Animal Care and Use Committee, and conform to the National Research Council’s Guide for the Care and Use of Laboratory Animals.
2.2. Estrous cycle synchronization and monitoring
To obtain desired estrous cycles on recording days, for most female subjects, estrous cycles were synchronized using [D-Trp6, Pro9-NEt]-gonadotropin releasing hormone (GnRH), a synthetic analog of GnRH, generously provided by Dr. Jean Rivier of The Salk Institute for Biological Studies. The peptide was dissolved in 0.1 N acetic acid (0.5 μg/μl), then diluted in sterile phosphate buffered saline to a concentration of 10 μg/ml. Two 2-μg doses of the GnRH analog were administered 5 h apart to mimic the onset of proestrus. Because fidelity of this synchronization protocol is imperfect (Ogilvie and Rivier, 1997), vaginal smears were obtained from all female rats under light anesthesia immediately prior to brain collection to confirm estrous cycle. All cycles were confirmed via methylene blue staining.
2.3. Electrophysiology
2.3.1. Slice preparation
To prepare slices for recording, rats were anesthetized with isoflurane and rapidly decapitated, and brains were quickly chilled in an oxygenated (95% O2/5% CO2) icy slurry of high-sucrose solution containing, in mM: 206 sucrose, 2.5 KCl, 0.5 CaCl2, 7 MgCl2, 1.2 NaH2PO4, 26 NaHCO3, 5 glucose, 5 HEPES. Coronal slices of 400 μm thickness were prepared on a vibrating microtome (VT1000S, Leica Biosystems Inc., Buffalo Grove, IL). Slices were transferred to a holding chamber of oxygenated (95% O2/5% CO2) artificial cerebrospinal fluid (aCSF), containing, in mM: 130 NaCl, 3.5 KCl, 1.25 NaH2PO4, 1.5 MgSO4·7 H2O, 2 CaCl2, 24 NaHCO3, 10 glucose. Slices were incubated in an interface configuration for ~15 min, then completely submerged and continuously superfused with aCSF (flow rate 2–4 ml/min). After immersion, slices recovered for at least 1 h prior to recording. This procedure was normally performed during the early portion of the light cycle so that rats would be near the nadir of the corticosterone (CORT) circadian cycle, within 4 h of the start of the light cycle.
2.3.2. Intracellular recording
Using sharp micropipettes, we recorded intracellularly from 126 CeA neurons, with 69 located in the lateral subdivision (CeL) and 57 located in the medial subdivision (CeM). Recording micropipettes (70–90 MΩ) were filled with 3 M KCl and recordings were performed using discontinuous current-clamp mode (Kallupi et al., 2014; Roberto et al., 2003; Roberto et al., 2004), with most neurons held near their resting membrane potentials. Postsynaptic responses were evoked using a bipolar stimulating electrode placed in the neighboring BLA. Compound evoked excitatory postsynaptic potentials (EPSPs) were pharmacologically isolated via addition of the GABAA receptor blocker bicuculline (30 μM) and the GABAB receptor blocker CGP 55845A (1 μM) to the superfused aCSF. Synaptic response parameters were determined for each cell using an input–output (I/O) protocol as described previously (Kallupi et al., 2014; Roberto et al., 2006; Roberto et al., 2004), with 5 stimuli [most were between 0.5 and 6.0 nA; range: 0.032 to 9.9 nA], delivered in succession with an 8-second interstimulus interval. Responses to paired stimuli separated by 50-, 100- and 200-msec intervals were assessed at the stimulus intensity that produced the half maximal amplitude of the EPSP, as determined by the I/O relationship. Hyperpolarizing and depolarizing current steps (200 pA increments, 750 msec duration) were applied to generate current-voltage (I/V) curves, with stability of membrane resistance across the experiment determined from the response to the 200-pA hyperpolarizing voltage step. Data were acquired with an Axoclamp-2A preamplifier and stored for later analysis using pClamp software (Axon Instruments, Foster City, CA). Cells with very low amplitude stimulus responses, defined as less than having a baseline maximal stimulus response at or below 3 mV, were excluded from the analyses; this eliminated 1 neuron (0.79% of all recorded cells).
2.3.3. Drug treatments
CGP 55845A, bicuculline and corticosterone (CORT; 100 nM) were purchased from Sigma Aldrich (St. Louis, MO) and ethanol (44 mM) was purchased from Remet (La Mirada, CA). All drugs were applied through the perfusion solution, with corticosterone experiments performed in the dark due to its light sensitivity. Corticosterone was dissolved in DMSO, then diluted 1:10,000 in aCSF. Stimulus responses at the half maximal intensity were collected every 30 sec throughout drug wash-on periods, averaged across 2-min bins for time course presentation.
2.4. Data analysis
Data were quantified using Clampfit Software (Axon Instruments) and analyzed using Systat and SigmaPlot software (Systat Software Inc., San Jose, CA). In all experiments, cells with greater than 20% shifts in series resistance over the course of the experiment were excluded from the analyses. Maximal treatment effects were calculated as the greatest difference observed over a 4-min period commencing at least 6 min after ethanol wash-on or at least 15 min after CORT addition, relative to an 8-min baseline average. The paired pulse response (PPR) was calculated as the ratio between the second and first EPSPs in response to temporally paired stimuli. Values are expressed as mean ± standard error of the mean (SEM). For each cell, maximal treatment effects and I/O data for the 3 middle intensity stimuli were normalized to average baseline response in the same condition. Normalized data were analyzed with one-sample t-tests to determine whether individual groups’ treatment responses differed from baseline. Raw values were used for statistical analyses by two-way or three-way ANOVA with repeated measures, as indicated.
3. Results
3.1. Male and female CeA neurons display similar baseline properties for BLA-evoked glutamatergic potentials
To investigate sex differences in the BLA-CeA circuit, compound glutamatergic EPSPs were evoked in the BLA and recorded from neurons in the medial (CeM) and lateral (CeL) subdivisions of the CeA, in the presence of bicuculline (30 μM) and CGP 55845A (1 μM). As this is the first investigation into sex differences in CeA neuronal responses, we first compared baseline evoked responses from males and diestrus females (n=90) to ascertain whether a priori sex or amygdala subdivision differences exist in this circuit. Females in the diestrus phase of the estrous cycle at euthanasia were specifically selected for this analysis as that cycle was common to all experiments and thus provided the greatest statistical power and comparable group sizes to the males. As shown in Table 1, I/O and PPR were similar for males and diestrus females in both CeM and CeL neurons. Analyses by two-way ANOVA with repeated measures indicated no significant effects for the between-subjects factor sex, nor interactions between sex and the within-subjects factors stimulus intensity (I/O) or interstimulus interval (PPR) in either subdivision (F’s<1.20, p’s>0.29). Significant main effects of stimulus intensity were observed in all I/O analyses (F’s>82.97, p’s<0.001), independent of sex. Direct testing of CeA subdivision differences in I/O responses using 3-way ANOVA with repeated measures yielded main effects of CeA subdivision (F1,86=4.84, p<0.05) and stimulus intensity (F4,344=216.69, p<0.001), as well as an interaction between those factors (F4,344=3.00, p<0.05). Although no significant main effects or interactions were observed for the factor sex, the effect of CeA subdivision was mainly supported by differences in CeL vs. CeM I/O responses in male neurons. Analysis of male neurons’ I/O responses by two-way ANOVA with repeated measures showed a main effect of stimulus intensity (F4,168=128.47, p<0.001), as well as an interaction between the stimulus intensity and CeA subdivision (F4,168>2.50, p<0.05), whereas similar analysis of female neurons’ I/O responses showed no significant effects or interactions for CeA subdivision. Unlike I/O, interrogation of CeA subdivision effects on PPR by 3-way ANOVA with repeated measures generated no significant main effects or interactions (F’s<2.89, p’s>0.05).
TABLE 1.
Baseline properties of compound glutamatergic responses in male and diestrus female medial and lateral central amygdala neurons across experiments.
| CeL | CeM | |||
|---|---|---|---|---|
|
|
||||
| Measure | Male | Female | Male | Female |
| Input/Output | N = 24 | N = 25 | N = 20 | N = 21 |
| Stimulus 1 | 4.08 ± 0.35 | 4.07 ± 0.50 | 2.90 ± 0.25 | 4.20 ± 0.39 |
| Stimulus 2 | 5.30 ± 0.37 | 6.15 ± 0.59 | 4.89 ± 0.42 | 5.25 ± 0.40 |
| Stimulus 3 | 7.27 ± 0.49 | 8.05 ± 0.67 | 6.54 ± 0.57 | 6.60 ± 0.53 |
| Stimulus 4 | 9.12 ± 0.62 | 9.62 ± 0.70 | 7.87 ± 0.67 | 8.28 ± 0.71 |
| Stimulus 5 | 11.58 ± 0.66 | 11.52 ± 0.90 | 9.17 ± 0.78 | 9.74 ± 0.86 |
| Paired-Pulse Ratio | N = 22 | N = 23 | N = 15 | N = 15 |
| 50 msec ISI | 1.14 ± 0.14 | 1.14 ± 0.16 | 1.36 ± 0.10 | 1.33 ± 0.14 |
| 100 msec ISI | 1.16 ± 0.08 | 1.03 ± 0.11 | 1.35 ± 0.09 | 1.09 ± 0.08 |
| 200 msec ISI | 1.14 ± 0.07 | 1.02 ± 0.08 | 1.18 ± 0.07 | 1.12 ± 0.10 |
To confirm that the lack of sexual dimorphism in baseline properties did not result from exclusion of estrous cycles, all female neurons included in the study were analyzed by two-way ANOVA with repeated measures. No significant impact of cycle on I/O responses was observed in either CeL neurons (cycle: F2,40=0.60, p=0.56; cycle x stimulus intensity, F8,160=1.30, p=0.25) or CeM neurons (cycle: F2,27=0.21, p=0.81; cycle x stimulus intensity, F8,108=0.46, p=0.88). Unlike I/O, differential cycle effects by CeA subdivision were observed for PPR. A significant interaction was found between cycle and interstimulus interval for CeM neurons (F4,34=3.86, p<0.05), whereas no significant cycle effects or interactions were observed in CeL neurons (F’s<0.56, p’s>0.69). Together, these data demonstrate that male and female CeA neurons do not display marked baseline differences in stimulus-response properties, although estrous cycle may modulate presynaptic release properties in the medial, but not lateral, CeA.
3.2. Sexual dimorphism in acute ethanol responses of CeA neurons
Previously we showed that acute ethanol treatment reduced the amplitude of locally evoked EPSPs in male CeM neurons (Roberto et al., 2004). Here we asked whether these effects would be reproduced with BLA stimulation, whether ethanol would similarly alter male CeL activity, and whether female neurons would be impacted by ethanol treatment similarly to male neurons. To address these gaps in knowledge, BLA-evoked compound glutamatergic responses were recorded before and during ethanol superfusion (44 mM, 6–12 min).
3.2.1. Acute ethanol treatment of CeL neurons
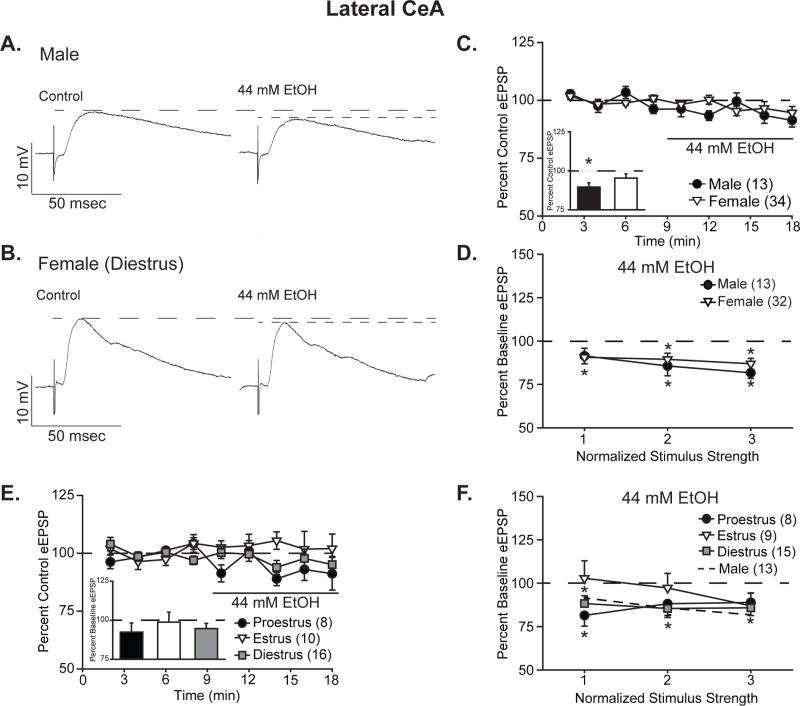
Ethanol reduced BLA-evoked EPSP amplitude in CeL neurons, as depicted in the representative traces from male (Fig. 1A) and female (Fig. 1B) neurons. Specifically, EPSPs (half-maximal intensity stimulation) were significantly reduced over the period of treatment, depicted in the time course (Fig. 1C), to 89.6 ± 2.7% of control in male CeL neurons (one-sample t-test, t12=−3.79, p<0.01), whereas in female CeL neurons, reduction to 95.4 ± 2.8% of control was not significant (one-sample t-test, t33=−1.69, p=0.10). Analysis by two-way ANOVA with repeated measures yielded a significant main effect of ethanol treatment (F1,45=11.46, p<0.01), but no main effect of sex or interaction between the factors (F’s<1.92, p’s>0.17). I/O curves displayed similar effects (Fig. 1D), with ethanol inhibition to 91.4 ± 4.5% to 81.7 ± 3.3% of control for males and 90.7 ± 3.8% to 86.9 ± 3.1% of control for females (t’s<−2.44 p’s<0.05 for male stimuli 2 and 3 and all female stimuli by one-sample t-test). Analysis by three-way ANOVA with repeated measures yielded main effects of treatment (F1,43=32.81, p<0.001) and stimulus intensity (F2,86=84.28, p<0.001), as well as an interaction between these factors (F2,86=8.52, p<0.001), without significant main effect or interactions for sex (F’s<1.11, p’s>0.33). Together these data show significant ethanol-induced reduction in CeL eEPSPs in both males and females, with possibly greater amplitude effects in males.
Figure 1. Ethanol acutely reduced the amplitude of BLA-evoked compound glutamatergic EPSPs in the lateral CeA.
A,B. Representative evoked glutamatergic-EPSPs (eEPSPs) in CeL, at baseline (Control) and during 44 mM ethanol (EtOH) superfusion onto slices obtained from male (A) and diestrus female (B) rats. C. Time course of treatment effects, with ethanol application following an 8-min baseline. Inset shows quantification of the peak alcohol-induced change in eEPSPs over a 4-minute bin beginning not less than 6 min into ethanol application in males vs. all females. Histograms depict mean ± standard error percent change in eEPSPs relative to control. D. Quantification of ethanol’s effect on I/O responses to the 3 middle intensity stimuli in males vs. all females. Data are depicted as mean ± standard error percent change in eEPSP after ethanol treatment, normalized to control. E. Time course of treatment effects by estrous cycle, with ethanol application following an 8-min baseline. No significant effects were observed in females based on estrous cycle status at the time of euthanasia. Histograms (inset) depict mean ± standard error percent changes in peak alcohol effect relative to control. F. Estrous cycle impacts stimulus responsiveness as measured by the I/O relationship at 3 stimuli of increasing intensity. Data are depicted as mean ± standard error percent change in eEPSP relative to control. n’s = 8–34, as listed on each panel of the graph; cells becoming unstable after drug wash-on were excluded from I/O analyses. *p<0.05 relative to control (one-sample t-test).
For statistical comparison to male data, female cells were considered as a single entity; however, estrogen itself as well as the estrous cycle of the female alter neuronal activity in multiple brain regions (Grassi et al., 2012; Schiess et al., 1988; Smejkalova and Woolley, 2010). To determine the impact of estrous cycle on ethanol’s modulation of CeL EPSPs (Fig. 1E) and I/O relationships (Fig. 1F), female data were analyzed with the inclusion of estrous cycle as a between-subjects factor. Ethanol minimally reduced EPSPs over the treatment time course (half-maximal intensity stimulation, Fig. 1E), so that no individual cycle was significantly changed relative to control, as assessed by one-sample t-tests (proestrus: 92.4 ± 5.9% of control, t7=−1.29; estrus: 98.8 ± 6.3% of control, t9=−0.19; diestrus: 94.7 ± 3.4% of control, t15=−1.58; all p’s>0.13). Nonetheless, two-way ANOVA with repeated measures yielded a trend towards a treatment effect (F1,31=3.70, p=0.06), without significant effect of estrous cycle or interaction between cycle and treatment (F’s<0.48, p’s>0.62). I/O curves displayed both larger amplitude effects and greater variability in response by cycle (Fig. 1F), with a maximal reduction by ethanol to 81.4 ± 6.1% of control observed during proestrus, whereas a mild increase to 102.9 ± 10.1% of control was observed at one stimulus intensity during estrus. Individual stimulus comparisons to control via one-sample t-test indicate significant reductions for all stimulus intensities during diestrus and for the lowest stimulus intensity in proestrus (t’s<−3.01, p’s<0.05), whereas no significance was observed for estrus or the higher stimulus intensities in proestrus (t’s>−2.02, p’s>0.08). As demonstrated for EPSP magnitude, I/O analysis via three-way ANOVA with repeated measures demonstrated a significant main effect of treatment (F1,29=17.96, p<0.001), as well as a main effect of stimulus intensity (F2,58=64.59, p<0.001) and an interaction between the two factors (F2,58=3.29, p<0.05). No main effect or interactions were observed for estrous cycle (F’s<1.46, p’s>0.22). Together these data indicate that in the CeL, the primary difference in sensitivity to acute ethanol’s effects is between males and females, but that there may be mild differences in ethanol responsiveness across the phases of the estrous cycle as well.
3.2.2 Acute ethanol treatment of CeM neurons
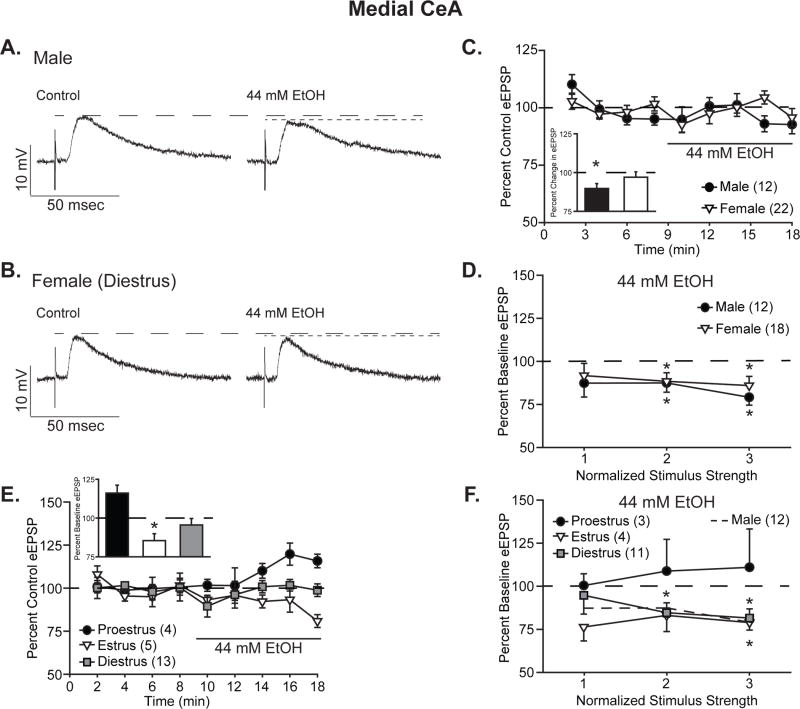
As in CeL neurons, representative traces (Fig. 2A–B) show that ethanol reduced BLA-evoked EPSP amplitude in CeM neurons over the course of drug application (Fig. 2C). Quantification of these effects at half maximal intensity stimulation (Fig. 2C, inset) showed significant reduction in EPSPs following ethanol treatment in male CeM neurons, to 89.7 ± 3.2% of control (one-sample t-test, t11=−3.24, p<0.05), but not in female CeM neurons, at 97.0 ± 3.5% of control (one-sample t-test, t21=−0.87, p=0.40). Analysis by two-way ANOVA with repeated measures yielded a main effect of ethanol treatment (F1,32=4.96, p<0.05) as well as trend towards an interaction between sex and treatment (F1,32=3.22, p=0.09). Examination of I/O data by three-way ANOVA with repeated measures (Fig. 2D) generated significant main effects of treatment (F1,28=19.22, p<0.001) and stimulus intensity (F2,56=39.66, p<0.001), but no main effect of sex or interactions between variables (F’s<1.29, p’s>0.26). Closer examination of CeM I/O data, relative to their respective baselines, showed slightly greater ethanol inhibition to 87.4% ± 8.1% to 79.2 ± 4.6% of control for males and 94.7 ± 6.9% to 88.4 ± 5.3% of control for females. For both males and females, responses to the two higher stimulus intensities were statistically significant relative to control (t’s<−2.32, p’s<0.05), whereas responses to the lowest stimulus intensity were not (t’s<‘−1.52, p’s>0.14). Together these data show that ethanol reduces CeM EPSPs in both males and females, with possibly greater amplitude effects in males.
Figure 2. Ethanol acutely reduced the amplitude of BLA-evoked compound glutamatergic EPSPs in the medial CeA, particularly in males.
A,B. Representative evoked glutamatergic-EPSPs (eEPSPs) in CeM, at baseline (Control) and during 44 mM ethanol (EtOH) superfusion onto slices obtained from male (A) and diestrus female (B) rats. C. Time course of treatment effects, with ethanol application following an 8-min baseline. Inset shows quantification of the peak ethanol-induced change in eEPSP over a 4-minute bin beginning not less than 6 min into ethanol application in males vs. all females. Data are depicted as mean ± standard error percent change in eEPSP relative to control. D. Quantification of ethanol’s effect on I/O responses to 3 intermediate intensity stimuli in males vs. all females. Data are depicted as mean ± standard error percent change in eEPSP after ethanol application, normalized to control. E. Time course of treatment effects by estrous cycle, with ethanol superfusion ollowing an 8-min baseline. Estrous cycle affects ethanol’s modulation of peak eEPSP amplitude. Histograms depict mean ± standard error percent changes in peak ethanol effect relative to control. F. Estrous cycle impacts CeM neurons’ stimulus responsiveness, as measured by the I/O relationship at 3 intermediate intensity stimuli. Data are depicted as mean ± standard error percent change in eEPSP relative to control. n’s = 3–22, as listed on each panel of the graph; cells becoming unstable after drug wash-on were excluded from I/O analyses. *p<0.05 relative to control (one-sample t-test).
Subdividing the female CeM data by estrous cycle revealed a striking difference in ethanol’s effects on EPSP amplitude according to estrous cycle, as shown in the treatment time course (Fig. 2E). Analysis of ethanol superfusion effects at half-maximal stimulus intensity (Fig. 2E, inset) via two-way ANOVA with repeated measures yielded a significant interaction between estrous cycle phase and treatment (F2,19=9.10, p<0.01). Post hoc analyses using Tukey’s test revealed significant differences between baseline and ethanol treatment during both proestrus (p<0.01) and estrus (p<0.05), but not diestrus (p=0.46). Particularly noteworthy is the increase in EPSPs following ethanol treatment of proestrus neurons, to 116.1 ± 5.1% of control (one-sample t-test, t3=3.17, p=0.05), as compared to no effect during diestrus, 94.7 ± 3.4% of control (p=0.14), and reduction to 85.4 ± 4.4% of control during estrous (one-sample t-test, t4=−3.29, p<0.05). Cyclic variability was also observed in I/O relationships (Fig. 2F): cells from proestrus females showed nonsignificant EPSP increases during ethanol treatment, ranging from 100.6 ± 6.9 to 111.1 ± 22.3% of control, whereas cells from diestrus and estrus females showed ethanol-induced reductions or no change in EPSP amplitude (diestrus: 94.9 ± 111.0% to 81.6 ± 5.3% of control, estrus: 83.2 ± 9.4% to 76.4 ± 8.1% of control; t’s<−2.95, p’s<0.05 for diestrus intensities 2 and 3 and estrus intensity 3). Despite this cyclic variability, analysis of I/O data by three-way ANOVA with repeated measures yielded main effects of treatment (F1,15=4.71, p<0.05) and stimulus intensity (F2,30=27.51, p<0.001), with no effect of cycle or interactions between any factors. Together these data suggest that male CeM neurons are more sensitive to ethanol’s inhibitory effects on BLA-evoked compound glutamatergic potentials than female neurons and that estrous cycle at the time of slice collection plays a significant role in female CeM neurons’ ethanol responses.
3.3. Ethanol modulation of BLA-evoked CeA EPSPs does not alter paired pulse facilitation
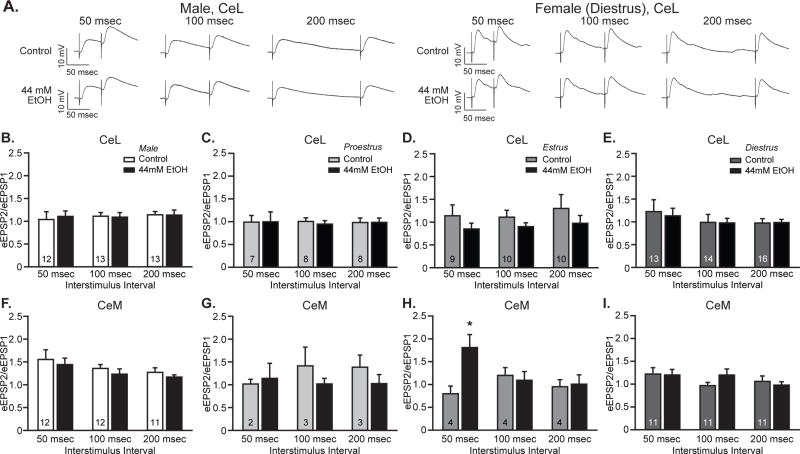
To investigate the presynaptic component of the observed reductions in EPSPs following acute ethanol treatment in the CeA, neuronal responses to temporally paired stimuli delivered 50, 100 and 200 msec apart were assessed. In the CeL, both male (Fig. 3A, left, B) and female (Fig. 3A, right, C-E) neurons displayed no significant difference in paired pulse ratios (PPRs) between control and ethanol treatment conditions at any interstimulus interval or estrous cycle tested (F’s<3.33, p’s>0.11), as measured by within-sex and within-cycle two-way ANOVA with repeated measures. Additional three-way ANOVA analyses to investigate the impact of sex on the relationship between treatment and interstimulus interval generated no main effect of sex (F1,37=0.59, p=0.45) or treatment (F1,37=0.67, p=0.42) and no interactions between sex, treatment and interstimulus interval (F’s<0.89, p’s>0.35). Estrous cycle also did not significantly affect PPR in CeL neurons, demonstrated by the lack of either main effects of cycle (F2,25=0.03, p=0.97) and treatment (F1,25=2.80, p=0.11) or any interactions between cycle, treatment and interstimulus interval (F’s<1.05, p’s>0.37). Thus, neither sex nor estrous cycle generated significant ethanol-related changes in CeL PPR, indicative of a postsynaptic locus of neuroadaptation. Analyses for male (Fig. 3F) and female (Fig. 3G–I) CeM neurons produced similar but distinct results. Three-way ANOVA with repeated measures yielded significant main effects of sex (F1,25=5.04, p<0.05) and interstimulus interval (F2,50=3.61, p<0.05), without other significant main effects or interactions (F’s<2.33, p’s>0.14). When analyzed by within-sex two-way repeated measures ANOVA, no significant main effects or interactions were observed for male or female neurons (F’s<2.08, p’s>0.14). Further investigation of estrous cycle effects in female CeM cells yielded a three-way interaction between interstimulus interval, ethanol treatment and estrous cycle (F4,26=5.26, p<0.01). Follow-up analyses at each interstimulus interval revealed significant facilitation by ethanol during estrus for the 50 msec interstimulus interval (Fig. 3H; F1,3=16.20, p<0.05) and a trend for facilitation by ethanol for the 100 msec interstimulus interval during diestrus (Fig. 3I; F1,10=4.60, p=0.06), without any additional ethanol treatment effects for other cycle phases or interstimulus intervals. Together these CeM results indicate that ethanol’s effects are primarily postsynaptic, although presynaptic changes might occur in females during various phases of the estrous cycle.
Figure 3. Ethanol did not significantly alter paired pulse ratios in lateral or medial CeA neurons of either sex.
A. Representative traces of paired pulse EPSPs evoked by two pulses delivered 50 msec (left), 100 msec (center) or 200 msec (right) apart, assessed at baseline (Control) and during superfusion of 44 mM ethanol (EtOH) onto slices from male and female rats. B-I. Quantification of the relationship between peak responses to the paired stimuli (paired pulse ratio), calculated as the ratio between the second stimulus response (eEPSP2) and the first stimulus responses (eEPSP1). Histograms depict mean ± standard error paired pulse ratios for CeL male (B) and proestrus (C), estrus (D), and diestrus females (E) and CeM male (F) and proestrus (G) estrus (H), and diestrus females (I) cells. n’s = 10–16, as listed on the graph panels.
3.4. Corticosterone has sexually dimorphic effects on CeA neurons
To simulate changes observed following stress exposure in vitro, we utilized corticosterone application to reproduce one molecular component of the stress response, thereby partially mimicking neuronal responses during a stressful encounter. To minimize activation of the endogenous corticosteroid system at the time of euthanasia, slices were prepared near the nadir of the circadian corticosteroid cycle. All female slices were collected during diestrus, the most representative phase of the estrous cycle, comprising 2–3 days of the 4–5-day cycle, or more than 50% of the female rat’s life. Diestrus is characterized by low progesterone levels throughout and low estrogen levels for most of the interval (Freeman, 2006). This allowed us to focus on corticosterone effects due to sex differences rather than cyclic hormone-specific effects.
3.4.1. Corticosterone occludes ethanol effects in female but not male CeL neurons
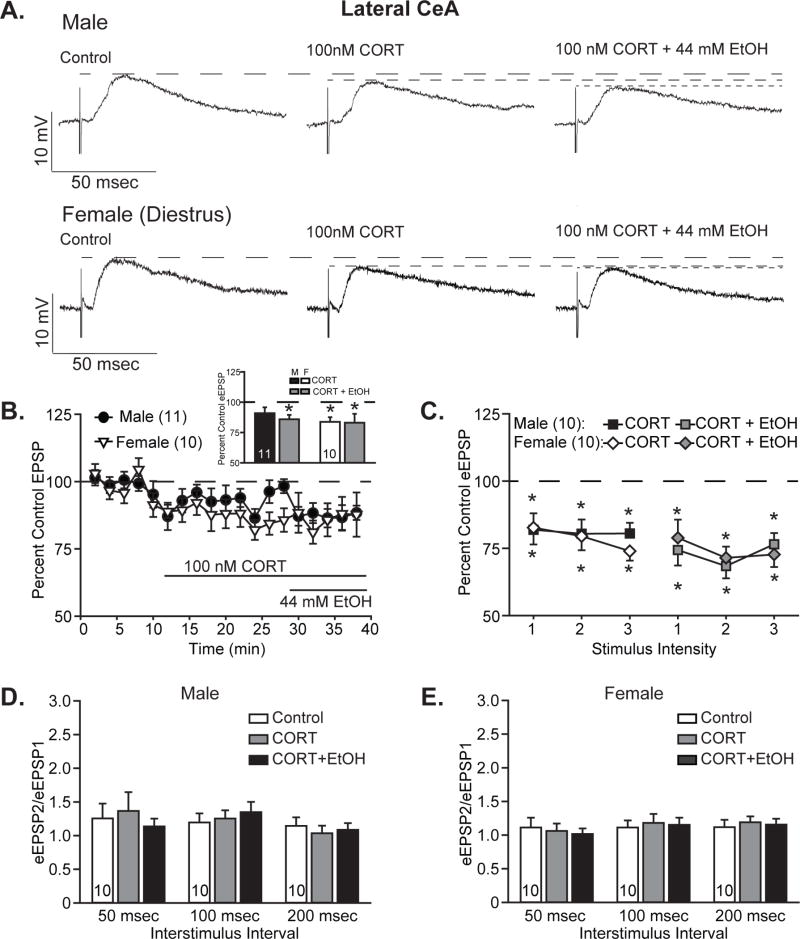
To assess the impact of corticosterone pretreatment on subsequent acute responses to ethanol, 100 nM corticosterone (CORT) was superfused onto slices for at least 15 min prior to co-application of 44 mM ethanol. Corticosterone alone did not reduce EPSPs in CeL neurons from males (representative traces, Fig. 4A, top), but did reduce EPSPs in female CeL neurons (representative traces, Fig. 4A, bottom). Maximal changes in EPSP amplitudes at half-maximal stimulation intensity over the course of drug wash-on (Fig. 4B) were significantly reduced to 83.8 ± 3.8% of control in female CeL neurons (one-sample t-test, t9=−4.28, p<0.01), whereas in male CeL neurons, EPSP reductions to 91.0 ± 4.9% of control were not statistically significant (one-sample t-test, t10=−1.86, p=0.09). Subsequent addition of ethanol in the presence of CORT additionally reduced male CeL EPSPs to 85.9% ± 3.6% of control, a significant change from the pretreatment baseline (one-sample t-test, t10=−3.91, p<0.01), while females remained at the same reduced EPSP magnitude observed after CORT treatment, 83.1± 7.3% of control (one-sample t-test vs. control, t9=−2.31, p<0.01). Evaluation of within-sex treatment effects by one-way ANOVA with repeated measures yielded significant main effects of treatment for both males (F2,20=5.87, p<0.05) and females (F2,18=8.40, p<0.01). However, post hoc analyses by Tukey’s test demonstrated that whereas for female CeL cells both CORT and CORT plus ethanol were significantly lower than untreated control, for male CeL cells only CORT plus ethanol was significantly reduced. Because of the similar direction of effects in both sexes, analysis by two-way ANOVA with repeated measures only yielded a main effect of treatment (F2,38=14.19, p<0.001) without main effect of sex or sex by treatment interaction, despite the differential CORT and ethanol responsiveness observed in male and female CeL cells.
Figure 4. Corticosterone acutely reduced lateral CeA EPSPs and occluded further ethanol effects in females, while males responded more to ethanol.
A. Representative evoked glutamatergic-EPSPs (eEPSPs) in CeL, at baseline (Control) and during superfusion of 100 nM corticosterone (CORT) and subsequent co-application of 44 mM ethanol (EtOH) onto slices obtained from male (top) and diestrus female (bottom) rats. B. Time course of treatment effects, with CORT application following an 8-min baseline and alcohol co-application beginning 20 min after CORT application. Inset shows quantification of the peak CORT- and ethanol-induced changes in eEPSPs over a 4-minute bin beginning not less than 15 min into CORT treatment and not less than 6 min into ethanol co-application. Histograms depict mean ± standard error percent change in eEPSP relative to control. C. Quantification of CORT and ethanol effects on I/O responses to 3 intermediate intensity stimuli in males (squares) vs. diestrus females (diamonds). Data are depicted as mean ± standard error percent change in eEPSP after CORT treatment (black and white shapes) and subsequent ethanol co-application (gray shapes), normalized to control. D–E. Histograms depict mean ± standard error paired pulse ratios for male (D) and female (E) cells. n’s = 9–11, as listed on the graph panels; cells becoming unstable after drug wash-on were excluded from I/O analyses. *p<0.05 relative to control (one-sample t-test).
CORT’s impact on I/O relationships (Fig. 4C) demonstrated a slightly different pattern than observed during drug wash-on, as males displayed greater sensitivity to CORT’s effects, with EPSPs decreased to 81.9 ± 5.5% to 80.4 ± 5.3% of control across the 3 stimuli, all of which were significant reductions (t’s<−3.32, p’s<0.05, one-sample t-tests vs. control). The addition of ethanol decreased EPSPs by an additional 4 to 12%, resulting in significant cumulative decreases to 76.5 ± 4.1 to 68.4 ± 4.5% of control (t’s<−4.43, p’s<0.01). Analysis of male I/O data by two-way ANOVA with repeated measures yielded main effects of stimulus intensity (F2,18=31.67, p<0.001) and treatment (F2,18 =16.03, p<0.001), as well as an interaction between the factors (F4,36=3.15, p<0.05). Post hoc analyses by Tukey’s test demonstrated that whereas the lowest stimulus intensity only significantly differed from control in the presence of CORT and ethanol (p<0.001), both CORT alone (p’s<0.05) and CORT plus ethanol (p’s<0.001) differed from control for the stronger stimuli. CORT with ethanol did not differ significantly from CORT alone for any of the stimulus intensities in the male cells (p’s>0.07). As observed during drug wash-on, female neurons displayed a greater reduction following CORT treatment than male neurons, to 82.6 ± 5.3% to 74.0 ± 3.6% of control (t’s<−3.25, p’s<0.05, one-sample t-tests). Co-application of ethanol marginally enhanced CORT’s effects by 3 to 8% (total combined treatment effect, 78.9 ± 6.8% to 71.4 ± 4.2% of control), but this was not significantly different from CORT treatment alone. Analysis by two-way ANOVA with repeated measures yielded main effects of stimulus intensity (F2,18=15.23, p<0.001) and treatment (F2,18=23.10, p<0.001), with only a trend towards interaction between the factors (F4,36=2.32, p=0.08). Post hoc analyses by Tukey’s test confirmed that both CORT and CORT plus ethanol significantly reduced EPSP magnitude relative to control (p<0.001), whereas CORT treatment did not differ from CORT plus ethanol treatment (p=0.63). Comparison of male and female treatment effects by three-way ANOVA with repeated measures confirmed the similar patterns observed across the sexes, with main effects of treatment (F2,36=37.66, p<0.001) and stimulus intensity (F2,36=39.95, p<0.001), as well as an interaction between the factors (F4,72=4.71, p<0.01), but no main effects or interactions for the factor sex.
As observed for acute ethanol treatment, no significant changes were found in PPRs for male (Fig. 4D) or female (Fig. 4E) neurons following CORT treatment alone or upon co-application of ethanol. This suggests a postsynaptic locus for the observed reductions in EPSPs.
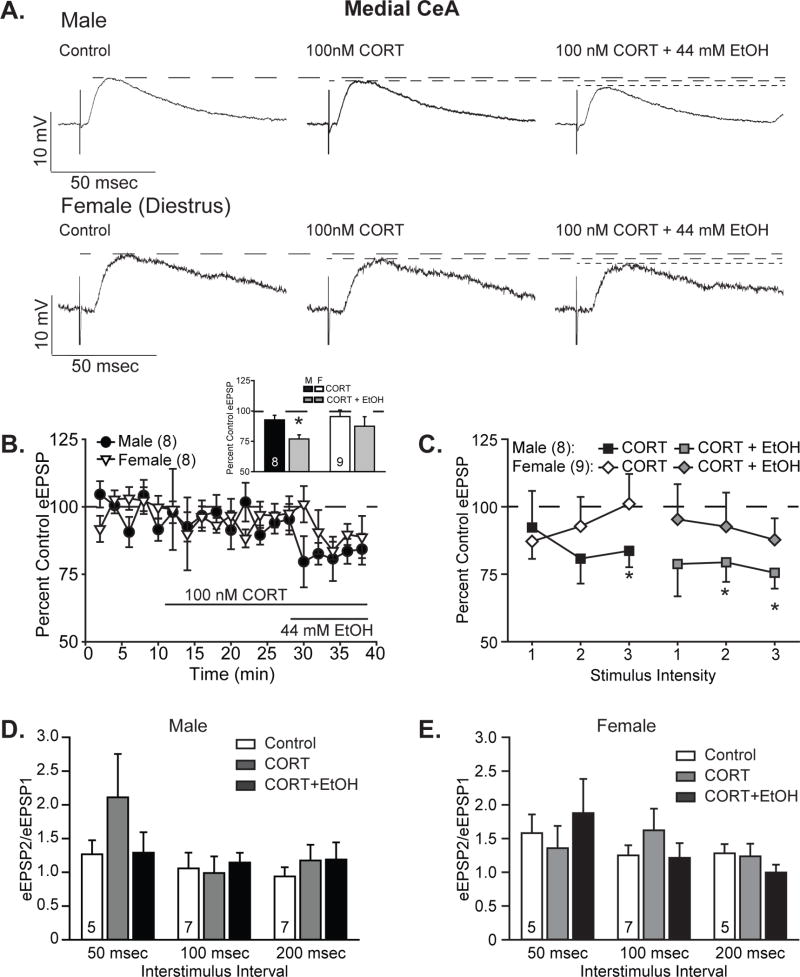
3.4.2. Female, but not male, CeM neurons are insensitive to corticosterone and ethanol
To assess the impact of acute corticosterone and subsequent response to ethanol co-application, EPSPs were recorded in CeM neurons as described above for CeL neurons. As shown in the representative traces in Figure 5A, male (Fig. 5A, top) and female (Fig. 5A, bottom) CeM neurons showed small changes in EPSP magnitude following acute corticosterone treatment at the half maximal intensity stimulation. The maximal reduction in EPSP magnitude during corticosterone wash-on, as quantified in Figure 5B (inset), was 92.7 ± 3.7% of control in male CeM neurons (one-sample t-test, t7=−1.96, p=0.09) and 95.4 ± 5.5% of control in female CeM neurons (one-sample t-test, t8=−0.85, p=0.42). Following co-application of ethanol, male CeM neurons displayed a significant reduction in EPSP amplitude to 77.0 ± 3.3% of control (one-sample t-test, t7=−6.92, p<0.001), whereas female CeM neurons continued to show no significant reductions relative to baseline responses, at 87.5 ± 7.8% of control (one-sample t-test, t8=−1.61, p=0.15). Comparison of drug treatments within sex by one-way ANOVA with repeated measures yielded a significant main effect of treatment in males (F2,14=15.70, p<0.001) but only a trend in females (F2,16=3.47, p=0.06). Post hoc Tukey’s test analyses demonstrated significant differences between drug co-application and both baseline and CORT treatment alone in males. Despite these differences in response patterns between the sexes, statistical analysis of sex differences by two-way ANOVA with repeated measures produced only a main effect of treatment (F2,30=14.00, p<0.001), but no main effects or interactions for the factor sex. Tukey post hoc tests showed significant differences between CORT/ethanol co-application and both control and CORT-only conditions.
Figure 5. Corticosterone minimally affects medial CeA responses but blocks ethanol’s effects in females, but not males.
A. Representative evoked glutamatergic-EPSPs (eEPSPs) in CeM at baseline (Control) and during superfusion of 100 nM corticosterone (CORT) and subsequent co-application of 44 mM ethanol (EtOH) onto slices obtained from male (top) and diestrus female (bottom) rats. B. Time course of treatment effects, with CORT treatment following an 8-min baseline and alcohol co-application beginning 20 min after CORT application. Inset shows quantification of the peak CORT and ethanol-induced changes in eEPSPs over a 4-minute bin beginning not less than 15 min into CORT treatment and not less than 6 min into ethanol co-application. Histograms depict mean ± standard error percent change in eEPSP relative to control. C. Quantification of CORT and ethanol effects on I/O responses to 3 intermediate intensity stimuli in males (squares) vs. diestrus females (diamonds). Data are depicted as mean ± standard error percent change in eEPSP after CORT treatment (black and white shapes) and subsequent ethanol co-application (gray shapes), normalized to control. D–E. Histograms depict mean ± standard error paired pulse ratios for male (D) and female (E) cells. n’s = 5–9, as listed on the graph panels; cells becoming unstable after drug wash-on were excluded from I/O analyses, and one female cell with incomplete data was omitted from the time course. *p<0.05 relative to control (one-sample t-test).
CORT modulation of I/O relationships (Fig. 5C) displayed a very similar pattern, although males showed slightly greater reductions in EPSPs following CORT treatment, to 92.3 ± 13.5% to 80.7 ± 9.2% of control across the 3 stimuli, yielding a significant reduction from control only for the highest stimulation intensity (t7=−2.67, p<0.05, one-sample t-test). Subsequent co-application of ethanol reduced EPSP magnitudes to 79.4 ± 7.2% to 75.5 ± 5.9% of control, yielding significant reductions relative to control for the middle and high intensity stimuli (t’s<−2.85, p’s<0.05, one-sample t-tests). Analysis of these data by two-way ANOVA with repeated measures produced significant main effects of stimulus intensity (F2,14=12.67, p<0.001) and treatment (F2,14=6.23, p<0.05), without interaction between the factors. Post hoc analyses by Tukey’s tests demonstrated significant differences between control and both CORT alone and CORT/ethanol co-application, but no significant differences between the two drug treatment conditions. Unlike the males, females displayed minimal sensitivity to either CORT or ethanol modulation of EPSP amplitude, similar to what was observed during drug wash-on, with responses ranging from 101.1 ± 11.1% to 87.2 ± 6.5% of control in the presence of CORT alone, and from 95.3 ± 13.0 to 87.8 ± 7.9% of control following co-application of ethanol. Analysis of these effects relative to the control condition by one-sample t-test indicated no significant changes in I/O following either application of CORT alone or co-application of ethanol (t’s>−1.97, p’s>0.08). Comparison of CORT and ethanol effects in female CeM neurons across all stimulus intensities by two-way ANOVA with repeated measures yielded a significant main effects of stimulus intensity (F2,16=7.07, p<0.05) but no main effect of treatment or interaction between the factors (F’s<2.70, p’s>0.09). Direct investigation of sex differences by three-way ANOVA with repeated measures yielded main effects of treatment (F2,30=9.32, p<0.01) and stimulus intensity (F2,30=15.60, p<0.001) with no main effect of sex or interactions between any factors.
As seen for acute ethanol treatment in all neurons and CORT/ethanol treatments in CeL neurons, no significant changes were observed in paired pulse ratios from male (Fig. 5D) or female (Fig. 5E) CeM neurons following either CORT treatment alone or CORT plus ethanol treatment. These data continue to support a postsynaptic locus for the observed effects of CORT and ethanol treatments.
4. Discussion
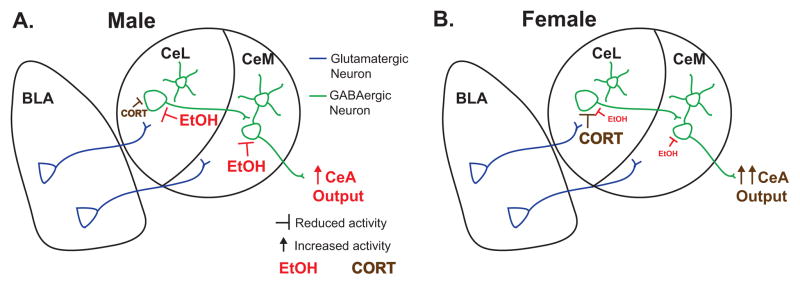
Here we demonstrate intrinsic sexual dimorphism in both ethanol and corticosterone modulation of CeA neuronal responses to stimulated glutamate release from BLA inputs. To our knowledge, this is the first study to investigate sex differences in CeA activity, as well as the first examination of CeA subdivision differences in acute ethanol and corticosterone modulation of BLA-evoked glutamatergic activity. As a region proposed to integrate ethanol and stress effects, these findings are central to understanding CeA function. Importantly, with the exception of ethanol effects in CeM during proestrus, the differences observed between males and females affected the sensitivity to drug effects, rather than the direction of change in neuronal activity. As depicted in the schematic of Fig. 6, acute ethanol effects were reduced and CORT effects were increased in the female CeL, relative to male neuronal responses, while female CeM neurons had reduced or absent responses to both treatments. Thus, male CeM and CeL neurons showed similar responses across the treatments, with ethanol, not CORT, primarily responsible for significantly reducing eEPSPs. Conversely, female CeM and CeL neurons displayed qualitatively different responsivity to acute ethanol, CORT and their co-application, with CeL neurons significantly inhibited by CORT alone, occluding any further efficacy of ethanol, whereas female CeM neurons showed minimal sensitivity to either CORT or ethanol. Despite these sexually dimorphic findings, since treatments reduced EPSPs relative to control in both sexes, we did not observe significant main effects of sex in our analyses. Nonetheless, we demonstrate sex differences in drug sensitivity, with greater responsivity of male CeL and CeM neurons to ethanol as well as enhanced responsivity of female CeL neurons to CORT, which occluded any subsequent response to ethanol treatment. Together these data support the hypothesis that males and females display differential neuronal responsivity to ethanol and CORT, which may underlie behavioral differences in alcohol- and stress-related behaviors between the sexes.
Figure 6. Schematic representation of observed sex differences.
Ethanol (EtOH) and corticosterone (CORT) differentially modulate male (A) and female (B) lateral (CeL) and medial (CeM) central amygdala synaptic responses to basolateral amygdala (BLA) stimulation. (A) In males, EtOH reduces EPSPs, whether the sole drug treatment or following CORT treatment, in both CeL and CeM, while CORT has a reduced effect on eEPSPs relative to EtOH and to CORT’s effects in females. (B) In females, CORT significantly reduces CeL, but not CeM, eEPSPs, whereas EtOH has a reduced ability to inhibit eEPSPs in females vs. males that is blocked in the presence of CORT.
Female rodents have long been known to release greater levels of CORT in response to behavioral stressors, including acute ethanol exposure (Rivier, 1993), and to have higher amplitude circadian fluctuations in CORT (Atkinson and Waddell, 1997), which might be expected to result in adaptative blunting of neuronal responses to CORT. Contrary to this hypothesis, corticosterone more effectively reduced BLA-evoked EPSPs in female vs. male CeL neurons. The corticosterone procedure utilized for our studies would more likely function though mineralocorticoid (MR), rather than glucocorticoid (GR), receptors, based on previous CORT slice treatment experiments in the basolateral amygdala, using the same dose and temporal parameters employed herein and in which MR deletion blocked the ability of acute CORT to increase BLA excitability (Karst et al., 2010). Different behavioral requirements for MR in males and females has been demonstrated for flexibility of fear extinction, with postnatal forebrain deletion of MR causing inflexible fear behavior in females but not males, such that female mice were unable to extinguish learned freezing behavior and instead showed generalization of freezing in the conditioning chamber even in the absence of the shock cue (Ter Horst et al., 2012). Future investigations will determine the involvement of MR vs. GR in the current treatment effects, as well as whether sex differences in MR and GR levels might contribute to the observed sexual dimorphism in electrophysiological efficacy of CORT.
In addition to the novel findings of sexual dimorphism in CORT modulation of the BLA-CeA circuit, the current results also extend previous findings from our laboratory regarding ethanol’s acute and chronic effects on glutamatergic postsynaptic responses in the medial CeA (Roberto et al., 2006; Roberto et al., 2004) in several ways. First, we have now shown that, in males, CeM and CeL neurons display similar reductions in glutamatergic postsynaptic responses following acute ethanol, acute CORT, and their co-application. Thus, CeM and CeL neurons are qualitatively similar in their pharmacological response to these treatments. Second, we have expanded greatly our understanding of ethanol’s effects on glutamatergic responses in CeA neurons to include direct BLA stimulation, which generates qualitatively similar effects as previously observed with local stimulation in the CeA. Finally and most importantly, we have studied females.
A key unexpected discovery in extending our work to females was the observation that ethanol increased EPSPs in all CeM neurons recorded during proestrus. This elevated EPSP magnitude suggests that cycling hormones may temporarily adjust postsynaptic glutamatergic receptor composition or signaling. While the effect of ovarian hormones on the synaptic composition of CeA neurons is not known, estrogen has been shown to enhance NMDA receptor-mediated activity and increase spine formation in hippocampal neurons (Woolley et al., 1997). This is proposed to involve increased expression of GluN2B subunits at synapses, although total protein expression or phosphorylation levels are not altered in hippocampal neurons by estrogen treatment (Snyder et al., 2011). While these hippocampal data suggest estrogen-induced alteration in NMDA receptor function might underlie the excitations by acute ethanol observed during proestrus in CeM neurons, it is of great interest for future studies to elucidate the molecular underpinnings of this and other sex differences described in the current studies.
5. Conclusions
The studies described herein demonstrate sex differences in neuronal responses to acute ethanol and acute corticosterone, one molecular component of the stress response. Our data suggest heightened corticosterone responsiveness in female CeL neurons, a possible source for enhanced female susceptibility to stress-related mental illnesses. Conversely, male neurons displayed greater inhibition by ethanol, which could contribute to the higher propensity towards alcohol use disorders in males vs. females. Overall, the data outline a role for the CeA as a locus for interaction between stress and alcohol, showing sexually dimorphic modulation of neuronal activity by these two treatments. These studies not only highlight intrinsic sex differences in the BLA-CeA circuit which is central to both anxiety and substance use disorders, but they also emphasize the need to identify how male and female brains differentially adapt to drug exposures in order to better design novel treatments for stress and alcohol use disorders.
HIGHLIGHTS.
Male CeA neurons are more sensitive to inhibition by ethanol than female neurons
Estrous cycle affects ethanol response: ethanol excites CeM neurons in proestrus
Female CeL neurons are inhibited by CORT, occluding ethanol’s effects
Female CeM neurons are insensitive to CORT and alcohol co-application
Acknowledgments
This research was supported by National Institutes of Health grants K99/R00 AA021802 (MLL) and P60 AA006420, AA015566, U01 AA013498 and AA017447 (MR) from the National Institute on Alcohol Abuse and Alcoholism. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Institute of Alcohol Abuse and Alcoholism. This is research publication number 29396 from The Scripps Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggleton JP, editor. The Amygdala: A Functional Analysis. Oxford University Press; 2000. [Google Scholar]
- Almeida OF, Shoaib M, Deicke J, Fischer D, Darwish MH, Patchev VK. Gender differences in ethanol preference and ingestion in rats. The role of the gonadal steroid environment. J Clin Invest. 1998;101:2677–2685. doi: 10.1172/JCI1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson HC, Waddell BJ. Circadian variation in basal plasma corticosterone and adrenocorticotropin in the rat: sexual dimorphism and changes across the estrous cycle. Endocrinology. 1997;138:3842–3848. doi: 10.1210/endo.138.9.5395. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Freedman LJ, Shi C. The intrinsic organization of the central extended amygdala. Ann N Y Acad Sci. 1999;877:217–241. doi: 10.1111/j.1749-6632.1999.tb09270.x. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Gray TS. Morphology of peptide-immunoreactive neurons in the rat central nucleus of the amygdala. J Comp Neurol. 1989;281:320–333. doi: 10.1002/cne.902810212. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Gray TS, Kiss JZ. Neuronal architecture in the rat central nucleus of the amygdala: a cytological, hodological, and immunocytochemical study. J Comp Neurol. 1986;246:478–499. doi: 10.1002/cne.902460406. [DOI] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, Muller C, Luthi A. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468:277–282. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Pare D. Glucocorticoids enhance the excitability of principal basolateral amygdala neurons. J Neurosci. 2007;27:4482–4491. doi: 10.1523/JNEUROSCI.0680-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou D, Hansson AC, Weiss F, Terasmaa A, Sommer WH, Cippitelli A, Fedeli A, Martin-Fardon R, Massi M, Ciccocioppo R, Heilig M. Dysregulation of nociceptin/orphanin FQ activity in the amygdala is linked to excessive alcohol drinking in the rat. Biol Psychiatry. 2008;64:211–218. doi: 10.1016/j.biopsych.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster KL, McKay PF, Seyoum R, Milbourne D, Yin W, Sarma PV, Cook JM, June HL. GABA(A) and opioid receptors of the central nucleus of the amygdala selectively regulate ethanol-maintained behaviors. Neuropsychopharmacology. 2004;29:269–284. doi: 10.1038/sj.npp.1300306. [DOI] [PubMed] [Google Scholar]
- Freeman ME. Neuroendocrine Control of the Ovarian Cycle of the Rat. In: Knobil E, Neill JD, editors. Knobil and Neill’s physiology of reproduction. 3. Academic Press; St. Louis, MO: 2006. pp. 2327–2388. [Google Scholar]
- Funk CK, O’Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Misra K, Herman MA, Cruz MT, Koob GF, Roberto M. Neuropeptide Y opposes alcohol effects on gamma-aminobutyric acid release in amygdala and blocks the transition to alcohol dependence. Biol Psychiatry. 2011;69:1091–1099. doi: 10.1016/j.biopsych.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi S, Frondaroli A, Scarduzio M, Dieni CV, Brecchia G, Boiti C, Pettorossi VE. Influence of sex and estrous cycle on synaptic responses of the medial vestibular nuclei in rats: role of circulating 17beta-estradiol. Brain Res Bull. 2012;87:319–327. doi: 10.1016/j.brainresbull.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Hartwell EE, Ray LA. Sex moderates stress reactivity in heavy drinkers. Addict Behav. 2013;38:2643–2646. doi: 10.1016/j.addbeh.2013.06.016. [DOI] [PubMed] [Google Scholar]
- Heffner JL, Blom TJ, Anthenelli RM. Gender differences in trauma history and symptoms as predictors of relapse to alcohol and drug use. Am J Addict. 2011;20:307–311. doi: 10.1111/j.1521-0391.2011.00141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Varodayan FP, Oleata CS, Luu G, Kirson D, Heilig M, Ciccocioppo R, Roberto M. Glutamatergic transmission in the central nucleus of the amygdala is selectively altered in Marchigian Sardinian alcohol-preferring rats: Alcohol and CRF effects. Neuropharmacology. 2016;102:21–31. doi: 10.1016/j.neuropharm.2015.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature. 2015;517:284–292. doi: 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallupi M, Varodayan FP, Oleata CS, Correia D, Luu G, Roberto M. Nociceptin/orphanin FQ decreases glutamate transmission and blocks ethanol-induced effects in the central amygdala of naive and ethanol-dependent rats. Neuropsychopharmacology. 2014;39:1081–1092. doi: 10.1038/npp.2013.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst H, Berger S, Erdmann G, Schutz G, Joels M. Metaplasticity of amygdalar responses to the stress hormone corticosterone. Proc Natl Acad Sci U S A. 2010;107:14449–14454. doi: 10.1073/pnas.0914381107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lack AK, Diaz MR, Chappell A, DuBois DW, McCool BA. Chronic ethanol and withdrawal differentially modulate pre- and postsynaptic function at glutamatergic synapses in rat basolateral amygdala. J Neurophysiol. 2007;98:3185–3196. doi: 10.1152/jn.00189.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehavot K, Stappenbeck CA, Luterek JA, Kaysen D, Simpson TL. Gender differences in relationships among PTSD severity, drinking motives, and alcohol use in a comorbid alcohol dependence and PTSD sample. Psychol Addict Behav. 2014;28:42–52. doi: 10.1037/a0032266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean CP, Asnaani A, Litz BT, Hofmann SG. Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J Psychiatr Res. 2011;45:1027–1035. doi: 10.1016/j.jpsychires.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie KM, Rivier C. Effect of alcohol on the proestrous surge of luteinizing hormone (LH) and the activation of LH-releasing hormone (LHRH) neurons in the female rat. J Neurosci. 1997;17:2595–2604. doi: 10.1523/JNEUROSCI.17-07-02595.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C. Female rats release more corticosterone than males in response to alcohol: influence of circulating sex steroids and possible consequences for blood alcohol levels. Alcohol Clin Exp Res. 1993;17:854–859. doi: 10.1111/j.1530-0277.1993.tb00853.x. [DOI] [PubMed] [Google Scholar]
- Roberto M, Bajo M, Crawford E, Madamba SG, Siggins GR. Chronic ethanol exposure and protracted abstinence alter NMDA receptors in central amygdala. Neuropsychopharmacology. 2006;31:988–996. doi: 10.1038/sj.npp.1300840. [DOI] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci U S A. 2003;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Schweitzer P, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Acute and chronic ethanol alter glutamatergic transmission in rat central amygdala: an in vitro and in vivo analysis. J Neurosci. 2004;24:1594–1603. doi: 10.1523/JNEUROSCI.5077-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarabdjitsingh RA, Kofink D, Karst H, de Kloet ER, Joels M. Stress-induced enhancement of mouse amygdalar synaptic plasticity depends on glucocorticoid and ss-adrenergic activity. PLoS One. 2012;7:e42143. doi: 10.1371/journal.pone.0042143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiess MC, Joels M, Shinnick-Gallagher P. Estrogen priming affects active membrane properties of medial amygdala neurons. Brain Res. 1988;440:380–385. doi: 10.1016/0006-8993(88)91012-8. [DOI] [PubMed] [Google Scholar]
- Seo D, Sinha R. The neurobiology of alcohol craving and relapse. Handb Clin Neurol. 2014;125:355–368. doi: 10.1016/B978-0-444-62619-6.00021-5. [DOI] [PubMed] [Google Scholar]
- Silberman Y, Shi L, Brunso-Bechtold JK, Weiner JL. Distinct mechanisms of ethanol potentiation of local and paracapsular GABAergic synapses in the rat basolateral amygdala. J Pharmacol Exp Ther. 2008;324:251–260. doi: 10.1124/jpet.107.128728. [DOI] [PubMed] [Google Scholar]
- Smejkalova T, Woolley CS. Estradiol acutely potentiates hippocampal excitatory synaptic transmission through a presynaptic mechanism. J Neurosci. 2010;30:16137–16148. doi: 10.1523/JNEUROSCI.4161-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder MA, Cooke BM, Woolley CS. Estradiol potentiation of NR2B-dependent EPSCs is not due to changes in NR2B protein expression or phosphorylation. Hippocampus. 2011;21:398–408. doi: 10.1002/hipo.20756. [DOI] [PubMed] [Google Scholar]
- Sonne SC, Back SE, Diaz Zuniga C, Randall CL, Brady KT. Gender differences in individuals with comorbid alcohol dependence and post-traumatic stress disorder. Am J Addict. 2003;12:412–423. [PubMed] [Google Scholar]
- Ter Horst JP, Carobrez AP, van der Mark MH, de Kloet ER, Oitzl MS. Sex differences in fear memory and extinction of mice with forebrain-specific disruption of the mineralocorticoid receptor. Eur J Neurosci. 2012;36:3096–3102. doi: 10.1111/j.1460-9568.2012.08237.x. [DOI] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Weiland NG, McEwen BS, Schwartzkroin PA. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J Neurosci. 1997;17:1848–1859. doi: 10.1523/JNEUROSCI.17-05-01848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Global status report on alcohol and health. 2014. [Google Scholar]
- Zhu PJ, Lovinger DM. Ethanol potentiates GABAergic synaptic transmission in a postsynaptic neuron/synaptic bouton preparation from basolateral amygdala. J Neurophysiol. 2006;96:433–441. doi: 10.1152/jn.01380.2005. [DOI] [PubMed] [Google Scholar]